Abstract
Somatostatin (6.11 nmol kg−1 i.p.) inhibited neurogenic plasma extravasation evoked by 1% mustard oil and non-neurogenic oedema induced by 5% dextran in the rat skin.
Cyclic synthetic octapeptide (TT-248 and TT-250) and heptapeptide (TT-232) somatostatin analogues proved to be more effective in reducing neurogenic and non-neurogenic inflammatory reactions but octreotide had no influence on either neurogenic or non-neurogenic inflammation.
TT-232 administered i.p. or i.v. (1.06 – 42.40 nmol kg−1) inhibited in a dose-dependent manner the plasma extravasation evoked by mustard oil in the rat's paw. Neither diclofenac (15.78 – 315.60 μmol kg−1) nor the selective COX-2 inhibitor meloxicam (2.95 – 569.38 μmol kg−1) attenuated the mustard oil-induced neurogenic plasma extravasation.
TT-232, diclofenac and meloxicam dose-dependently diminished non-neurogenic dextran-oedema of the paw the ED35 values were 1.73 nmol kg−1 for TT-232 and 34.37 μmol kg−1 for diclofenac.
TT-232 inhibited in the dose range of 1.06 – 21.21 nmol kg−1 the bradykinin-induced plasma extravasation in the skin of the chronically denervated paw.
Mustard oil-induced cutaneous plasma extravasation was dose-dependently diminished by s.c. TT-232 1, 2, 4, 6 or 16 h after the treatment. TT-232 (2×106, 2×212 and 2×530 nmol kg−1 per day s.c. for 18 days) caused dose-dependent inhibition of chronic Freund adjuvant-induced arthritis during the experimental period.
TT-232 (200 and 500 nM) inhibited the release of SP, CGRP and somatostatin from the rat isolated trachea induced by electrical field stimulation (40 V, 0.1 ms, 10 Hz, 120 s) or by capsaicin (10−7 M), but did not influence the basal, non-stimulated peptide release.
It is concluded that somatostatin analogues without endocrine functions as TT-232 are promising compounds with a novel site of action for inhibition of non-neurogenic and neurogenic inflammatory processes.
Keywords: Neurogenic inflammation, anti-inflammatory effect, neuropeptide release, mustard oil, dextran-oedema, Freund adjuvant, somatostatin analogues, TT-232, diclofenac, meloxicam
Introduction
It has been shown that activation of nociceptors sensitive to capsaicin, noxious heat or inflammatory mediators (Szolcsányi, 1996a, 1996b; Caterina et al., 1997) results in not only pain sensation but a release of sensory neuropeptides. Among these neuropeptides calcitonin gene-related peptide (CGRP) and substance P (SP) elicit local neurogenic inflammation (vasodilatation and plasma extravasation) (Chahl, 1991; Holzer, 1992; Maggi, 1995; Geppetti & Holzer, 1996; Szolcsányi, 1988; 1996a). Neurogenic inflammation participates in this sense in various extent in all inflammatory responses where nociception or pain sensation occurs. This type of inflammation is resistant to the inhibitory effect of non-steroidal anti-inflammatory agents like indomethacin, phenylbutazone, amidopyridine or flufenamic acid (Jancsó-gábor & Szolcsányi, 1970). It has been described that somatostatin is also stored in the capsaicin-sensitive subpopulation of nociceptors from where it can be released and depleted (Gamse et al., 1981; Holzer, 1988; Szolcsányi et al., 1994). It has been known for a long time that somatostatin inhibits neurogenic inflammation (Lembeck et al., 1982; Karalis et al., 1994; Fioravanti et al., 1995) and nociception but our studies were the first to reveal that suitable amount of somatostatin could be released from the activated primary afferent nerve terminals to elicit a systemic anti-inflammatory (Szolcsányi et al., 1998a, 1998b) and anti-nociceptive (Helyes et al., 2000) action.
Somatostatin is widely expressed in the central nervous system (Gamse et al., 1981) and the peripheral tissues in 14 and 28 amino acid-containing forms (Patel et al., 1995; ten Bokum et al., 2000). A great variety of its effects has already been described including modulation of hormone (growth hormone, glucagon, insulin) and neurotransmitter (SP, dopamine, 5-hydroxytriptamine, acetylcholine and somatostatin) release, cognitive and behavioural processes, the gastrointestinal tract, the cardiovascular system and tumour-cell proliferation (ten Bokum et al., 2000). These effects are mediated via five different somatostatin receptor subtypes which have been cloned and which are known to be members of the G-protein associated receptor family (Hoyer et al., 1995; Reisine & Bell, 1995). The therapeutic use of native somatostatin is limited by its broad range of effects at these somatostatin receptors and also by its very short (3 min) plasma half life-time (ten Bokum et al., 2000). Functional characterization of these receptor subtypes has revealed two main groups: SRIF1 group comprising the sst2, sst3 and sst5 receptors and SRIF2 group comprising sst1 and sst4 receptors (Reisine & Bell, 1995; Hofland et al., 1995). Recently a series of new potent, stable, analogues (Table 1) has been synthesized in our laboratories to study the relative importance of specific substitutions in selectivity between these receptor subtypes. An analogue with a cyclopenta-ring structure, called TT-232, was found to be unique because it had no endocrine activity. This analogue failed to inhibit growth hormone (GH) release or gastrin secretion in vivo but had a strong antiproliferative and apoptotic effect on tumour cells in vivo and in vitro (Kéri et al., 1993; 1996).
Table 1.
Amino acid sequence and molecular weight of the somatostatin analogues under investigation (Ind: indolinil)
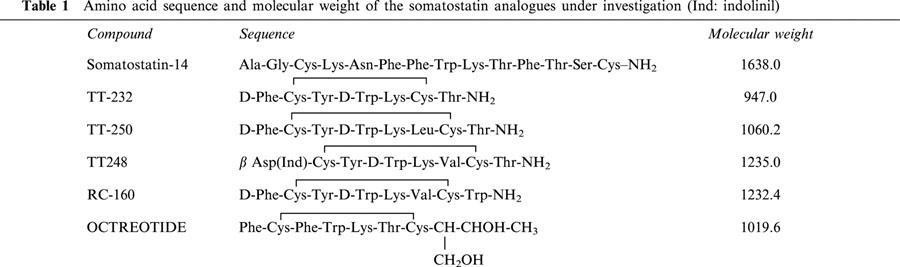
The aim of the present study was to analyse the effect of somatostatin and its cyclic, synthetic analogues, particularly the heptapeptide TT-232 (Kéri et al., 1993; 1996) on neurogenic and non-neurogenic inflammatory reactions in vivo and on sensory neuropeptide release in vitro.
