Abstract
Thymidylate synthase (TS) is a target for several anticancer drugs. We previously showed that an antisense oligodeoxynucleotide (ODN) directed against TS mRNA down-regulated TS protein and enhanced cytotoxicity of TS-targeting drugs [including 5-fluorodeoxyuridine (5-FUdR)] in HeLa cells. Patient tumours with increased TS expression are resistant to TS-targeting drugs. It was hypothesized that TS mRNA and consequently TS protein could be down-regulated in 5-FUdR-resistant cells that overexpress TS, sensitizing them to 5-FUdR cytotoxicity. In this study we assessed the capacity of an anti-TS antisense ODN to circumvent resistance dependent on TS overexpression.
Variant HeLa clones exhibiting 2 – 20 fold resistance to 5-FUdR were selected by exposing cultured cells to drug. Clones FUdR-5a, -25b, and -50a expressed TS protein levels 10 fold, 10 fold, and 17 fold higher (respectively) than parental cells. Cells were treated with antisense ODN 83 (a 2′-methoxy-ethoxylated, phosphorothioated 20-mer, complementary to a portion of the 3′-untranslated region of TS mRNA), or ODN 32 (a control ODN with the same base composition as ODN 83, but in randomized order). Twenty-four and 48 h following transfection (50 – 100 nM ODN, plus polycationic liposome), TS mRNA levels (by RT – PCR) and protein levels (by radiolabelled 5-FUdR-monophosphate binding) were decreased by at least 60% in ODN 83-treated cells compared with control ODN 32-treated cells. ODN 83 enhanced the cytotoxicity of 5-FUdR by up to 85% in both parental and 5-FUdR-resistant cell lines.
Antisense ODN can be used to down-regulate TS and attenuate drug resistance in TS-overexpressing cells.
Keywords: Antisense, thymidylate synthase, drug resistance, 5-fluorodeoxyuridine
Introduction
Thymidylate synthase (5,10-methylenetetrahydrofolate:dUMP C-methyltransferase; EC 2.1.1.45) (TS) is important in DNA precursor synthesis and repair, and is an important target for anticancer chemotherapy. The enzyme is a highly conserved homodimer of 35 kDa subunits, and catalyses the synthesis of thymidylate from deoxyuridylate and 5,10-methylene-tetrahydrofolate (Me-FH4) (Chu & Allegra, 1996; Danenberg, 1977). The TS protein exerts tight control over its own synthesis during cell cycle by binding to TS mRNA both at the translational start site (TSS) and at a specific coding region, thereby regulating translational processing of the message (Johnson, 1994; Chu et al., 1991; 1993b). TS protein has been reported to bind to mRNAs of at least nine other proteins important in cell cycling and resistance to toxicity, including p53 (Chu et al., 1996) and c-myc (Chu et al., 1994), and modulates the translatability of p53 mRNA in vitro (Chu et al., 1999; Ju et al., 1999). Direct TS-targeting drugs also arrest cells in vitro in early S phase (Inaba & Mitsuhashi, 1994; Matsui et al., 1996; Yin et al., 1999). Therefore, altering the level of active TS protein changes the ability of cells to replicate DNA and progress through the cell cycle.
Direct inhibitors of the TS enzyme include the nucleoside analogue 5-fluorodeoxyuridine monophosphate (5-FdUMP) [a metabolite of 5-fluorouracil (5-FU) and 5-fluorodeoxyuridine (5-FUdR) produced within mammalian cells] and folate analogues including N-(5-[N-(3,4-dihydro-2-methyl-4-oxoquinazolin-6-ylmethyl-N-methylamino]-2-thenoyl)-L-glutamic acid (raltitrexed, Tomudex, ICID 1694) (Jackman et al., 1991). In cell lines selected for resistance to drugs of both these types, a common mechanism of resistance is increased cellular expression of TS (Zhang et al., 1992), possibly resulting directly from increased TS mRNA (Murakami et al., 2000; Kitchens et al., 1999; Shibata et al., 1998). In patient tumours, therefore, preferential survival of variant tumour cells with increased TS levels in the presence of drug, or induction of TS gene transcription leading to increased TS levels, is a potential impediment to antitumour drug treatment. In addition, a novel drug-dependent ‘translational release' leading to transiently increased TS levels has been described. Raltitrexed, 5-FU or 5-FUdR, and Me-FH4 are all able to relieve the repression of translation caused by binding of TS protein to TS mRNA (Chu & Allegra, 1996; Chu et al., 1991; Lin et al., 2000; Mader et al., 1997; Parr et al., 1998; Peters et al., 2000; Welsh et al., 2000). This results in a transient increase in TS mRNA translation and TS protein levels, a phenomenon observed in vitro (Chu et al., 1990; 1991; 1993a; Keyomarsi et al., 1993; Mader et al., 1997; Parr et al., 1998; Van der Wilt et al., 1992), and in animals (Van der Wilt et al., 1992) and patients (Peters et al., 1994; Swain et al., 1989). This induction of TS synthesis [upwards of 3 – 5 fold (Chu et al., 1990; 1993a; Van der Wilt et al., 1992)] would serve to at least partially circumvent the cytotoxic effect of the drug (Berne et al., 1986). Conversely, a favourable response by colon carcinomas to treatment with 5-FU correlates directly with lower levels of TS in patient tumours (Edler et al., 2000; Mini et al., 1999; Peters et al., 1994; Salonga et al., 2000; Van Triest & Peters, 1999).
Given the importance of regulation of mRNA in TS production, we hypothesized that the use of an antisense strategy that targeted TS mRNA in combination with drugs targeting TS protein would be effective in wholly or partially circumventing resistance to treatment.
A number of antisense oligodeoxynucleotides (ODNs) and vectors are reported to enhance the cytotoxicity of various drugs, by targeting modulators of drug activity and cell response as opposed to the drug target per se, with mdr1 and bcl-2 being the most common targets of antisense (Cucco & Calabretta, 1996; Kitada et al., 1994; Li et al., 1997; Luo et al., 1999; Miayake et al., 2000; Quattrone et al., 1994; Thierry et al., 1993). Based on the success of in vivo screening of antisense ODNs, at least seven of these agents are currently in clinical trial for anti-cancer treatment, with targets including bcl-2 (Chen et al., 2000; Scher et al., 2000; Waters et al., 2000) and p53 (Bishop et al., 1996). The success of antisense strategies to reduce specific cellular proteins and improve disease therapies led us to generate a protocol to reduce the cellular levels of TS mRNA through the use of specific antisense ODNs. An antisense ODN was designed to anneal with a 20-base region in the 3′-untranslated region (UTR) of TS mRNA and induce RNase H-dependent cleavage (Bennett, 1998; Binder et al., 1994; Stein et al., 1988; Stewart et al., 1996). The ODN used in these experiments is a phosphorothioated oligomer of 20 bases, modified by methoxyethoxylation at the 2′-positions of the six sugars on either end of the ODN. This structure is very resistant to nuclease digestion (Dean et al., 1994; 1996; Shaw et al., 1991; Stein & Cheng, 1993; Stein et al., 1988).
We previously demonstrated that antisense-induced decreases in cellular TS mRNA in HeLa cells: (a) reduced the cellular content of TS protein; (b) inhibited cellular proliferation; and (c) enhanced the cytotoxicity of TS inhibitors (Ferguson et al., 1999). However, elevated TS levels expressed in some patient tumours of various tissue etiologies (Mini et al., 1999; Peters et al., 1994; Suzuki et al., 1999; Takenoue et al., 2000) might make them resistant to antisense therapy. To determine the potential for anti-TS antisense therapy to overcome drug resistance associated with TS, it was necessary to determine whether the antisense protocol used against parental HeLa cells, unselected for resistance to TS-targeting drugs, could abrogate resistance due to TS overexpression. Therefore, a series of cell lines was selected for increasing resistance to 5-FUdR in order to establish a cell culture model in which the ability to down-regulate TS-overexpression could be tested. We report here that, in these TS-overexpressing, 5-FUdR-resistant cell lines, an antisense ODN against TS mRNA was able to decrease the amount of TS enzyme and increase the sensitivity of the cells to 5-FUdR.
