Abstract
The role of the pro-inflammatory cytokine interleukin-1β (IL-1β) in the mechanism of cell death induced by the human immunodeficiency virus type 1 (HIV-1) recombinant coat glycoprotein, gp120 IIIB, has been studied in the human CHP100 neuroblastoma cell line maintained in culture.
Death of neuroblastoma cells typically elicited by 10 pM gp120 or by human recombinant IL-1β (10 ng ml−1) has been minimized by the antagonist of IL-1 receptor, i.e. IL-1ra (0.5 and 50 ng ml−1, respectively), an endogenous molecule that antagonizes most of the biological actions of IL-1β, or by an antibody (5 and 50 ng ml−1) which blocks the human IL-1 receptor type I (IL-1RI).
ELISA experiments have established that gp120 enhances immunoreactive IL-1β levels in the culture medium and this is prevented by exposure to the IL-1 converting enzyme (ICE) inhibitor t-butoxycarbonyl-L-aspartic acid benzyl ester-chloromethylketone [Boc-Asp(OBzl)-CMK] used at a concentration (2.5 μM) which significantly (P<0.001) reduces cell death.
Death of CHP100 cells induced by gp120 is also prevented by acetyl-Tyr-Val-Ala-Asp-chloromethylketone (Ac-YVAD-CMK; 10 – 100 μM), a second inhibitor of ICE, supporting the concept that the viral protein stimulates the conversion of the 31 kDa pro-IL-1β in to the 17 kDa mature cytokine which is then secreted to cause death.
In conclusion, our present data demonstrate that gp120 stimulates the secretion of IL-1β which then triggers CHP100 neuroblastoma cell death via stimulation of IL-1 receptor type I.
Keywords: HIV-1 recombinant gp120 IIIB, CHP100 neuroblastoma cells, IL-1β secretion, ICE inhibitors, Ac-YVAD-CMK, Boc-Asp(OBzl)-CMK, IL-1ra, IL-1 receptor type I, cell death
Introduction
Some 30% of patients suffering from AIDS develops a neurological syndrome, referred to as AIDS dementia complex, characterized by cognitive impairment, postural disorders and tremor which can culminate in paralysis (Price et al., 1988). Loss of cortical neurones has been described at post mortem in the brain of AIDS patients (Everall et al., 1991) and the human immunodeficiency virus type 1 (HIV-1) coat glycoprotein gp120 has been implicated in the mechanisms of the observed brain neuronal loss because it causes death of several types of neurones maintained in culture. In fact, in vitro exposure to gp120 causes death of rodent cortical (Dawson et al., 1993) and hippocampal (Dreyer et al., 1990) neurones, retinal ganglion cells (Lipton et al., 1991), cerebellar granule cells (Savio & Levi, 1993) and human CHP100 neuroblastoma cells (Corasaniti et al., 1995). The mechanism underlying cell death involves excessive Ca2+ entry into neurones via N-methyl-D-aspartate (NMDA) receptor associated cation channel and through voltage operated Ca2+ channels since NMDA antagonists and Ca2+ channels blockers prevent both the rise in the intracellular level of the cation and the cytotoxicity (Dreyer et al., 1990; Lipton et al., 1991) suggesting an excitotoxic, glutamate-mediated, type of death (see Choi, 1988). In agreement with the latter hypothesis, death of human CHP100 neuroblastoma cells induced by gp120 is prevented by selective, competitive (CGP37849 and LY274614) and non-competitive (MK801, 7-chlorokinurenic acid) antagonists of the NMDA receptor complex, by the dihydropyridine, nicardipine, an L-type Ca2+ channel blocker, and by removal of calcium ions from the culture medium (Corasaniti et al., 1995).
Several experimental data demonstrate that excitotoxic stimuli increase the expression of the pro-inflammatory cytokine, IL-1β, and this has been implicated in neuronal damage (see Rothwell, 1999). In particular, enhanced expression of IL-1β mRNA has been reported in the brain of rats undergoing transient forebrain ischaemia (Minami et al., 1992) and of rats receiving systemic administration of chemical convulsants, such as kainic acid and pentylenetetrazole (Minami et al., 1990). Furthermore, focal intracerebral injection of NMDA, given at a dose which causes neuronal cell death, has been shown to enhance IL-1β expression in the brain of perinatal rats and this is prevented by a neuroprotective dose of the NMDA receptor antagonist MK801 (Hagan et al., 1996). In addition, intrastriatal injection of human recombinant IL-1β exacerbates brain damage caused by ischaemia in the adult rat (Stroemer & Rothwell, 1998). Further support for a role of IL-1β in excitotoxic neuronal damage stems from the evidence that inhibition of its biological actions by the naturally occurring IL-1 receptor antagonist (IL-1ra) reduces ischaemic brain damage (Relton & Rothwell, 1992; Loddick & Rothwell, 1996) and damage caused by brain trauma (Toulmond & Rothwell, 1995), excitotoxins (Relton & Rothwell, 1992) and by hypoxia-ischaemia insults (Martin et al., 1994). More recently, it has been reported that brain ischaemia induces the expression of IL-1ra mRNA and inhibition of its biological action markedly enhances ischaemic brain damage (Loddick et al., 1997). Altogether, these data strongly implicate abnormal expression of IL-1β in the mechanisms underlying neuronal cell death caused by excitotoxic stimuli. Here we now report experimental evidence demonstrating that the HIV-1 coat protein gp120 stimulates the secretion of IL-1β from neuroblastoma cells and this contributes to the mechanisms of cytotoxicity elicited by the viral coat protein because pharmacological manipulations reducing the biological actions of IL-1β prevent death of human CHP100 neuroblastoma cells.