Methods
Animals
Experiments were performed on female Wistar rats weighing 150 – 250 g. The animals were kept in the Laboratory Animal Centre of the University Medical School of Pécs under pathogen free conditions at 24 – 25°C and provided with standard rat chow and water ad libitum. All experimental procedures used in this study were in agreement with the rules of the Ethics Committee on Animal Research of Pécs University.
Induction and determination of acute cutaneous inflammation
Experiments were carried out under sodium pentobarbitone (40 mg kg−1 i.p.) anaesthesia.
Neurogenic inflammation
Neurogenic inflammation in the skin of the acutely denervated hindleg was evoked by topical application of 1% mustard oil dissolved in paraffin oil. Both saphenous and sciatic nerves were exposed and cut 30 min before the experiments in order to avoid the interference of autonomic reflexes. Extravasation of plasma albumin was measured by the Evans blue leakage method. Evans blue (50 mg kg−1) was injected i.v. and neurogenic inflammation was induced 10 min later. Rats were killed by exsanguination 20 min after the application of the inflammatory agent. The skin of the hindpaws were removed and the extravasated dye was extracted with formamide for 72 h at room temperature for photometric determination at 620 nm (Spectromom 195). The amount of the accumulated Evans blue, which quantitatively correlates with the intensity of neurogenic inflammation, was expressed as μg dye g−1 wet tissue.
Non-neurogenic inflammation
Non-neurogenic inflammation was elicited by dextran (100 μl, 5%) or bradykinin (50 μl, 0.25 μg) administered s.c. under the plantar skin of the chronically denervated hindleg to produce tissue oedema and plasma extravasation. The hindlimbs were denervated 5 days prior to dextran or bradykinin injection to elicit degeneration of the leg's nerve supply and therefore exclude the neurogenic part of the inflammation (Jancsó et al., 1967). Oedema formation in the rat hindpaw was measured by plethysmometry (Ugo Basile 7140). The transducer of the instrument records small differences in water level caused by volume displacement. The paw volumes were measured prior to s.c. injection of 0.1 ml 5% dextran (control value) and 10, 20, 30 min after the treatment. The extent of the oedema was expressed as a percentage of control. Non-neurogenic plasma extravasation elicited by bradykinin was determined by the Evans blue technique.
Somatostatin in a dose of 10 μg kg−1 i.p. (6.11 nmol kg−1), 10 μg kg−1 i.p. of its cyclic, synthetic analogues, TT-248, TT-250, RC-160 and octreotide (8.12, 9.43 and 8.11 nmol kg−1, respectively); 1, 2.5, 5, 10, 20 and 40 10 μg kg−1 TT-232 i.p. and i.v. (1.06, 2.65, 5.30, 10.60, 21.2, 42.40 nmol kg−1); 5, 10, 20 i.v. and 100 mg kg−1 i.m. diclofenac (15.75, 31.56, 63.12 μmol kg−1 and 314.4 μmol kg−1) or 1, 10, 20, 200 mg kg−1 i.v. selective cyclo-oxygenase-2 (COX-2) inhibitor meloxicam (2.95, 29.5, 56.94 and 569.38 μmol kg−1) were given 10 min before the induction of neurogenic or non-neurogenic inflammation. In a subset of experiments 10, 20, 40, 80, 160, 320 μg kg−1 TT-232 (10.6, 21.2, 42.4, 84.8, 169.6, 339.2 nmol kg−1) was administered s.c. 1, 2, 4 and 6 h before mustard oil smearing. Animals of the control groups were pretreated with the solvent of the respective compound.
Induction and determination of chronic arthritis
Oedema of the ankle joint was measured by plethysmometry before and on the 2nd, 5th, 8th, 12th, 15th and 18th day after 0.1 ml (1 mg ml−1) injection of complete Freund-adjuvant (mixture of killed Mycobacteria and liquid paraffin) into the plantar skin of the left hindpaw. In order to enhance the systemic effects additional intradermal injections (0.1 ml, 1 mg ml−1) were given into the root of the tail on the same and on the following day. Joint swelling was expressed as a percentage of pre-injection control values. TT-232 in doses of 2×100, 2×200 or 2×500 μg kg−1 (2×106, 2×212 or 2×530 nmol kg−1, respectively) per day was administered s.c. throughout the experimental period of 18 days.
Measurement of sensory neuropeptide release in vitro
After exsanguination the tracheae of 2 – 2 female Wistar rats were removed and perfused (1 ml min−1) in an organ bath (1.8 ml) at 37°C for 60 min with oxygenated (95% O2 and 5% CO2) Krebs solution of the following composition (in mM): NaCl 119, NaHCO3 25, KH2PO4 1.2, MgSO4 1.5, KCl 4.7, CaCl2 2.5, glucose 11. After stopping the flow the solution was changed three times for 8 min (prestimulated – stimulated – poststimulated). Electrical field stimulation (40 V, 0.1 ms, 10 Hz, 120 s) and chemical stimulation (capsaicin 10−7 M) were performed to induce release of sensory neuropeptides from the tissue pieces in the presence or absence of TT-232 (200, 500 or 1500 nM). The fractions were collected in ice-cold tubes and the wet weight of the tracheae were measured. Concentrations of SP, CGRP and somatostatin were determined by specific radioimmunoassay (RIA) methods developed in our laboratory (Németh et al., 1996; 1998a, 1998b), and were expressed as the released amount of peptide per tissue weight.
Drugs
Sodium pentobarbitone was obtained from May and Baker (England, U.K.), mustard oil (allyllisothiocyanate) and dextran from Fluka (Buchs, Switzerland), complete Freund adjuvant, Evans blue dye, capsaicin (8-methyl-N-vanillyl-6-nonenamide), bradykinin, and somatostatin-14 from Sigma (St. Louis, MO, U.S.A.), rat α-CGRP, [Tyr1]somatostatin-14 and Tyr-α-CGRP(23 – 37) from Bachem (Bubendorf, Switzerland), substance P RIA-tracer from Amersham (Amersham, U.K.), octreotide (Sandostatin) from Sandoz (U.K.), diclofenac sodium from RBI (U.S.A.) and meloxicam from Boehringer Ingelheim (Biberach, Germany). Capsaicin was dissolved in 10% ethanol, 10% Tween 80 (Reanal, Hungary) and 80% isotonic saline. TT-248, TT-250 and TT-232 were synthetized in the Central Research Institute for Chemistry of the Hungarian Academy of Sciences. Somatostatin and the analogues were dissolved in isotonic saline with 200 μl 0.1 M acetic acid for i.p. application. For i.v. and s.c. administration TT-232 (1 mg) was dissolved in 1 ml acetate-acetic acid buffer (0.2 mol l−1, pH: 3.4) as a stock solution, further dilutions were made in the same buffer and the injection form of the solution contained 5% mannitol. Diclofenac in low doses and meloxicam were dissolved in saline, but owing to the limited solubility of diclofenac, the 100 mg kg−1 dose was dissolved in DMSO and made up to the final volume with 0.9% saline. Substance P antiserum was kindly provided by Prof D.J. Dockray, University of Liverpool and C-terminal sensitive somatostatin antiserum and CGRP antiserum by Dr T. Görcs, Semmelweis University Medical School of Budapest. 125I-labelled Tyr-α-CGRP(23 – 37) and 125I-labelled [Tyr1]somatostatin-14 were prepared in our laboratory.
