Abstract
This study examined the effects of chronic exposure of rats to 3,4-methylenedioxymethamphetamine (MDMA) on [3H]5-hydroxytryptamine ([3H]5-HT) re-uptake into purified rat brain synaptosomes, 5-HT-induced isometric contraction of aortic rings and [3H]5-HT re-uptake into rat aorta.
Rats were administered MDMA (20 mg kg−1 i.p.) twice daily over 4 days. One, 7, 14 or 21 days post treatment, whole brain synaptosomes and descending thoracic aortic rings were prepared for investigation.
Chronic MDMA treatment significantly reduced the maximum rate (Vmax) of specific high-affinity [3H]5-HT re-uptake 1 day after treatment and for up to 21 days post-final administration of MDMA. Direct application of MDMA (100 μM) abolished synaptosomal re-uptake of [3H]5-HT in vitro.
Chronic MDMA administration significantly reduced the maximum contraction (Emax) to 5-HT at 1 and 7 days after treatment, but not at 14 or 21 days.
Chronic MDMA administration had no effect on sodium-dependent [3H]5-HT re-uptake into aorta 1 day after treatment, nor did 100 μM MDMA have any direct effect on [3H]5-HT uptake into aortic rings in vitro.
These results show, for the first time, an altered responsiveness of vascular tissue to MDMA after chronic administration. In addition, they demonstrate a difference in the sensitivity of central and peripheral 5-HT uptake systems to chronic MDMA exposure, and suggest that the action of MDMA in the cardiovascular system does not arise from a direct effect of MDMA on peripheral 5-HT transport.
Keywords: MDMA, 5-HT, synaptosomal re-uptake, 5-HT-induced aortic contraction, aortic [3H]5-HT re-uptake
Introduction
Much of the research to date on the psychoactive ring-substituted amphetamine 3,4-methylenedioxymethamphetamine (MDMA) has focused on its neurotoxic effects, arising from its use as the recreational drug, ‘Ecstasy'. Acute effects of MDMA in the central nervous system include release of 5-hydroxytryptamine (5-HT; Nichols et al., 1982; Schmidt et al., 1987; McKenna et al., 1991) and an increase in the CSF levels of 5-HT and its major metabolite 5-hydroxyindoleacetic acid (5-HIAA) in man (Ricaurte et al., 1990). In addition, MDMA irreversibly inhibits tryptophan hydroxylase (TPH), the rate-limiting enzyme of 5-HT synthesis (Schmidt & Taylor, 1987). MDMA targets both plasma membrane and vesicular 5-HT transporters, and induces 5-HT release via MDMA – 5-HT heteroexchange (Hekmatpanah & Peroutka, 1990; Rudnick & Wall, 1992). Furthermore, Bengel et al. (1998) have shown that in 5-HT transporter-deficient mice, MDMA-induced hyperactivity is not observed.
In the cardiovascular system, relatively little is known regarding the influence of MDMA on vascular function, although there are reports of adverse cardiac effects in humans. These include the induction of arrhythmias (Dowling et al., 1987; Milroy et al., 1996) and sudden cardiac death (Suarez & Riemersma, 1988). Common non-fatal consequences of MDMA ingestion in humans include ventricular tachycardia, hypertension (Hayner & McKinney, 1986; Vollenweider et al., 1998, Mas et al., 1999) and impaired parasympathetic activity (Brody et al., 1998), suggestive of enhanced sympathetic tone. In rats, reported cardiovascular actions of MDMA include arrhythmogenic effects in isolated perfused hearts (Fitzgerald & Reid, 1994) and elevations of both temperature and heart rate (Gordon et al., 1991). Somewhat paradoxically however, MDMA reportedly acts as an α2-adrenoceptor agonist in isolated rat atria (Lavelle et al., 1999) and at central α2-adrenoceptors mediating depressor responses (McDaid & Docherty, 2001).
Recreational abuse of MDMA often occurs in the context of chronic exposure, thus there is a need to understand fully the long-term consequences of MDMA administration in a wide-range of 5-HT-associated systems. A prolonged reduction in [3H]5-HT uptake in the brain following chronic exposure to MDMA is well-documented (Cummins, 1986; Sabol et al., 1996), yet there is little information on how peripheral functions of 5-HT are altered by MDMA. Unlike in the brain, repeated injections of MDMA to rats (twice daily for 4 days) had no significant effect on platelet 5-HT uptake sites 7 days later (Nash et al., 1991), from which it can be concluded that brain and peripheral 5-HT transporters differ in their sensitivity to MDMA. It is not known whether the extraneuronal 5-HT uptake sites in rat aorta, identified by Fukuda et al. (1986), are affected by MDMA. There is also no information on whether vascular responses to 5-HT are altered by chronic exposure to MDMA.
In this study, we have compared the effects of chronic administration of MDMA (20 mg kg−1, twice daily for 4 days) on central and vascular 5-HT uptake, and on vascular responses to 5-HT, using the rat as an experimental model.
Methods
Animals
During and after treatments, male Wistar rats weighing 240 – 260 g were housed separately at room temperature under a 12 h light/dark cycle with food and water in open supply. Between three and five animals were used for each set of experiments, and brain synaptosomes and vascular tissue were obtained or prepared, respectively, from different animals.
Synthesis and purification of MDMA
Synthesis of MDMA.HCl was carried out following the method of Braun et al. (1980). Briefly, isosafrole was oxidized to 3,4-methylenedioxyphenylacetone, which was then reacted with methylamine. The product of this reaction was treated with sodium cyanoborohydride to yield the corresponding MDMA derivative. This product was prepared as its (±)-HCl salt and its structure confirmed by NMR, microanalysis and mass spectroscopy. All doses and concentrations of MDMA used in the study refer to the HCl salt.