Methods
Antisense oligonucleotides
Fully phosphorothioated 20-base ODNs were synthesized by ISIS Pharmaceuticals (Carlsbad, CA, U.S.A.) as described previously (Dean et al., 1996). The six nucleotides on either end of the ODN were methoxyethoxylated in the 2′-position to enhance hybridization to TS mRNA and ODN resistance to exonuclease digestion (Dean et al., 1994). The central eight nucleotides were left without methoxyethoxylation to allow access by RNase H and degradation of mRNA hybridized to the ODN (Dean et al., 1994). ODN 83 is complementary to TS mRNA, starting from a position 136 bases downstream of the translational stop site (5′-GCCAGTGGCAACATCCTTAA-3′). ODN 32 is a randomized sequence of ODN 83 (5′-ATGCGCCAACGGTTCCTAAA-3′), with the same base constituents in random order. A search of available mRNA sequences using the NCBI BLAST search tool, assuming a requirement for 10 or more hybridizable bases (adjacent or not) to constitute complementarity, revealed that ODN 83 was complementary only to human TS mRNA, and ODN 32 was not complementary to any known mRNA.
Radioisotopes
[6-3H]5-FdUMP (specific activity 18.6 Ci mmol−1) was purchased from Moravek Biochemicals (Brea, CA, U.S.A.). This isotope was 99.98% pure upon initial production, with a degradation rate of 0.5 – 1% per month at −20°C. It was used within 6 months of manufacture.
Chemotherapy reagent
5-FUdR was purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.).
Other supplies
Cell culture chemicals and nutrients were obtained from Canadian Life Technologies (CLT/GIBCO/BRL) (Burlington, ON, Canada). Oligonucleotides for use as polymerase chain reaction primers were synthesized at the LRCC Cancer Research Laboratories Core DNA Synthesis facility, using a Beckman Oligo 1000 M DNA Synthesizer and cyanethyl-phosphoramidite chemistry. All other chemicals were obtained from commercial sources. Plasticware was purchased from Canadian Life Technologies (NUNC), VWR Canlab (Mississauga, ON, Canada) and Fisher Scientific (Uniondale, ON, Canada).
Cell culture
Cell lines
Human cervical carcinoma HeLa cells were maintained in Dulbecco's modified Eagle's medium (D-MEM) plus 10% foetal bovine serum and penicillin (50 units ml−1)/streptomycin (50 μg ml−1) (growth medium). Cultures were incubated in a humidified atmosphere of 5% CO2 at 37°C. Rapidly proliferating cells were utilized for establishing cultures of experimental cells, which were allowed to incubate overnight prior to manipulation.
Establishment of 5-FUdR-resistant lines
5-FUdR-resistant variants were selected by growing HeLa cells in the continuous presence of 5, 10 or 15 nM FUdR for 3 weeks. Medium was replaced weekly with growth medium containing fresh FUdR. Colonies propagated from single cells were selected and expanded in medium containing drug. Cells from one clone (HeLa/FUdR-5a) were used as starting material for selection of variants resistant to higher drug concentrations, by propagation in the presence of 25, 50, 100 or 200 nM FUdR.
Transfection of ODNs
Cultures for experimentation were established from rapidly proliferating populations of cells 24 h prior to manipulation. Resistant cell lines were cultured for at least 4 days in the absence of drug prior to experiments. Transfection was performed using Lipofectamine 2000 (LFA-2K) [CLT/GIBCO, Burlington, ON, Canada], a polycationic liposome formulation. Cells were transfected for 4 h with 50 – 100 nM ODN in the appropriate concentration of LFA-2K, in growth medium. For proliferation experiments, the starting cell number was between 0.6 and 1×105 cells per 25-cm2 flask, and LFA-2K was used at 0.5 μg ml−1. For cells in 75-cm2 flasks, to be harvested and extracted for assay of mRNA or TS content, the starting cell number was approximately 8 – 10×105, and the LFA-2K concentration was 2 μg ml−1. The 4-h incubation of the cells with ODN/LFA-2K was followed immediately by dilution by addition of one volume of growth medium without ODN/LFA-2K. Cells assessed for relative TS mRNA levels and TS protein levels were harvested 24 and 48 h after transfection. In cultures that were subsequently exposed to 5-FUdR, drug exposure was initiated by addition of 0.25-volume of growth medium containing the drug at five times the final concentration. Cell numbers were determined after 4 days of growth in medium with or without chemotherapeutic drug, by enumerating with a particle counter (Coulter Electronics, Hialeah, FL, U.S.A.). The proliferation of drug-treated cells (fold-increase in cell number) was calculated as a percentage of that of the control cells grown in the absence of 5-FUdR. IC50 values (concentration of drug that inhibited proliferation by 50%) were determined by interpolation of plotted data.
Reverse transcriptase – polymerase chain reaction (RT – PCR) to measure TS mRNA
Measurement of TS and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) mRNA in the same ODN-transfected cell populations was conducted using RT – PCR, as previously described (Ferguson et al., 1999). RNA was isolated from transfected cells using Trizol® (CLT/GIBCO/BRL). PCR products were separated on a 1.0% agarose gel, and visualized by ethidium bromide staining, using an ImageMaster VDS gel documentation system and LISCAP Capture Utility software (Amersham Pharmacia Biotech). The intensity of staining of individual bands was quantitated using ImageQuant software (Molecular Dynamics/Amersham Pharmacia).
TS binding assay
Cellular content of TS was assayed by binding of [6-3H]5-FdUMP, according to previous methodology (Spears & Gustavsson, 1988), with slight modifications in the final steps of removing activated charcoal (Ferguson et al., 1999). This method lables total TS unless the cells are pretreated with 5-FU or 5-FUdR (Chu et al., 1990), and correlates well with in situ activity assays (Ju et al., 1998; van Triest et al., 1999) and Western blots (Kitchens et al., 1999; van Triest et al., 2000).
Statistical analysis
Data for cell proliferation after treatment with ODNs alone, or in combination with 5-FUdR, are presented as the mean±standard deviation, and assayed for significance using Student's t-test. For determinations of [6-3H]5-FdUMP binding, differences between paired samples from cells transfected with different ODNs were assessed using a paired t-test. In all cases, significance was chosen a priori to be indicated by differences at a confidence level of P⩽0.05.
Results
Establishment of FUdR-resistant variants of HeLa
It was the intent of this study to determine whether an antisense ODN targeted against TS could downregulate this enzyme in cells that overexpress it as a consequence of selection for growth in TS-targeting drugs. Based on the previous finding of enhancement of cytotoxicity of TS inhibitors in HeLa cells, it was expected that drug resistance could be circumvented in TS-overexpressing cells using an antisense ODN. To this end, it was necessary to establish a model system in which cell lines overexpressed TS to various degrees, to determine the magnitude of resistance that could be overcome. The variants of HeLa that were established by continuous exposure to 5-FUdR are listed in Table 1. Three cell lines (FUdR-5a, -10g, and -15a) were propagated from colonies that grew among HeLa cells cultured in the presence of 5-FUdR at the respective concentrations (5, 10 and 15 nM). The remaining four cell lines were each selected from FUdR-5a by a single selection step, in the presence of the indicated concentrations of 5-FUdR. The first three cell lines displayed a similar level of resistance to 5-FUdR, but the TS protein level in the FUdR-5a line was approximately six times greater than that measured in FUdR-10g or -15a. The lines selected from FUdR-5a had at least a 10 fold increase in TS protein level compared to parental HeLa cells, accompanied by a slight increase in relative TS mRNA concentration.
Table 1.