Methods
Materials
Lyophilized, full-length glycosylated recombinant HIV-1 gp120 IIIB (>90% pure as by SDS – PAGE; tested randomly for endotoxin contamination), was from Intracel (London, U.K., Catalogue no 120011). Human recombinant IL-1β (hrIL-1β; biological activity, given as ED50 in a murine T cell line proliferation assay, is 5 – 10 pg ml−1 and endotoxin content is <0.1 ng μg−1 of cytokine) and the anti-human IL-1 RI neutralizing antibody (lot no MU03; cross-reactivity with rhIL-1 RII is 5 – 10%) were from R&D Systems (Milan, Italy). IL-1ra was a kind gift from Dr D. Boraschi (Dompè, Italy). ELISA reagents were from Endogen (MA, U.S.A.): coating antibody (mouse monoclonal anti-human IL-1β) specific for natural and recombinant human IL-1β (clone ILB1-H67); mouse monoclonal biotin-labelled anti-human IL-1β specific for natural and recombinant human IL-1β (clone ILB1-H6). The ICE inhibitors acetyl-Tyr-Val-Ala-Asp-chloromethylketone (Ac-YVAD-CMK) and t-butoxycarbonyl-L-aspartic acid benzyl ester-chloromethylketone [(Boc-Asp(OBzl)-CMK)] were from Bachem (Bubendorf, Switzerland).
Cell cultures and treatments
Human CHP100 neuroblastoma cells were cultured as previously described (Corasaniti et al., 1992) in a 1 : 1 mixture of MEM (Eagle's minimal essential medium with Earle's salts) and Ham's F-12 media, supplemented with 10% heat-inactivated foetal bovine serum, at 37°C in a 5% CO2 atmosphere. Cells were subcultured from confluent 75 cm2 flasks and seeded in 35 mm 6-well plates. Twenty-four hours after plating, the growth medium was replaced with fresh normal medium (control cultures) or with medium supplemented with gp120; exposure to gp120 was carried out for 24 h. The viral protein was used at 10 pM as this concentration has been shown to induce significant cytotoxic effects in CHP100 cells (Corasaniti et al., 1995; 1996; 1998; Maccarrone et al., 1998). In experiments involving IL-1ra, Ac-YVAD-CMK or Boc-Asp(OBzl)-CMK and the anti-human IL-1 RI neutralizing antibody, these were applied to CHP100 cells 30 min or 1 h before, respectively, the addition of the viral protein and they were present during the 24 h exposure time. Cell viability was assessed by cell exclusion of trypan blue (0.4% w v−1) and cell death was reported as the percentage of stained (non viable) vs total cells counted (Corasaniti et al., 1995; 1996; 1998; Maccarrone et al., 1998). The data were expressed as mean±s.e.mean percentage cell death.
IL-1β ELISA
For the determination of intracellular IL-1β concentrations, cells, treated as described above, were resuspended in phosphate buffer saline (PBS; 0.01 M phosphate buffer, 0.0027 M KC1, 0.137 M NaCl, pH 7.4) containing a cocktail of protease inhibitors (Sigma, Milan, Italy) and subjected to three freeze-thaw cycles, then the lysates were sonified and centrifuged at 30,000×g for 30 min to remove insoluble material. The resulting supernatants were assayed for protein content (Bio-Rad DC Protein Assay, Bio-Rad Laboratories, Milan, Italy) and employed for the determination of IL-1β levels by ELISA. Measurement of secreted IL-1β was carried out using aliquots of culture medium from control and treated cultures following centrifugation at 30,000×g for 20 min to sediment cell debris. The mouse monoclonal anti-human IL-1β coating antibody (1 μg ml−1), the anti-human IL-1β mouse monoclonal biotin-labelled antibody (0.05 μg ml−1) as well as the human recombinant IL-1β used to prepare the standard curve (0.0 – 128 pg ml−1) were from Endogen (MA, U.S.A.). Poly-horseradish peroxidase-conjugated streptavidin (CLB, Amsterdam, Holland) was used at 1 : 5000 dilution and the colour developed by using the chromogen o-phenylenediamine (Sigma, Milan, Italy). Optical densities were read at 492 nm by using an automated plate reader (Multiscan MS, Labsystems) and cytokine levels calculated by interpolation from the standard curve (0.0 – 128 pg ml−1). Data were corrected for protein concentration of the sample and the values expressed as picograms of IL-1β mg−1 of protein.
Statistical analysis
Data are expressed as mean±s.e.mean and the resulting means evaluated statistically for differences using one-way analysis of variance (ANOVA) followed by Tukey – Kramer multiple comparison test. Values of P<0.05 were considered significant.
Results
Blockade of IL-1 receptors prevents HIV-1 gp120-induced cell death
To characterize the role of IL-1β in gp120-induced cell death this has been studied in human CHP100 neuroblastoma cell cultures exposed to the IL-1 receptor antagonist (IL-1ra), an endogenous protein which binds IL-1 receptors and prevents most of the biological effects of the cytokine (Hannum et al., 1990). The experiments were carried out by incubation with the viral protein at a concentration (10 pM) known to produce significant cytotoxic effects in human CHP100 cultures (Corasaniti et al., 1995; 1996; 1998; Maccarrone et al., 1998) and complete dose-effect relations are reported elsewhere (Corasaniti et al., 1995). Under these experimental conditions, exposure to 10 pM gp120 (n=8 experiments) yielded a 22.4±1.25% cell death (P<0.001 vs control cell death; n=8 experiments) (Figure 1). As shown in Figure 1, IL-1ra (0.5 – 500 ng ml−1) significantly reduced cell death elicited by the viral coat protein. In particular, an approximately 80% (80.6±9.3%; n=4 experiments) inhibition of gp120-induced cell death is produced by IL-1ra applied at a concentration of 0.5 ng ml−1; no further protection is provided by higher concentrations of the antagonist (5 – 500 ng ml−1; n=4 – 8 experiments per concentration) whereas at 0.05 ng ml−1 (n=4 experiments) IL-1ra was ineffective against gp120-induced cell death (Figure 1). IL-1ra given alone (0.05 – 500 ng ml−1; n=4 experiments per concentration) does not affect the viability of CHP100 cultures (Figure 1).
