Abstract
The hepatic CYP4A-dependent ω-hydroxylation of arachidonic acid and CYP2C11-dependent 2α-/16α-hydroxylations of testosterone were decreased to 74 and 60% of respective control in microsomal fractions from vitamin A-deficient rats. Decreases in the rates of arachidonic acid ω-1-hydroxylation and testosterone 6β-, 7α- and 17α-hydroxylations were less pronounced.
Corresponding decreases in microsomal CYP4A and CYP2C11 immunoreactive protein expression to 64 and 68% of respective control were observed in vitamin A-deficient rat liver. Expression of CYP3A proteins was unchanged from vitamin A-adequate control.
Northern analysis revealed a selective decrease in CYP4A2 mRNA expression in vitamin A-deficient rat liver to ∼5% of control; expression of the related CYP4A1/4A3 mRNAs was not decreased. CYP2C11 mRNA expression was also decreased in vitamin A-deficient male rat liver to 39% of control levels.
Intake of the deficient diet containing all-trans-retinoic acid (ATRA) during the final week of the experiment restored CYP4A2 mRNA and CYP4A protein. Administration of exogenous androgen or episodic growth hormone was ineffective. In contrast, CYP2C11 expression was restored by ATRA and androgen, but not by growth hormone.
From these studies it emerges that CYP4A2, a fatty acid ω-hydroxylase in rat liver, is highly dependent on vitamin A for optimal expression, whereas CYP2C11 is indirectly down regulated by androgen deficiency resulting from vitamin A-deficiency. Altered CYP expression in vitamin A-deficiency provides insights into the relationship between dietary constituents and the intracellular formation of vasoactive eicosanoids as well as the clearance of androgenic steroids.
Keywords: Cytochrome P450, vitamin A, all-trans-retinoic acid, hepatic microsomal fatty acid hydroxylation, hepatic microsomal steroid hydroxylation
Introduction
Male-specific cytochromes P450 (CYPs) in rat liver, including the steroid 2α-/16α-hydroxylase CYP2C11 (Figure 1) and the fatty acid ω-hydroxylase CYP4A2, are regulated by growth hormone (GH) acting in conjunction with androgen and other hormones (Gustafsson et al., 1983; Morgan et al., 1985; Skett, 1987; Sundseth & Waxman, 1992). Many CYP-mediated drug and endobiotic oxidations are deactivation processes and factors that influence hepatic CYP expression modify the duration of action of such agents. However, it is also apparent that CYP-mediated arachidonic acid oxidation generates terminal hydroxyeicosatetraenoic acids (HETEs; Figure 1) that modulate vascular reactivity in tissues (Fitzpatrick & Murphy, 1989; Harder et al., 1997; Wang et al., 1999).
Figure 1.
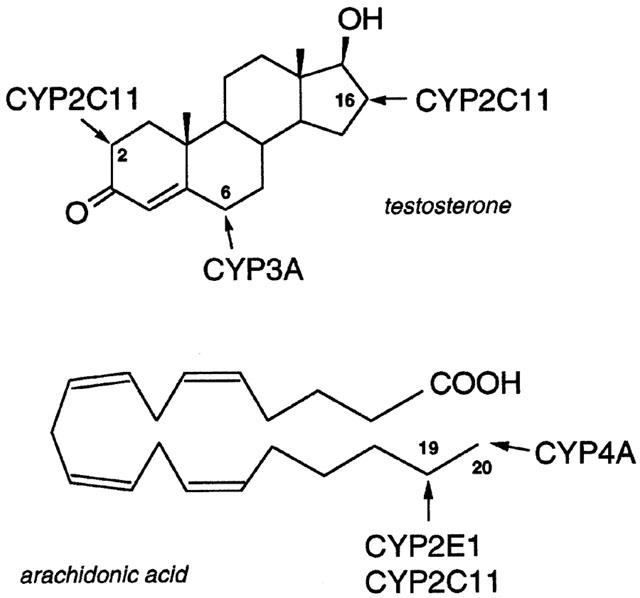
Oxidation of testosterone and arachidonic acid by rat hepatic CYPs.
Dietary factors also modulate hepatic CYP expression and function, but there is little information on the specific CYPs involved or the mechanisms underlying these processes. Down regulation of the androgen-dependent CYP2C11 has been documented in vitamin A-deficient male rat liver, which coincided with a decline in circulating testosterone concentrations (Martini et al., 1995). Replacement of dietary vitamin A esters with all-trans-retinoic acid (ATRA) prevented the loss of hepatic CYP2C11 expression and circulating androgen produced by vitamin A-deficiency (Martini et al., 1995). Subsequently, administration of androgen to vitamin A-deficient male rats was found to restore hepatic CYP2C11 expression and function (Murray et al., 1996).
Three members of the CYP4A subfamily have been identified in rat liver (Kimura et al., 1989a, 1989b; Tamburini et al., 1984). On the basis of the limited information that is available, these proteins appear to possess similar functional properties and also appear to be regulated similarly (Aoyama et al., 1990; Sundseth & Waxman, 1992; Wang et al., 1999). Thus, the microsomal CYPs 4A are fatty acid hydroxylases that are inducible in rat liver by ligands of the peroxisome proliferator-activated receptors (PPARs) (Bell et al., 1991; Sharma et al., 1989; Sundseth & Waxman, 1992; Tamburini et al., 1984), which are members of the steroid hormone receptor superfamily that act, in conjunction with the 9-cis-retinoic acid receptor (retinoid X receptor, RXR), as transcriptional activators of a number of target genes (Kliewer et al., 1992). Previous studies have reported that vitamin A status modulates the response of hepatic genes such as CYPs 4A to peroxisome proliferators (PPs), like perfluorooctanoic acid (Sohlenius et al., 1996), and the expression of nuclear hormone receptors, including those for vitamin A and thyroid hormone (Higueret et al., 1992). The present study was undertaken because of the association between dietary vitamin A intake, which might modulate the intracellular availability of 9-cis-retinoic acid for binding to RXR, and the expression of PPAR-responsive genes such as CYPs 4A.
The major finding to emerge from the present study was that CYP4A2 expression is acutely sensitive to dietary vitamin A intake, more so than the related CYPs 4A1 and 4A3. Down regulation of CYP4A mRNAs in vitamin A-deficient male rat liver also decreased CYP4A immunoreactive protein and the formation of 20-hydroxyeicosatetraenoic acid (20-HETE) from arachidonic acid. Inclusion of ATRA in diets that were otherwise deficient in vitamin A restored CYP4A2 expression and function to levels found in vitamin A-adequate control rat liver. Thus, the present findings establish a mechanistic link between dietary factors and the expression of CYP4A proteins that are modulators of vasoactivity in a range of tissues (Fitzpatrick & Murphy, 1989; Harder et al., 1997; Wang et al., 1999).
Methods
Chemicals
[α-32P]-dCTP (specific activity 3000 Ci mmol−1), Hyperfilm, ACS II, Hybond-N+ filters, [4-14C]-testosterone (specific activity ca. 55 mCi mmol−1) and reagents for enhanced chemiluminescence were obtained from Amersham Pharmacia Biotech Australia (Castle Hill, NSW, Australia). All-trans-retinoic acid (ATRA), retinyl acetate and unlabelled steroids were from the Sigma Chemical Co. (St. Louis, MO, U.S.A.). Deoxynucleotidyl transferase and other biochemicals were obtained from Sigma or Boehringer-Mannheim (Castle Hill, NSW, Australia) and methyltrienolone (17β-hydroxy-17-methylestra-4,9,11-trien-3-one; MT) was purchased from DuPont-New England Nuclear (North Ryde, NSW, Australia). Human recombinant growth hormone (hGH) was provided generously by Aza Research (North Ryde, NSW, Australia). Reagents used in gel electrophoresis were obtained from Bio-Rad (Richmond, CA, U.S.A.). HPLC grade solvents were obtained from Rhone-Poulenc Chemicals (Baulkham Hills, Australia) and analytical grade reagents were from Ajax Chemicals (Sydney, Australia).