Characteristics of FUdR-Resistant variants of HeLa
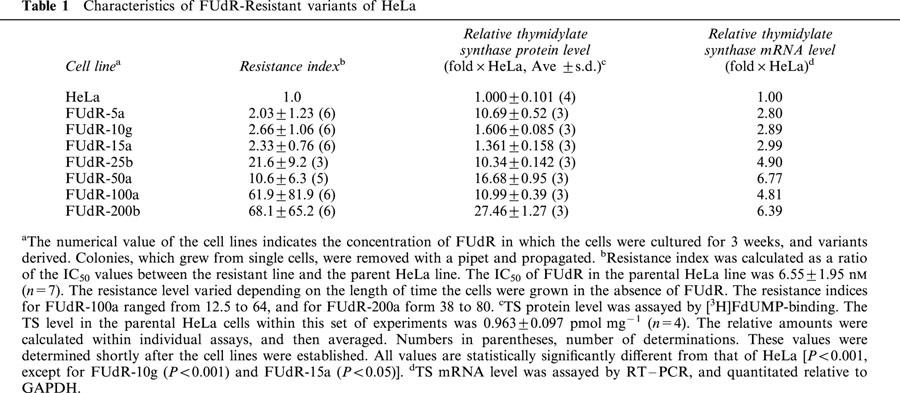
Three 5-FUdR-resistant lines representative of different levels of TS overexpression, and three levels of resistance to 5-FUdR, were chosen for further study: FUdR-5a, -25b, and -50a. The effectiveness of anti-TS antisense ODN 83 in downregulating TS and overcoming resistance to 5-FUdR was assessed in these three lines. Optimal conditions were established within which antisense ODN treatment alone significantly decreased TS protein levels, but did not inhibit proliferation more than 30%. This permitted sufficient remaining growth potential to clearly measure the effect of subsequent 5-FUdR treatment on proliferation. Transfection conditions were also adjusted to minimize non-specific short-term toxicity, depending on whether cells were transfected at low density (0.6 – 1×105 cells per 25-cm2 flask) for assays of proliferation, or higher density (8 – 10×105) for assays of protein or RNA content. These conditions were: for low cell density, 0.5 μg ml−1 LFA-2K for all lines (except FUdR-50a and -100a, where 0.25 μg ml−1 LFA-2K/ml was used), plus 50 nM ODN; for high cell density, 2 μg ml−1 LFA-2K, plus 100 nM ODN.
Effect of antisense ODN on TS mRNA and protein expression
It was expected that anti-TS antisense ODN 83 would decrease TS protein levels by specifically interacting with TS mRNA to promote degradation through the action of RNase H. Therefore, ODN-treated cells were assayed for TS mRNA content, relative to control GAPDH mRNA levels, 1 and 2 days following treatment. Total cellular RNA was isolated, reverse-transcribed, and selected regions of TS and control GAPDH cDNA products amplified by PCR and characterized by agarose gel electrophoresis. Figure 1 is representative of assays performed on extracts from three different sets of ODN-treated cultures, and clearly demonstrates the specific decrease in TS mRNA. RNA from three independent antisense transfection experiments was quantitated using image analysis software, the results of which are summarized in Figure 2. The decrease in TS mRNA level (greatest on day 2 after ODN transfection) was mirrored by the decreased level of TS protein (measured by [3H]FdUMP-binding). (In this set of experiments, the relative TS protein content of the FUdR-5a line was less than that presented in Table 1, probably as a result of incubation of the cell line in the absence of drug during the period of these experiments. Cultures were maintained drug-free prior to experiments to avoid interference of 5-FUdR and its metabolites in assays of TS protein and of inhibition of proliferation by exogenous 5-FUdR. To enable comparison of the antisense ODN effect between experiments, the relative amounts of TS mRNA and TS protein were determined within each experiment.) Under these treatment conditions, proliferation was inhibited to varying degrees depending on the cell line being treated. In two experiments in which proliferation was evaluated over 2 days following transfection, relative proliferation of antisense-treated cells (compared with control ODN 32) was, respectively, 33.5±21.2%, 41.0±1.1%, 52.8±15.2%, and −17±15.3% (a negative value indicating a drop in the number of cells below the starting number) for HeLa, FUdR-5a, FUdR-25b, and FUdR-50a.
Figure 1.
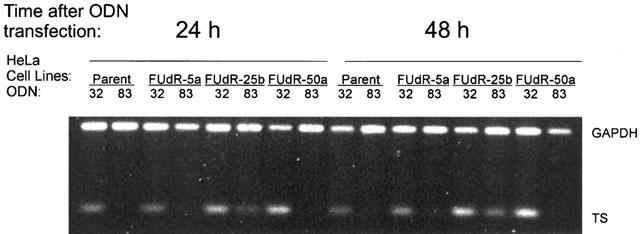
Reduction of cellular levels of TS mRNA in HeLa cells and FUdR-resistant variants following treatment with antisense ODN 83. Following administration of ODNs to cultures, cells were harvested on days 1 and 2. Relative TS mRNA was visualized by RT – PCR, using GAPDH as a control for RNA integrity. This image is typical and representative of assays of cells harvested from three separate experiments.
Figure 2.
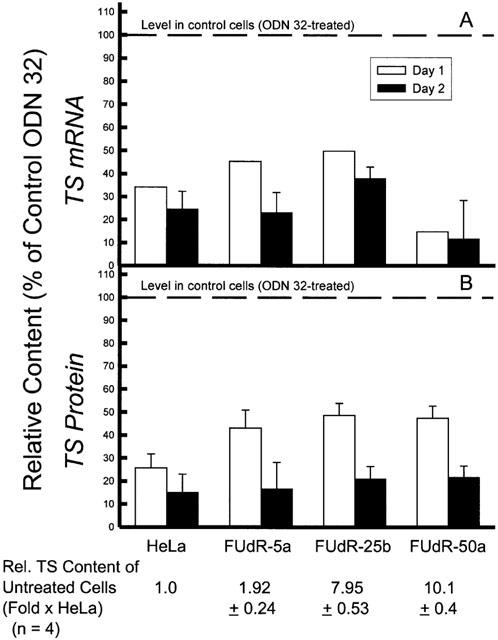
Reduction in thymidylate synthase mRNA and protein levels by treatment of HeLa cells and FUdR-resistant variants with antisense ODN 83. Following a 4 h transfection of cells with ODN at the concentration indicated, one volume of medium was added, and cells were further incubated in ODN at 50% of the original concentration. Cells were harvested 1 and 2 days following initiation of exposure to ODN. (A) relative TS mRNA was assayed by RT – PCR, and compared with the level of the housekeeping gene product GAPDH. Data are summarized from the analysis of two separate determinations for day 1 and 3 for day 2, as represented by Figure 1. (B) TS protein level was measured by [3H]-FdUMP-binding. The TS content in the ODN 32-treated parental HeLa line was 0.983±0.132 pmol mg−1 (n=5) on Day 1, and 0.792±0.232 pmol mg−1 (n=5) on Day 2. All values presented are significantly different from the respective ODN 32-treated control (P<0.01).
Effect of antisense ODN on sensitivity of 5-FUdR-resistant cell lines to 5-FUdR
Since antisense ODN 83 was able to downregulate the intracellular content of TS, it was expected that this treatment would enhance sensitivity of the cells to 5-FUdR. As demonstrated by the representative experiment presented in Figure 3, the antisense treatment enhanced drug sensitivity, at concentrations of ODN 83 that inhibited proliferation by only 10 – 20% on their own. (At 100 nM ODN 83 plus 2 μg ml−1 LFA-2K, proliferation of all the cell lines was inhibited by approximately 50% (data not shown).) The proliferation rates shown specifically reveal the enhancing effect of antisense ODN 83 on drug sensitivity, and exclude the effect of ODN 83 alone on cell proliferation. Cells treated with ODN 83 or ODN 32 were assigned a proliferation value of 100% and all data points showing proliferation in the presence of drug after ODN treatment are relative to that 100% value. The results of three independent experiments are summarized in Figure 4. The 70 – 80% enhancement of 5-FUdR cytotoxicity was significant (P<0.001) for all cell lines. There was no significant difference in the degree of enhancement of sensitivity to FUdR by anti-TS antisense ODN 83 between any TS-overexpressing, FUdR-resistant cell line and the parental, FUdR-sensitive HeLa cells with low TS levels.
Figure 3.