Statistical analysis
Results are presented as means±s.e.mean. Non-parametric (Mann – Whitney) test was used for statistical evaluation of the in vivo data and Student's t-test for paired comparison for the in vitro experiments. Probability values P<0.05 or less were regarded as significant. For evaluation of the anti-inflammatory effects of i.v. or s.c. TT-232, diclofenac and meloxicam dose-response curves of per cent inhibitions with 95% confidence intervals were calculated and presented with the ID50 or ID35 values.
Results
Effect of i.p. administered somatostatin and its analogues on neurogenic and dextran-induced non-neurogenic inflammation
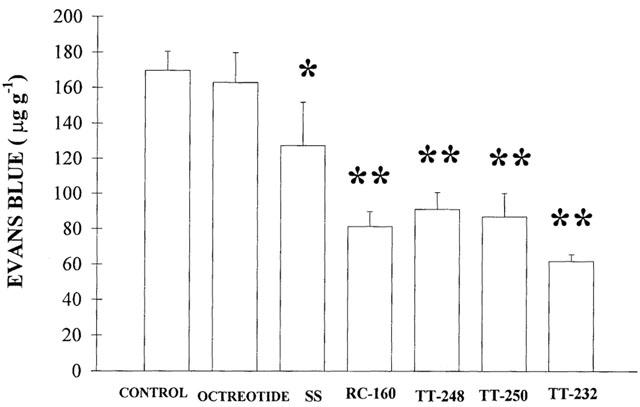
Somatostatin (6.11 nmol kg−1 i.p.) inhibited the 1% mustard oil-induced neurogenic plasma extravasation in the skin of the acutely denervated hindlegs by 24.85% (n=7). This inhibition was 46.26% (n=7), 48.86% (n=5), 53.98% (n=6) and 63.62% (n=5), after TT-248 (8.12 nmol kg−1 i.p), TT-250 (9.43 nmol kg−1 i.p), RC-160 (8.11 nmol kg−1 i.p) and TT-232 (10.6 nmol kg−1 i.p) pretreatments, respectively. Octreotide did not show inhibitory action compared to the solvent treated group (n=5) (Figure 1). Oedema formation of the chronically denervated hindlegs elicited by subplantar injection of 100 μl 5% dextran was diminished by 30.96% (n=7), 22.2% (n=7), 24.93% (n=5), 20.74% (n=5) and 51.92% (n=5) 30 min after the induction of inflammation in rats pretreated with somatostatin, TT-248, TT-250, RC-160 and TT-232 (6.11, 8.12, 9.43, 8.11 and 10.6 nmol kg−1 i.p), respectively. Octreotide had no influence on non-neurogenic oedema either (n=5) (Figure 2).
Figure 1.
Effect of 10 μg kg−1 i.p. somatostatin (SS) and its cyclic, synthetic analogues, octreotide, TT-248, TT-250, RC-160 and TT-232 (6.11, 8.12, 9.43, 8.11 and 10.6 nmol kg−1, respectively) on 1% mustard oil-induced neurogenic Evans blue accumulation in the skin of the acutely denervated hindlegs. In the control group saline (solvent) was applied i.p. in the same volume. Each column shows the mean of n=5 – 7 experiments with s.e.mean. *P<0.05; **P<0.01.
Figure 2.
Effect of 10 μg kg−1 i.p. somatostatin (SS), octreotide and its new peptidomimetic analogues, TT-248, TT-250, RC-160 and TT-232 (6.11, 8.12, 9.43, 8.11 and 10.6 nmol kg−1, respectively) on non-neurogenic oedema induced by subplantar injection of dextran (100 μl 5%) in the chronically denervated hindleg 30 min after the induction of inflammation. In the control group of rats the same volume of saline was given. Oedema was measured by plethysmometry before and 30 min after the administration of dextran. Columns indicate per cent increase of the volume of the hindpaws as compared to the original values (means±s.e.mean, n=5 – 7 per group). *P<0.05; **P<0.01.
Efficacy and time-course of anti-inflammatory action of TT-232 on neurogenic inflammation and ineffectiveness of diclofenac and meloxicam
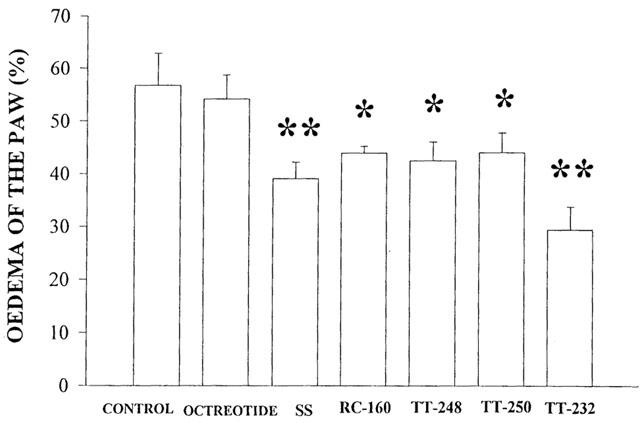
The heptapeptide TT-232 administered i.p. or i.v. (1.06, 2.65, 5.30, 10.6, 21.21, 42.42 nmol kg−1) inhibited in a dose-dependent manner the plasma extravasation evoked by mustard oil in the skin of the rat's paw. Dose-response curves of per cent inhibitions with 95% confidence intervals for i.p. and i.v. administrations gave almost identical values. The ID50 values were 4.58 (3.60 – 5.76) nmol kg−1 (i.p.) (n=29) and 4.56 (2.55 – 9.34) nmol kg−1 (i.v.) (n=28) (Figure 3a,b). The anti-inflammatory effect lasted for 2 h after a dose of 2.65 nmol kg−1 i.v. (n=15). Neither diclofenac given i.v. in doses of 15.72, 31.56, 63.12 μmol kg−1 and given i.m. in a high dose of 314.37 μmol kg−1 nor the selective COX-2 inhibiting agent meloxicam (2.95, 29.50, 56.91 and 569.38 μmol kg−1 i.v.) inhibited the mustard oil-induced neurogenic plasma extravasation. Mustard oil-induced cutaneous plasma extravasation was dose-dependently diminished by s.c. TT-232 (10.6, 21.21, 42.42, 84.84, 169.86, 339.36 and 2×530 nmol kg−1) 1, 2, 4, 6 and 16 h after the pretreatment. Although the dose-response curves for s.c. administrations were not steep enough to determine the 95% confidence intervals, the 1, 2, 4 and 6 h ID35 values were 21.75, 34.31, 42.09 and 82.60 nmol kg−1, respectively. Administration of 2×530 μg kg−1 per day TT-232 s.c. caused 52.09% inhibition of neurogenic plasma extravasation evoked by mustard oil 16 h after the second injection (Table 2).