Animal treatments
Studies were performed in accordance with the guidelines of the Australian National Health and Medical Research Council and were approved by the Institutional Animal Care and Ethics Committee. Male Wistar rats (ca. 3 weeks of age, ca. 60 g) were held under conditions of constant temperature and lighting (12 h light – dark cycle). Animals had free access to the vitamin A-deficient diet (ICN biochemicals, Seven Hills, NSW, Australia) over the 10 week experimental period; vitamin A-adequate control rats received the deficient diet that was supplemented with retinyl acetate (25 IU g−1). After body weight had plateaued in the vitamin A-deficient animals (by 8 – 9 weeks of dietary conditioning), subsequent in vivo treatments were initiated. In one experiment animals received the deficient diet that was supplemented with 12 μg ATRA g−1 (Martini et al., 1995) for a further 7 days. In another study MT was administered to vitamin A-deficient rats at a dose of 0.625 mgkg−1 in propylene glycol by subcutaneous injection once daily for 5 days; treatment controls received solvent alone (Murray et al., 1996). In a further experiment vitamin A-deficient rats were administered recombinant human growth hormone (hGH; Aza Research, West Ryde, NSW, Australia) in saline at a dose of 12.5 μg 100 g−1 body weight by subcutaneous injection at 12 h intervals for 7 days (Waxman et al., 1991), during which animals were allowed free access to water and the appropriate diet.
Animals were killed by exsanguination under enflurane anaesthesia, livers were removed and perfused with ice cold saline, and part of the tissue (∼1 gram) was frozen in liquid nitrogen for the subsequent extraction of total RNA and determination of hepatic CYP mRNAs. The remainder was used in the preparation of hepatic microsomes by differential ultracentrifugation. Briefly, tissue was homogenized in 50 mM potassium phosphate buffer, (pH 7.4) containing 1 mM EDTA and 100 mM sucrose using a Potter-Elvehjem homogenizer and centrifuged at 10,000×g for 10 min. The supernatant was ultracentrifuged at 105,000×g for 60 min, followed by resuspension in buffer and resedimentation at 105,000×g for 30 min. The final microsomal pellets obtained were resuspended in 50 mM potassium phosphate buffer, pH 7.4, that contained 20% glycerol and 1 mM EDTA, snap frozen in liquid nitrogen and stored at −70°C until required in experiments (Murray et al., 1999).
Microsomal arachidonic acid hydroxylation and analysis of HETE formation by gas chromatography-mass spectrometry
The microsomal hydroxylation of arachidonic acid (30 μM) was determined in similar fashion to that described by Schwartzmann et al. (1990), except that unlabelled substrate was employed. Incubations (0.25 ml) contained 25 μg microsomal protein, were conducted for 5 min at 37°C in 0.1 M phosphate buffer, pH 7.4) and were initiated with NADPH (1 mM). Reactions were terminated by the addition of glacial acetic acid (0.2 ml), placed in an ice bath, 20-HETE-d2 (10 ng) was added (internal standard) and the mixture was extracted with ethyl acetate (1 ml). The extract was evaporated under a stream of nitrogen and the residue was treated with a mixture of pentafluorobenzyl bromide (40 μl, 10% in acetonitrile) and DIPEA (20 μl, 10% in acetonitrile) at room temperature for 30 min, followed by evaporation under nitrogen to yield the HETE pentafluorobenzyl esters. The sample was then treated with bis(trimethylsilyl)-trifluoroacetamide (20 μl) and anhydrous pyridine (20 μl) at 45°C for 20 min to yield the trimethylsilylethers. For analysis samples were run on a Hewlett-Packard 5890 Series II gas chromatograph coupled to a 5989B Mass Spectrometer. Samples were injected onto a HP-5MS column (30 m×0.25 mm, 0.25 μm film thickness) using helium as carrier gas. Samples were run using methane as the reagent gas for electron capture chemical ionization. The initial column temperature was 160°C programmed to 300°C at 20°C min−1. The mass spectrometer was operated in selected ion monitoring mode and HETEs were detected by monitoring m/z 391 (which corresponds to the loss of pentafluorobenzyl radical (M-181) from the molecular ion) and the corresponding m/z 393 ion for 20-HETE-d2 (internal standard). Peaks were identified by comparison with the retention times of authentic standards.
Microsomal steroid hydroxylation
Microsomal testosterone hydroxylation activities were determined by methods described previously (Martini et al., 1995). The 14C-labelled substrate (50 μM, 0.18 μCi) was incubated with 0.15 mg microsomal protein and NADPH (1 mM) at 37°C (0.1 M phosphate buffer, pH 7.4; 0.4 ml). Chloroform (5 ml) was added after 2.5 min, the tubes were transferred to an ice bath and shaken for 10 min. After centrifugation the chloroform phase was evaporated under N2. The residue was applied to thin-layer chromatography (TLC) plates (Merck silica gel 60 F254 type; Darmstadt, Germany) in a small volume of chloroform, run in dichloromethane:acetone (4 : 1) and then chloroform:ethyl acetate:ethanol (4 : 1 : 0.7) with air drying between (Waxman et al., 1983). Radioactive metabolites were located on TLC plates by autoradiography (Hyperfilm-MP; Amersham) over 48 – 60 h and quantified by scintillation counting (ACS II; Amersham). To confirm the identity and homogeneity of the products of microsomal metabolism of 14C-labelled testosterone, radioactive regions corresponding to individual metabolites were scraped from the TLC plates and eluted from the silica with methanol. After application to new TLC plates along with the appropriate unlabelled standard hydroxytestosterone metabolite, the plates were resolved in dichloromethane:acetone (9 : 5) and subjected to autoradiography. Thus, individual metabolites comigrated in two separate solvent systems and radioactivity was localized to one metabolite region. Intra-assay variation was estimated at 3% after analysis of three samples in triplicate. Inter-assay variation was 8% when microsomal fractions (n=3) were analysed on three consecutive days. The linearity of product formation with protein concentration and time was confirmed for each metabolite; substrate conversion was 15±2% in microsomes from control rat liver.
Immunoquantitation of CYPs in rat liver microsomes
The properties of the rabbit anti-rat CYP2C11 immunoglobulin G (IgG) used in this study has been described previously (Murray et al., 1992). The goat anti-rat CYP4A1 and rabbit anti-human CYP2E1 IgGs were generously provided by Professor G.G. Gibson (School of Biological Sciences, University of Surrey, U.K.) and Professor M. Ingelman-Sundberg (Department of Medical Biochemistry and Biophysics, Karolinska Institute, Sweden), respectively. The anti-rat CYP3A IgG was raised in female rabbits against CYP3A that was isolated from hepatic microsomes from dexamethasone-induced female rats according to established methods (Murray et al., 1992). The purified CYP3A catalysed testosterone 2β- and 6β-hydroxylation in reconstituted enzyme systems and the IgG effectively inhibited testosterone 6β-hydroxylation in microsomal fractions from control rat liver.
Rat hepatic microsomes (5 μg per lane; Lowry et al., 1951) were incubated at 100°C for 5 min with 2% sodium dodecyl sulphate and 5% 2-mercaptoethanol and electrophoresed overnight at constant voltage (25 V) through 7.5% polyacrylamide gels (Laemmli, 1971) with minor modifications (Murray et al., 1986). Microsomal proteins were then transferred electrophoretically (1.5 h at 70 V) to nitrocellulose sheets (Towbin et al., 1979) and incubated with the anti-CYP IgG fractions (3.7 μg ml−1) for 120 min. Immunoreactive proteins were detected on nitrocellulose sheets (Protran, Medos Co., Lidcombe, NSW, Australia) by enhanced chemiluminescence (ECL RPN 2106 kit, Amersham Pharmacia Biotech Australia) and autoradiography on Hyperfilm-ECL (Amersham Pharmacia Biotech Australia) and the resultant signals were analysed by densitometry (Bio-Rad GS-700 Imaging Densitometer, Richmond, CA, U.S.A.). The linearity of the response under these conditions was confirmed for each CYP by loading several different quantities of microsomal protein over a 10 fold range and a 4 fold range of IgG concentrations.