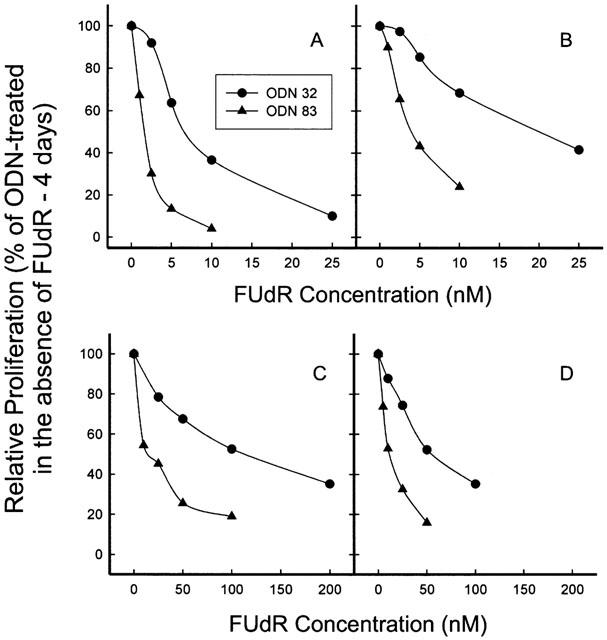
Enhancement of FUdR cytotoxicity in HeLa cells and FUdR-resistant variants by treatment with antisense ODN 83. Cells were treated with ODNs for 4 h, followed by administration of FUdR without changing the medium. Cell number was determined after 4 days, and proliferation calculated as a percentage of that of control ODN-treated cells. (A) HeLa; (B) FUdR-5a; (C) FUdR-25b; (D) FUdR-50a. Error bars are smaller than the symbols.
Figure 4.
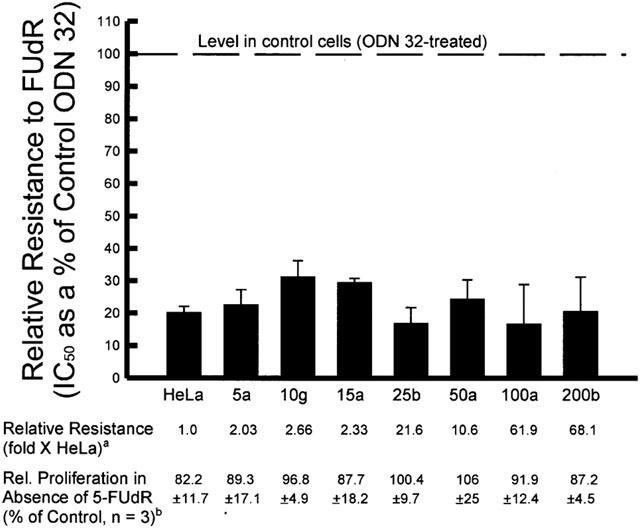
Enhancement of FUdR cytotoxicity in HeLa cells and in FUdR-resistant variants by antisense ODN 83. Cells were treated with antisense ODN 83 or scrambled control ODN 32 for 4 h, followed immediately by coincubation with FUdR for 4 days. Proliferation was determined by increase in cell number. IC50 values were interpolated from plotted data, from a series of experiments represented by Figure 3. The value presented is the IC50 of the ODN 83-treated cells as a percentage of that of the control ODN 32-treated cells, calculated within individual experiments. All values for relative resistance are significantly different from that of control ODN-treated cells, P<0.001. aThis value is reproduced from Table 1 (see table for s.d. and n values for these averages). Resistance index was calculated by dividing the IC50 of the FUdR-resistant line by that of the parent HeLa cell line, within each individual experiment. bRelative proliferation is the relative increase in cell number, as a percentage of the control ODN-treated cells, of the cultures treated with antisense ODN 83 alone.
Discussion
The amount of anticancer drug administered to patients is limited by debilitating, dose-dependent side effects, including potentially fatal toxicity. Therefore, the acquisition of some form of resistance by a tumour, including low level, incremental decreases in drug sensitivity, is a major obstacle to the success of treatment. A minor increase in the cellular content of a target of a specific drug could impede the ability of that drug to lethally damage tumour cells. Therefore, it would be of benefit to chemotherapeutic outcome if a drug target could be down-regulated in tumour cells in order to increase the effective drug : target ratio. The first step toward specifically decreasing drug-target levels in tumour cells has been the development of antisense technology. Antisense ODNs of 15 – 21 bases in length that are complementary to various regions of a particular mRNA can effectively and specifically downregulate a selected gene product (Bennett, 1998). Although the use of antisense RNA expression vectors have been tested as a strategy to generate a prolonged antisense effect on a specific mRNA target (Dean et al., 1994; 1996), the stability of phosphorothioated ODNs, and their capacity to be taken up into animal-borne tumours without a requirement for transfection agents, has resulted in their superseding antisense RNA vectors as the agents of choice in targeting specific mRNAs in vivo (Dean et al., 1994; 1996; Shaw et al., 1991; Stein & Cheng, 1993; Stein et al., 1988).
Many proteins have been down-regulated by antisense with resulting enhancement in drug toxicity. Of those, however, only tubulin (Kyu-Ho Han et al., 2000; Kavallaris et al., 1999) and TS have been tested as targets for both antisense nucleic acids (at the mRNA level) and traditional drug (at the protein level). We previously demonstrated that a 20-mer ODN antisense to the 3′UTR of TS mRNA (ODN 83) specifically down-regulated TS expression and inhibited HeLa cell proliferation (Ferguson et al., 1999). In addition, ODN 83 specifically enhanced cell sensitivity to drugs that target TS (raltitrexed, 5-FU, and 5-FUdR). This has since been extended to animal studies, in which the same anti-TS antisense ODN inhibits human colon tumour growth in nude mice (Berg et al., 2001).
To date there have been few attempts to sensitize cells to TS inhibitors using antisense technology, and those reported in the literature describe only limited success. Transfection of an anti-TS antisense expression vector sensitized cells to 5-FUdR (Ju et al., 1998). However, an antisense ODN against TS induced resistance to 5-FUdR, possibly due to a rebound in TS expression following an initial transient decrease in TS mRNA (Ju et al., 1998). Antisense against a number of other mRNAs has been used to alter cytotoxicity to TS inhibitors. Sensitivity to 5-FU and/or 5-FUdR was enhanced following treatment of cultured cells with an antisense ODN against c-jun (Kakutani et al., 1998) and antisense vectors against cyclin D1 (Kornmann et al., 1999) and proline-directed protein kinase-FA (Yang et al., 2000). In contrast, antisense against thymidine phosphorylase or uridine phosphorylase antagonizes cytotoxicity of 5-FU (Mader et al., 1997), as does pre-administration of antisense ODN against TGFα mRNA (De Luca et al., 1997), although the latter has no effect if administered after the drug (De Luca et al., 1997). Antisense ODN against c-myc (Mizutani et al., 1994) and antisense vectors against heat shock protein 27 (Garrido et al., 1996) or the antimetastatic protein nm23-H1 (Iizuka et al., 1999) did not affect 5-FU cytotoxicity.
The level of TS in tumour cells correlates well with sensitivity to cytotoxicity of TS-inhibitory drugs in vitro and in vivo. A low basal level of TS has been shown to yield greater sensitivity of cultured cells to TS inhibitors (Nita et al., 1998; van Triest et al., 1999), and a more favourable response of patient tumours to 5-FU (Edler et al., 2000; Mini et al., 1999; Peters et al., 1994; Salonga et al., 2000; van Triest & Peters, 1999). Therefore, down-regulation of basal levels of TS could lead to improved responses in drug-naïve tumours. However, antisense could also be used to downregulate TS that for some reason becomes overexpressed in tumour cells. The etiology of such altered expression can be considered as: (1) responsive, or epigenetic, resistance; or (2) acquired, or mutational, resistance.
Responsive, or epigenetic, resistance to TS inhibitors
TS regulates its own production by binding to its own mRNA to inhibit translation (Kaneda et al., 1987; Keyomarsi et al., 1993). Removal of TS protein from TS mRNA, possibly triggered by an increase in the pool of substrates of TS (Chu & Allegra, 1996), or by binding of inhibitors of TS enzyme activity (Chu et al., 1990; 1991; 1993a; Keyomarsi et al., 1993; Lin et al., 2000; Mader et al., 1997; Parr et al., 1998; Peters et al., 2000; Van der Wilt et al., 1992; Welsh et al., 2000), can restore TS protein synthesis from the mRNA template. This drug-induced increase in TS protein has been observed to enhance resistance to 5-FU therapy in mice (Van der Wilt et al., 1992), and has been reported following treatment with TS-targeted chemotherapeutics in cancer patients (Peters et al., 1994; Swain et al., 1989).