Figure 3.
Dose-response curves of TT-232 after (a) i.p. and (b) i.v. administration for inhibiting the 1% mustard oil-induced neurogenic Evans blue accumulation in the skin of the acutely denervated hindlegs. Results are expressed in per cent inhibition as compared to the solvent-treated control group. The per cent inhibitions with 95% confidence intervals are calculated and presented with the ID50 values, n=5 – 7 animals in each group.
Table 2.
Effects of different s.c. doses (nmol kg −1) of TT-232 on neurogenic plasma extravasation 1, 2, 4, 6 and 16 h after 1% mustard oil smearing on the skin of the hindleg
Effect of TT-232, diclofenac and meloxicam on non-neurogenic dextran-oedema and bradykinin-evoked Evans blue accumulation
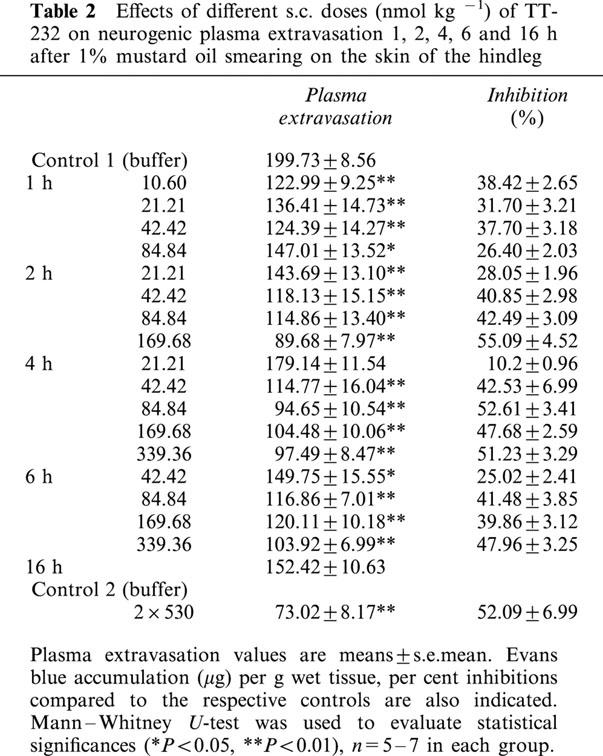
TT-232, diclofenac and meloxicam dose-dependently diminished non-neurogenic dextran-oedema of the paw. The respective ID35 (i.v.) values for TT-232 and diclofenac were 1.73 (1.61 – 6.22) nmol kg−1 and 34.37 (22.41 – 59.43) μmol kg−1, respectively. The slope of the dose-response curve of meloxicam was not steep enough to determine this value (Figure 4a,b,c) and 50% inhibition in the applied dose range of the three drugs could neither be reached.
Figure 4.
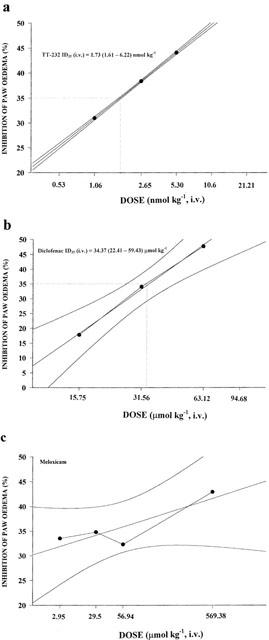
Dose-response curves of i.v. given TT-232 (a) diclofenac (b) and meloxicam (c) for inhibiting the dextran-induced oedema of the chronically denervated hindlegs. Tissue swelling was measured by plethysmometry 30 min after the induction of inflammation and the results were expressed in per cent inhibition as compared to the solvent-treated control group. The per cent inhibitions with 95% confidence intervals were calculated and presented with the ID35 values, n=5 – 7 animals in each group.
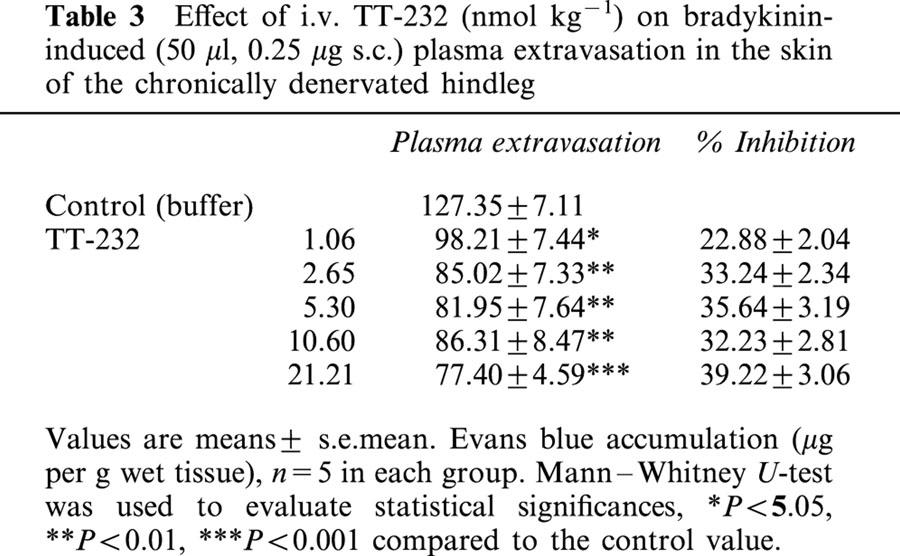
TT-232 inhibited in the dose range of 1.06 – 21.21 nmol kg−1 the bradykinin-induced plasma extravasation in the skin of the chronically denervated paw. Although the inhibition was statistically significant at all doses the dose-response curve was flat and did not reach the ID35 level (Table 3).