Figure 1.
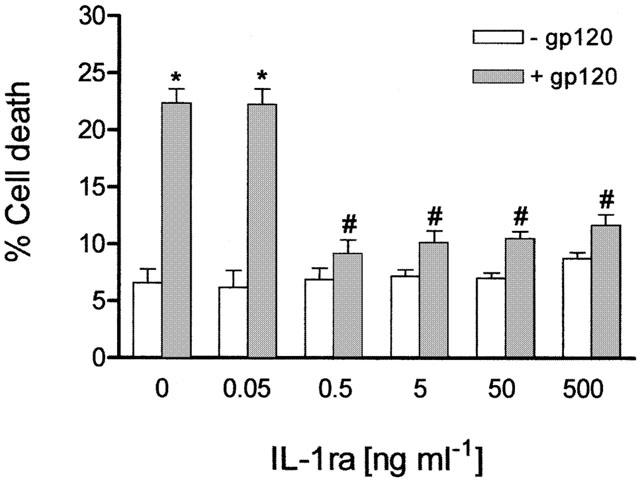
Inhibition of HIV-1 gp120-induced death of human CHP100 neuroblastoma cells by IL-1 receptor antagonist. Incubation of neuroblastoma cells with 10 pM gp120 for 24 h in the presence of IL-1 receptor antagonist (IL-1ra; 0.5 – 500 ng ml−1) prevents the cell death induced by the viral protein. A lower concentration of IL-1ra (0.05 ng ml−1) is ineffective. Each value is the mean±s.e.mean of 4 – 8 experiments. *Denotes P<0.001 vs control cell death; # denotes P<0.001 vs cell death produced by gp120 given alone (one-way ANOVA followed by Tukey – Kramer multiple comparison test).
Prevention of gp120 (10 pM)-induced cell death is also afforded by an antibody which blocks the human IL-1 receptor type I (IL-1 RI) (Figure 2). This neutralizing antibody is effective at concentrations of 5 and 50 ng ml−1 (n=4 – 6 experiments per concentration) whereas no prevention of gp120-induced cell death is conferred by a lower concentration (0.05 ng ml−1; n=6 experiments) of the antibody (Figure 2). As shown in Figure 2, the anti-IL-1 RI neutralizing antibody given alone does not affect CHP100 viability (P>0.05 vs control cell death).
Figure 2.
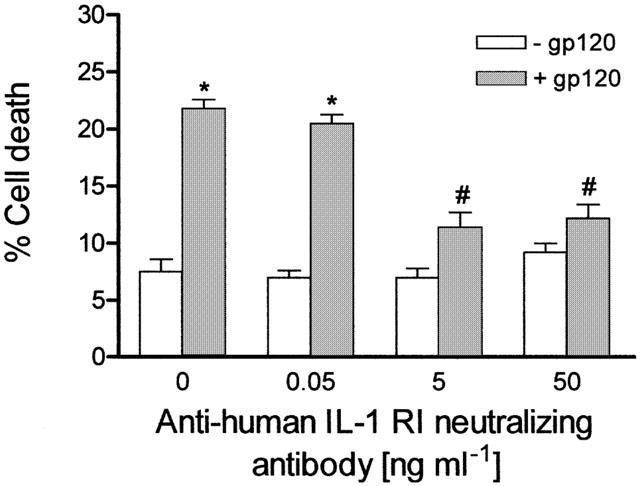
Blockade of IL-1 receptor type I (IL-1 RI) protects against HIV-1 gp120-induced death of human CHP100 neuroblastoma cells. Incubation of neuroblastoma cells with 10 pM gp120 for 24 h in the presence of an anti-human IL-1 RI neutralizing antibody (5 and 50 ng ml−1) prevents the cell death induced by the viral protein. Each value is the mean±s.e.mean of 4 – 8 experiments. *Denotes P<0.001 vs cell death of untreated, control, cultures; # denotes P<0.001 vs gp120 given alone (one-way ANOVA followed by Tukey – Kramer multiple comparison test).
Recombinant IL-1β induces death of CHP100 cells and this is reduced by IL-1ra
To further evaluate the cytotoxic potential of IL-1β in CHP100 cultures, these have been exposed to human recombinant IL-1β (hrIL-1β) for 24 h alone or in the presence of IL-1ra. As reported in Figure 3, hrIL-1β (10 ng ml−1; n=4 experiments) has cytotoxic effects (P<0.001 vs control) that are significantly (P<0.01) reduced by IL-1ra (50 ng ml−1; n=4 experiments). A lower concentration (0.1 ng ml−1; n=4 experiments) of the human recombinant cytokine does not affect cell viability (P>0.05 vs control; Figure 3).
Figure 3.