Hepatic RNA extraction and analysis of CYP mRNAs
Total RNA was extracted from male rat liver by the guanidinium thiocyanate/CsCl method and quantified spectrophotometrically (Sambrook et al., 1989). Synthetic oligonucleotides were purchased from Bresatec (Adelaide, South Australia). Sequences were: CYP4A1 (5′-TAT-GGG-AAG-GGT-GCT-GGC-TT-3′ (Sundseth & Waxman, 1992), corresponding to the complement of the 3′-untranslated region of the gene from nucleotides 1701 – 1720 (Kimura et al., 1989a), CYP4A2 (5′-GCT-GGG-AAG-GTG-TCT-GGA-GT-3′ (Sundseth & Waxman, 1992), corresponding to the complement of the genomic sequence (Kimura et al., 1989a)), CYP4A3 (5′-ACT-GGG-ATG-GAG-TCT-GGA-GG-3′ (Sundseth & Waxman, 1992), corresponding to the complement of the 3′-untranslated region of the gene from nucleotides 1691 – 1710 (Kimura et al., 1989b)), CYP2C11 (5′-ATC-CAC-GTG-TTT-CAG-CAG-CAG-CAG-GAG-TCC-3′ (Ram & Waxman, 1991), corresponding to the complement of the coding region of the gene from nucleotides 945 – 974 (Yoshioka et al., 1987)), and 18S RNA (5′-CGG-CAT-GTA-TTA-GCT-CTA-GAA-TTA-CCA-CAG-3′, corresponding to complement of the coding region of the gene from nucleotides 151 – 180 (Chan et al., 1984)). Oligonucleotides were labelled with [α-32P]-dCTP and deoxynucleotidyl transferase.
For Northern analysis, RNA (10 μg lane−1) was electrophoresed on 1% agarose in the presence of 2.2 M formaldehyde and then transferred to Hybond-N+ nylon filters (0.45 μm, Amersham). Hybridization and washing conditions were as described previously (Jiang et al., 1994), and signals corresponding to CYP mRNAs were quantified after autoradiography (Hyperfim MP, Amersham Pharmacia Biotech Australia) using a Fuji Phosphorimager. To demonstrate equivalence of RNA loading between samples, filters were stripped and rehybridized to the 18S ribosomal RNA α-32P-labelled probe.
Statistics
Data are presented throughout as means±s.e.mean. All measurements were made in samples from individual rats: n=6 for catalytic measurements, n=4 for protein data and n=3 for mRNA data. Differences between means values from control and treatment groups were detected using one-way analysis of variance in conjunction with the Dunnett's q'-test.
Results
CYP-dependent oxidation of arachidonic acid and testosterone in hepatic microsomes from vitamin A-deficient male rats
Intake of vitamin A-deficient diets by male rats over a 10 week period decreased hepatic and serum stores of vitamin A. Thus, in control rats hepatic retinyl palmitate and serum retinol levels were 150±20 nmol g liver−1 and 1.2±0.1 nmol ml−1, respectively, and, in vitamin A-deficient animals, were <0.3 nmol g liver−1 and <0.02 nmol ml−1, respectively. Several CYP-dependent pathways of arachidonic acid and testosterone hydroxylation (Figure 1) were impaired in hepatic microsomes from vitamin A-deficient male rats. As indicated by the data in Table 1 CYP4A-dependent formation of 20-HETE from arachidonic acid, estimated by GC-MS (Figure 2), was decreased in vitamin A-deficient rat liver to 74% of control (1.34±0.08 nmol mg protein−1 min−1 versus 1.80±0.15 in vitamin A-adequate control; P<0.05) and was restored by inclusion of ATRA in the experimental diet (Table 1). CYP2C11/CYP2E1-mediated ω-1-hydroxylation of arachidonic acid (to 19-HETE) was decreased in vitamin A-deficient rat liver microsomes to 88% of control (P<0.01); this was also reversed by inclusion of ATRA in diets (Table 1). CYP2C11-mediated 2α- and 16α-hydroxylations of testosterone were decreased in microsomes from vitamin A-deficient rat liver (Figure 3) to 58 and 60% of the corresponding activities in vitamin A-adequate control liver (P<0.01 in both cases; Table 1). Inclusion of ATRA in the deficient diet during the final week of the study reversed this defect in CYP2C11 function (Table 1). Two other major pathways of testosterone hydroxylation were differentially affected by dietary vitamin A status. Thus, CYP3A-mediated testosterone 6β-hydroxylation was decreased in hepatic microsomes from vitamin A-deficient rats to about 73% of control (P<0.05), whereas the apparent decrease in microsomal 17α-hydroxylation (to produce androstenedione) did not attain statistical significance. Testosterone was also converted to a number of minor products by microsomal CYPs and oxidoreductases; in total these products constituted approximately 20% of total testosterone biotransformation. Vitamin A-deficiency decreased the formation of these metabolites to 78% of control (P<0.05; Table 1).
Table 1.
Arachidonic acid and testosterone hydroxylation in hepatic microsomes from rats that received vitamin A modified diets
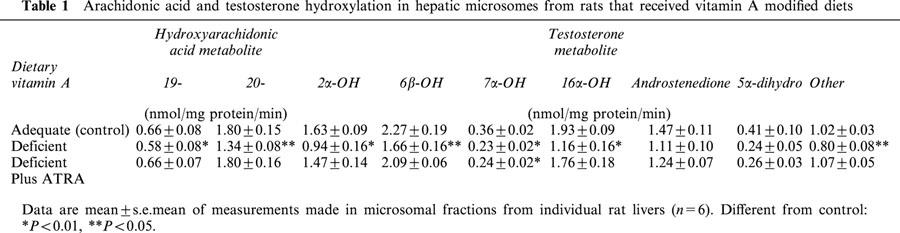
Figure 2.
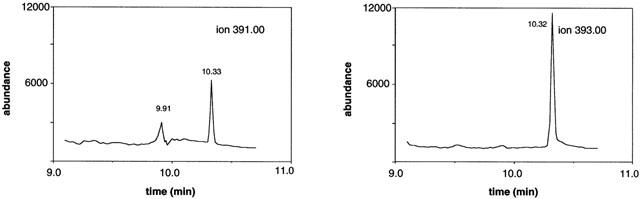
Representative ion chromatogram showing the elution and analysis of (left panel) 19-HETE (ion 391.00; retention time 9.91 min), 20-HETE (ion 391.00; retention time 10.33 min) and (right panel) 20-HETE-d2 (internal standard; ion 393.00; retention time 10.32 min) during gas chromatography-mass spectrometry.
Figure 3.
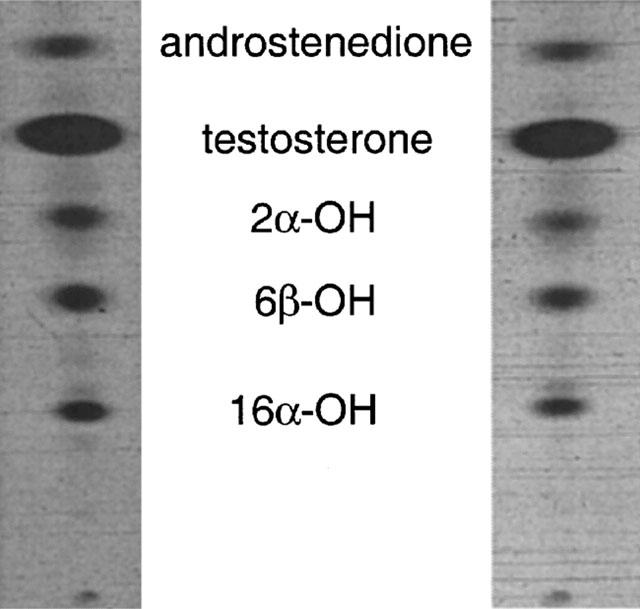
Formation of oxidized testosterone metabolites in hepatic microsomes from (left panel) control and (right panel) vitamin A-deficient rats.
CYP expression in microsomal fractions from control and vitamin A-deficient male rats
Immunoblotting experiments were undertaken with CYP-specific IgG fractions to corroborate the findings from arachidonic acid and testosterone hydroxylation measurements (Figure 4). In agreement with measurements of microsomal 20-HETE formation, expression of CYP4A immunoreactive protein was decreased to 64±5% of adequate control in vitamin A-deficient male rat liver (P<0.01; Figure 4); inclusion of ATRA in diets restored CYP4A expression (82±7% of control). In contrast, CYP2E1 expression in vitamin A-deficient rat liver was unchanged from control but, consistent with earlier reports from this laboratory, CYP2C11 apoprotein content was decreased to 68±9% of control (P<0.05; Figure 4). Down regulation of CYP2C11 in microsomes from vitamin A-deficient rat liver was reversed by inclusion of ATRA in the deficient diet during the final week of the study (week 10). Thus, impaired expression of CYP2C11 and not CYP2E1 appears more likely to be responsible for the decreased rates of arachidonic acid 19-hydroxylation as well as the decline in testosterone 2α- and 16α-hydroxylations. CYP3A apoprotein levels in vitamin A-deficient rat liver were decreased slightly to 85±21% of control, but this effect did not attain statistical significance. CYP4A and CYP2C11, but not CYP2E1 or CYP3A, are therefore important endobiotic-metabolizing CYPs that are down regulated in vitamin A-deficiency.
