Abstract
Roles of histamine in the production of vascular endothelial growth factor (VEGF) in the carrageenin-induced granulation tissue in rats were analysed in vitro and in vivo.
Incubation of the minced granulation tissue in the presence of histamine (1 and 10 μM) increased the content of VEGF protein in the conditioned medium in a time- and concentration-dependent manner. The levels of VEGF mRNA in the minced granulation tissue were also increased by histamine in a concentration-dependent manner.
The increase in the content of VEGF protein in the conditioned medium by histamine (10 μM) was suppressed by the H2 receptor antagonist cimetidine (IC50 0.37 μM), but not by the H1 receptor antagonist pyrilamine maleate, the H3 receptor antagonist thioperamide or the cyclo-oxygenase inhibitor indomethacin.
The histamine-induced increase in the content of VEGF protein in the conditioned medium was inhibited by the cyclic AMP antagonist Rp-cAMP (IC50 6.8 μM), and the protein kinase A inhibitor H-89 (IC50 12.5 μM), but not by the protein kinase C inhibitors Ro 31-8425 and calphostin C or the tyrosine kinase inhibitor genistein.
Simultaneous injection of cimetidine (400 μg) and indomethacin (100 μg) into the air pouch of rats additively reduced the carrageenin-induced increase in VEGF protein levels and angiogenesis in the granulation tissue as assessed by using carmine dye.
These findings indicate that histamine has an activity to induce VEGF production in the granulation tissue via the H2 receptor-cyclic AMP-protein kinase A pathway and augments angiogenesis in the granulation tissue.
Keywords: Histamine, H2 receptor, VEGF, angiogenesis, granulation tissue
Introduction
Histamine plays a variety of roles as an autacoid which regulates allergic inflammatory reactions (Beer et al., 1984; Falus & Meretey, 1992), differentiation of leucocytes precursors (Nakaya & Tasaka, 1988) and gastric acid secretion (Miyata et al., 1990), and as a neurotransmitter in the central nervous system (Schwartz et al., 1980). In addition, histamine is produced in rapidly growing tissues, and suggested to promote neoplastic growth and angiogenesis (Beer et al., 1984; Hellstrand & Hermodsson, 1986; Nielsen & Kikuchi, 1993; Suonio et al., 1993). Histamine is also reported as an angiogenic factor in vivo (Zauberman et al., 1969; Fraser & Simpson, 1983). However, the mechanism by which histamine promotes these responses has not been clarified in detail.
In a carrageenin-induced air pouch-type inflammation model in rats, we found that angiogenesis markedly occurs during the formation of the granulation tissue, which produces vascular endothelial growth factor (VEGF) (Ghosh et al., 2000), a most potent angiogenic factor in physiological and pathological angiogenesis (Breier et al., 1992). We also demonstrated that cyclo-oxygenase-2-dependent prostaglandin (PG) E2 plays a role in the production of VEGF and angiogenesis in the granulation tissue (Ghosh et al., 2000). However, the cyclo-oxygenase inhibitor indomethacin and the selective cyclo-oxygenase-2 inhibitor NS-398 (Futaki et al., 1994) only partially inhibited VEGF production (Ghosh et al., 2000).
Because we found that histamine is continuously produced at the chronic phase in the same model (Hirasawa et al., 1991), we analysed roles of histamine in the production of VEGF and the angiogenesis in the granulation tissue in the carrageenin-induced air pouch-type inflammation model in rats.
Methods
Induction of air pouch-type inflammation by carrageenin in rats
The rats were treated in accordance with procedures approved by the Animal Ethics Committee of the Graduate School of Pharmaceutical Sciences, Tohoku University, Japan. Air pouch-type inflammation was induced by carrageenin in male Sprague-Dawley rats, specific pathogen-free, weighing 160 – 170 g (Charles River Japan, Inc., Kanagawa, Japan) according to the procedure described by Tsurufuji et al. (1978). Eight milliliters of air was injected subcutaneously into the back to make an air pouch oval in shape. Twenty-four hours later, 4 ml of a 2% (w v−1) solution of carrageenin (Seakem No. 202, Marine Colloids Inc., Springfield, NJ, U.S.A.) in saline was injected into the air pouch. The carrageenin solution had been sterilized by autoclaving at 121°C for 15 min and supplemented with antibiotics (0.1 mg of penicillin G potassium (Meiji Seika, Tokyo, Japan) and 0.1 mg of dihydrostreptomycin sulphate (Meiji Seika) per ml of the solution) after cooling to 40 – 45°C.
Culture of minced granulation tissue
Indomethacin (Sigma Chemical Co., St. Louis, MO, U.S.A.) was dissolved in 99.5% of ethanol and diluted with saline. Five-hundred microliters of the solution containing 0.1 mg of indomethacin was injected into the pouch of each rat just after and 2 days after the carrageenin injection to minimize the effect of endogeous PGE2. Three days after injection of the carrageenin solution, the rats were sacrificed by cutting the carotid artery and the granulation tissue was excised and minced into 1 – 2-mm pieces with a pair of small scissors. The minced granulation tissue was washed twice with 5 volumes of ice-cold phosphate-buffer saline (PBS) (mM): NaCl 137, Na2HPO4 8.1, KCl 2.68, KH2PO4 1.47, (pH 7.4). Four-hundred milligrams of the minced tissue was placed in a 60×15-mm tissue culture dish (Corning Costar, Cambridge, MA, U.S.A.) and incubated in 4 ml of Eagle's minimal essential medium (EMEM) (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 10% (v v−1) calf serum (Dainippon Pharmaceutical, Osaka, Japan), penicillin G potassium (18 μg ml−1) and streptomycin sulphate (50 μg ml−1) for 3 h at 37°C under an atmosphere of 95% air and 5% CO2. The tissues were then washed three times with calf serum-free medium and incubated at 37°C for the periods indicated in 4 ml of the medium containing various concentrations of histamine (0.1 – 10 μM, Sigma Chemical Co.) in the presence or absence of drugs. After incubation, the minced granulation tissue and the conditioned medium were collected, and centrifuged at 220×g and 4°C for 5 min. The supernatant fraction of the conditioned medium was stored at −40°C. The minced granulation tissue was homogenized in 0.5 mM sodium hydroxide using the Vir-Tis 45 homogenizer (Virtis Company, Gardiner, NY, U.S.A.) for 4 min at scale 40 on an ice bed. The homogenates were centrifuged at 14,000×g and 4°C for 30 min and the supernatant fractions were stored at −40°C.
Preparation and culture of rat peritoneal macrophages and fibroblasts
A solution containing soluble starch (Wako Pure Chemicals, Osaka, Japan) and Bacto peptone (Difco Laboratories, Detroit, MI, U.S.A.) (5% each) was injected i.p. into male Sprague-Dawley rats (400 – 600 g, specific pathogen-free, Charles River Japan, Kanagawa, Japan) at a dose of 5 ml per 100 g body weight. Four days after the injection, the rats were sacrificed and peritoneal cells were harvested according to the procedure described by Ohuchi et al. (1985). The peritoneal cells were suspended in EMEM (Nissui Pharmaceutical Co.) supplemented with 10% (v v−1) calf serum (Dainippon Pharmaceutical), penicillin G potassium (18 μg ml−1) and streptomycin sulphate (50 μg ml−1). The peritoneal macrophages (1.5×107 cells) were plated in a 100-mm tissue culture dish (Corning Costar) and incubated at 37°C for 2 h in 10 ml medium. The dishes were washed three times to remove non-adherent cells and the adherent cells were further incubated for 20 h at 37°C, after which the cells were washed and incubated at 37°C for the periods indicated in 10 ml of EMEM supplemented with 10% (v v−1) calf serum containing various concentrations of histamine (0.1 – 10 μM).