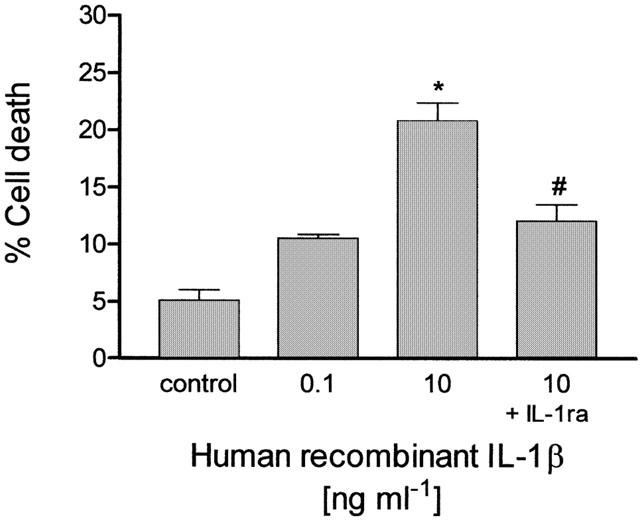
Human recombinant IL-1β (hrIL-1β) induces death of human CHP100 neuroblastoma cells and this is reduced by IL-1 receptor antagonist. Exposure of neuroblastoma cultures to hrIL-1β (10 ng ml−1) for 24 h induces significant cytotoxic effects, as estimated by trypan blue uptake assay. At a lower concentration (0.1 ng ml−1), hrIL-1β does not affect significantly CHP100 cell viability (P>0.05 vs control cell death). The cell death induced by 10 ng ml−1 hrIL-1β is significantly (P<0.01) reduced by IL-1 receptor antagonist (IL-1 ra; 50 ng ml−1). Each value is the mean±s.e.m. of four experiments. *Denotes P<0.001 vs control cell death; # denotes P<0.01 vs cell death produced by 10 ng ml−1 hrIL-1β given alone (one-way ANOVA followed by Tukey – Kramer multiple comparison test).
HIV-1 gp120 enhances the secretion of IL-1β from CHP100 cells
The finding that hrIL-1β likewise gp120 causes neuroblastoma cell death and that the latter is minimized by blockade of cell surface IL-1β receptors strongly supports the deduction that the viral protein stimulates the secretion of IL-1β from CHP100 cells. To test the latter hypothesis, measurement of IL-1β levels by ELISA was carried out in cellular lysates and in the extracellular medium of control- and gp120-treated cultures. In comparison with untreated, control, cultures treatment with 10 pM gp120 significantly (P<0.05) increases IL-1β levels in the culture supernatant (from 18.0±4.8 to 39.9±5.9 pg mg−1 of protein; n=5 experiments) but does not affect intracellular content of the cytokine (3.37±0.77 and 3.49±0.59 pg mg−1 of protein, respectively; n=5 experiments). Thus, exposure to gp120 stimulates CHP100 neuroblastoma cells to produce and release IL-1β.
Inhibition of ICE minimizes gp120-induced cell death via reduction of secreted IL-1β
IL-1β is synthesized as a 31 kDa inactive precursor, pro-IL-1β, that to yield the mature, 17 kDa biologically active form of the cytokine has to be cleaved by IL-1 converting enzyme (ICE) (Black et al., 1988; Thornberry et al., 1992), also known as caspase-1, a member of the caspase family of proteases that are known to be implicated in cell death (Yuan & Yankner, 2000). To assess whether blockade of IL-1β production from its inactive precursor could affect the induction of cell death by gp120, we examined the ability of Ac-YVAD-CMK, an irreversible inhibitor of ICE (see Milligan et al., 1995), to prevent CHP100 neuroblastoma cell death caused by the viral protein. Incubation of CHP100 cells with 10 pM gp120 for 24 h in the presence of Ac-YVAD-CMK (10 and 100 μM; n=5 – 7 experiments per concentration) significantly (P<0.001) reduces the per cent cell death elicited by the viral protein (Figure 4); a lower concentration (1 μM; n=4 experiments) of Ac-YVAD-CMK resulted ineffective (Figure 4). At concentrations of 10 and 100 μM, Ac-YVAD-CMK given alone (n=4 experiments per concentration) induces a slight but statistically significant (P<0.05 and P<0.01, respectively) increase over control cell death, a finding this that may explain why this enzyme inhibitor reduces but does not prevent cell death induced by gp120.
Figure 4.
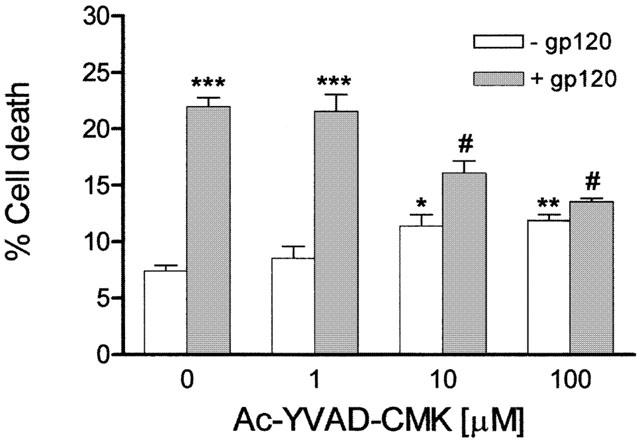
The inhibitor of ICE, Ac-YVAD-CMK, reduces gp120-induced death of human CHP100 neuroblastoma cells. Exposure of CHP100 cells to 10 pM gp120 for 24 h in the presence of Ac-YVAD-CMK (10 and 100 μM) significantly reduces the cell death caused by the viral protein. No protection is afforded by the enzyme inhibitor applied at a lower concentration (1 μM). Each value is the mean±s.e.mean of 4 – 6 experiments. *,** and ***denote P<0.05, P<0.01 and P<0.001 vs control cell death, respectively; # denotes P<0.001 vs control cell death and P<0.001 vs cell death induced by gp120 given alone (one-way ANOVA followed by Tukey – Kramer multiple comparison test).
Protection from gp120-induced cell death was also afforded by another inhibitor of ICE, Boc-Asp(OBzl)-CMK (Boudreau et al., 1995). In particular, a significant (P<0.001) reduction of gp120-induced death was provided by Boc-Asp(OBbzl)-CMK applied at a concentration of 2.5 μM (n=8 experiments; Figure 5), whereas at a lower concentration (0.25 μM, n=5 experiments) the ICE inhibitor was ineffective. As illustrated in Figure 5, the ICE inhibitor given alone (0.25 and 2.5 μM; n=5 experiments per concentration) does not affect cell viability.