Table 3.
Effect of i.v. TT-232 (nmol kg−1) on bradykinin-induced (50 μl, 0.25 μg s.c.) plasma extravasation in the skin of the chronically denervated hindleg
Effect of TT-232 on Freund-adjuvant induced arthritis
In control animals definite oedema developed in the Freund-adjuvant treated left leg and slight swelling on the contralateral side. Chronic arthritis was dose-dependently diminished by TT-232 (2×106, 2×212 and 2×530 nmol kg−1 per day s.c.) throughout the period of 18 days, more dominant inhibition was observed in the left leg (Table 4, Figure 5). Systemic symptoms (decreased mobility and appetite, fever, weariness) and local hyperalgesia were seen during the experiment.
Table 4.
Effect of TT-232 s.c. treatment (2×106, 2×212 and 2×530 nmol kg−1 per day) on Freund adjuvant-induced arthritis
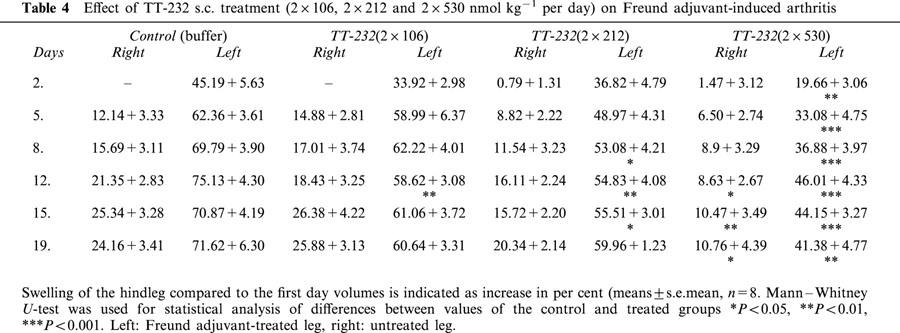
Figure 5.
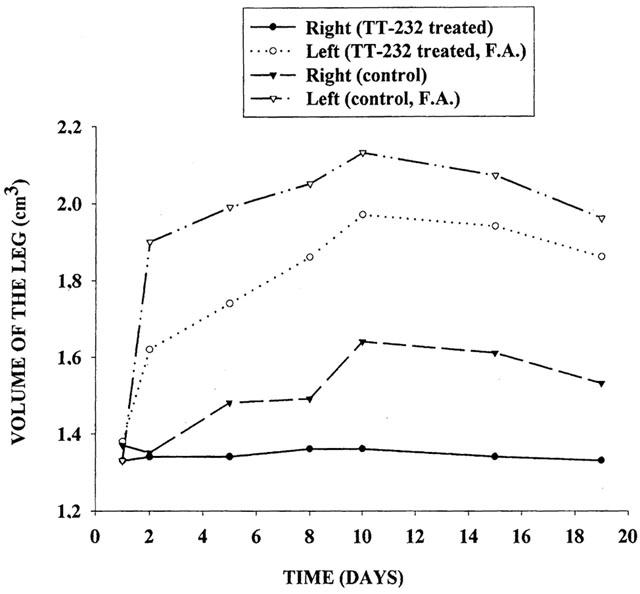
Freund-adjuvant induced oedema of the hindlegs in control and TT-232 (2×530 nmol kg−1 per day s.c.) pretreated rats (n=5 – 7 per group) throughout the experimental period of 18 days. In control animals pronounced swelling developed in the treated left leg and slight swelling on the contralateral side. Responses of both legs were significantly inhibited by TT-232.
Effect of TT-232 on sensory neuropeptide release from isolated rat trachea
Both capsaicin (10−7 M) and electrical field stimulation (40 V, 0.1 ms, 10 Hz, 120 s) evoked a significant increase in SP, CGRP and somatostatin release from isolated rat tracheae in control samples. TT-232 (500 nM) reduced the release of these neuropeptides elicited by capsaicin by 79% (SP), 48% (CGRP), 74% (somatostatin), respectively (Figure 6). The corresponding values in the presence of 500 nM TT-232 in response to electrical field stimulation were 36, 48 and 42% (Figure 7). TT-232 did not influence the basal, non-stimulated peptide release.
Figure 6.
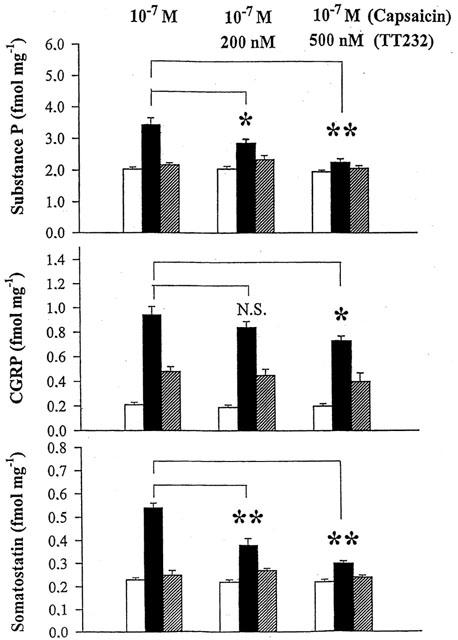
Effect of TT-232 (200 and 500 nM) on substance P, CGRP and somatostatin release from isolated rat tracheae in response to 10 M−7 capsaicin. Columns indicate prestimulated (open), stimulated (solid) and poststimulated (hatched) values. Results are shown as means±s.e.mean obtained from six experiments (*P<0.05; **P<0.01).
Figure 7.
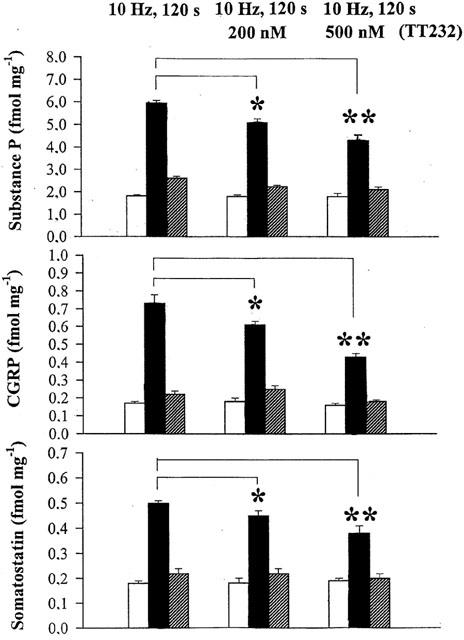
Effect of TT-232 (200 and 500 nM) on substance P, CGRP and somatostatin release from isolated rat tracheae in response to electrical field stimulation (40 V, 0.1 ms, 10 Hz, 120 s). Columns indicate prestimulated (open), stimulated (solid) and poststimulated (hatched) values. Results are shown as means±s.e.mean obtained from six experiments (*P<0.05; **P<0.01).