For isolation of fibroblasts, the granulation tissue dissected 3 days after the injection of a 2% (w v−1) solution of carrageenin was minced into 1 – 2-mm pieces, and 1 g of the minced granulation tissue was incubated in a 100-mm tissue culture dish (Corning Costar) in 10 ml of Dulbecco's modified Eagle's medium (DMEM) (Nissui Pharmaceutical Co.) supplemented with 10% (v v−1) foetal bovine serum (Dainippon Pharmaceutical), penicillin G potassium (18 μg ml−1) and streptomycin sulphate (50 μg ml−1) at 37°C for 8 days under an atmosphere of 95% air and 5% CO2. The medium was changed each 48 h interval and the minced granulation tissues were removed from the dish. The adherant fibroblasts were washed three times with PBS and detached from the dish by the addition of 0.05% trypsin and 0.02 mM EDTA in PBS. After washing, fibroblasts (1.5×107 cells) were incubated in a 100-mm tissue culture dish (Corning Costar) at 37°C for 2 h in 10 ml of DMEM containing 10% (v v−1) foetal bovine serum. The cells were then washed three times with foetal bovine serum-free DMEM and incubated at 37°C for the periods indicated in 10 ml of DMEM supplemented with 10% (v v−1) foetal bovine serum containing various concentrations of histamine (0.1 to 10 μM).
In vitro drug treatment
Drugs used were pyrilamine maleate (Sigma Chemical Co.), cimetidine (Sigma Chemical Co.), thioperamide (a gift from Dr J.C. Schwartz at Unite de Neurobiologie et Pharmacologie Moleculaire (U.109) de l'INSERM, Paris, France), indomethacin (Sigma Chemical Co.), Rp-cAMPs triethylamine salt (Research Biochemicals International, Natick, MA, U.S.A.), Ro 31-8425 (Amersham Pharmacia Biotech Co., Piscataway, NJ, U.S.A.), calphostin C (Kyowa Medex, Tokyo, Japan), H-89 (Biomol Research Lab., Polymouth Meeting, PA, U.S.A.), genistein (Wako Pure Chemical Ind.), brefeldin A (Sigma Chemical Co.), forskolin (Seikagaku Co., Tokyo, Japan), histamine (Sigma Chemical Co.), and PGE2 (Sigma Chemical Co.). Histamine, pyrilamine maleate, cimetidine, thioperamide, and Rp-cAMPs triethylamine salt were dissolved in EMEM. PGE2, brefeldin A, forskolin and indomethacin were dissolved in 99.5% of ethanol. Ro 31-8425, calphostin C, H-89, and genistein were dissolved in dimethyl sulphoxide. Final concentration of ethanol and dimethyl sulphoxide in medium was adjusted to 0.1% (v v−1). The control medium contained the same amount of ethanol and dimethyl sulphoxide.
Western blot analysis of VEGF protein
Protein levels in the supernatant fractions were determined according to the method described by Bradford (1976). Proteins, at 100 μg aliquot for the conditioned medium, 4.6 μg aliquot for the granulation tissue and 1.4 μg aliquot for the pouch fluid, were separated by electrophoresis on a 12% (w v−1) sodium dodecylsulphate-polyacrylamide gel and transferred onto a nitrocellulose membrane (Schleicher and Schuell Inc., Dassel, Germany). The membrane was incubated at 4°C for 12 h with mouse monoclonal anti-VEGF (1 : 200, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.). It was then incubated at 4°C for 3 h with biotinylated anti-mouse IgG (1 : 2000, Vector Laboratories Inc., CA, U.S.A.) and in avidin-biotin-peroxidase complex (Vector Laboratories Inc.) for 30 min at room temperature. The reaction product was visualized with an ECL kit (ECL system; Amersham, Arlington Heights, IL, U.S.A.).
Quantification of VEGF mRNA levels in the granulation tissue by reverse transcription-polymerase chain reaction (RT – PCR)
After incubation, the minced granulation tissue was collected and homogenized in D-solution (47.3% (w v−1) guanidine thiocyanate, 0.5% (w v−1) sarcosyl, 2.5% (v v−1) 1 M sodium citrate and 0.1% (v v−1) 2-mercaptoethanol) using the Vir-Tis 45 homogenizer for 3 min at scale 40 on an ice bed. Total RNA was prepared from each sample by guanidinium-phenol-chloroform extraction (Chomczynski & Sacchi, 1987) and the yield of RNA extracted was determined by spectrophotometry. The primers for VEGF mRNA were designed as described by Asano et al. (1997); forward (5′-CGCGAATTCCATGAACTTTCTGCTCTCT-3′) and reverse (5′-TGAGAATTCTAGTTCCCGAAACCCTGA-3′). These primers amplify three isoforms of VEGF mRNA, 711 bp (VEGF 188), 636 bp (VEGF 164) and 504 bp (VEGF 120). The rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene (a housekeeping gene) was used as an internal standard gene. The primers were designed as described by Robbins & McKinney (1992); forward (5′-TGATGACAAGAAGGTGGTGAAG-3′) and reverse (5′-TCCTTGGAGGCCATGTAGGCCAT-3′). Two micrograms of RNA from each sample and 20 pmol of reverse primers of GAPDH and VEGF were incubated at 80°C for 5 min, cooled immediately and reverse-transcribed in 20 μl of a buffer (mM): Tris-HCl (pH 8.3) 50, KCl 75, MgCl2 3, 200 units of the reverse-transcriptase from moloney murine leukaemia virus (Life Technologies, Gaithersburg, MD, U.S.A.), deoxyribonucleotide triphosphates (Amersham Pharmacia Biotech Co.) 0.5 and dithiothreitol 10, at 37°C for 1 h. For VEGF mRNA, PCR amplification was performed in 50 μl of a PCR buffer (Tris-HCl (pH 8.3) 2.5 mM, KCl 50 mM, MgCl2 1.5 mM, 2 μl of the reverse transcribed RNA solution, 20 pmol of each primer, 170 μM dNTPs and 1.25 units of Taq polymerase (Takara Shuzo, Shiga, Japan)) with a thermal cycler (GeneAmp PCR system 2400, Perkin Elmer Cetus, Norwalk, CT, U.S.A.). PCR was performed at 94°C for 3 min for denaturation, 57°C for 1 min for annealing and 72°C for 3 min for extension in the first round, 94°C for 1 min, 57°C for 1 min and 72°C for 3 min in the next 38 rounds, and 94°C for 1 min, 57°C for 1 min and 72°C for 10 min in the last round. For GAPDH mRNA, PCR amplification was performed for 27 cycles at 30 s denaturation at 94°C, 1 min annealing at 57°C and 2 min extension at 72°C. The other conditions were the same as those used for VEGF mRNA. After PCR, 10 μl of the PCR reaction mixture was loaded onto a 2% (w v−1) agarose minigel and the PCR products were visualized by ethidium bromide staining after electrophoresis at 100 V for 40 min.