Figure 5.
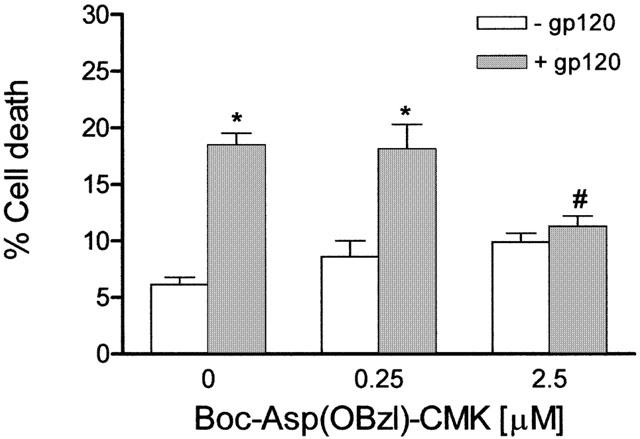
The inhibitor of ICE, Boc-Asp(OBzl)-CMK, reduces death of human CHP100 neuroblastoma cells induced by HIV-1 coat protein gp120. Incubation of CHP100 cells with 10 pM gp120 for 24 h in the presence of Boc-Asp(OBzl)-CMK (2.5 μM), significantly reduces death caused by the viral protein. No protection is afforded by the enzyme inhibitor applied at a lower concentration (0.25 μM). Each value is the mean±s.e.mean of 5 – 8 experiments. *Denotes P<0.001 vs control cell death; # denotes P<0.001 vs cell death induced by gp120 given alone (one-way ANOVA followed by Tukey – Kramer multiple comparison test).
To strengthen the concept that protection afforded by Boc-Asp(OBzl)-CMK relates to inhibition of ICE and consequent reduction of the secreted, mature cytokine, IL-1β expression was studied in gp120-treated cells and in cells exposed to the viral protein in the presence of a concentration (2.5 μM) of the ICE inhibitor known to rescue neuroblastoma cells from gp120-induced cytotoxicity. As shown in Figure 6, exposure of CHP100 cultures to Boc-Asp(OBzl)-CMK (n=5 experiments) prevents gp120-stimulated release of IL-1β.
Figure 6.
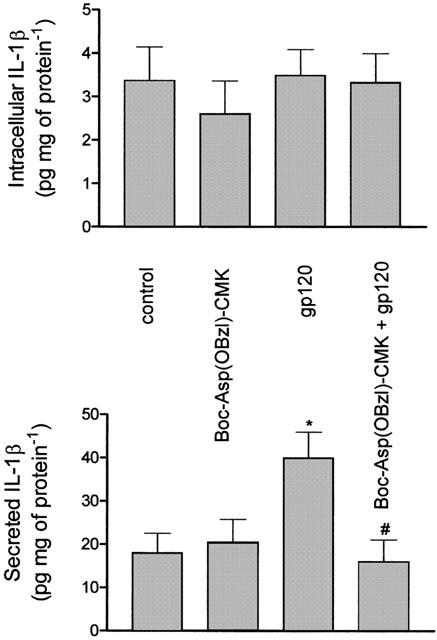
The inhibitor of ICE, Boc-Asp(OBzl)-CMK, prevents gp120-stimulated release of IL-1β in human CHP100 neuroblastoma cells. CHP100 cell cultures were incubated with 10 pM gp120 for 24 h alone or in the presence of Boc-Asp(OBzl)-CMK applied at a concentration (2.5 μM) able to rescue neuroblastoma cells from death caused by the viral protein. At the end of the incubation time, IL-1β levels were measured by ELISA in cell lysates (intracellular IL-1β) and in the corresponding culture medium (secreted IL-1β) (see Methods). Under these conditions, the inhibitor of ICE prevents the enhancement of IL-1β release induced by gp120. Each value is the mean±s.e.mean of five experiments. *Denotes P<0.05 vs control; # denotes P<0.05 vs gp120 given alone (one-way ANOVA followed by Tukey – Kramer multiple comparison test).
Discussion
Whilst the present experiments have confirmed that exposure of human CHP100 neuroblastoma cultures to the HIV-1 coat protein gp120 causes cell death (Corasaniti et al., 1995) they have established that the pro-inflammatory cytokine IL-1β is involved. In fact, a pretreatment with IL-1ra, a molecule which inhibits most of the biological actions of IL-1β (Dripps et al., 1991; Hagan et al., 1996; Hannum et al., 1990), or with a neutralizing antibody for IL-1 RI, the receptor that mediates IL-1 signalling (Sims et al., 1993), abolished the cytotoxic effects of the viral coat protein suggesting that CHP100 neuroblastoma cells exposed to gp120 secrete IL-1β. This hypothesis is confirmed by the results of ELISA experiments carried out on aliquots from the extracellular medium of neuroblastoma cell cultures exposed to gp120; under these experimental conditions, in fact, IL-1β immunoreactive levels were significantly enhanced by gp120 as compared to control. Incubation of CHP100 cells with inhibitors of group 1 caspases (see Alnemri et al., 1996), e.g. Ac-YVAD-CMK or Boc-Asp(OBzl)-CMK (Milligan et al., 1995; Boudreau et al., 1995; Nicholson, 2000), prevents cell death; interestingly, Boc-Asp(OBzl)-CMK also prevented IL-1β release elicited by the viral coat protein suggesting that cleavage of the 31 kDa inactive precursor, pro-IL-1β, to yield the mature, 17 kDa biologically active form of the cytokine (Black et al., 1988; Thornberry et al., 1992) is important. However, it cannot be excluded that inhibition of IL-1β release by the relatively low selective group 1 caspase inhibitor Boc-Asp(OBzl)-CMK may stem from reduced cell death attributable to blockade of other members of the caspase family of proteases (see Nicholson, 2000 for further discussion). Collectively, these data, together with the observation that human recombinant IL-1β causes death of neuroblastoma cells sensitive to the blockade of IL-1ra, suggest that gp120 activates an autocrine loop leading to secretion of IL-1β which then stimulates IL-1 RI to trigger human CHP100 neuroblastoma cell death.