Figure 4.
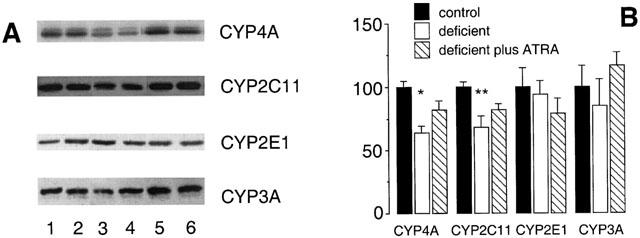
Western analysis of CYP proteins in rat hepatic microsomal fractions after intake of vitamin A modified diets for 10 weeks. (A) Lanes 1,2 (vitamin A-adequate control), lanes 3,4 (vitamin A-deficient diet) and lanes 5,6 (vitamin A- deficient diet for 9 weeks followed by the deficient diet supplemented with ATRA 12 mg kg−1 for a further 7 days). (B) CYP protein expression after densitometric analysis (percentage of control). Different from control; *P<0.01, **P<0.05; data are means±s.e.mean of estimates in four individual animals per group.
Expression of CYP mRNAs in vitamin A-deficient male rat liver
The effect of vitamin A-deficiency on CYP4A and CYP2C11 expression was explored further at the mRNA level. CYP4A2 mRNA was found to be highly dependent on vitamin A status, whereas the hepatic expression of the closely related CYP4A1 and CYP4A3 mRNAs was less dependent on the vitamin (Figure 5A). Inclusion of ATRA in the deficient diet over the final week of dietary manipulation reversed the down regulation of hepatic CYP4A2 expression produced by dietary vitamin A-deficiency (5±1% of vitamin A-adequate control; P<0.05; Figure 5B). By comparison, effects of dietary vitamin A-deficiency on CYP4A1 mRNA (77±8% of adequate control) and CYP4A3 mRNA (91±5% of adequate control; Figure 5B) were not significant. Intake of the vitamin A-deficient diet for 10 weeks also decreased the expression of CYP2C11 mRNA (to 39±15% of control; P<0.01; Figure 5B), an effect that was also reversed by intake of the ATRA-containing vitamin A-deficient diet, during the final week of the experiment.
Figure 5.
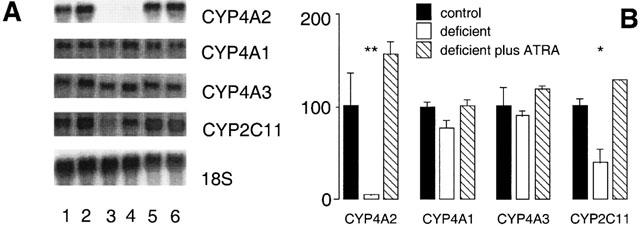
Northern analysis of CYP mRNAs in liver of rats after intake of vitamin A modified diets for 10 weeks. (A) Lanes 1,2 (vitamin A-adequate control), lanes 3,4 (vitamin A-deficient diet) and lanes 5,6 (vitamin A-deficient diet for 9 weeks followed by the deficient diet supplemented with ATRA 12 mg kg−1 for a further 7 days). The 18S rRNA signal was used to calibrate RNA loading between lanes. (B) Relative expression of CYP mRNAs in rat liver (percentage of control). Different from control; *P<0.01, **P<0.05; data are means±s.e.mean of estimates in three individual animals per group.
In vivo modulation of CYP expression in liver of vitamin A-deficient rats by exogenous androgen and GH
The expression of CYP4A2 and CYP2C11 in rat liver is male gender specific (Sundseth & Waxman, 1992) and dependent on androgen and episodic GH secretion by the pituitary. Since testicular androgen output is partially dependent on vitamin A (Appling & Chytil, 1981; Martini et al., 1995), androgen (MT) was administered to vitamin A-deficient rats during the final week of dietary conditioning. The decrease in hepatic CYP4A2 mRNA content in vitamin A-deficiency was unaffected by exogenous androgen (remained at 22±3% of control), whereas, consistent with earlier findings (Murray et al., 1996), CYP2C11 mRNA expression was restored to control levels (Figure 6). These findings were complemented with data from immunoblotting experiments. CYP4A apoprotein was again decreased in vitamin A-deficient rat liver (to 68±6% of control; P<0.01; Figure 7) but expression remained impaired after androgen administration (60±4% of control; P<0.01). In contrast, the decrease in microsomal CYP2C11 protein elicited by dietary vitamin A-deficiency (to 63±3% of control; Figure 7) was reversed by androgen (99±10% of control). Consistent with earlier experiments, CYP2E1 and CYP3A apoprotein contents were not influenced significantly by dietary vitamin A intake and were similarly unaffected by MT administration (Figure 7).
Figure 6.
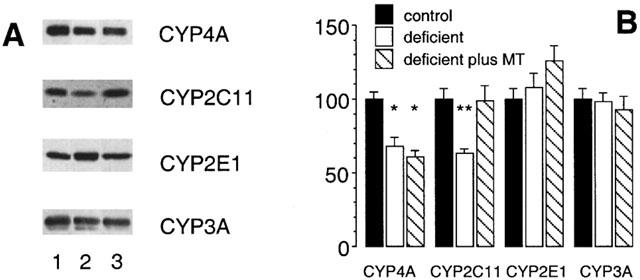
Northern analysis of CYP mRNAs in liver of rats after intake of vitamin A modified diets for 10 weeks. (A) Lane 1 (vitamin A-adequate control), lane 2 (vitamin A-deficient diet) and lane 3 (vitamin A-deficient diet for 10 weeks and MT administration 0.625 mg kg−1 s.c. daily during week 10 of the experiment). (B) Relative expression of CYP mRNAs in rat liver (percentage of control). Different from control; *P<0.01, **P<0.05; data are means±s.e.mean of estimates in three individual animals per group.
Figure 7.
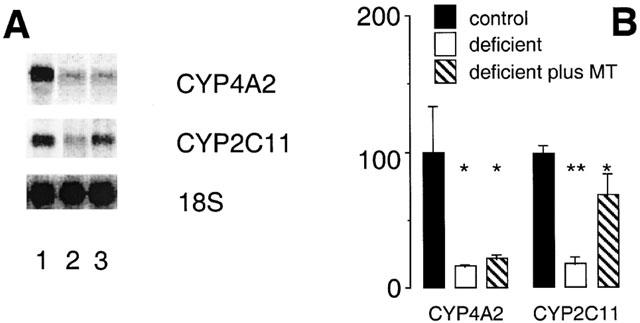
Western analysis of CYP proteins in rat hepatic microsomal fractions after intake of vitamin A modified diets for 10 weeks. (A) Lane 1 (vitamin A-adequate control), lane 2 (vitamin A-deficient diet) and lane 3 (vitamin A-deficient diet for 10 weeks and MT administration 0.625 mg kg−1 s.c. daily during week 10 of the experiment). (B) CYP protein expression after densitometric analysis (percentage of control). Different from control; *P<0.01, **P<0.05; data are means±s.e.mean of estimates in four individual animals per group.
GH also participates in the regulation of several CYPs in rat liver, including CYP4A2 (Sundseth & Waxman, 1992). In the present study GH was administered to vitamin A-adequate and vitamin A-deficient male rats to test whether GH deficiency may contribute to the impaired expression of CYP4A2 and CYP2C11 in vitamin A-deficient rat liver. A regimen involving the episodic administration of GH by subcutaneous injection, which has been documented previously to restore CYP2C11 expression in hypophysectomized male rats (Waxman et al., 1991), was employed during the final week of the experimental period. However, 12-hourly administration of GH in order to mimic the pulsatile release of GH by the male rat pituitary did not restore CYP4A or CYP2C11 immunoreactive protein (Figure 8), and was also without significant effect on the expression of either CYP2E1 or CYP3A (Figure 8). Further, the decrease in CYP4A2 mRNA expression produced by vitamin A-deficiency (to 17±1% of adequate control; P<0.01; Figure 9) was not reversed by exogenous GH (26±3% of adequate control; P<0.01; Figure 9). Similarly, exogenous GH did not reverse the decrease in CYP2C11 mRNA expression produced by vitamin A-deficiency (Figure 9B).
Figure 8.