Acquired, or mutational, resistance
As in the case of resistance involving other enzyme drug targets, cell lines selected for resistance to 5-FU or 5-FUdR have elevated levels of TS mRNA, usually due to gene amplification (Kitchens et al., 1999), and subsequently increased TS protein (Kitchens et al., 1999; Murakami et al., 2000; Shibata et al., 1998). However, other mechanisms of resistance are possible, and may or may not accompany TS overexpression. These are: (a) acquisition of a mutant TS with reduced affinity for drug, but having decreased stability (Kitchens et al., 1999); or (b) an increase in the half-life of the wild-type TS, putatively due to a mutated or adapted cellular component that stabilizes TS or is responsible for degrading it (Kitchens et al., 1999). TS overexpression due to any of the above phenomena presents a potentially important target for use of an anti-TS antisense ODN to enhance the ability of TS inhibitors to kill tumour cells.
The cell culture model used in this study is comprised of TS-overexpressing cell lines selected in one or two steps of exposure to 5-FUdR. The FUdR-5a, -10g and -15a lines all express a similar level of resistance to 5-FUdR, and a similar level of TS mRNA. However, FUdR-5a has 10 fold more TS protein than parental HeLa cells compared to a maximum 2 fold elevation in TS protein in FUdR-10g and -15a. Upon a second selection step, the increased level of TS protein expressed by FUdR-5a was, at a minimum, maintained in the derived variants, and increased by 0.6 – 1.7-fold in the FUdR-50a and -200b lines, respectively. Conversely, resistance to 5-FUdR increased substantially in the FUdR-25b and -100a lines without a significant increase in TS protein. The disparity in the ratio of TS mRNA : TS protein among these drug-resistant cell lines suggests that, in addition to increased TS mRNA levels, other changes (including regulation of translation of TS mRNA; stability/drug affinity of TS protein; altered intracellular drug availability; or other events not directly related to regulation of TS (Kitchens et al., 1999)) may be involved in mediating resistance to one or more of the 5-FUdR-resistant HeLa lines.
Because of the different levels of TS overexpression and of resistance displayed by FUdR-5a, -25b and -50a (including the potential for differences in the overall mechanisms mediating 5-FUdR resistance among them), these lines were chosen as representative of tumours in which different mechanisms of 5-FUdR resistance might be encountered, and as models to test the potential clinical use of anti-TS antisense ODNs against TS-overexpressing tumours. In the parental HeLa cells, sensitivity to several TS inhibitors was enhanced up to 70% by treatment with antisense ODN 83 (Ferguson et al., 1999). In the present study, an 80% enhancement of 5-FUdR toxicity rendered the 5-FUdR-resistant FUdR-5a, -10g and -15a cell lines even more sensitive than the non-ODN-treated, parental HeLa cells. Therefore, human tumours that had acquired 2 – 3-fold resistance imparted by an incremental increase in TS content could potentially be sensitized by antisense treatment to a sufficient degree to completely abolish resistance.
In cell lines with higher levels of 5-FUdR-resistance, the antisense ODN enhanced sensitivity to 5-FUdR to the extent that resistance of the FUdR-25b and -50a lines (relative to parental cells) was reduced from between 10 – 20-fold to between 2 – 3-fold. This suggests that, for human tumours expressing a high level of resistance, it may be possible to achieve a lethal combination of antisense and drug. Under the transfection conditions used in these experiments, the cells were sensitized using a dose of ODN 83 that did not substantially slow proliferation on its own. However, as described above, antisense ODN 83 alone (at appropriate concentrations) can inhibit proliferation. Thus, the use of TS inhibitors together with higher concentrations of ODN 83 would be expected to inhibit proliferation due to a combined effect of antisense alone plus antisense-mediated enhancement of drug sensitivity, as previously reported in the parent HeLa line under these conditions (Ferguson et al., 1999). Direct measurement of this combination effect in vitro is hindered by the non-specific toxicity of higher concentrations of ODN and LFA-2K against the 5-FUdR-resistant cell lines.
The degree of enhancement of sensitivity of cells to FUdR by ODN 83 (70 – 80% reduction in IC50) was similar in all 5-FUdR-resistant cell lines, regardless of the level of resistance. This is likely due to the fact that, in the presence of 5-FUdR, the rate of proliferation is due to the remaining amount of active TS, and this amount, for a given rate of proliferation, is the same regardless of the original level of resistance. Therefore, since the antisense ODN causes a similar relative decrease in TS mRNA and protein among the parental and drug-resistant cell lines (Figure 2), the relative reduction in active TS is also the same among the different lines. A given percentage reduction in active TS requires approximately the same percentage less 5-FUdR to achieve a cytotoxic effect, regardless of the absolute, initial amount of TS in the cell. The question remains why the antisense ODN can reduce the TS mRNA by 80% in both a highly-overexpressing cell line and a similar amount in the parental cell line. This is possibly due to the kinetics of ODN binding to mRNA and of RNase enzyme action.
The consequence of the anti-TS ODN treatment may potentially extend beyond the immediate effect on drug cytotoxicity. In fact, we have reported that antisense ODN 83 induces a block at G2/M in HeLa cells, without appreciable apoptosis (Berg et al., 2001). In addition, TS protein has been reported to bind to, and inhibit translation of, p53 mRNA in vitro (Ju et al., 1999; Chu et al., 1999), and antisense downregulation of TS protein resulted in a 7 fold increase in p53 protein in a human colon cancer cell line (Schmitz et al., 2001). This increased p53 expression could permit easier induction of cell death by cytotoxic agents. Altered expression of proteins capable of mediating cell cycling and apoptosis in response to antisense targeting of TS mRNA warrants further investigation.
In summary, use of an anti-TS antisense ODN targeting TS mRNA, in combination with a TS inhibitor targeting TS protein, effectively enhanced the cytotoxicity of the protein-targeting drug in cells with low TS levels and in cells selected for TS overexpression by selection for growth in drug-containing media. Furthermore, enhancement of cytotoxicity was effective in drug-resistant cell lines that appear to employ more than one mechanism to mediate 5-FUdR resistance. These data indicate that use of anti-TS antisense reagents against tumours that overexpress TS may ultimately be of clinical utility, particularly as an adjunct to enhance the effectiveness of drugs targeting TS protein.
Acknowledgments
Financial support for this study was provided by AstraZeneca Inc., Mississauga, Ontario, Canada, and Imperial Oil Canada, Ltd. We gratefully acknowledge the kind gift of oligonucleotides from Isis Pharmaceuticals, Carlsbad, CA, U.S.A.