Previous in vitro studies have shown that exposure to HIV-1 or to the viral coat protein, gp120, induces IL-1β production in blood monocytes (Wahl et al., 1989) and in brain microglia and/or astrocytes (Merril et al., 1992) and this has been implicated in the mechanisms of death of co-cultured neuronal cells (Genis et al., 1992). Interestingly, increased expression of IL-1β mRNA (Ilyin & Plata-Salaman, 1997) and gene product (Bagetta et al., 1999) have also been reported in the brain of adult rat following administration of gp120. In particular, double-labelling immunofluorescence experiments have established that neuronal as well as non-neuronal cells, very likely microglial cells, represent the main source for enhanced IL-1β expression which then is secreted in the extracellular space (Bagetta et al., 1999). These data in conjunction with the present demonstration that gp120 enhances IL-1β and causes cell death in a neuroectodermal tumour cell line, e.g. in the absence of other, contaminating, cell types, support the hypothesis that cells of the myelomonocytic lineage and neuronal cells are both important sources for the expression of the pro-inflammatory cytokine, IL-1β. In addition, it is conceivable that this cytokine might be the signal through which neurones contribute to recruitment and activation of immune cells and to their own demise.
The mechanism through which gp120 stimulates the secretion of IL-1β from CHP100 cells is not known; however, the observation that in P388D1 murine macrophage and human Jurkat T cell lines (Siders & Mizel, 1995; Siders et al., 1993) and in human activated monocytes (Rubartelli et al., 1990) the secretion of mature IL-1β is enhanced by calcium ionophores raises the possibility that elevation of intracellular calcium may play a role in the secretory process. We have previously reported that L-type voltage-dependent Ca2+ channel blockers and the NMDA receptor associated cation channel blocker, MK801, prevent gp120-induced neuroblastoma cell death (Corasaniti et al., 1995). More recently, we have established that, at least in part, gp120 causes death of CHP100 cells via activation of CXCR4 and CCR5 chemokine receptors which are constitutively expressed by this human neuroblastoma cell line (Catani et al., 2000) and, like gp120, are known to affect the regulation of Ca2+ signalling in neurones (Meucci et al., 1998). Therefore, it can be assumed that different cellular mechanisms contribute to increase [Ca2+]i which in turn enhances IL-1β availability in CHP100 cells exposed to the viral protein.
It is well established that in the mammalian brain IL-1β is endowed with neurotrophic as well as neurotoxic properties (see Strijbos & Rothwell, 1995). Under our experimental conditions, increased IL-1β availability has a clear neurotoxic significance though, the lack of a glial cell component in our culture system may conceivably limit the extent of cytotoxicity. Interestingly, increased IL-1β levels have been reported in the CNS of patients affected by AIDS dementia complex (Perrella et al., 1992; Tyor et al., 1992), Alzheimer disease and Down syndrome (Griffin et al., 1989) though its neuronal origin and pathophysiological role in these chronic neurodegenerative diseases remain to be established.
Acknowledgments
Partial finantial support from the MURST-DAE ‘Cofin 1998', the II National Programme on AIDS, Istituto Superiore di Sanità, Rome (Italy) and The European Fund for Regional Development (Calabria Region, P.O.P. 1994/99) is gratefully acknowledged.
Abbreviations
- Ac-YVAD-CMK
acetyl-Tyr-Val-Ala-Asp-chloromethylketone
- Boc-Asp(OBzl)-CMK
t-butoxycarbonyl-L-aspartic acid benzyl ester-chloromethylketone
- HIV-1
human immunodeficiency virus type 1
- ICE
Interleukin-1 converting enzyme
- IL-1β
interleukin-1β
- IL-1ra
IL-1 receptor antagonist
- IL-1 RI
IL-1 receptor type I
References
- ALNEMRI E.S., LIVINGSTON D.J., NICHOLSON D.W., SALVESEN G., THORNBERRY N.A., WONG W.W., YUAN J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- BAGETTA G., CORASANITI M.T., BERLIOCCHI L., NISTICÒ R., GIAMMARIOLI A.M., MALORNI W., ALOE L., FINAZZI-AGRÒ A. Involvement of interleukin-1β in the mechanism of human immunodeficiency virus type 1 (HIV-1) recombinant protein gp120-induced apoptosis in the neocortex of rat. Neuroscience. 1999;89:1051–1066. doi: 10.1016/s0306-4522(98)00363-7. [DOI] [PubMed] [Google Scholar]
- BLACK R.A., KRONHEIM S.R., CANTRELL M., DEELEY M.C., MARCH C.J., PRICKETT K.S., WIGNALL J., CONLON P.J., COSMAN D., HOPP T.P., MOCHIZUKI D.Y. Generation of biologically active interleukin-1 beta by proteolytic cleavage of the inactive precursor. J. Biol. Chem. 1988;263:9437–9442. [PubMed] [Google Scholar]