VEGF immunostaining
The minced granulation tissue was incubated at 37°C for 6 h in the presence of histamine (10 μM) and brefeldin A (50 μM, Sigma Chemical Co.). Brefeldin A reversibly blocks protein translocation from endoplasmic reticulum to the Golgi apparatus (Strous et al., 1993) resulting in the inhibition of the release of VEGF. After the incubation, the tissue was fixed in 10% (v v−1) formalin in PBS for 72 h at 4°C and then dehydrated through three changes of 70% (v v−1), 80% (v v−1), 90% (v v−1), and 95% (v v−1) alcohol, three changes of absolute alcohol and three changes of pure chloroform for 12 h each. The tissues were then embedded in paraffin. The sections (5 μm) from the paraffin embedded tissues were mounted on glass slides and fixed by warming at 60°C for 30 min. The tissue sections were deparaffined with xylene and ethanol, treated with 0.3% (v v−1) hydrogen peroxide in methanol to inactivate endogenous peroxidase activity, and incubated in 3% (w v−1) bovine serum albumin (BSA) (Sigma Chemical Co.) in PBS for 1 h at room temperature to block nonspecific staining. The slides were then incubated with mouse monoclonal anti-VEGF (Santa Cruz Biotechnology) in 3% BSA-PBS at a concentration of 1 μg ml−1 for 1 h at 4°C. After being washed with PBS, the slides were treated with biotinylated anti-mouse IgG (1 : 2000, Vector Laboratories Inc.) in 3% BSA-PBS for 1 h at 4°C and then treated with avidin-biotin peroxidase complex for 30 min at room temperature. The reaction product was detected with 0.05% (w v−1) 3,3′-diaminobenzidine-tetrahydrochloride (Dojindo Laboratories, Kumamoto, Japan), and 0.04% (w v−1) nickel chloride in 50 mM Tris-HCl buffer (pH 7.4) containing 0.033% (v v−1) hydrogen peroxide.
In vivo studies
Drugs used were cimetidine (Sigma Chemical Co.) and indomethacin (Sigma Chemical Co.). Cimetidine was dissolved in saline and indomethacin was dissolved in 99.5% of ethanol, and diluted with saline. Concentration of ethanol was adjusted to 0.1% (v v−1). Five-hundred microliters saline containing 400 μg cimetidine, 100 μg indomethacin, or both was injected into the pouch of each rat just after carrageenin injection and then once a day for five consecutive days. Control rats received the same amount of saline containing 0.1% (v v−1) ethanol. Six days after the injection of the carrageenin solution, total pouch fluid was collected and its volume was measured. The infiltrating leucocytes in the pouch fluid were enumerated using a hemocytometer. The population of infiltrating leucocytes in the pouch fluid were analysed by May-Gruenwald-Giemsa staining. The granulation tissue was dissected, weighed and homogenized as described above. The pouch fluid was centrifuged at 10,000×g and 4°C for 10 min. VEGF protein levels in the supernatant fractions of the homogenates and the pouch fluid were determined by Western blot analysis as described above.
Evaluation of angiogenesis in the granulation tissue
The angiogenesis in the granulation tissue was assessed using carmine dye (Natural Red 4, Sigma Chemical Co.) according to the method described by Kimura et al. (1986) and Colville-Nash et al. (1995) with slight modifications (Ghosh et al., 2000). Three milliliters of 5% (w v−1) carmine dye in 5% (w v−1) gelatin (Sankoh Jun-yaku, Tokyo, Japan) in saline at 37°C was injected into the tail vein of each rat. The carcasses were chilled on ice for 3 h, and the entire granulation tissue was dissected and weighed. After 3 washes with PBS, the granulation tissue was homogenized in 2 volumes of 0.5 mM sodium hydroxide using the Vir-Tis 45 homogenizer for 4 min at scale 40 on an ice bed. The tissue homogenate was centrifuged at 10,000×g and 4°C for 30 min. Five-hundred microliters of the supernatant was diluted 2 fold with 0.5 mM sodium hydroxide and centrifuged again at 14,000×g and 4°C for 30 min. The dye content in 200 μl of the supernatant was determined spectrophotometrically by measuring absorbance at 490 nm. For the standard curve, known amounts of carmine dye were added to the final supernatant of the granulation tissue of control rats which were injected with 3 ml of a 5% (w v−1) gelatin solution in saline without carmine dye, and the absorbance determined. The amount of carmine dye in the whole granulation tissue was then calculated.
Statistical analysis
The statistical significance of the results was analysed by Dunnett's test for multiple comparisons and Student's t-test for unpaired observations.
Results
Induction of VEGF expression by histamine in the minced granulation tissue
As shown in Figures 1b and 2a, histamine increased the VEGF protein content in the conditioned medium in a time- and concentration-dependent manner. mRNA (504, 636 and 711 bp) levels for the three isoforms of VEGF in the minced granulation tissue were also increased by histamine with a maximum at 1 h (Figure 1c), and in a concentration-dependent manner (Figure 2b). VEGF protein levels in the minced granulation tissue reached a maximum at 3 h and then declined (Figure 1a). VEGF protein levels in the conditioned medium of the minced granulation tissue were also increased by incubating the granulation tissue in the presence of PGE2 (1 μM). The effect of PGE2 (1 μM) was almost the same as that of histamine at 1 – 10 μM (Figure 2a).
Figure 1.
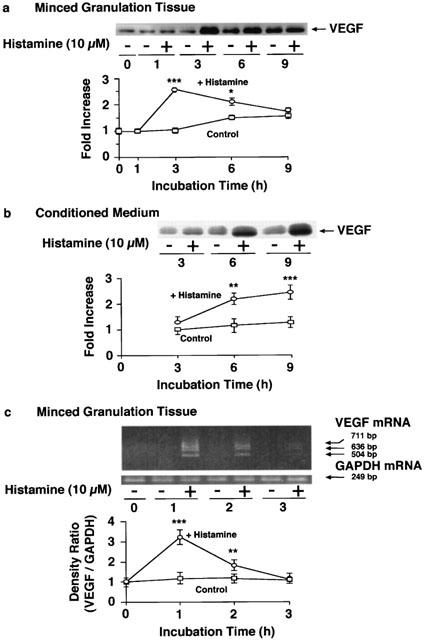
Time course of the histamine-induced VEGF production in the minced granulation tissue. The minced granulation tissue (0.4 g) was incubated in 4 ml of EMEM containing 10% (v v−1) calf serum at 37°C for 3 h. After three washes, the tissue was further incubated at 37°C for the periods indicated in medium with or without histamine (10 μM). VEGF protein in the minced granulation tissue (a) and in the conditioned medium (b) was detected by immunoblotting and the levels were analysed densitometrically. Representative immunoblots are shown at the top in (a) and (b). The mean VEGF protein level in the minced granulation tissue at time 0 h in the control group (a) or in the conditioned medium at time 3 h in the control group (b) is set to 1.0. VEGF mRNA levels in the minced granulation tissue were determined by RT – PCR and analysed densitometrically (c). Representative VEGF mRNA bands are shown at the top in (c). The mean density ratio of mRNA for VEGF to that for GAPDH at time 0 h in the control group is set to 1.0. Values are the means from four samples with s.e.mean shown by vertical bars. Statistical significance: *P<0.05, **P<0.01 and ***P<0.001 versus the corresponding control.
Figure 2.
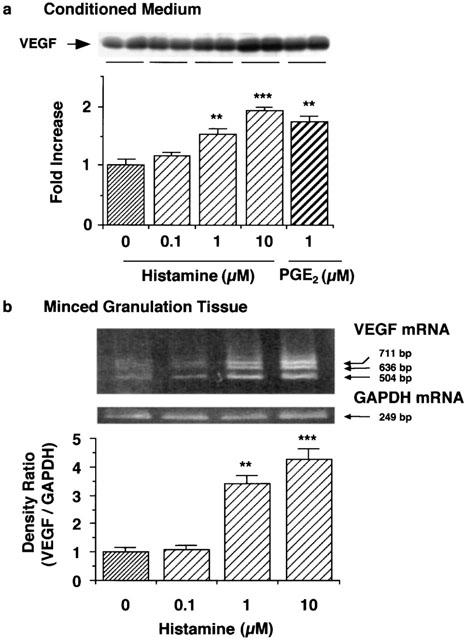
Effects of various concentrations of histamine on VEGF production in the minced granulation tissue. The minced granulation tissue (0.4 g) was incubated in 4 ml of EMEM containing 10% (v v−1) calf serum at 37°C for 3 h. After three washes, the tissue was further incubated at 37°C for 6 h (a) or 1 h (b) in medium containing the indicated concentrations of histamine and PGE2. (a) VEGF protein in the conditioned medium was detected by immunoblotting and analysed densitometrically. Representative immunoblots from two samples of each group are shown at the top. The mean VEGF protein level in the conditioned medium in the control group is set to 1.0. (b) VEGF mRNA levels in the minced granulation tissue were determined by RT – PCR and analysed densitometrically. Representative VEGF mRNA bands are shown at the top. The mean density ratio of mRNA for VEGF to that for GAPDH in the control group is set to 1.0. Values are the means from four samples with s.e.mean shown by vertical bars. Statistical significance: **P<0.01 and ***P<0.001 versus the corresponding control.