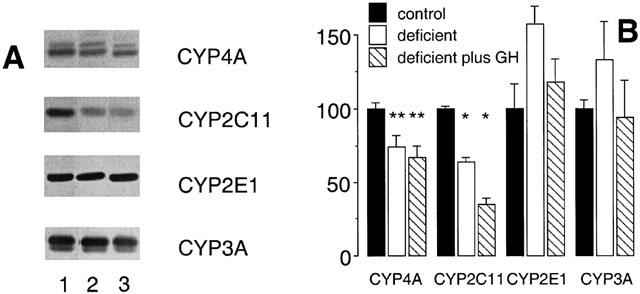
Western analysis of CYP proteins in rat hepatic microsomal fractions after intake of vitamin A modified diets for 10 weeks. (A) Lane 1 (vitamin A-adequate control), lane 2 (vitamin A-deficient diet) and lane 3 (vitamin A-deficient diet) for 10 weeks and GH administration 0.125 mg kg−1 s.c. 12 hourly during week 10 of the experiment). (B) CYP protein expression after densitometric analysis (percentage of control). Different from control; *P<0.01, **P<0.05; data are means±s.e.mean of estimates in four individual animals per group.
Figure 9.
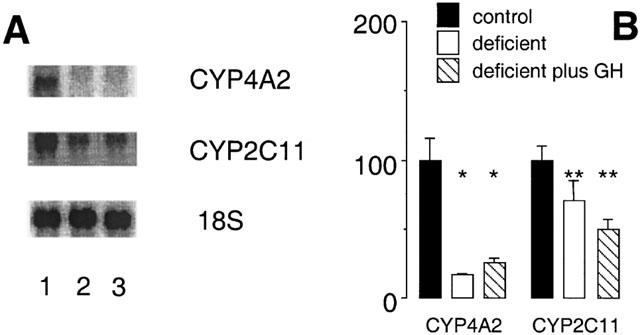
Northern analysis of CYP mRNAs in liver of rats after intake of vitamin A modified diets for 10 weeks. (A) Lane 1 (vitamin A-adequate control), lane 2 (vitamin A-deficient diet) and lane 3 (vitamin A-deficient diet for 10 weeks GH administration 0.125 mg kg−1 s.c. 12 hourly during week 10 of the experiment). (B) Relative expression of CYP mRNAs in rat liver (percentage of control). Different from control; *P<0.01, **P<0.05; data are means±s.e. mean of estimates in three individual animals per group.
Discussion
Dietary factors have been shown previously to modulate the activity of hepatic CYPs involved in endobiotic and xenobiotic oxidation (Walter-Sack & Klotz, 1996), but there is a deficiency of mechanistic information to account for such changes. From the present study the microsomal CYP-dependent oxidations of endobiotics, such as fatty acids and steroids that have important physiological roles in intracellular signalling pathways, are impaired in the liver of vitamin A-deficient rats. Thus, the microsomal CYP4A-mediated formation of 20-HETE from arachidonic acid and CYP2C11-dependent 2α-/16α-hydroxylations of testosterone were decreased in rat liver after intake of the vitamin A-deficient diet. Consistent with these observations the microsomal expression of CYP4A and CYP2C11 proteins was also decreased in vitamin A-deficient male rat liver. Northern analysis indicated that the decrease in CYP4A immunoreactive protein was due to a selective impairment of CYP4A2 mRNA; the closely related CYPs 4A1 and 4A3 were essentially unchanged from control. As reported in earlier studies, CYP2C11 mRNA is decreased in vitamin A-deficient rat liver (Martini et al., 1995). Thus, the dysregulation of endobiotic oxidizing CYPs in rat liver caused by dietary vitamin A-deficiency occurs at a pretranslational level.
The microsomal formation of 19-HETE from arachidonic acid was also decreased significantly in rat liver, although this was somewhat less pronounced than the decrease in 20-HETE formation. Arachidonic acid 19-hydroxylation activity has been attributed to several CYPs in rat liver, most notably CYP2E1 and CYP2C11 (Falck et al., 1990; Laethem et al., 1993). Since CYP2E1 expression was refractory to vitamin A-deficiency it appears that the decline in CYP2C11 expression is most likely to be responsible for the observed effects on rates of 19-HETE formation. This decrease in CYP2C11 expression is also responsible for the impaired capacity of microsomal androgen 2α-/16α-hydroxylation in vitamin A-deficient rat liver. Apart from CYP2C11, the function of other CYP-dependent steroid hydroxylases was also decreased in vitamin A-deficient rat liver. Thus, intake of the deficient diet decreased the rate of CYP3A-mediated testosterone 6β-hydroxylation, although this was less pronounced than the loss of CYP2C11-mediated oxidation pathways. CYP3A immunoreactive protein appeared to be slightly decreased in vitamin A-deficient rat liver but this did not attain statistical significance. This may be due to the multiplicity of the CYP3A subfamily. Thus, at least four members of this CYP subfamily have been identified in rat liver (Mahnke et al., 1997; Miyata et al., 1994; Nagata et al., 1990; 1999), and it is possible that individual CYP3A proteins that are more active in steroid 6β-hydroxylation but that are expressed at relatively low levels in liver may be down regulated by dietary vitamin A-deficiency.
Factors that influence the expression of CYP4A protein may contribute to marked effects on hepatocellular homeostasis because of the established role of 20-HETE in vasoactivity (Wang et al., 1999). The finding that hepatic CYP4A expression was impaired in vitamin A-deficiency may provide insight into the mechanisms by which diet influences the formation of vasoactive eicosanoids from arachidonic acid that elicit consequent effects on hepatic vascular tone. Similarly, CYP2C11 has been shown to be down regulated in models of chronic liver disease (Murray et al., 1987a,1987b), portal bypass (Cantrill et al., 1989) and inflammatory processes mediated by cytokines (Morgan, 1989; Wright & Morgan, 1990). Impaired hepatic androgen extraction because of decreased expression of CYP-hydroxylases increases the extrahepatic availability of substrate for oestrogen formation by aromatase activity in adipose tissue, thus contributing to hypogonadism and other hormonal imbalances that have been observed in liver disease (Gordon et al., 1975). Decreased activity of CYP2C11 in vitamin A-deficient rat liver may contribute by a similar mechanism to the impaired testicular function and androgen production reported previously in these animals (Rich et al., 1979; Martini et al., 1995).
The mechanisms that are responsible for CYP down regulation in dietary vitamin A-deficiency were evaluated in a series of in vivo restitution experiments. ATRA, the biologically active form of vitamin A that serves as the endogenous ligand for retinoic acid receptors (Glass et al., 1991), but which is normally absent from the diet, effectively substituted for dietary vitamin A esters to restore CYP4A2 and CYP2C11 in vitamin A-deficient rat liver. In these studies the deficient diet was supplemented with ATRA during the final (tenth) week of the experiment. This is complementary to previous studies in which supplementation of the deficient diet with ATRA over the entire period prevented the decline in CYP2C11 produced by vitamin A-deficiency (Martini et al., 1995). Thus, both short- and long-term ATRA can substitute for dietary vitamin A in the maintenance of CYP expression in liver.
CYP4A2 exhibits a male-specific pattern of expression in rat liver and, like CYP2C11, its basal expression is regulated by hormonal factors (Sundseth & Waxman, 1992). In male rats androgen modulates the pulsatility of pituitary GH release mediated via the hypothalamo-pituitary-gonadal axis (Legraverend et al., 1992; Waxman et al., 1991; 1995). The present study provides evidence of a link between vitamin A intake and hormonal perturbations that lead to impaired expression of male-specific hepatic CYPs. Vitamin A status has been shown previously to influence androgen biosynthesis by testicular Leydig cells (Chaudhary et al., 1989) and vitamin A-deficiency in male rats causes Leydig cell dysfunction (Rich et al., 1979). The decrease in serum testosterone that has been reported in vitamin A-deficient male rats (Martini et al., 1995) is consistent with the down regulation of male-specific CYPs. Indeed, administration of androgen to vitamin A-deficient male rats has been found to restore CYP2C11 without influencing vitamin A status: the animals remained vitamin A-deficient (Murray et al., 1996). However, from the present study, regulation of CYP4A2 mRNA by vitamin A appears more complex and administration of the exogenous androgen MT did not restore its expression in rat liver.