- BOUDREAU N., SYMPSON C.J., WERB Z., BISSELL M.J. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATANI M.V., CORASANITI M.T., NAVARRA M., NISTICÒ G., FINAZZI-AGRÒ A., MELINO G. gp120 induces cell death in human neuroblastoma cells through the CXCR4 and CCR5 chemokine receptors. J. Neurochem. 2000;74:2373–2379. doi: 10.1046/j.1471-4159.2000.0742373.x. [DOI] [PubMed] [Google Scholar]
- CHOI D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- CORASANITI M.T., MELINO G., NAVARRA M., GARACI E., FINAZZI-AGRÒ A., NISTICÒ G. Death of cultured human neuroblastoma cells induced by HIV-1 gp120 is prevented by NMDA receptor antagonists and inhibitors of nitric oxide and cyclooxygenase. Neurodegeneration. 1995;4:315–321. doi: 10.1016/1055-8330(95)90021-7. [DOI] [PubMed] [Google Scholar]
- CORASANITI M.T., NAVARRA M., CATANI M.V., MELINO G., NISTICÒ G., FINAZZI-AGRÒ A. NMDA and HIV-1 coat protein, gp120, produce necrotic but not apoptotic cell death in human CHP100 neuroblastoma cultures via a mechanism involving calpain. Biochem. Biophys. Res. Commun. 1996;229:299–304. doi: 10.1006/bbrc.1996.1796. [DOI] [PubMed] [Google Scholar]
- CORASANITI M.T., NAVARRA M., NISTICÒ S., ROTIROTI D., MACCARRONE M., MELINO G., FINAZZI-AGRÒ A. Requirement for membrane lipid peroxidation in HIV-1 gp120-induced neuroblastoma cell death. Biochem. Biophys. Res. Commun. 1998;246:686–689. doi: 10.1006/bbrc.1998.8687. [DOI] [PubMed] [Google Scholar]
- CORASANITI M.T., TARTAGLIA R.L., MELINO G., NISTICO' G., FINAZZI-AGRO' A. Evidence that CHP100 neuroblastoma cell death induced by N-methyl-D-aspartate involves L-arginine-nitric oxide pathway activation. Neurosci. Lett. 1992;147:221–223. doi: 10.1016/0304-3940(92)90600-c. [DOI] [PubMed] [Google Scholar]
- DAWSON V.L., DAWSON T.M., UHL G.R., SNYDER S.H. Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical cultures. Proc. Natl. Acad. Sci. U.S.A. 1993;90:3256–3259. doi: 10.1073/pnas.90.8.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREYER E.B., KAISER P.K., OFFERMANN J.T., LIPTON S.A. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- DRIPPS D.J., BRANDHUBER B.C., THOMPSON R.C., EISENBERG S.P. Interleukin-1 receptor antagonist binds to the 80 kDa interleukin-1 receptor, but does not initiate interleukin-1 signal transduction. J. Biol. Chem. 1991;266:10331–10336. [PubMed] [Google Scholar]
- EVERALL I.P., LUTHERT P.J., LANTOS P.L. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991;337:1119–1121. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- GENIS P., JETT M., BERNTON E.W., BOYLE T., GELBARD H.A., DZENKO K., KEANE R.W., RESNICK L., MIZRACHI Y., VOLSKY D., EPSTEIN L.G., GENDELMAN H.E. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interaction: implications for the neuropathogenesis of HIV disease. J. Exp. Med. 1992;176:1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN W.S., STANLEY L.C., LING C., WHITE L., MACLEOD V., PERROT L.J., WHITE C.L., ARAOZ C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGAN P., POOLE S., BRISTOW A.F., TILDERS F., SILVERSTEIN F.S. Intracerebral NMDA injection stimulates production of interleukin-1β in perinatal rat brain. J. Neurochem. 1996;67:2215–2218. doi: 10.1046/j.1471-4159.1996.67052215.x. [DOI] [PubMed] [Google Scholar]
- HANNUM C.H., WILCOX C.J., AREND W.P., JOSLIN F.G., DRIPPS D.J., HEIMDAL P.L., ARMES L.G., SOMMER A., EISENBERG S.P., THOMPSON R.C. Interleukin-1 receptor antagonist activity of a human interleukin-1 inhibitor. Nature. 1990;343:336–340. doi: 10.1038/343336a0. [DOI] [PubMed] [Google Scholar]
- ILYIN S.E., PLATA-SALAMAN C.R. HIV-1 envelope glycoprotein 120 regulates brain IL-1β system and TNF-α mRNAs in vivo. Brain Res. Bull. 1997;44:67–73. doi: 10.1016/s0361-9230(97)00091-9. [DOI] [PubMed] [Google Scholar]
- LIPTON S.A., SUCHER N.J., KAISER P.K., DREYER E.B. Synergistic effects of HIV-1 coat protein and NMDA receptor-mediated neurotoxicity. Neuron. 1991;7:111–118. doi: 10.1016/0896-6273(91)90079-f. [DOI] [PubMed] [Google Scholar]
- LODDICK S.A., ROTHWELL N.J. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J. Cereb. Blood Flow. Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- LODDICK S.A., WONG MA-LI., BONGIORNO P.B., GOLD P.W., LICINIO J., ROTHWELL N.J. Endogenous interleukin-1 receptor antagonist is neuroprotective. Biochem. Biophys. Res. Commun. 1997;234:211–215. doi: 10.1006/bbrc.1997.6436. [DOI] [PubMed] [Google Scholar]
- MACCARRONE M., NAVARRA M., CORASANITI M.T., NISTICÒ G., FINAZZI-AGRÒ A. Cytotoxic effect of HIV-1 coat glycoprotein gp120 on human neuroblastoma CHP100 cells involves activation of the arachidonate cascade. Biochem. J. 1998;333:45–49. doi: 10.1042/bj3330045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN D., CHINOOKOSWONG N., MILLER G. The interleukin-1 receptor antagonist (rhIL-1ra) protects against cerebral infarction in a rat model of hypoxia-ischemia. Exp. Neurol. 1994;130:362–367. doi: 10.1006/exnr.1994.1215. [DOI] [PubMed] [Google Scholar]