Effects of histamine receptor antagonists and indomethacin on VEGF protein expression induced by histamine
As shown in Figure 3, the histamine-induced increase in VEGF protein levels in the conditioned medium of the minced granulation tissue was suppressed by the H2 receptor antagonist cimetidine, but not by the H1 receptor antagonist pyrilamine maleate (100 μM) or the H3 receptor antagonist thioperamide (100 μM). The IC50 value of cimetidine was calculated to be 0.37 μM (Figure 4). The possibility that histamine induces VEGF production by increasing the production of PGE2, which is one of the inducers of VEGF protein, was excluded because indomethacin at 1 μM which inhibits PGE2 production almost completely did not inhibit the histamine-induced VEGF production in the minced granulation tissues (Figure 3a).
Figure 3.
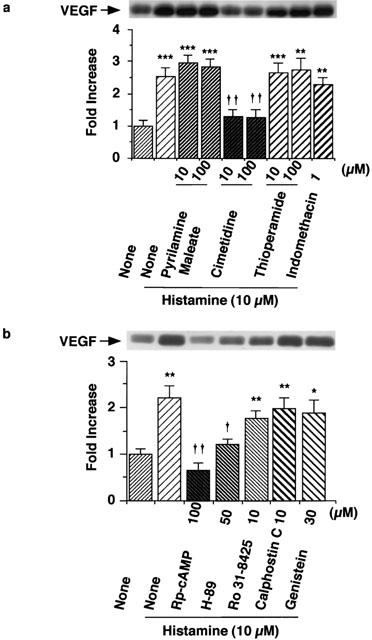
Effects of histamine receptor antagonists, indomethacin, and inhibitors of PKA and PKC on VEGF protein expression induced by histamine. The minced granulation tissue (0.4 g) was incubated in 4 ml of EMEM containing 10% (v v−1) calf serum at 37°C for 3 h. After three washes, the tissue was further incubated at 37°C for 6 h in medium containing histamine (10 μM) in the presence or absence of each histamine receptor antagonist or indomethacin (a), and Rp-cAMP, H-89, Ro 31-8415, calphostin C or genistein (b) at the concentrations indicated. VEGF protein in the conditioned medium was detected by immunoblotting and analysed densitometrically. Representative immunoblots are shown at the top. The mean VEGF protein level in the conditioned medium in the control group is set to 1.0. Values are the means from four samples with s.e.mean shown by vertical bars. Statistical significance: *P<0.05, **P<0.01 and ***P<0.001 versus control, and †P<0.05 and ††P<0.01 versus histamine (10 μM) alone.
Figure 4.
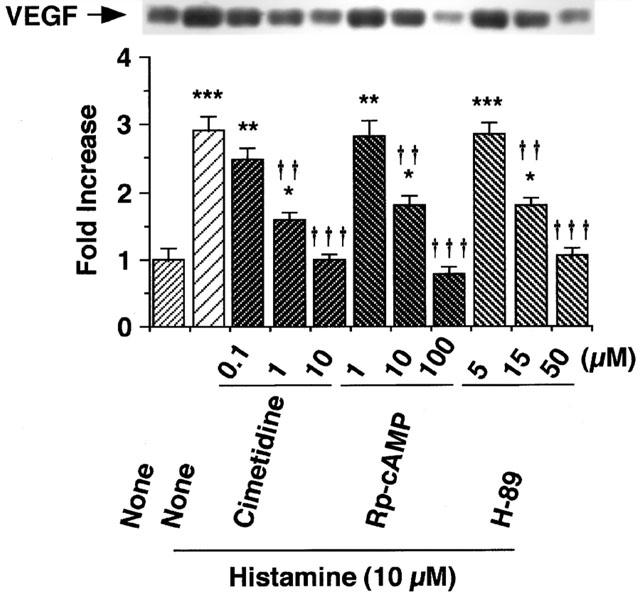
Effects of various concentrations of cimetidine, Rp-cAMP and H-89 on histamine-induced VEGF production in the granulation tissue. The minced granulation tissue (0.4 g) was incubated in 4 ml of EMEM containing 10% (v v−1) calf serum at 37°C for 3 h. After three washes, the tissue was further incubated at 37°C for 6 h in medium containing histamine (10 μM) in the presence of cimetidine (0.1 – 10 μM), Rp-cAMP (1 – 100 μM) or H-89 (5 – 50 μM). VEGF protein levels in the conditioned medium were determined by immunoblotting and analysed densitometrically. Representative immunoblots are shown at the top. The mean VEGF protein level in the conditioned medium in the control group is set to 1.0. Values are the means from four samples with s.e.mean shown by vertical bars. Statistical significance: *P<0.05, **P<0.01 and ***P<0.001 versus control, and ††P<0.01 and †††P<0.001 versus histamine (10 μM) alone.
Effects of inhibitors of PKA and PKC on histamine-induced VEGF protein expression
The histamine-induced VEGF production in the minced granulation tissue was markedly inhibited by the protein kinase A (PKA) inhibitor H-89 (15 – 50 μM) in a concentration-dependent manner, with IC50 12.5 μM, and the cyclic AMP antagonist Rp-cAMP (10 – 100 μM) in a concentration-dependent manner, with IC50 6.8 μM (Figure 4). In contrast, the protein kinase C (PKC) inhibitors Ro 31-8425 (10 μM) and calphostin C (10 μM) did not inhibit the histamine-induced VEGF production (Figure 3b). The protein tyrosine kinase inhibitor genistein (30 μM) also showed no effect (Figure 3b). To confirm the role of the cyclic AMP-PKA pathway in the histamine-induced VEGF production, effects of the adenylate cyclase activator forskolin by itself was examined. In the absence of histamine, forskolin (10 μM) increased VEGF protein expression at 6 h in the minced granulation tissue (Fold Increase: Control 1.00±0.12, Forskolin (10 μM) 1.86±0.24*, the means from four samples with s.e.mean, *P<0.05 versus the Control). These findings indicated that the cyclic AMP-PKA pathway participates in VEGF protein expression.
Immunohistochemical analysis of VEGF-producing cells in the minced granulation tissue
The minced granulation tissue was incubated at 37°C for 6 h in medium containing brefeldin A (50 μM) in the presence or absence of histamine (10 μM) and the expression of VEGF protein was analysed by immunostaining. Brefeldin A was added to block protein translocation from endoplasmic reticulum to the Golgi apparatus resulting in the inhibition of the release of VEGF into the conditioned medium. Histochemical analysis demonstrated that histamine increased the number of cells stained by anti-VEGF (Figure 5) and the VEGF-positive cells were mainly macrophages, endothelial cells and fibroblasts (Figure 5).
Figure 5.