Abbreviations
- D-MEM
Dulbecco's modified Eagle's medium
- 5-FdUMP
5-fluorodeoxyuridine monophosphate
- 5-FU
5-fluorouracil
- 5-FUdR
5-fluorodeoxyuridine
- GAPDH
glyceraldehyde-3-phosphate-dehydrogenase
- IC50 value
concentration of drug that inhibited proliferation by 50%
- LFA-2K
Lipofectamine 2000®
- Me-FH4
5,10-methylene-tetrahydrofolate
- ODN
oligodeoxynucleotide
- PBS
phosphate-buffered saline (0.15 M NaCl+0.67 mM KH2PO4, pH 7.4)
- RT – PCR
reverse transcriptase-polymerase chain reaction
- TS
thymidylate synthase (5,10-methylenetetrahydrofolate : dUMP C-methyltransferase
- EC 2.1.1.45); UTR
untranslated region
References
- BENNETT C.F. Antisense oligonucleotides: is the glass half full or half empty. Biochem. Pharmacol. 1998;55:9–19. doi: 10.1016/s0006-2952(97)00214-1. [DOI] [PubMed] [Google Scholar]
- BERG R.W., WERNER M., FERGUSON P.J., POSTENKA C., VINCENT M., KOROPATNICK D.J., BEHREND E. Tumor growth inhibition in vivo and G2/M cell cycle arrest induced by antisense oligodeoxynucleotide targeting thymidylate synthase. J. Pharmacol. Exp. Ther. 2001;298:477–484. [PubMed] [Google Scholar]
- BERNE M.H.O., GUSTAVSSON B.G., ALMERSJO O., SPEARS P.C., FROSING R. Sequential methotrexate/5-FU: FdUMP formation and TS inhibition in a transplantable rodent colon adenocarcinoma. Cancer Chemother. Pharmacol. 1986;16:237–242. doi: 10.1007/BF00293984. [DOI] [PubMed] [Google Scholar]
- BINDER R., HOROWITZ J.A., BASILION J.P., KOELLER D.M., KLAUSNER R.D., HARFORD J.B. Evidence that the pathway of transferrin receptor mRNA degradation involves an endonucleolytic cleavage within the 3′ UTR and does not involve poly(A) tail shortening. EMBO J. 1994;13:1969–1980. doi: 10.1002/j.1460-2075.1994.tb06466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP M.R., IVERSEN P.L., BAYEVER E., SHARP J.G., GREINER T.C., COPPLE B.L., RUDDON R., ZON G., SPINOLO J., ARNESON M., ARMITAGE J.O., KESSINGER A. Phase I trial of an antisense oligonucleotide OL(1)p53 in hematologic malignancies. J. Clin. Oncol. 1996;14:1320–1326. doi: 10.1200/JCO.1996.14.4.1320. [DOI] [PubMed] [Google Scholar]
- CHEN H.X., MARSHALL J.L., TROCKY N., LING Y., BAIDAS S., RIZVI N., BHARGAVA P., LIPPMAN M.E., YANG D., HAYES D.F. A phase I study of bcl-2 antisense G3139 (GENTA) and weekly docetaxel in patients with advanced breast cancer and other solid tumors. Proc. Amer. Soc. Clin. Oncol. 2000;19:178. doi: 10.1093/annonc/mdh317. [DOI] [PubMed] [Google Scholar]
- CHU E., ALLEGRA C.J. The role of thymidylate synthase in cellular regulation. Advan. Enzyme Regul. 1996;36:143–163. doi: 10.1016/0065-2571(95)00004-6. [DOI] [PubMed] [Google Scholar]
- CHU E., COGLIATI T., COPUR S.M., BORRE A., VOELLER D.M., ALLEGRA C.J., SEGAL S. Identification of in vivo target RNA sequences bound by thymidylate synthase. Nucl. Acids Res. 1996;24:3222–3228. doi: 10.1093/nar/24.16.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU E., COPUR S.M., JU J., CHEN T.M., KHLEIF S., VOELLER D.M., MIZUNUMA N., PATEL M., MALEY G.F., MALEY F., ALLEGRA C.J. Thymidylate synthase protein and p53 mRNA form an in vivo ribonucleoprotein complex. Mol. Cell. Biol. 1999;19:1582–1594. doi: 10.1128/mcb.19.2.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU E., KOELLER D.M., CASEY J.L., DRAKE J.C., CHABNER B.A., ELWOOD P.C., ZINN S., ALLEGRA C.J. Autoregulation of human thymidylate synthase messenger RNA translation by thymidylate synthase. Proc. Natl. Acad. Sci. U.S.A. 1991;88:8977–8981. doi: 10.1073/pnas.88.20.8977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU E., KOELLER D.M., JOHNSTON P.G., ZINN S., ALLEGRA C.J. Regulation of thymidylate synthase in human colon cancer cells treated with 5-fluorouracil and interferon γ. Molec. Pharmacol. 1993a;43:527–533. [PubMed] [Google Scholar]
- CHU E., VOELLER D.M., JONES K.L., TAKECHI T., MALEY G.F., MALEY F., SEGAL S., ALLEGRA C.J. Identification of a thymidylate synthase ribonucleoprotein complex in human colon cancer cells. Molec. Cell. Biol. 1994;14:207–213. doi: 10.1128/mcb.14.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU E., VOELLER D., KOELLER D.M., DRAKE J.C., TAKIMOTO C.H., MALEY G.F., MALEY F., ALLEGRA C.J. Identification of an RNA binding site for human thymidylate synthase. Proc. Natl. Acad. Sci. U.S.A. 1993b;90:517–521. doi: 10.1073/pnas.90.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU E., ZINN S., BOARMAN D., ALLEGRA C.J. Interaction of interferon and 5-fluorouracil in the H630 human colon carcinoma cell line. Cancer Res. 1990;50:5834–5840. [PubMed] [Google Scholar]
- CUCCO C., CALABRETTA B. In vitro and in vivo reversal of multidrug resistance in a human leukemia-resistant cell line by mdr1 antisense oligodeoxynucleotides. Cancer Res. 1996;56:4332–4337. [PubMed] [Google Scholar]
- DANENBERG P.V. Thymidylate synthase–a target enzyme in cancer chemotherapy. Biochim. Biophys. Acta. 1977;473:73–92. doi: 10.1016/0304-419x(77)90001-4. [DOI] [PubMed] [Google Scholar]
- DEAN N.M., MCKAY R., CONDON T.P., BENNET C.F. Inhibition of protein kinase Cα expression in a human A549 cells by antisense oligonucleotides inhibits induction of intercellular adhesion molecule 1 (ICAM-1) mRNA by phorbol esters. J. Biol. Chem. 1994;269:16416–16424. [PubMed] [Google Scholar]
- DEAN N., MCKAY R., MIRAGLIA L., HOWARD R., COOPER S., GIDDINGS J., NICKLIN P., MEISTER L., ZIEL R., GEIGER T., MULLER M., FABBRO D. Inhibition of growth of human tumor cell lines in nude mice by an antisense oligonucleotide inhibitor of protein kinase Cα expression. Cancer Res. 1996;56:3499–3507. [PubMed] [Google Scholar]
- DE LUCA A., SELVAM M.P., SANDOMENICO C., PEPE S., BIANCO A.R., CIARDIELLO F., SALOMON D.S., NORMANNO N. Anti-sense oligonucleotides directed against EGF-related growth factors enhance anti-proliferative effect of conventional anti-tumor drugs in human colon-cancer cells. Int. J. Cancer. 1997;73:277–282. doi: 10.1002/(sici)1097-0215(19971009)73:2<277::aid-ijc19>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- EDLER D., HALLSTROM M., JOHNSTON P.G., MAGNUSSON I., RAGNHAMMAR P., BLOMGREN H. Thymidylate synthase expression: an independent prognostic factor for local recurrence, distant metastasis, disease-free and overall survival in rectal cancer. Clin. Cancer Res. 2000;6:1378–1384. [PubMed] [Google Scholar]
- FERGUSON P.J., COLLINS O., DEAN N.M., DEMOOR J., CHEN S.-L., VINCENT M.D., KOROPATNICK J. Antisense down-regulation of thymidylate synthase to suppress growth and enhance cytotoxicity of 5-FUdR, 5-FU, and Tomudex in HeLa cells. Br. J. Pharmacol. 1999;127:1777–1786. doi: 10.1038/sj.bjp.0702728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARRIDO C., MEHLEN P., FROMENTIN A., HAMMANN A., ASSEM M., ARRIGO A.P., CHAUFFERT B. Inconstant association between 27-kDa heat-shock protein (Hsp27) content and doxorubicin resistance in human colon cancer cells. The doxorubicin-protecting effect of Hsp27. Eur. J. Biochem. 1996;237:653–659. doi: 10.1111/j.1432-1033.1996.0653p.x. [DOI] [PubMed] [Google Scholar]