Discussion
Somatostatin and its novel cyclic synthetic analogues (the octapeptides RC-160, TT-248 and TT-250 and the heptapeptide TT-232) inhibited in nmol kg−1 dose range both the mustard oil-induced neurogenic plasma extravasation and the non-neurogenic dextran oedema. Mustard oil in concentrations under 5% causes pure neurogenic inflammation without involvement of mast cells (Inoue et al., 1997; Szolcsányi et al., 1998b) by selectively stimulating the capsaicin-sensitive sensory nerve endings (Jancsó et al., 1967). On the other hand dextran is known to exert oedema formation through mast cell degranulation (Selye, 1965). Thus, these data show that somatostatin and its analogues elicit acute anti-inflammatory effect either by acting directly on venular plasma extravasation process or by inhibition the release of inflammatory mediators both from the peptidergic sensory nerve terminals and also from the mast cells. Experiments with TT-232 indicate a slight diminution of bradykinin-induced plasma extravasation in chronically denervated hindleg. However, in contrast to the potent anti-inflammatory action of TT-232 against neurogenic plasma extravasation (ID50=4.56 nmol kg−1 i.v.) the significant inhibition of the bradykinin response did not reach the ID50 level up to a dose of 21.2 nmol kg−1 i.v. It is concluded that the potent acute anti-inflammatory action of the compound is mainly due to the inhibition of proinflammatory mediator release both from capsaicin-sensitive nociceptors (Szolcsányi, 1996a, 1996b) and also from mast cells. Similar dual inhibition was observed in the case of another neuropeptide, nociceptin which inhibited simultaneously the release of sensory neuropeptides and mast cell degranulation, producing similar anti-inflammatory effect as somatostatin (Helyes et al., 1997; Németh et al., 1998b).
TT-232 failed to inhibit growth hormone release or gastrin secretion in vivo, but it had a strong antiproliferative, apoptotic (Kéri et al., 1996) and anti-nociceptive effect (Helyes et al., 2000). The octapeptide analogue, octreotide, which is effectively used in the treatment of hormone-secreting tumours (Pincus et al., 1989), in the applied dose had no influence on neurogenic inflammation and dextran oedema, although there is some evidence, that octreotide inhibits carrageenin-induced oedema and leukocyte accumulation (Karalis et al., 1994) and formalin-induced nociception (Carlton et al., 2001). Consequently, it is concluded that the receptor subtypes being responsible for the anti-inflammatory and endocrine effects of somatostatin are different. Octreotide which has been reported to mediate its hormone secretion inhibitory action through the sst2, sst3 and sst5 receptors (Hofland et al., 1995; Siehler & Hoyer, 1999) proved to be less effective than TT-248, TT-250 and RC-160 compounds, which also inhibit GH secretion (Jaspers et al., 1994). Structure-activity relationship study of these compounds on somatostatin receptor subtypes has not been done, but the heptapeptide TT-232 without endocrine effect showed the greatest anti-inflammatory potency, indicating pivotal role for sst1 and/or sst4 respors in this response. TT-232 in contrast to the other compounds contains a five-residue ring, which makes the molecular structure more rigid (Jaspers et al., 1994). This seems to be favourable to enhance its receptor selectivity. The present data has revealed that TT-232 inhibited the release of sensory neuropeptides from the rat trachea in vitro providing biochemical evidence for involvement of somatostatin receptors on capsaicin-sensitive sensory nerve endings in inhibition of neurogenic inflammation. Nevertheless, an additional neurokinin 1 (NK 1) receptor blocking activity described in the case of vapreotide (RC-160), a long-lasting octapeptide somatostatin analogue with analgesic effect (Betoin et al., 1995; Helyes et al., 2000) cannot be excluded. In rat lung tissues mRNA of two somatostatin receptor subtypes were indentified. Predominantly sst4 receptor, and a lesser extent sst1 subtype were expressed (Schloos et al., 1997). Therefore it is tempting to assume that sst4 and probably sst1 receptors are responsible for the anti-inflammatory effect of somatostatin and TT-232 on the neurogenic inflammation. Although recently a non-peptide somatostatin agonist with sst4 selectivity was described the action of this compound on neurogenic inflammation has not been tested (Ankersen et al., 1998).
The classical non-steroidal anti-inflammatory agents (Jancsó-Gábor & Szolcsányi, 1970), diclofenac and the selective COX-2 inhibitor meloxicam (Engelhardt et al., 1995) diminished non-neurogenic oedema in a dose-dependent manner but did not influence neurogenic plasma extravasation. High dose steroids are able to attenuate neurogenic inflammation (Piedimonte et al., 1990), but their many serious side-effects limit their usage in clinical practice. Neurogenic and non-neurogenic components simultaneously take part in most of the inflammatory processes, and neurogenic inflammation plays significant role in the pathogenesis of rheumatoid arthritis, allergic rhinitis and conjunctivitis, asthma bronchiale, urticaria, psoriasis, migraine (Geppetti & Holzer, 1996) and also in adjuvant arthritis in the rat (Donaldson et al., 1995), and murine delayed-type hypersensitivity reactions (Girolomoni & Tigelaar, 1990).
Daily s.c. pretreatment of rats with doses of TT-232 which inhibited neurogenic inflammation for 6 – 16 h diminished also the development of Freund adjuvant-induced bilateral arthritis. It has been described in several experimental models that somatostatin suppresses a number of immune functions among others the release of proinflammatory cytokines, lymphocyte proliferation, and immunoglobulin production (ten Bokum et al., 2000). In the Freund adjuvant arthritis model activation of T-cell subtypes by Mycobacterium cross-reacts with articular tissues causing in this way joint destruction and enhancement of local immune responses (Wooley, 1991). The fact that this type of systemic chronic inflammatory response is also inhibited by somatostatin analogue which devoid of endocrine effects opens new horizons for development of broad spectrum anti-inflammatory agents which are effective also against neurogenic inflammation. TT-232 is a promising, effective, stable and selective somatostatin analogue for parenteral application. It is proposed as a lead molecule for a new class of anti-inflammatory, analgesic agents.