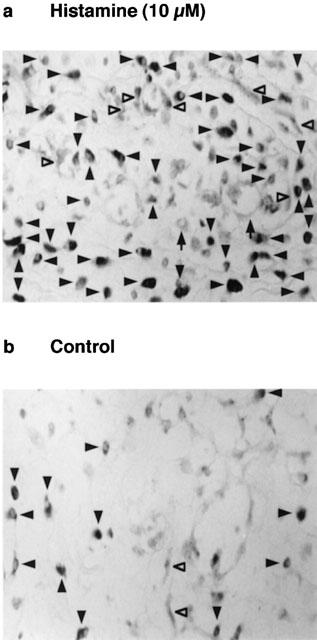
Immunohistochemical analysis of VEGF-producing cells in the minced granulation tissue. The minced granulation tissue (0.4 g) was incubated in 4 ml of EMEM containing 10% (v v−1) calf serum at 37°C for 3 h. After three washes, the tissue was further incubated at 37°C for 6 h in medium containing brefeldin A (50 μM) with (a) or without (b) histamine (10 μM). The minced granulation tissue was dehydrated and sliced to 5 μM in thickness. VEGF-producing cells were immunostained as described in the Methods, and observed with a light microscope (400× magnification). VEGF-producing cells are indicated by closed arrowheads (macrophages), open arrowheads (endothelial cells) and arrows (fibroblasts). Representative micrographs are shown from four samples.
Effects of histamine on the levels of VEGF mRNA in rat peritoneal macrophages and fibroblasts isolated from the granulation tissue
In rat peritoneal macrophages, 1 h incubation with histamine increased the level of VEGF mRNA at 1 and 10 μM in a concentration-dependent manner (Figure 6a). In fibroblasts obtained from the granulation tissue 3 days after the carrageenin injection, the levels of VEGF mRNA were also increased by histamine at 1 and 10 μM (Figure 6b). In both cases, the levels of three isoforms of VEGF mRNA (504, 636 and 711 bp) were increased.
Figure 6.
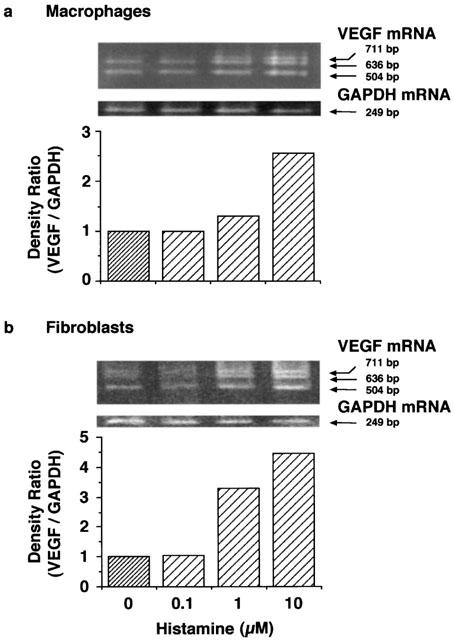
Effects of various concentrations of histamine on VEGF production in peritoneal macrophages and fibroblasts isolated from the granulation tissue. Rat peritoneal macrophages (1.5×107 cells) and fibroblasts (1.5×107 cells) isolated from the granulation tissue were incubated at 37°C for 3 h in 10 ml of EMEM containing 10% (v v−1) calf serum and 10 ml DMEM cotaining 10% (v v−1) foetal bovine serum, respectively. After three washes, the cells were further incubated at 37°C for 1 h in medium containing the indicated concentrations of histamine. VEGF mRNA levels in peritoneal macrophages (a) and fibroblasts (b) were determined by RT – PCR and analysed densitometrically. Representative VEGF mRNA bands are shown at the top. The mean density ratio of mRNA for VEGF to that for GAPDH in the control group is set to 1.0. The results were confirmed by repeating two independent sets of experiments.
Effects of cimetidine and indomethacin on carrageenin-induced granulation tissue formation and angiogenesis in vivo
Carrageenin-induced pouch fluid accumulation, leucocyte infiltration, and formation of granulation tissue 6 days after carrageenin injection were significantly inhibited by the administration of cimetidine (400 μg) into the air-pouch (Figure 7). Effects of indomethacin (100 μg) were more potent than those of cimetidine (400 μg), and the additive effects were observed by the combined treatment with cimetidine (400 μg) and indomethacin (100 μg) (Figure 7). The angiogenesis in the granulation tissue was evaluated by the carmine dye method. Cimetidine (400 μg) inhibited the angiogenesis in the whole granulation tissue at day 6 and the inhibition by indomethacin (100 μg) was more potent than that by cimetidine (400 μg) (Figure 8). The combined treatment with indomethacin (100 μg) and cimetidine (400 μg) further suppressed the angiogenesis (Figure 8).
Figure 7.
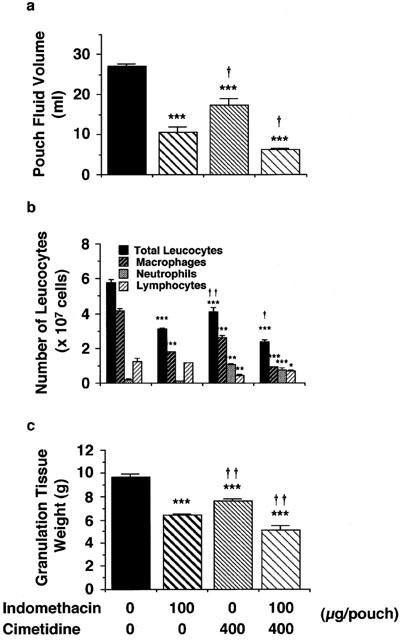
Effects of cimetidine and indomethacin on pouch fluid volume, total number of leucocytes infiltrating into the pouch fluid and granulation tissue weight 6 days after carrageenin injection. Four milliliters of a 2% (w v−1) carrageenin solution in saline was injected into the air pouch. Five-hundred microliters saline containing 400 μg cimetidine, 100 μg indomethacin or both was injected into the pouch every day after the injection of carrageenin. Control rats received the same amount of saline. Pouch fluid volume (a), total number of leucocytes in the pouch fluid (b), and granulation tissue weight (c) were determined 6 days after injection of the carrageenin solution. Number of infiltrating eosinophils was too small to depict. Values are the means from five rats with s.e.mean shown by vertical bars. Statistical significance: *P<0.05, **P<0.01 and ***P<0.001 versus the corresponding control, and †P<0.05 and ††P<0.01 versus indomethacin (100 μg).
Figure 8.
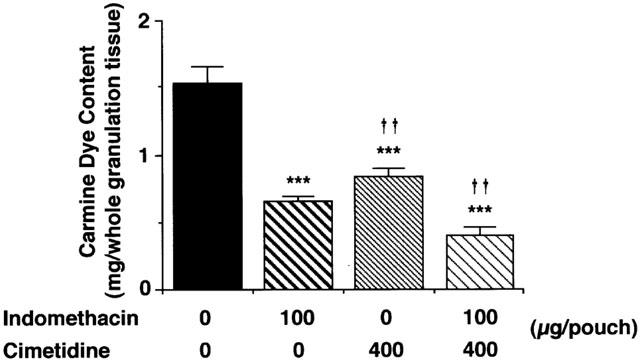
Effects of cimetidine and indomethacin on the angiogenesis in the granulation tissue 6 days after carrageenin injection. Four milliliters of a 2% (w v−1) carrageenin solution in saline was injected into the air pouch. Five-hundred microliters saline containing 400 μg cimetidine, 100 μg indomethacin or both was injected into the pouch every day after the injection of carrageenin. Six days after injection of the carrageenin solution, 3 ml of prewarmed saline at 37°C containing 5% (w v−1) carmine dye and 5% (w v−1) gelatin was injected intravenously into each anaesthetized rat. The granulation tissue was dissected, and total carmine dye content was determined as described in the Methods. Values are the means from five rats with s.e.mean shown by vertical bars. Statistical significance: ***P<0.001 versus control, and ††P<0.01 versus indomethacin (100 μg).