- IIZUKA N., HIROSE K., NOMA T., HAZAMA S., TANGOKU A., HAYASHI H., ABE T., YAMAMOTO K., OKA M. The nm23-H1 gene as a predictor of sensitivity to chemotherapeutic agents in oesophageal squamous cell carcinoma. Br. J. Cancer. 1999;81:469–475. doi: 10.1038/sj.bjc.6690717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INABA M., MITSUHASHI J. Flow cytometric analysis of cell-killing actions of 5-fluorouracil in human colorectal cancer cells. Oncol. Res. 1994;6:303–309. [PubMed] [Google Scholar]
- JACKMAN A.L., TAYLOR G.A., GIBSON W., KIMBELL R., BROWN M., CALVERT A.H., JUDSON I.R., HUGHES L.R. ICI D1694, a quinazoline antifolate thymidylate synthase inhibitor that is a potent inhibitor of L1210 tumor cell growth in vitro and in vivo: a new agent for clinical study. Cancer Res. 1991;51:5579–5586. [PubMed] [Google Scholar]
- JOHNSON L.F. Posttranscriptional regulation of thymidylate synthase gene expression. J. Cell. Biochem. 1994;54:387–392. doi: 10.1002/jcb.240540405. [DOI] [PubMed] [Google Scholar]
- JU J., KANE S.E., LENZ H.-J., DANENBERG K.D., CHU E., DANENBERG P.V. Desensitization and sensitization of cells to fluoropyrimidines with different antisenses directed against thymidylate synthase messenger RNA. Clin. Cancer Res. 1998;4:2229–2236. [PubMed] [Google Scholar]
- JU J., PEDERSEN-LANE J., MALEY F., CHU E. Regulation of p53 expression by thymidylate synthase. Proc. Natl. Acad. Sci. U.S.A. 1999;96:3769–3774. doi: 10.1073/pnas.96.7.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAKUTANI T., EBARA Y., KANJA K., TAKAHASHI K., WATAYA Y. Activation of c-jun and c-fos genes in dNTP imbalance cell death induced with 5-fluoro-2′-deoxyuridine in mouse mammary tumor FM3A cell line. Nucleosides Nucleotides. 1998;17:1299–1308. doi: 10.1080/07328319808003468. [DOI] [PubMed] [Google Scholar]
- KANEDA S., TAKEISHI K., AYUSAWA D., SHIMIZU K., SENO T., ALTMAN S. Role in translation of a triple tandemly repeated sequence in the 5′-untranslated region of human thymidylate synthase mRNA. Nucl. Acids Res. 1987;15:1259–1270. doi: 10.1093/nar/15.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAVALLARIS M., BURKHART C.A., HORWITZ S.B. Antisense oligonucleotides to class IIIβ tubulin sensitize drug-resistant cells to Taxol. Br. J. Cancer. 1999;80:1020–1025. doi: 10.1038/sj.bjc.6690507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYOMARSI K., SAMET J., MOLNAR G., PARDEE A.B. The thymidylate synthase inhibitor, ICI D1694, overcomes translational detainment of the enzyme. J. Biol. Chem. 1993;268:15142–15149. [PubMed] [Google Scholar]
- KITADA S., TAKAYAMA S., DE RIEL K., TANAKA S., REED J.C. Reversal of chemoresistance of lymphoma cells by antisense-mediated reduction of bcl-2 gene expression. Antisense Res. Dev. 1994;4:71–79. doi: 10.1089/ard.1994.4.71. [DOI] [PubMed] [Google Scholar]
- KITCHENS M.E., FORSTHOEFEL A.M., BARBOUR K.W., SPENCER H.T., BERGER F.G. Mechanisms of acquired resistance to thymidylate synthase inhibitors: the role of enzyme stability. Mol. Pharmacol. 1999;56:1063–1070. doi: 10.1124/mol.56.5.1063. [DOI] [PubMed] [Google Scholar]
- KORNMANN M., DANENBERG K.D., ARBER N., BEGER H.G., DANENBERG P.V., KORC M. Inhibition of cyclin D1 expression in human pancreatic cancer cells is associated with increased chemosensitivity and decreased expression of multiple chemoresistance genes. Cancer Res. 1999;59:3505–3511. [PubMed] [Google Scholar]
- KYU-HO HAN E., GEHRKE L., TAHIR S.K., CREDO R.B., CHERIAN S.P., SHAM H., ROSENBERG S.H., NG S. Modulation of drug resistance by β tubulin in paclitaxel-resistant human lung cancer cell lines. Eur. J. Cancer. 2000;36:1565–1571. doi: 10.1016/s0959-8049(00)00145-3. [DOI] [PubMed] [Google Scholar]
- LI X., SMYTH A.P., BARRETT D.J., IVY S.P., VON HOFE E. Sensitization of multidrug-resistant human leukemia cells with MDR1-targeted antisense and inhibition of drug-mediated MDR1 induction. Leukemia. 1997;11:950–957. doi: 10.1038/sj.leu.2400696. [DOI] [PubMed] [Google Scholar]
- LIN X., PARSELS L.A., VOELLER D.M., ALLEGRA C.J., MALEY G.F., MALEY F., CHU E. Characterization of a cis-acting regulatory element in the protein coding region of thymidylate synthase mRNA. Nucleic Acids Res. 2000;28:1381–1389. doi: 10.1093/nar/28.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUO D., CHENG S.C., XIE H., XIE Y. Chemosensitivity of human hepatocellular carcinoma cell line QGY-7703 is related to bcl-2 protein levels. Tumour Biol. 1999;20:331–340. doi: 10.1159/000030097. [DOI] [PubMed] [Google Scholar]
- MADER R.M., SIEDER A.E., BRAUN J., RIZOVSKI B., KALIPCIYAN M., MUELLER M.W., JAKESZ R., RAINER H., STEGER G.G. Transcription and activity of 5-fluorouracil converting enzymes in fluoropyrimidine resistance in colon cancer in vitro. Biochem. Pharmacol. 1997;54:1233–1242. doi: 10.1016/s0006-2952(97)00330-4. [DOI] [PubMed] [Google Scholar]
- MATSUI S.I., ARREDONDO M.A., WRZOSEK C., RUSTUM Y.M. DNA damage and p53 induction do not cause ZD1694-induced cell cycle arrest in human colon carcinoma cells. Cancer Res. 1996;56:4715–4723. [PubMed] [Google Scholar]
- MIAYAKE H., TOLCHER A., GLEAVE M.E. Chemosensitization and delayed androgen-independent recurrence of prostate cancer with the use of antisense Bcl-2 oligodeoxynucleotides. J. Natl. Cancer Inst. 2000;92:34–41. doi: 10.1093/jnci/92.1.34. [DOI] [PubMed] [Google Scholar]
- MINI E., BIONDI C., MORGANTI M., NAPOLI C., MAZZONI P., CIANCHI F., TONELLI F., CORTESINI C., CAPACCIOLI S., FICARI F., QUATTRONE A., ROSSI S., MAZZEI T. Marked variation of thymidylate synthase and folylpolyglutamate synthetase gene expression in human colorectal tumors. Oncol. Res. 1999;11:437–445. [PubMed] [Google Scholar]
- MIZUTANI Y., FUKUMOTO M., BONAVIDA B., YOSHIDA O. Enhancement of sensitivity of urinary bladder tumor cells to cisplatin by c-myc antisense oligonucleotide. Cancer. 1994;74:2546–2554. doi: 10.1002/1097-0142(19941101)74:9<2546::aid-cncr2820740924>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- MURAKAMI Y., KAZUNO H., EMURA T., TSUJIMOTO H., SUZUKI N., FUKUSHIMA M. Different mechanisms of acquired resistance to fluorinated pyrimidines in human colorectal cancer cells. Int. J. Oncol. 2000;17:277–283. doi: 10.3892/ijo.17.2.277. [DOI] [PubMed] [Google Scholar]
- NITA M.E., TOMINAGA O., NAGAWA H., TSURUO T., MUTO T. Dihydropyrimidine dehydrogenase but not thymidylate synthase expression is associated with resistance to 5-fluorouracil in colorectal cancer. Hepatogastroenterol. 1998;45:2117–2122. [PubMed] [Google Scholar]
- PARR A.L., DRAKE J.C., GRESS R.E., SCHWARTZ G., STEINBERG S.M., ALLEGRA C.J. 5-fluorouracil-mediated thymidylate synthase induction in malignant and nonmalignant human cells. Biochem. Pharmacol. 1998;56:231–235. doi: 10.1016/s0006-2952(98)00152-x. [DOI] [PubMed] [Google Scholar]
- PETERS G.J., VAN DER WILT C.L., VAN GROENINGEN C.J., SMID K., MEIJER S., PINEDO H.M. Thymidylate synthase inhibition after administration of fluorouracil with or without leucovorin in colon cancer patients: implications for treatment with fluorouracil. J. Clin. Oncol. 1994;12:2035–2042. doi: 10.1200/JCO.1994.12.10.2035. [DOI] [PubMed] [Google Scholar]