Pituitary GH gene transcription in regulated in part by ATRA (Bedo et al., 1989), and circulating GH profiles are modulated by dietary vitamin A intake (Mallo et al., 1992). The restoration of hepatic CYP2C11 in vitamin A-deficient rats by exogenous androgen clearly suggests that GH pulsatility was restituted adequately. However, attempts to restore CYP2C11 and CYP4A2 in vitamin A-deficient rat liver with exogenous GH according to a regimen that stimulated the expression of GH-regulated CYPs in liver of hypophysectomized male rats were unsuccessful. These findings are consistent with the assertion that vitamin A-deficiency is associated with perturbation, but not the abolition, of GH secretion by the rat pituitary. Thus, superimposition of exogenous GH pulses over circulating levels of endogenous GH was inadequate for the restoration of CYP expression in rat liver.
Considered together, several possibilities may contribute to the observations from restitution experiments. Androgen may regulate hepatic CYP4A2 mRNA expression more tightly than CYP2C11 mRNA. A number of studies have successfully restored CYP2C11 expression in liver of hypophysectomized male rats by several regimen even though the resultant plasma GH profiles were unphysiological (Waxman et al., 1991); CYP4A2 expression may be more sensitive to circulating GH pulses. In this regard the studies of Sundseth & Waxman (1992) highlight several differences between the expression of CYP4A2 and other male-specific CYPs.
The basal regulation of hepatic CYPs 4A has not been explored. In contrast, a number of studies have established their inducibility by PP chemicals, such as clofibric acid (Gibson et al., 1982; Tamburini et al., 1984), which are ligands for PPARα. PPARs are known to form heterodimers with the RXR, a common partner for many nuclear receptors, to activate target genes (Kliewer et al., 1992). Heterodimers between PPARα and RXRα are unusual in that the RXRα ligand 9-cis-retinoic acid enhances the peroxisome proliferator-dependent activation of PPARα-responsive genes (Kliewer et al., 1992). The possible association between dietary vitamin A intake, which might influence 9-cis-retinoic acid availability, and PPAR-mediated gene activation was investigated in the present study. Although CYPs 4A1-3 are all responsive to PPs (Sundseth & Waxman, 1992), the present study found that CYP4A2 was selectively impaired in vitamin A-deficiency. Moreover, studies in a PPARα-null mouse line established that, although induction by PP chemicals was lost, the basal hepatic expression of CYPs 4A was unchanged from wild type control (Peters et al., 1996). Thus, it appears clear that signalling pathways, distinct from PPARα/RXRα which mediates CYP4A induction, are operative in the basal expression of these genes.
The JAK-STAT system is implicated in the regulation of GH-responsive genes, such as CYP2C11, in rat liver (Waxman et al., 1995). Thus, GH pulses received at the hepatocyte activate JAK kinase trans-autophosphorylation, which in turn promotes the nuclear accumulation of tyrosine phosphorylated STAT5b (Darnell, 1997; Waxman et al., 1991). The action of nuclear and/or cytosolic SH2-containing phosphatases returns the tyrosine phosphorylated STAT5b to a GH-responsive state following activation of target gene transcription (Ram & Waxman, 1997; Yu et al., 2000). Androgen plays a significant role in this process in that it maintains the episodic nature of pituitary GH release. In androgen deficiency continuous GH pulses would be expected to impair the responsiveness of the JAK-STAT system, leading to CYP2C11 down regulation. A similar process may contribute to the regulation of CYP4A2, which is also male gender-specific and GH responsive (Sundseth & Waxman, 1992).
The absorption of vitamin A via the oral route and the intracellular concentration of ATRA are tightly controlled (Blomhoff et al., 1992). However, retinol and ATRA have been shown to upregulate the expression of certain CYPs in rat liver after in vivo injection, which bypasses intestinal regulation of absorption, (Wright et al., 1997) or in primary hepatocytes cultured on matrigel (Westin et al., 1993). From the present experiments the augmentation of vitamin A-adequate diets with ATRA and the intraperitoneal administration of ATRA did not increase hepatic CYP4A2 mRNA expression in rat liver (not shown). Thus, vitamin A does not appear to have a direct role in the maintenance of CYP4A2 expression in male rat liver.
In summary, it is apparent from the present findings that individual hormone-regulated CYPs in rat liver are regulated differently by dietary micronutrients. CYP2C11 is down regulated in vitamin A-deficiency by androgen deficiency that may be overcome by exogenous androgen. In contrast, CYP4A2 appears to be down regulated by vitamin A-deficiency per se since the administration of ATRA, but not exogenous MT, restores expression in liver. It is possible that CYP4A2 is regulated directly by an intermediary signalling molecule that is dependent on vitamin A but not androgen for expression.
Acknowledgments
This study was supported by a grant from the Australian National Health and Medical Research Council. The gifts of anti-CYP4A1 and anti-CYP2E1 IgGs from Professors G. Gibson, University of Surrey, England and M. Ingelman-Sundberg, Karolinska Institute, Sweden, respectively, are gratefully acknowledged. The recombinant human growth hormone was generously provided by Aza Research Pty Ltd. The technical assistance of Kim Lorenzo in the GCMS analysis and Louise Nadin in the preparation of the rat CYP3A protein and anti-CYP3A IgG is also acknowledged.
Abbreviations
- ATRA
all-trans-retinoic acid
- CYP
cytochrome P450
- GH
growth hormone
- HETE
hydroxyeicosatetraenoic acid
- IgG
immunoglobulin G
- MT
methyltrienolone (17β-hydroxy-17-methylestra-4,9,11-trien-3-one)
- PP
peroxisome proliferator
- PPAR
peroxisome proliferator-activated receptor
- RXR
retinoid X (9-cis-retinoic acid) receptor
References
- AOYAMA T., HARDWICK J.P., IMAOKA S., FUNAE Y., GELBOIN H.V., GONZALEZ F.J. Clofibrate-inducible rat hepatic P450s IVA1 and IVA3 catalyze the ω- and (ω-1)-hydroxylation of fatty acids and the ω-hydroxylation of prostaglandins E1 and F2a. J. Lipid Res. 1990;31:1477–1482. [PubMed] [Google Scholar]
- APPLING D.R., CHYTIL F. Evidence of a role for retinoic acid (vitamin A-acid) in the maintenance of testosterone production in male rats. Endocrinology. 1981;108:2120–2123. doi: 10.1210/endo-108-6-2120. [DOI] [PubMed] [Google Scholar]
- BEDO G., SANTISTEBAN P., ARANDA A. Retinoic acid regulates growth hormone gene expression. Nature. 1989;339:231–234. doi: 10.1038/339231a0. [DOI] [PubMed] [Google Scholar]
- BELL D.R., BARS R.G., GIBSON G.G., ELCOMBE C.R. Localization and differential induction of cytochrome P450IVA and acyl-CoA oxidase in rat liver. Biochem. J. 1991;275:247–252. doi: 10.1042/bj2750247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOMHOFF R., GREEN M.H., NORUM K.R. Vitamin A: physiological and biochemical processing. Ann. Rev. Nutr. 1992;12:37–57. doi: 10.1146/annurev.nu.12.070192.000345. [DOI] [PubMed] [Google Scholar]