Effects of cimetidine and indomethacin on VEGF protein content in the granulation tissue and in the pouch fluid
Administration of cimetidine (400 μg) and indomethacin (100 μg) into the air pouch lowered the VEGF protein levels in the granulation tissue (Figure 9a) and in the pouch fluid (Figure 9b) at day 6. The effect of cimetidine was less potent than that of indomethacin (Figure 9a,b), and the combined treatment with cimetidine and indomethacin further decreased the VEGF production.
Figure 9.
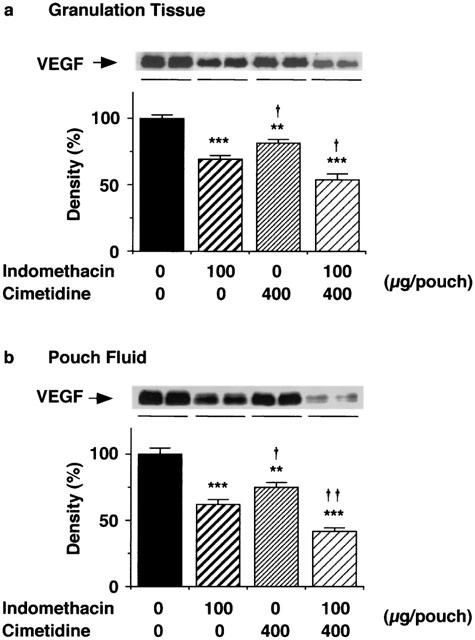
Effects of cimetidine and indomethacin on VEGF protein levels in the granulation tissue and the pouch fluid 6 days after carrageenin injection. Four milliliters of a 2% (w v−1) carrageenin solution in saline was injected into the air pouch. Five-hundred microliters saline containing 400 μg cimetidine, 100 μg indomethacin or both was injected into the pouch every day after the injection of carrageenin. The granulation tissue 6 days after injection of the carrageenin solution was dissected, homogenized and centrifuged as described in the Methods. VEGF protein in the supernatant fraction of the homogenate of the granulation tissue (a) and the pouch fluid (b) was immunoblotted and analysed densitometrically. The immunoblots of VEGF proteins in the granulation tissue and the pouch fluid from two rats in each group are shown at the top respectively. Values are the means from six rats with s.e.mean shown by vertical bars. The mean density in the control group is set to 100%. Statistical significance: **P<0.01 and ***P<0.001 versus the corresponding control, and †P<0.05 and ††P<0.01 versus indomethacin (100 μg).
Discussion
We demonstrated that PGE2 partially participates in the production of VEGF in the carrageenin-induced granulation tissue in rats (Ghosh et al., 2000). The participation of PGE2 in VEGF production is also demonstrated in the sponge-induced granulation tissue in rats (Majima et al., 2000) and in human colon cancer cells (Tsujii et al., 1998). The present findings indicate that histamine also contributes to VEGF protein expression and angiogenesis in the carrageenin-induced granulation tissue in rats. In this model, histamine concentration in the pouch fluid increases time-dependently after injection of the carrageenin solution, and the maximum concentration reaches 3 μM at 24 h (Hirasawa et al., 1991). Our findings in the present study indicate that histamine at such concentration participates in VEGF production in the granulation tissue.
VEGF is a secreted protein (Houck et al., 1991) with several isoforms translated from alternatively spliced mRNAs (Tischer et al., 1991). In rats, there are three isoforms of VEGF protein, VEGF188, VEGF164 and VEGF120, of which the mRNA are 711, 636 and 504 bp, respectively (Bacic et al., 1995). In the minced granulation tissue, incubation with histamine (1 and 10 μM) for 1 h increased the levels of VEGF protein and three isoforms of VEGF mRNA (Figures 1 and 2). In the ovarian epithelial carcinoma tissue in humans, Sowter et al. (1997) reported that VEGF164 and VEGF120 play a significant role in the angiogenesis. However, in rats, we could not clarify the roles of the three isoforms of VEGF in the angiogenesis in the carrageenin-induced granulation tissue. The VEGF-producing cells in the granulation tissue stimulated by histamine were macrophages, endothelial cells and fibroblasts (Figure 5), as reported in basic fibroblast growth factor-induced granulation tissue in rats (Majima et al., 2000). In isolated macrophages and fibroblasts, histamine at 1 and 10 μM increased the levels of VEGF mRNA (Figure 6).
In the carrageenin-induced granulation tissue, histamine induced expression of VEGF protein through the H2 receptor, because the H2 receptor antagonist cimetidine but not the H1 receptor antagonist pyrilamine or the H3 receptor antagonist thioperamide, inhibited the histamine-induced VEGF production (Figures 3a and 4). The possibility that histamine induces the production of VEGF by increasing PGE2 production by the minced granulation tissue was excluded because indomethacin at 1 μM, high enough to inhibit PGE2 production by macrophages almost completely (Yamashita et al., 1999), did not inhibit the histamine-induced VEGF production (Figure 4). In addition, although histamine induces PGE2 production in various cells, the action is not mediated by H2 receptors (Niisato et al., 1996). Therefore, it is likely that histamine directly induces VEGF production via H2 receptors. In general, it is accepted that the stimulation of H2 receptor increases cyclic AMP levels (Reinhardt & Ritter, 1979; Nishibori, 1981; Shayo et al., 1997). We found that the induction of VEGF protein by histamine in the granulation tissue is cyclic AMP-mediated, because the histamine-induced VEGF production was markedly inhibited by the cyclic AMP antagonist Rp-cAMP and the PKA inhibitor H-89 (Figures 3b and 4). In contrast, PKC and the protein tyrosine kinase do not play critical roles in the VEGF induction by histamine because the PKC inhibitors Ro 31-8425 (10 μM) and calphostin C (10 μM), and the protein tyrosine kinase inhibitor genistein (30 μM) did not inhibit the histamine-induced VEGF production (Figure 4). Such concentrations of Ro 31-8425 and calphostin C, or genistein are high enough to inhibit PKC (Kobayashi et al., 1989; Muid et al., 1991) or protein tyrosine kinase (Akiyama et al., 1987), respectively. These findings suggest that the histamine-induced production of VEGF in the granulation tissue is dominantly mediated by the cyclic AMP-PKA pathway. This hypothesis is supported by the observation that histamine modulates the expression of c-fos through the production of cyclic AMP via H2 receptors in a human promonocytic cell line U937 (Shayo et al., 1997) and c-fos participates in the induction of VEGF (Marconcini et al., 1999). PGE2 also induces VEGF production in a human monocytic cell line and lung tissues through the cyclic AMP-PKA pathway (Hoper et al., 1997). Therefore, histamine and PGE2 might activate a common signal pathway for VEGF production.
Histamine at 1 μM and above, increases cyclic AMP levels via H2 receptors in rabbit mesenteric artery (Reinhardt & Ritter, 1979), canine parotid gland (Nishibori, 1981), and human promonocytic cell line U937 (Shayo et al., 1997). Histamine also stimulates IL-10 production in human monocytes (Elenkov et al., 1998) and IL-6 production in human endothelial cells (Delneste et al., 1994) via H2 receptors at 1 and 10 μM. The concentration of histamine employed in the present study is almost the same range as in those reports.