Acknowledgments
This work was supported by Hungarian Grants: OTKA T-029428, and ETT 03-382/2000, NRDP 1/047/2001 and the Hungarian Academy of Sciences. Special thanks to Dr Judit Horváth for useful ideas and Mrs Csilla Zádor and Mrs Mária Zsoldos for expert technical assistance.
Abbreviations
- CGRP
calcitonin gene-related peptide
- COX
cyclo-oxygenase
- GH
growth hormone
- NK1
neurokinin1
- RIA
radioimmunoassay
- SP
substance P
- SRIF
somatotropin release inhibiting factor
- sstr
somatostatin receptor
References
- ANKERSEN M., CRIDER M., LIU S., HO B., ANDERSEN H.S., STIDSEN C. Discovery of a novel non-peptide somatostatin agonist with sst4 selectivity. J. Am. Chem. Soc. 1998;120:1368–1373. [Google Scholar]
- BETOIN F., ADVENIER C., FARDIN V., WILCOX G., LAVARENNE J., ESCHALIER A. In vitro and in vivo evidence for a tachykinin NK1 receptor antagonist effect of vapreotide, an analgesic cyclic analog of somatostatin. Eur. J. Pharmacol. 1995;279:241–249. doi: 10.1016/0014-2999(95)00168-k. [DOI] [PubMed] [Google Scholar]
- CARLTON S.M., DU J., DAVIDSON E., ZHOU S., COGGESHALL R.E. Somatostatin receptors on peripheral primary afferent terminals: inhibition on sensitized nociceptors. Pain. 2001;90:233–244. doi: 10.1016/S0304-3959(00)00407-3. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHAHL L.A.Antidromic vasodilatation and neurogenic inflammation Novel Peripheral Neurotransmitters 1991New York: Pergamon Press; 161–192.Bell, C., ed. pp [Google Scholar]
- DONALDSON L.F., MCQUEEN D.S., SECKL J.R. Neuropeptide gene expression and capsaicin-sensitive primary afferents: maintenance and spread of adjuvant arthritis in the rat. J. Physiol. 1995;486:473–482. doi: 10.1113/jphysiol.1995.sp020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGELHARDT G., HOMMA D., SCHLEGEL K., UTZMANN R., SCHNITZLER C. Anti-inflammatory, analgesic, antipyretic, and related properties of meleoxicam a new non-steroidal anti-inflammatory agent with favourable gastrointestinal tolerance. Inflamm. Res. 1995;44:423–433. doi: 10.1007/BF01757699. [DOI] [PubMed] [Google Scholar]
- FIORAVANTI A., GOVONI M., LA MONTAGNA G., PERPIGNANO G., TIRRI G., TROTTA F., BOGLIOLO A., CIOCCI A., TAUCERI M.T., MARCOLONGO R. Somatostatin-14 and joint inflammation: evidence for intraarticular efficacy of prolonged administration in rheumatoid arthritis. Drugs Exp. Clin. Res. 1995;21:97–103. [PubMed] [Google Scholar]
- GAMSE R., LEEMAN S.E., HOLZER P., LEMBECK F. Differential effect of capsaicin on the content of somatostatin, substance P and neurotensin in the nervous system of the rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1981;317:140–148. doi: 10.1007/BF00500070. [DOI] [PubMed] [Google Scholar]
- GEPPETTI P., HOLZER P. Neurogenic Inflammation. Boca Raton, U.S.A.: CRC Press; 1996. [Google Scholar]
- GIROLOMONI G., TIGELAAR R.E. Capsaicin-sensitive primary sensory neurones are potent modulators of murine delayed-type hypersensitivity reactions. J. Immunol. 1990;145:1105–1112. [PubMed] [Google Scholar]
- HELYES Z.S., NÉMETH J., PINTÉR E., SZOLCSÁNYI J. Inhibition by nociceptin of neurogenic inflammation and the release of SP and CGRP from sensory nerve terminals. Br. J. Pharmacol. 1997;121:613–615. doi: 10.1038/sj.bjp.0701209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELYES Z.S., THÁN M., OROSZI G., PINTÉR E., NÉMETH J., KÉRI G.Y., SZOLCSÁNYI J. Anti-nociceptive effect induced by somatostatin released from sensory nerve terminals and by synthetic somatostatin analogues in the rat. Neurosci. Lett. 2000;278:185–188. doi: 10.1016/s0304-3940(99)00936-2. [DOI] [PubMed] [Google Scholar]
- HOFLAND L.J., VISSER-WISSELAAR H.A., LAMBERTS S.W. Somatostatin analogues: clinical application in relation to human somatostatin receptor subtypes. Biochem. Pharmacol. 1995;50:287–297. doi: 10.1016/0006-2952(95)00066-9. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Peptidergic sensory neurones in the control of vascular functions: mechanisms and significance in the cutaneous and splanchnic vascular bed. Rev. Physiol. Biochem. Pharmacol. 1992;121:49–146. doi: 10.1007/BFb0033194. [DOI] [PubMed] [Google Scholar]
- HOYER D., BELL G.J, , BERELOWITZ M., EPELBAUM J., FERNIUK W., HUMPHREY P.P., O'CARROLL A.-M., PATEL Y.C., SCHONBRUNN A., TAYLOR J.E., REISINE T. Classification and nomenclature of somatostatin receptors. Trends. Pharmacol. Sci. 1995;16:86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- INOUE H., ASAKA T., NAGATA N., KOSHIHARA Y. Mechanism of mustard oil-induced skin inflammation in mice. Eur. J. Pharmacol. 1997;333:231–240. doi: 10.1016/s0014-2999(97)01040-6. [DOI] [PubMed] [Google Scholar]
- JANCSÓ N., JANCSÓ-GÁBOR A., SZOLCSÁNYI J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pre-teatment with capsaicin. Br. J. Pharmacol. 1967;31:138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANCSÓ-GÁBOR A., SZOLCSÁNYI J. Action of rare earth metal complexes on neurogenic as well as on bradykinin-induced inflammation. J. Pharm. Pharmacol. 1970;22:366–371. doi: 10.1111/j.2042-7158.1970.tb08539.x. [DOI] [PubMed] [Google Scholar]
- JASPERS H., HORVÁTH A., MEZÔ I., KÉRI G.Y., VAN MINST G. Conformational study of a series of somatostatin analogues with antitumor and/or GH inhibitory activity. Int. J. Peptide Protein Res. 1994;43:271–276. doi: 10.1111/j.1399-3011.1994.tb00390.x. [DOI] [PubMed] [Google Scholar]