- CANTRILL E., MURRAY M., MEHTA I., FARRELL G.C. Down-regulation of the male-specific steroid 16α-hydroxylase, cytochrome P-450 UT-A, in male rats with portal bypass: Relevance to estradiol accumulation and impaired drug metabolism in hepatic cirrhosis. J. Clin. Invest. 1989;83:1211–1216. doi: 10.1172/JCI114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN Y.L., GUTELL R., NOLLER H.F., WOOL I.F. The nucleotide sequence of a rat ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18S ribosomal ribonucleic acid. J. Biol. Chem. 1984;259:224–230. [PubMed] [Google Scholar]
- CHAUDHARY L.R., HUTSON J.C., STOCCO D.M. Effect of retinol and retinoic acid on testosterone production by rat Leydig cells in primary culture. Biochem. Biophys. Res. Comm. 1989;158:400–406. doi: 10.1016/s0006-291x(89)80061-0. [DOI] [PubMed] [Google Scholar]
- DARNELL J.E., JR STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- FALCK J.R., LUMIN S., BLAIR I., DISHMAN E., MARTIN M.V., WAXMAN D.J., GUENGERICH F.P., CAPDEVILA J.H. Cytochrome P-450-dependent oxidation of arachidonic acid to 16-, 17-, and 18-hydroxyeicosatetraenoic acids. J. Biol. Chem. 1990;265:10244–10249. [PubMed] [Google Scholar]
- FITZPATRICK F.A., MURPHY R.C. Cytochrome P-450 metabolism of arachidonic acid: Formation and biological actions of “epoxygenase”-derived eicosanoids. Pharmacol. Rev. 1989;40:229–241. [PubMed] [Google Scholar]
- GIBSON G.G., ORTON T.C., TAMBURINI P.P. Cytochrome P-450 induction by clofibrate. Purification and properties of a hepatic cytochrome P-450 relatively specific for the 12- and 11-hydroxylation of dodecanoic acid (lauric acid) Biochem. J. 1982;203:161–168. doi: 10.1042/bj2030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASS C.K., DIRENZO J., KUROKAWA R., HAN Z. Regulation of gene expression by retinoic acid receptors. DNA Cell. Biol. 1991;10:623–638. doi: 10.1089/dna.1991.10.623. [DOI] [PubMed] [Google Scholar]
- GORDON G.G., OLIVO J., RAFII F., SOUTHREN A.L. Conversion of androgens to estrogens in cirrhosis of the liver. J. Clin. Endocrinol. Metab. 1975;40:1018–1026. doi: 10.1210/jcem-40-6-1018. [DOI] [PubMed] [Google Scholar]
- GUSTAFSSON J.-Å., MODE A., NORSTEDT G., SKETT P. Sex steroid induced changes in hepatic enzymes. Ann. Rev. Physiol. 1983;45:51–60. doi: 10.1146/annurev.ph.45.030183.000411. [DOI] [PubMed] [Google Scholar]
- HARDER D.R., LANGE A.R., GEBREMEDHIN D., BIRKS E.K., ROMAN R.J. Cytochrome P450 metabolites of arachidonic acid as intracellular signaling molecules in vascular tissue. J. Vasc. Res. 1997;34:237–243. doi: 10.1159/000159228. [DOI] [PubMed] [Google Scholar]
- HIGUERET P., PALLET V., COUSTAUT M., AUDOUIN I., BEGUERET J., GARCIN H. Retinoic acid decreases retinoic acid and triiodothyronine nuclear receptor expression in the liver of hyperthyroidic rats. FEBS Lett. 1992;310:101–105. doi: 10.1016/0014-5793(92)81306-7. [DOI] [PubMed] [Google Scholar]
- JIANG X.-M., CANTRILL E., FARRELL G.C., MURRAY M. Pretranslational down-regulation of male specific hepatic P450s after portal bypass. Biochem. Pharmacol. 1994;48:701–708. doi: 10.1016/0006-2952(94)90047-7. [DOI] [PubMed] [Google Scholar]
- KIMURA S., HANIOKA N., MATSUNAGA E., GONZALEZ F.J. The rat clofibrate-inducible CYP4A gene subfamily I. Complete intron and exon sequence of the CYP4A1 and CYP4A2 genes, unique exon organization, and identification of a conserved 19-bp upstream element. DNA Cell. Biol. 1989a;8:503–516. doi: 10.1089/dna.1.1989.8.503. [DOI] [PubMed] [Google Scholar]
- KIMURA S., HARDWICK J.P., KOZAK C.A., GONZALEZ F.J. The rat clofibrate-inducible CYP4A subfamily II. cDNA sequence of IVA3, mapping of the Cyp4a locus to mouse chromosome 4, and coordinate and tissue-specific regulation of the CYP4A genes. DNA Cell. Biol. 1989b;8:517–525. doi: 10.1089/dna.1.1989.8.517. [DOI] [PubMed] [Google Scholar]
- KLIEWER S.A., UMESONO K., NOONAN D.J., HEYMAN R.A., EVANS R.M. Convergence of 9-cis-retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:559–567. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAEMMLI U.K. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1971;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LAETHEM R.M., BALAZY M., FALCK J.R., LAETHEM C.L., KOOP D.R. Formation of 19(S)-, 19(R)-, and 18(R)-hydroxyeicosatetraenoic acids by alcohol-inducible cytochrome P450 2E1. J. Biol. Chem. 1993;268:12912–12918. [PubMed] [Google Scholar]
- LEGRAVEREND C., MODE A., WELLS T., ROBINSON I., GUSTAFSSON J.-Å. Hepatic steroid hydroxylating enzymes are controlled by the sexually dimorphic pattern of growth hormone secretion in normal and dwarf rats. FASEB J. 1992;6:711–718. doi: 10.1096/fasebj.6.2.1537461. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–270. [PubMed] [Google Scholar]
- MAHNKE A., STROTKAMP D., ROOS P.H., HANSTEIN W.G., CHABOT G.G., NEF P. Expression and inducibility of cytochrome P450 3A9 (CYP3A9) and other members of the CYP3A subfamily in rat liver. Arch. Biochem. Biophys. 1997;337:62–68. doi: 10.1006/abbi.1996.9752. [DOI] [PubMed] [Google Scholar]
- MALLO F., LAMAS J.A., CASANUEVA F.F., DIEGUEZ C. Effect of retinoic acid deficiency on in vivo and in vitro GH responses to GHRH in male rats. Neuroendocrinology. 1992;55:642–647. doi: 10.1159/000126183. [DOI] [PubMed] [Google Scholar]
- MARTINI R., BUTLER A.M., JIANG X.-M., MURRAY M. Pretranslational down regulation of cytochrome P450 2C11 in vitamin A-deficient male rat liver: Prevention by dietary inclusion of retinoic acid. J. Pharmacol. Exp. Ther. 1995;273:427–434. [PubMed] [Google Scholar]
- MIYATA M., NAGATA K., SHIMADA M., YAMAZOE Y., KATO R. Structure of a gene and cDNA of a major constitutive form of testosterone 6β-hydroxylase (P450/6βA) encoding CYP3A2: Comparison of the cDNA with P450PCN2. Arch. Biochem. Biophys. 1994;314:351–359. doi: 10.1006/abbi.1994.1453. [DOI] [PubMed] [Google Scholar]
- MORGAN E.T. Suppression of constitutive cytochrome P-450 gene expression in livers of rats undergoing an acute phase response to endotoxin. Mol. Pharmacol. 1989;36:699–707. [PubMed] [Google Scholar]
- MORGAN E.T., MACGEOCH C., GUSTAFSSON J.-Å. Hormonal and developmental regulation and expression of the hepatic microsomal steroid 16α-hydroxylase cytochrome P-450 apoprotein in the rat. J. Biol. Chem. 1985;260:11895–11898. [PubMed] [Google Scholar]
- MURRAY M., BUTLER A.M., AGUS C. Restoration of cytochrome P450 2C11 in vitamin A-deficient rat liver by exogenous androgen. FASEB J. 1996;10:1058–1063. doi: 10.1096/fasebj.10.9.8801167. [DOI] [PubMed] [Google Scholar]
- MURRAY M., HUDSON A.M., YASSA V. Hepatic microsomal metabolism of the anthelmintic benzimidazole fenbendazole: Enhanced inhibition of cytochrome P450 reactions by oxidized metabolites of the drug. Chem. Res. Toxicol. 1992;5:60–66. doi: 10.1021/tx00025a010. [DOI] [PubMed] [Google Scholar]
- MURRAY M., SEFTON R.M., MARTINI R., BUTLER A.M. Comparative induction of CYP3A and CYP2B in rat liver by 3-benzoylpyridine and meyrapone. Chem.- Biol. Interact. 1999;113:161–173. doi: 10.1016/s0009-2797(98)00017-9. [DOI] [PubMed] [Google Scholar]