We also indicated that endogenous histamine plays a role in the angiogenesis in granulation tissue in vivo. The intra-pouch injection of indomethacin and cimetidine resulted in the decrease in the granulation tissue weight, VEGF protein levels both in the granulation tissue and in the pouch fluid, and the angiogenesis in the granulation tissue (Figures 7, 8 and 9). Consistent with our previous findings that treatment with cimetidine, 6 and 12 h after the injection of carrageenin solution, increased the number of infiltrating neutraphils in the pouch fluid at 24 h (Hirasawa et al., 1991), the intra-pouch injection of cimetidine once a day for six consecutive days also increased the number of infiltrating neutrophils at day 6 (Figure 7). Although cimetidine increased neutrophil infiltration, it decreased infiltration of macrophages and lymphocytes, pouch fluid accumulation and angiogenesis. Since indomethacin inhibits neutrophil infiltration and vascular permeability increase at the acute phase of this model (Hirasawa et al., 1987), the inhibition of angiogenesis by indomethacin might be caused both by inhibition of VEGF production and by inhibition of acute inflammatory responses. Cimetidine also inhibited pouch fluid accumulation, infiltration of macrophages and granulation tissue formation (Figure 7). Therefore, the possibility remains that the inhibition of angiogenesis by cimetidine was also caused both by inhibition of histamine-induced VEGF production and by inhibition of the infiltration of macrophages in which VEGF mRNA levels were increased by histamine (Figure 6). Because cimetidine and indomethacin showed additive inhibitory effects on VEGF production (Figure 9) and angiogenesis (Figure 8), it is suggested that histamine in addition to PGE2 participates in VEGF production and angiogenesis in the granulation tissue in carrageenin-induced inflammation in rats.
Although there are several reports describing that cimetidine delays wound healing (Tsuchida et al., 1990; Tsukamoto et al., 1992; Hahm et al., 1996; Lawson et al., 1996), the mechanism of action has not been clarified. However, it is possible that cimetidine inhibits the histamine-mediated upregulation of angiogenesis, and thus delays wound healing. Our finding is the first to demonstrate the novel action of histamine on VEGF production in granulation tissue through the H2 receptor-cyclic AMP-PKA pathway.
Acknowledgments
We are grateful to Dr J.C. Schwartz (Unite de Neurobiologie et Pharmacologie Moleculaire (U.109) de l'INSERM, Paris, France) for providing the H3 receptor antagonist thioperamide. We thank Dr T. Terui and Prof H. Tagami (Department of Dermatology, Tohoku University, Graduate School of Medicine, Sendai, Japan) for use of the microscopy facility. The present study was supported in part by Grants-in-Aid for Exploratory Research (11877381) and Scientific Research (11470481) from the Ministry of Education, Science, Sports and Culture of Japan and by Nishinomiya Basic Research Fund (Japan).
Abbreviations
- BSA
bovine serum albumin
- DMEM
Dulbecco's modified Eagle's medium
- EMEM
Eagle's minimal essential medium
- PBS
phosphate-buffered saline
- PGE2
prostaglandin E2
- VEGF
vascular endothelial growth factor
References
- AKIYAMA T., ISHIDA J., NAKAGAWA S., OGAWARA H., WATANABE S., ITOH N., SHIBUYA M., FUKAMI Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- ASANO A., MORIMATSU M., NIKAMI H., YOSHIDA T., SAITO M. Adrenergic activation of vascular endothelial growth factor mRNA expression in rat brown adipose tissue: implication in cold-induced angiogenesis. Biochem. J. 1997;328:179–183. doi: 10.1042/bj3280179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACIC M., EDWARDS N.A., MERRILL M.J. Differential expression of vascular endothelial growth factor (vascular permeability factor) forms in rat tissues. Growth Factors. 1995;12:11–15. doi: 10.3109/08977199509003209. [DOI] [PubMed] [Google Scholar]
- BEER D.J., MATLOFF S.M., ROCKLIN R.E. The influence of histamine on immune and inflammatory responses. Adv. Immunol. 1984;35:209–268. doi: 10.1016/s0065-2776(08)60577-5. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BREIER G., ALBRECHT U., STERRER S., RISAU W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992;114:521–532. doi: 10.1242/dev.114.2.521. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- COLVILLE-NASH P.R., ALAM C.A., APPLETON I., BROWN J.R., SEED M.P., WILLOUGHBY D.A. The pharmacological modulation of angiogenesis in chronic granulomatous inflammation. J. Pharmacol. Exp. Ther. 1995;274:1463–1472. [PubMed] [Google Scholar]
- DELNESTE Y., LASSALLE P., JEANNIN P., JOSEPH M., TONNEL A.B., GOSSET P. Histamine induces IL-6 production by human endothelial cells. Clin. Exp. Immunol. 1994;98:344–349. doi: 10.1111/j.1365-2249.1994.tb06148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELENKOV I.J., WEBSTER E., PAPANICOLAOU D.A., FLEISHER T.A., CHROUSOS G.P., WILDER R.L. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J. Immunol. 1998;161:2586–2593. [PubMed] [Google Scholar]
- FALUS A., MERETEY K. Histamine: an early messenger in inflammatory and immune reactions. Immunol. Today. 1992;13:154–156. doi: 10.1016/0167-5699(92)90117-p. [DOI] [PubMed] [Google Scholar]
- FRASER R.A., SIMPSON J.G. Role of mast cells in experimental tumour angiogenesis. Chiba Foundation Symposium. 1983;100:120–131. doi: 10.1002/9780470720813.ch8. [DOI] [PubMed] [Google Scholar]
- FUTAKI N., TAKAHASHI S., YOKOYAMA M., ARAI I., HIGUCHI S., OTOMO S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX2) activity in vitro. Prostaglandins. 1994;47:55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- GHOSH A.K., HIRASAWA N., NIKI H., OHUCHI K. Cyclooxygenase-2-mediated angiogenesis in carrageenin-induced granulation tissue in rats. J. Pharmacol. Exp. Ther. 2000;295:802–809. [PubMed] [Google Scholar]
- HAHM K.B., PARK I.S., KIM H.C., LEE K.J., KIM J.H., CHO S.W., LEE S.I. Comparison of antiproliferative effects of 1-histamine-2 receptor antagonists, cimetidine, ranitidine, and famotidine, in gastric cancer cells. Int. J. Immunopharmacol. 1996;18:393–399. doi: 10.1016/s0192-0561(96)00044-6. [DOI] [PubMed] [Google Scholar]
- HELLSTRAND K., HERMODSSON S. Histamine H2-receptor-mediated regulation of human natural killer cell activity. J. Immunol. 1986;137:656–660. [PubMed] [Google Scholar]
- HIRASAWA N., OHUCHI K., WATANABE M., TSURUFUJI S. Machanism of the inhibitory action of cyclooxygenase inhibitors on leukocyte infiltration: involvement of endogenous histamine. Eur. J. Pharmacol. 1987;144:267–275. doi: 10.1016/0014-2999(87)90379-7. [DOI] [PubMed] [Google Scholar]
- HIRASAWA N., WATANABE M., MUE S., TSURUFUJI S., OHUCHI K. Downward regulation of neutrophil infiltration by endogenous histamine without affecting vascular permeability responses in air-pouch-type carrageenin inflammation in rats. Inflammation. 1991;15:117–126. doi: 10.1007/BF00917506. [DOI] [PubMed] [Google Scholar]