- KARALIS K., MASTOKAROS G., CHROUSOS G.P., TOLIS G. Somatostatin analogues suppress the inflammatory reaction in vivo. J. Clin. Invest. 1994;93:2000–2006. doi: 10.1172/JCI117193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KÉRI G.Y., MEZÖ I., VADÁSZ Z.S., HORVÁTH A., IDEI M., VÁNTUS Á., BALOGH G., BÖKÖNYI G., BAJOR T., TEPLÁN I., TAMÁS J., MÁK M., HORVÁTH J., CSUKA O. Structure-activity relationship studies of novel somatostatin analogs with antitumor activity. Peptide Research. 1993;6:281–288. [PubMed] [Google Scholar]
- KÉRI G.Y., ÉRCHEGYI J., HORVÁTH A., MEZÖ I., IDEI M., VÁNTUS T., BALOGH Á., VADÁSZ Z.S., BÖKÖNYI G.Y., SEPRÖDI J., TEPLÁN I., CSUKA O., TEJEDA M., GAÁL D., SZEGEDI Z.S., SZENDE B., ROZE C., KALTHOFF H., ULLRICH A. A tumor-selective somatostatin analog (TT-232) with strong in vitro and in vivo anti-tumor activity. Proc. Natl. Acad. Sci. U.S.A. 1996;93:12513–12518. doi: 10.1073/pnas.93.22.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMBECK F., DONNERER J., BARTHÓ L. Inhibition of neurogenic vasodilatation by substance P antagonists, somatostatin and (D-met2, pro5)enkephalinamide. Eur. J. Pharmacol. 1982;85:171–176. doi: 10.1016/0014-2999(82)90462-9. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Prog. Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- NÉMETH J., GÖRCS T., HELYES Z.S., OROSZI G., KOCSY T., PINTÉR E., SZOLCSÁNYI J. Development of a new sensitive CGRP radioimmunoassay for neuropharmacological research. Neurobiology. 1998a;6:473–475. [PubMed] [Google Scholar]
- NÉMETH J., HELYES Z.S., PINTÉR E., GÖRCS T., GARDI J., SZOLCSÁNYI J. Development of somatostatin radioimmunoassay for the measurement of plasma and tissue contents of hormone. Acta Physiol. Hung. 1996;84:221–223. [PubMed] [Google Scholar]
- NÉMETH J., HELYES Z.S., OROSZI G., THÁN M., PINTÉR E., SZOLCSÁNYI J. Inhibition of nociceptin on sensory neuropeptide release and mast cell-mediated plasma extravasation in rats. Eur. J. Pharmacol. 1998b;347:101–104. doi: 10.1016/s0014-2999(98)00216-7. [DOI] [PubMed] [Google Scholar]
- PATEL Y.C., GREEENWOOD M.T., PANETTA R., DEMCHYSHYN L., NIZNIK L., SRIKANT C.B. Minireview. The somatostatin receptor family. Life Sci. 1995;57:1249–1265. doi: 10.1016/0024-3205(95)02082-t. [DOI] [PubMed] [Google Scholar]
- PIEDIMONTE G., MCDONALD D.M., NADEL J.A. Glucocorticoids inhibit neurogenic plasma extravasation and prevent virus-potentiated extravasation in the rat trachea. J. Clin. Invest. 1990;86:1409–1415. doi: 10.1172/JCI114855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINCUS T., SMITH S., OATES J.A. Somatostatin analogue octreotide is effective in revealing musculoskeletal pain associated with carcinoid syndrome. Arthritis Rheum. 1989;32 Suppl.4:S71. [Google Scholar]
- REISINE T., BELL G.I. Molecular properties of somatostatin receptors. Neuroscience. 1995;67:777–790. doi: 10.1016/0306-4522(95)00072-q. [DOI] [PubMed] [Google Scholar]
- SCHLOOS J., RAULF F., HOYER D., BRUNS C. Identification and pharmacological characterization of somatostatin receptors in rat lung. Br. J. Pharmacol. 1997;121:963–971. doi: 10.1038/sj.bjp.0701205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELYE H. The mast cells. Washington, Butterworths; 1965. [Google Scholar]
- SIEHLER S., HOYER D. Characterization of human recombinant somatostatin receptors. Modulation of phospholipase C activity. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:488–499. doi: 10.1007/s002109900141. [DOI] [PubMed] [Google Scholar]
- SZOLCSÁNYI J. Antidromic vasodilatation and neurogenic inflammation. Agents Actions. 1988;23:4–11. doi: 10.1007/BF01967170. [DOI] [PubMed] [Google Scholar]
- SZOLCSÁNYI J.Neurogenic inflammation: reevaluation of axon reflex theory Neurogenic Inflammation 1996aBoca Raton, U.S.A.: CRC Press; 33–42.Gepetti, G. & Holzer, P., eds. pp [Google Scholar]
- SZOLCSÁNYI J.Capsaicin-sensitive sensory nerve terminals with local and systemic efferent functions: facts and scopes of an unorthodox neuroregulatory mechanism Progress in Brain Research 1996bVol. 113Amsterdam: Elsevier; 343–359.Kumazawa, T., Kruger, L. & Mizumura, K., eds [DOI] [PubMed] [Google Scholar]
- SZOLCSÁNYI J., PÓRSZÁSZ R., PETHÕ G.Capsaicin and pharmacology of nociceptors Peripheral Neurons in Nociception: Physio-pharmacological Aspects 1994Paris: John Libbey, Eurotext; 109–124.Besson, J.M., Guilbaud, G. & Ollat, H., eds. pp [Google Scholar]
- SZOLCSÁNYI J., HELYES Z.S., OROSZI G., NÉMETH J., PINTÉR E. Release of somatostatin and its role in mediation of the anti-inflammatory effect induced by antidromic stimulation of sensory fibres of the rat sciatic nerve. Br. J. Pharmacol. 1998a;123:936–942. doi: 10.1038/sj.bjp.0701685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZOLCSÁNYI J., PINTÉR E., HELYES Z.S., OROSZI G., NÉMETH J. Systemic anti-inflammatory effect induced by counter-irritation through a local release of somatostatin from nociceptors. Br. J. Pharmacol. 1998b;125:916–922. doi: 10.1038/sj.bjp.0702144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEN BOKUM A.M.C., HOFLAND L.J., VAN HAGEN P.M. Somatostatin and somatostatin receptors in the immune system: a review. Eur. Cytokine Netw. 2000;11:161–176. [PubMed] [Google Scholar]
- WOOLEY P.H. Animal models of rheumatoid arthritis. Curr. Opin. Rheumatol. 1991;3:407–420. doi: 10.1097/00002281-199106000-00013. [DOI] [PubMed] [Google Scholar]