- MERRIL J.E., KOYANAGI Y., ZACK J., THOMAS L., MARTIN F., CHEN I.S.Y. Induction of interleukin-1 and tumor necrosis factor alfa in brain cultures by human immunodeficiency virus type 1. J. Virol. 1992;66:2217–2225. doi: 10.1128/jvi.66.4.2217-2225.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEUCCI O., FATATIS A., SIMEN A.A., BUSCHELL T.J., GRAY T.W., MILLER R.J. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc. Natl. Acad. Sci. U.S.A. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLIGAN C.E., PREVETTE D., YAGINUMA H., HOMMA S., CARDWELL C., FRITZ L.C., TOMASELLI K.J., OPPENHEIM R.W., SCHWARTZ L.M. Peptide inhibitors of ICE protease family arrest programmed cell death of motoneurons in vivo and in vitro. Neuron. 1995;15:385–393. doi: 10.1016/0896-6273(95)90042-x. [DOI] [PubMed] [Google Scholar]
- MINAMI M., KURAISHI Y., YABUUCHI K., YAMAZAKI A., SATOH M. Induction of interleukin-1β mRNA in rat brain after transient forebrain ischemia. J. Neurochem. 1992;58:390–392. doi: 10.1111/j.1471-4159.1992.tb09324.x. [DOI] [PubMed] [Google Scholar]
- MINAMI M., KURAISHI Y., YAMAGUCHI T., NAKAI S., HIRAI Y., SATOH M. Convulsants induce interleukin-1β messenger RNA in rat brain. Biochem. Biophys. Res. Commun. 1990;171:832–837. doi: 10.1016/0006-291x(90)91221-d. [DOI] [PubMed] [Google Scholar]
- NICHOLSON D.W. From bench to clinic with apoptosis-based therapeutic agents. Nature. 2000;407:810–816. doi: 10.1038/35037747. [DOI] [PubMed] [Google Scholar]
- PERRELLA O., CARRIERI P.B., GUARNACCIA D., SOSCIA M. Cerebrospinal fluid cytokines in AIDS dementia complex. J. Neurol. 1992;239:387–388. doi: 10.1007/BF00812156. [DOI] [PubMed] [Google Scholar]
- PRICE R.W., BREW B., SIDTIS J., ROSENBLUM M., SCHECK A.C., CLEARY P. The brain and AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988;239:586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- RELTON J.K., ROTHWELL N.J. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain. Res. Bull. 1992;23:243–246. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- ROTHWELL N.J. Cytokines - killers in the brain. J. Physiol. Lond. 1999;514:3–17. doi: 10.1111/j.1469-7793.1999.003af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBARTELLI A., COZZOLINO F., TALIO M., SITIA R. A novel secretory pathway for IL-1β, a protein lacking a signal sequence. EMBO J. 1990;9:1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAVIO T., LEVI G. Neurotoxicity of HIV coat protein gp120, NMDA receptors and protein kinase C: a study with rat cerebellar granule cell cultures. J. Neurosci. Res. 1993;34:265–272. doi: 10.1002/jnr.490340303. [DOI] [PubMed] [Google Scholar]
- SIDERS W.M., KLIMOVITZ J.C., MIZEL S.B. Characterization of the structural requirements and cell type specificity of IL-1α and IL-1β secretion. J. Biol. Chem. 1993;268:22170–22174. [PubMed] [Google Scholar]
- SIDERS W.M., MIZEL S.B. Interleukin-1β secretion. A possible multistep process that is regulated in a cell type-specific manner. J. Biol. Chem. 1995;270:16258–16264. doi: 10.1074/jbc.270.27.16258. [DOI] [PubMed] [Google Scholar]
- SIMS J.E., GAYLE M.A., SLACK J.L., ALDERSON M.R., BIRD T.A., GIRI J.G., COLOTTA F., RE F., MANTOVAN I.A., SHANEBECK K., GRABSTEIN K.H., DOWER S.K. Interleukin-1 signaling occurs exclusively via the type I receptor. Proc. Natl. Acad. Sci. U.S.A. 1993;90:6155–6159. doi: 10.1073/pnas.90.13.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRIJBOS P.J., ROTHWELL N.J. Interleukin-1beta attenuates excitatory amino acid induced neurodegeneration in vitro: involvement of nerve growth factor. J. Neurosci. 1995;15:3468–3474. doi: 10.1523/JNEUROSCI.15-05-03468.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STROEMER R.P., ROTHWELL N.J. Exacerbation of ischemic brain damage by localized striatal injection of interleukin-1β in the rat. J. Cereb. Blood Flow Metab. 1998;18:833–839. doi: 10.1097/00004647-199808000-00003. [DOI] [PubMed] [Google Scholar]
- THORNBERRY N.A., BULL H.G., CALAYCAY J.R., CHAPMAN K.T., HOWARD A.D., KOSTURA M.J., MILLER D.K., MOLINEAUX S.M., WEIDNER J.R., AUNINS J., et al. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- TOULMOND S., ROTHWELL N.J. Interleukin-1 receptor antagonist inhibits neuronal damage caused by fluid percussion injury in the rat. Brain Res. 1995;671:261–266. doi: 10.1016/0006-8993(94)01343-g. [DOI] [PubMed] [Google Scholar]
- TYOR W.R., GLASS J.D., GRIFFIN J.W., BECKER S.P., MCARTHUR J.C., BEZMAN L., GRIFFIN D.E. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann. Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- WAHL L.M., CORCORAN M.L., PYLE S.W., ARTHUR L.O., HARELBELLAN A., FARRAR W.L. Human immunodeficiency virus glycoprotein (gp120) induction of monocyte arachidonic acid metabolites and interleukin 1. Proc. Natl. Acad. Sci. U.S.A. 1989;86:621–625. doi: 10.1073/pnas.86.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUAN J., YANKNER B.A. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]