- HOPER M.M., VOELKEL N.F., BATES T.O., ALLARD J.D., HORAN M., SHEPHERD D., TUDER R.M. Prostaglandins induce vascular endothelial growth factor in a human monocytic cell line and rat lungs via cAMP. Am. J. Respir. Cell. Mol. Biol. 1997;17:748–756. doi: 10.1165/ajrcmb.17.6.2888. [DOI] [PubMed] [Google Scholar]
- HOUCK K.A., FERRARA N., WINER J., CACHIANES G., LI B., LEUNG D.W. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol. Endocrinol. 1991;5:1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- KIMURA M., AMEMIYA K., YAMADA T., SUZUKI J. Quantitative method for measuring adjuvant-induced granuloma angiogenesis in insulin-treated diabetic mice. J. Pharmacobio-Dyn. 1986;9:442–446. doi: 10.1248/bpb1978.9.442. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI E., NAKANO H., MORIMOTO M., TAMAOKI T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem. Biophys. Res. Commun. 1989;159:548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- LAWSON J.A., ADAMS W.J., MORRIS D.L. Ranitidine and cimetidine differ in their in vitro and in vivo effects on human colonic cancer growth. Br. J. Cancer. 1996;73:872–876. doi: 10.1038/bjc.1996.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJIMA M., HAYASHI I., MURAMATSU M., KATADA J., YAMASHINA S., KATORI M. Cyclo-oxygenase-2 enhances basic fibroblast growth factor-induced angiogenesis through induction of vascular endothelial growth factor in rat sponge implants. Br. J. Pharmacol. 2000;130:641–649. doi: 10.1038/sj.bjp.0703327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCONCINI L., MARCHIO S., MORBIDELLI L., CARTOCCI E., ALBINI A., ZICHE M., BUSSOLINO F., OLIVIERO S. C-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc. Natl. Acad. Sci. U.S.A. 1999;96:9671–9676. doi: 10.1073/pnas.96.17.9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYATA K., KAMATO T., FUJIHARA A., TAKEDA M. Selective and competitive histamine H2-receptor blocking effect of famotidine on the blood pressure response in dogs and the acid secretory response in rats. Jpn. J. Pharmacol. 1990;54:197–204. doi: 10.1254/jjp.54.197. [DOI] [PubMed] [Google Scholar]
- MUID R.E., DALE M.M., DAVIS P.D., ELLIOTT L.H., HILL C.H., KUMAR H., LAWTON G., TWOMEY B.M., WADSWORTH J., WILKINSON S.E., NIXON J.S. A novel conformationally restricted protein kinase C inhibitor, Ro 31-8425, inhibits human neutrophil superoxide generation by soluble, particulate and post-receptor stimuli. FEBS Lett. 1991;293:169–172. doi: 10.1016/0014-5793(91)81178-b. [DOI] [PubMed] [Google Scholar]
- NAKAYA N., TASAKA K. The influence of histamine on precursors of granulocytic leukocytes in murine bone marrow. Life Sci. 1988;42:999–1010. doi: 10.1016/0024-3205(88)90430-4. [DOI] [PubMed] [Google Scholar]
- NIELSEN H.J., KIKUCHI Y. Histamine-2 receptor antagonists as potential adjuvant treatment of malignant diseases. Adv. Biosci. 1993;89:319–334. [Google Scholar]
- NIISATO N., OGATA Y., FURUYAMA S., SUGIYA H. Histamine H1 receptor-induced Ca2+ mobilization and prostaglandin E2 release in human gingival fibroblasts. Biochem. Pharmacol. 1996;52:1015–1023. doi: 10.1016/0006-2952(96)00417-0. [DOI] [PubMed] [Google Scholar]
- NISHIBORI M. Cyclic AMP increase in canine parotid gland mediated by histamine H2-receptors. Eur. J. Pharmacol. 1981;76:309–316. doi: 10.1016/0014-2999(81)90101-1. [DOI] [PubMed] [Google Scholar]
- OHUCHI K. , WATANABE M. , YOSHIZAWA K. , TSURUFUJI S. , FUJIKI H. , SUGANUMA M. , SUGIMURA T., LEVINE L. Stimulation of prostaglandin E2 production by 12-O-tetradecanoylphorbol 13-acetate (TPA)-type and non-TPA-type tumor promoters in macrophages and its inhibition by cycloheximide. Biochim. Biophys. Acta. 1985;834:42–47. doi: 10.1016/0005-2760(85)90174-2. [DOI] [PubMed] [Google Scholar]
- REINHARDT D., RITTER E. Hypothermia-induced potentiation of histamine H2-receptor-mediated relaxation and cyclic AMP increase in the isolated mesenteric artery of the rabbit. Agents Actions. 1979;9:9–14. doi: 10.1007/BF02024089. [DOI] [PubMed] [Google Scholar]
- ROBBINS M., MCKINNEY M. Transcriptional regulation of neuromodulin (GAP-43) in mouse neuroblastoma clone NIE-115 as evaluated by the RT/PCR method. Brain Res. 1992;13:83–92. doi: 10.1016/0169-328x(92)90047-f. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ J.C., POLLARD H., QUACH T.T. Histamine as a neurotransmitter in mammalian brain: neurochemical evidence. J. Neurochem. 1980;35:26–33. doi: 10.1111/j.1471-4159.1980.tb12485.x. [DOI] [PubMed] [Google Scholar]
- SHAYO C., DAVIO C., BRODSKY A., MLADOVAN A.G., LEGNAZZI B.L., RIVERA E., BALDI A. Histamine modulates the expression of c-fos through cyclic AMP production via the H2 receptor in the human promonocytic cell line U937. Mol. Pharmacol. 1997;51:983–990. doi: 10.1124/mol.51.6.983. [DOI] [PubMed] [Google Scholar]
- SOWTER H.M., CORPS A.N., EVANS A.L., CLARK D.E., CHARNOCK-JONES D.S., SMITH S.K. Expression and localization of the vascular endothelial growth factor family in ovarian epithelial tumors. Lab. Invest. 1997;77:607–614. [PubMed] [Google Scholar]
- STROUS G.J., VANKERKHOF P., VANMEER G., RIJINBOUTT S., STOORVOGEL W. Differential effects of brefeldin A on transport of secretory and lysosomal proteins. J. Biol. Chem. 1993;268:2341–2347. [PubMed] [Google Scholar]
- SUONIO E., TUOMISTO L., KANGAS L. The role of histamine in cancer growth as assessed by the mouse subrenal capsule assay. Adv. Biosci. 1993;89:349–374. [Google Scholar]
- TISCHER E., MITCHELL R., HARTMAN T., SILVA M., GOSPODAROWICZ D., FIDDES J.C., ABRAHAM J.A. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J. Biol. Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- TSUCHIDA T., TSUKAMOTO Y., SEGEWA K., GOTO H., HASE S. Effects of cimetidine and omeprazole on angiogenesis in granulation tissue of acetic acid-induced gastric ulcers in rats. Digestion. 1990;47:8–14. doi: 10.1159/000200469. [DOI] [PubMed] [Google Scholar]
- TSUJII M., KAWANO S., TSUJI S., SAWAOKA H., HORI M., DUBOIS R.N. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–716. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- TSUKAMOTO Y., NIWA Y., ARISAWA T. Relapse of peptic ulcer after quick healing induced by proton pump inhibitors. Jpn. J. Clin. Med. 1992;50:174–180. [PubMed] [Google Scholar]
- TSURUFUJI S., SATO H., MIN K.R., OHUCHI K. Difference in the anti-inflammatory effect of indomethacin between acute and chronic stages of carrageenin-induced inflammation. J. Pharmacobio-Dyn. 1978;1:8–14. [Google Scholar]
- YAMASHITA M., ICHINOWATARI G., YAMAKI K., OHUCHI K. Inhibition by auranofin of the production of prostaglandin E2 and nitric oxide in rat peritoneal macrophages. Eur. J. Pharmacol. 1999;368:251–258. doi: 10.1016/s0014-2999(99)00053-9. [DOI] [PubMed] [Google Scholar]
- ZAUBERMAN H., MICHAELSON I.C., BERGMAN F., MAURICE D.M. Stimulation of neovascularization of the cornea by biogenic amines. Exp. Eye Res. 1969;8:77–83. doi: 10.1016/s0014-4835(69)80083-7. [DOI] [PubMed] [Google Scholar]