- PETERS G.J., VAN TRIEST B., BACKUS H.H., KUIPER C.M., VAN DER WILT C.L., PINEDO H.M. Molecular downstream events and induction of thymidylate synthase in mutant and wild-type p53 colon cancer cell lines after treatment with 5-fluorouracil and the thymidylate synthase inhibitor raltitrexed. Eur. J. Cancer. 2000;36:916–924. doi: 10.1016/s0959-8049(00)00026-5. [DOI] [PubMed] [Google Scholar]
- QUATTRONE A., PAPUCCI L., MORGANTI M., CORONNELLO M., MINI E., MAZZEI T., COLONNA F.P., GARBESI A., CAPACCIOLI S. Inhibition of MDR1 gene expression by antimessenger oligonucleotides lowers multiple drug resistance. Oncol. Res. 1994;6:311–320. [PubMed] [Google Scholar]
- SALONGA D., DANENBERG K.D., JOHNSON M., METZGER R., GROSHEN S., TSAO-WEI D.D., LENZ H.J., LEICHMAN C.G., LEICHMAN L., DIASIO R.B., DANENBERG P.V. Colorectal tumors responding to 5-fluorouracil have low gene expression levels of dihydropyrimidine dehydrogenase, thymidylate synthase, and thymidine phosphorylase. Clin. Cancer Res. 2000;6:1322–1327. [PubMed] [Google Scholar]
- SCHER H.I., MORRIS M.J., TONG W.P., CORDON-CARDO C., DROBNJAK M., KELLY W.K., SLOVIN S.F., TERRY K.L., DIPAOLA R.S., RAFI M., ROSEN N. A phase I trial of G3139, a bcl2 antisense drug, by continuous infusion (CI) as a single agent and with weekly taxol (T) Proc. Amer. Soc. Clin. Oncol. 2000;19:199. [Google Scholar]
- SCHMITZ J.C., YU D., AGRAWAL S., CHU E.Effect of 2′-O-methyl antisense ORNs on expression of thymidylate synthase in human colon cancer RKO cells Nucleic Acids Res. 200129415–422.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAW J.-P., KENT K., BIRD J., FISHBACK J., FROEHLER B. Modified deoxyoligonucleotides stable to exonuclease degradation in serum. Nucl. Acids Res. 1991;19:747–750. doi: 10.1093/nar/19.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHIBATA J., AIBA K., SHIBATA H., MINOWA S., HORIKOSHI N. Detection and quantitation of thymidylate synthase mRNA in human colon adenocarcinoma cell line resistant to 5-fluorouracil by competitive PCR. Anticancer Res. 1998;18:1457–1463. [PubMed] [Google Scholar]
- SPEARS C.P., GUSTAVSSON B.G. Methods for thymidylate synthase pharmacodynamics: serial biopsy, free and total TS, FdUMP and dUMP, and H4PTEGLU and CH2-H4PTEGLU assays. Adv. Exp. Med. Biol. 1988;244:97–104. doi: 10.1007/978-1-4684-5607-3_9. [DOI] [PubMed] [Google Scholar]
- STEIN C.A., CHENG Y.-C. Antisense oligonucleotides as therapeutic agents - is the bullet really magical. Science. 1993;261:1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- STEIN C.A., SUBASINGHE C., SHINOZUKA K., COHEN J.S. Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucl. Acids Res. 1988;16:3209–3221. doi: 10.1093/nar/16.8.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART A.J., CANITROT Y., BARACCHINI E., DEAN N.M., DEELEY R.G., COLE S.P. Reduction of expression of the multidrug resistance protein (MRP) in human tumor cells by antisense phosphorothioate oligonucleotides. Biochem. Pharmacol. 1996;51:461–469. doi: 10.1016/0006-2952(95)02220-1. [DOI] [PubMed] [Google Scholar]
- SUZUKI M., TSUKAGOSHI S., SAGA Y., OHWADA M., SATO I. Enhanced expression of thymidylate synthase may be of prognostic importance in advanced cervical cancer. Oncology. 1999;57:50–54. doi: 10.1159/000012000. [DOI] [PubMed] [Google Scholar]
- SWAIN S.M., LIPPMAN M.E., EGAN E.F., DRAKE J.C., STEINBERG S.M., ALLEGRA C.J. Fluorouracil and high-dose leucovorin in previously treated patients with metastatic breast cancer. J. Clin. Oncol. 1989;7:890–899. doi: 10.1200/JCO.1989.7.7.890. [DOI] [PubMed] [Google Scholar]
- TAKENOUE T., NAGAWA H., MATSUDA K., FUJII S., NITA M.E., HATANO K., KITAYAMA J., TSURUO T., MUTO T. Relation between thymidylate synthase expression and survival in colon carcinoma, and determination of appropriate application of 5-fluorouracil by immunohistochemical method. Ann. Surg. Oncol. 2000;7:193–198. doi: 10.1007/BF02523653. [DOI] [PubMed] [Google Scholar]
- THIERRY A.R., RAHMAN A., DRITSCHILO A. Overcoming multidrug resistance in human tumor cells using free and liposomally encapsulated antisense oligodeoxynucleotides. Biochem. Biophys. Res. Commun. 1993;190:952–960. doi: 10.1006/bbrc.1993.1142. [DOI] [PubMed] [Google Scholar]
- VAN DER WILT C., PINEDO H.M., SMID K., PETERS G.J. Elevation of thymidylate synthase following 5-fluorouracil treatment is prevented by the addition of leucovorin in murine colon tumors. Cancer Res. 1992;52:4922–4928. [PubMed] [Google Scholar]
- VAN TRIEST B., LOFTUS B.M., PINEDO H.M., BACKUS H.H., SCHOENMAKERS P., TELLEMAN F., TADEMA T., AHERNE G.W., VAN GROENINGEN C.J., ZOETMULDER F.A., TAAL B.G., JOHNSTON P.G., PETERS G.J. Thymidylate synthase expression in patients with colorectal carcinoma using a polyclonal thymidylate synthase antibody in comparison to the TS 106 monoclonal antibody. J. Histochem. Cytochem. 2000;48:755–760. doi: 10.1177/002215540004800604. [DOI] [PubMed] [Google Scholar]
- VAN TRIEST B., PETERS G.J. Thymidylate synthase: a target for combination therapy and determinant of chemotherapeutic response in colorectal cancer. Oncology. 1999;57:179–194. doi: 10.1159/000012030. [DOI] [PubMed] [Google Scholar]
- VAN TRIEST B., PINEDO H.M., VAN HENSBERGEN Y., SMID K., TELLEMAN F., SCHOENMAKERS P.S., VAN DER WILT C.L., VAN LAAR J.A., NOORDHUIS P., JANSEN G., PETERS G.J. Thymidylate synthase level as the main predictive parameter for sensitivity to 5-fluorouracil, but not for folate-based thymidylate synthase inhibitors, in 13 nonselected colon cancer cell lines. Clin. Cancer Res. 1999;5:643–654. [PubMed] [Google Scholar]
- WATERS J.S., WEBB A., CUNNINGHAM D., CLARKE P.A., RAYNAUD F., DI STEFANO F., COTTER F.E. Phase I clinical and pharmacokinetic study of bcl-2 antisense oligonucleotide therapy patients with non-Hodgkin's lymphoma. J. Clin. Oncol. 2000;18:1812–1823. doi: 10.1200/JCO.2000.18.9.1812. [DOI] [PubMed] [Google Scholar]
- WELSH S.J., TITLEY J., BRUNTON L., VALENTI M., MONAGHAN P., JACKMAN A.L., AHERNE G.W. Comparison of thymidylate synthase (TS) protein up-regulation after exposure to TS inhibitors in normal and tumor cell lines and tissues. Clin. Cancer Res. 2000;6:2538–2546. [PubMed] [Google Scholar]
- YANG C.C., HSU C.P., YANG S.D. Antisense suppression of proline-directed protein kinase FA enhances chemosensitivity in human prostate cancer cells. Clin. Cancer Res. 2000;6:1024–1030. [PubMed] [Google Scholar]
- YIN M.B., GUO B., PANADERO A., FRANK C., WRZOSEK C., SLOCUM H.K., RUSTUM Y.M. Cyclin E-cdk2 activation is associated with cell cycle arrest and inhibition of DNA replication induced by the thymidylate synthase inhibitor Tomudex. Exp. Cell Res. 1999;247:189–199. doi: 10.1006/excr.1998.4346. [DOI] [PubMed] [Google Scholar]
- ZHANG Z.-G., HARSTRICK A., RUSTUM Y.M. Mechanisms of resistance to fluoropyrimidines. Sem. Oncol. 1992;19:4–9. [PubMed] [Google Scholar]