- MURRAY M., ZALUZNY L., FARRELL G.C. Drug metabolism in cirrhosis: Selective changes in cytochrome P-450 isozymes in the choline-deficient rat model. Biochem. Pharmacol. 1986;35:1817–1824. doi: 10.1016/0006-2952(86)90298-4. [DOI] [PubMed] [Google Scholar]
- MURRAY M., ZALUZNY L., FARRELL G.C. Impaired androgen 16α-hydroxylation in hepatic microsomes from carbon tetrachloride-cirrhotic male rats. Gastroenterology. 1987a;93:141–147. doi: 10.1016/0016-5085(87)90326-x. [DOI] [PubMed] [Google Scholar]
- MURRAY M., ZALUZNY L., DANNAN G.A., GUENGERICH F.P., FARRELL G.C. Altered regulation of cytochrome P-450 enzymes in choline-deficient cirrhotic male rat liver: Impaired regulation and activity of the male-specific androst-4-ene-3,17-dione 16α-hydroxylase, cytochrome P-450UT-A, in hepatic cirrhosis. Mol. Pharmacol. 1987b;31:117–121. [PubMed] [Google Scholar]
- NAGATA K., GONZALEZ F.J., YAMAZOE Y., KATO R. Purification and characterization of four catalytically active testosterone 6β-hydroxylase P-450s from rat liver microsomes: Comparison of a novel form with three structurally and functionally related forms. J. Biochem. 1990;107:718–725. doi: 10.1093/oxfordjournals.jbchem.a123115. [DOI] [PubMed] [Google Scholar]
- NAGATA K., OGINO M., SHIMADA M., MIYATA M., GONZALEZ F.J., YAMAZOE Y. Structure and expression of the rat CYP3A1 gene: Isolation of the gene (P450/6βB) and characterization of the recombinant protein. Arch. Biochem. Biophys. 1999;362:242–253. doi: 10.1006/abbi.1998.1030. [DOI] [PubMed] [Google Scholar]
- PETERS J.M., ZHOU Y.C., RAM P.A., LEE S.S.T., GONZALEZ F.J., WAXMAN D.J. Peroxisome proliferator-activated receptor-α required for gene induction by dehydroepiandrosterone-3β-sulfate. Mol. Pharmacol. 1996;50:67–74. [PubMed] [Google Scholar]
- RAM P.A., WAXMAN D.J. Hepatic P450 expression in hypothyroid rats: differential responsiveness of male-specific P450 forms 2a (IIIA2), 2c (IIC11), and RLM2 (IIA2) to thyroid hormone. Mol. Endocrinol. 1991;5:13–20. doi: 10.1210/mend-5-1-13. [DOI] [PubMed] [Google Scholar]
- RAM P.A., WAXMAN D.J. Interaction of growth hormone-activated STATs with SH2-containing phosphotyrosine phosphatase SHP-1 and nuclear JAK2 tyrosine kinase. J. Biol. Chem. 1997;272:17694–17702. doi: 10.1074/jbc.272.28.17694. [DOI] [PubMed] [Google Scholar]
- RICH K.A., KERR J.B., DE KRETSER D.M. Evidence for Leydig cell dysfunction in rats with seminiferous tubule damage. Mol. Cell. Endocrinol. 1979;13:123–135. doi: 10.1016/0303-7207(79)90013-3. [DOI] [PubMed] [Google Scholar]
- SAMBROOK J., FRITSCH E.F., MANIATIS T. Molecular cloning: a Laboratory Manual 1989Cold Spring Harbor Laboratory Press; 7.37–7.52.2nd Ed. pp [Google Scholar]
- SCHWARTZMANN M.L., MARTASEK P., RIOS A.R., LEVERE R.D., SOLANGI K., GOODMAN A.I., ABRAHAM N.G. Cytochrome P450-dependent arachidonic acid metabolism in human kidney. Kidney Int. 1990;37:94–99. doi: 10.1038/ki.1990.13. [DOI] [PubMed] [Google Scholar]
- SHARMA R.K., LAKE B.G., KAKOWSKI R., BRADSHAW T., EARNSHAW D., DALE J.W., GIBSON G.G. Differential induction of peroxisomal and microsomal fatty acid-oxidising enzymes by peroxisome proliferators in rat liver and kidney. Eur. J. Pharmacol. 1989;184:69–78. doi: 10.1111/j.1432-1033.1989.tb14991.x. [DOI] [PubMed] [Google Scholar]
- SKETT P. Hormonal regulation and sex differences of xenobiotic metabolism. Prog. Drug Metab. 1987;10:85–140. [Google Scholar]
- SOHLENIUS A.K., REINFELDT M., BACKSTROM K., BERGSTRAND A., DEPIERRE J.W. Hepatic peroxisome proliferation in vitamin A-deficient mice without a simultaneous increase in peroxisomal acyl-CoA oxidase activity. Biochem. Pharmacol. 1996;51:821–827. doi: 10.1016/0006-2952(95)02231-7. [DOI] [PubMed] [Google Scholar]
- SUNDSETH S., WAXMAN D.J. Sex-dependent expression and clofibrate inducibility of cytochrome P450 4A fatty acid ω-hydroxylases. Male specificity of liver and kidney CYP4A2 mRNA and tissue-specific regulation by growth hormone and testosterone. J. Biol. Chem. 1992;267:3915–3921. [PubMed] [Google Scholar]
- TAMBURINI P.P., MASSON H.A., BAINS S.K., MAKOWSKI R.J., MORRIS B., GIBSON G.G. Multiple forms of hepatic cytochrome P-450: purification, characterization and comparison of a novel clofibrate-induced isozyme with other major forms of cytochrome P-450. Eur. J. Biochem. 1984;139:245–246. doi: 10.1111/j.1432-1033.1984.tb07999.x. [DOI] [PubMed] [Google Scholar]
- TOWBIN H., STAEHELIN T., GORDON J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl Acad. Sci. U.S.A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTER-SACK I., KLOTZ U. Influence of diet and nutritional status on drug metabolism. Clin. Pharmacokinet. 1996;31:47–64. doi: 10.2165/00003088-199631010-00004. [DOI] [PubMed] [Google Scholar]
- WANG M.H., GUAN H., NGUYEN X., ZAND B.A., NASJLETTI A., SCHWARTZMANN M.L. Contribution of cytochrome P-450 4A1 and 4A2 to vascular 20-hydroxyeicosatetraenoic acid synthesis in rat kidneys. Am. J. Physiol. 1999;276:F246–F253. doi: 10.1152/ajprenal.1999.276.2.F246. [DOI] [PubMed] [Google Scholar]
- WAXMAN D.J., KO A., WALSH C. Regioselectivity and stereoselectivity of androgen hydroxylations catalysed by cytochrome P-450 isozymes purified from phenobarbital-induced rat liver. J. Biol. Chem. 1983;258:11937–11947. [PubMed] [Google Scholar]
- WAXMAN D.J., PAMPORI N.A., RAM P.A., AGRAWAL A.K., SHAPIRO B.H. Interpulse interval in circulating growth hormone patterns regulates sexually dimorphic expression of hepatic cytochrome P450. Proc. Natl Acad. Sci. U.S.A. 1991;88:6868–6872. doi: 10.1073/pnas.88.15.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAXMAN D.J., RAM P.A., PARK S.H., CHOI H.K. Intermittent plasma growth hormone triggers tyrosine phosphorylation and nuclear translocation of a liver-expressed, Stat 5-related DNA binding protein. Proposed role as an intracellular regulator of male-specific liver gene transcription. J. Biol. Chem. 1995;270:13262–13270. doi: 10.1074/jbc.270.22.13262. [DOI] [PubMed] [Google Scholar]
- WESTIN S., MODE A., MURRAY M., CHEN R., GUSTAFSSON J.-Å. Growth hormone and vitamin A induce P4502C7 mRNA expression in primary rat hepatocytes. Mol. Pharmacol. 1993;44:997–1002. [PubMed] [Google Scholar]
- WRIGHT K., MORGAN E.T. Transcriptional and post-transcriptional suppression of P450IIC11 and P450IIC12 by inflammation. FEBS Lett. 1990;271:59–61. doi: 10.1016/0014-5793(90)80371-o. [DOI] [PubMed] [Google Scholar]
- WRIGHT M.C., ALLENBY G., PAINE A.J. Effect of vitamin A deficiency on the expression of low affinity glucocorticoid binding site activity and glucocorticoid-dependent induction of CYP3A2 in rat liver. Biochem. Biophys. Res. Comm. 1997;237:211–216. doi: 10.1006/bbrc.1997.7114. [DOI] [PubMed] [Google Scholar]
- YOSHIOKA Y., MOROHASHI K.I., SOGAWA K., MIYATA T., KAWAJIRI K., HIROSE T., INAYAMA S., FUJII-KURIYAMA Y., OMURA T. Structural analysis and specific expression of microsomal cytochrome P-450 (M-1) mRNA in male rat livers. J. Biol. Chem. 1987;262:1706–1711. [PubMed] [Google Scholar]
- YU C.L., JIN Y.J., BURAKOFF S.J. Cytosolic tyrosine dephosphorylation of STAT5. Potential role of SHP-2 in STAT5 regulation. J. Biol. Chem. 2000;275:599–604. doi: 10.1074/jbc.275.1.599. [DOI] [PubMed] [Google Scholar]