Drug administration
Chronic treatment with MDMA
MDMA (20 mg kg−1, dissolved in 1 ml 0.9% saline) was administered eight times (i.p.) in total (twice daily for 4 consecutive days). Animals were allowed to recover for periods of 1, 7, 14 or 21 days after the last dose, before tissue isolation. Control rats were injected with 1 ml of 0.9% saline at the same times that the experimental animals received MDMA and were held in the same manner.
Direct administration of MDMA
Addition of MDMA (100 μM, final concentration) was made to the incubation mixture of the synaptosomal and aortic uptake experiments 10 min prior to initiation of each assay. MDMA was present in the incubation mixture for the duration of each assay: 4 min (synaptosomes) or 5 min (aortic rings).
Synaptosomal uptake procedure
Saline- and MDMA-treated animals were killed by cervical dislocation; brains were rapidly removed and placed in ice-cold homogenization medium containing 0.32 M sucrose, 1 mM EDTA and 0.25 mM dithiothreitol. Following homogenization of the whole brain, the synaptosomal fraction was purified by Percoll density gradient centrifugation according to the method of Dunkley et al. (1988). The synaptosomal fraction (layer 4) was removed using a Pasteur pipette, washed twice in ice-cold Krebs' bicarbonate medium (composition mM): NaCl 109.6, KCl 4.72, KH2PO4 1.2, MgSO4.7H2O 1.2, NaHCO3 25, D-(+)-glucose 11 and CaCl2.2H2O 2.5, pH 7.4, gassed with 95% O2/5% CO2 by centrifugation (16,000×g for 15 min at 4°C) followed by gentle re-suspension, and maintained on ice until required.
Transport of [3H]5-HT was determined under conditions of initial velocity, and was initiated by the addition of 25 μl synaptosomal fraction to pre-warmed (37°C) tubes containing 975 μl of Krebs' bicarbonate medium and 25 μl [3H]5-HT (final specific activity 18.3 – 9.17×103 Bq pmol−1, diluted with unlabelled 5-HT to give a final concentration range of 1 – 500 nM 5-HT). Uptake of [3H]5-HT was determined in triplicate at each concentration of the substrate, and transport was linear between 0 – 20 min at 37°C. A 4 min incubation period was used for all subsequent experiments. Transport assays were terminated by addition of an excess of ice-cold 5-HT and placement of the tubes on ice followed by centrifugation at 16,000×g for 10 min at 4°C. The results are expressed as the rate of specific uptake of 5-HT (pmol mg protein−1 min−1), which was obtained by subtraction of the non-specific transport (defined using 100 μM alaproclate) from the total transport. Separate experiments determined that sodium-independent transport was less than the non-specific transport defined by alaproclate. Protein was determined by the method of Markwell et al. (1978).
Measurement of [3H]5-HT uptake into aortic rings
The transport of [3H]5-HT into aortic rings was determined according to the method of Fukuda et al. (1986), with minor modifications. Briefly, thoracic aortic segments, 40 mm in length, were incubated in Krebs' bicarbonate medium containing 0.1 mM pargyline for 30 min at 25°C. After rinsing in fresh Krebs' medium, segments of 3 mm were prepared, which were then placed in individual tubes in a final volume of 250 μl Krebs' medium at 37°C in a shaking water bath. After equilibration of the rings for 5 min, uptake of [3H]5-HT was initiated by the addition of [3H]5-HT (1 μM; final specific activity 28×103 Bq pmol−1) and the incubation was continued for an additional 5 min. The assay was terminated by removing the rings from the incubation medium, and blotting them between pieces of filter paper. Each ring was extracted in 4 ml of scintillation fluid for 48 h in the dark, and the quantity of radioactivity measured by liquid scintillation spectroscopy. Transport was determined in both normal Krebs' and sodium-free medium, and the quantity of sodium-dependent uptake determined by subtraction. Previous work has shown both sodium-dependent and -independent uptake of [3H]5-HT (0.1 – 9 μM) in aortic rings (Fukuda et al., 1986), and a concentration of 1 μM was selected for these experiments. The results are expressed as the sodium-dependent transport of 5-HT (nmol [3H]5-HT taken up mg tissue−1 min−1).
Measurement of aortic contraction
Descending thoracic aortae were dissected, mounted in a tissue bath, attached to a Grass force displacement (FT03) transducer and maintained at 37°C in Krebs'-Hensleit bicarbonate buffer of the following composition (mM): NaCl 112.6, KCl 4.7, KH2PO4 1.2, MgSO4-7H2O 1.2, NaHCO3 25, (D)-(+)-glucose 12, CaCl2-2H2O 1.9, gassed with 95% O2/5% CO2. After equilibration for 1 h at a basal tension of 1.3 g, changes in isometric tension were recorded using a Powerlab 400 data acquisition system.
Materials
[3H]5-hydroxytryptamine (Specific Activity=1017 GBq (27.5 Ci) mmol−1) was purchased from NEN, Amsterdam. Alaproclate.HCl was purchased from RBI (U.K.). All other chemicals and compounds were purchased from Sigma Chemical Co., Dorset, U.K. and were of the highest grade commercially available.
Data analysis
Data presented are mean values±s.e.mean obtained from n experiments as indicated in Figure legends. Data were analysed using the software package Graphpad Prism under non-linear regression analysis and fitting to either a one site rectangular hyperbola (Michaelis Menten curve) or sigmoidal dose-response curve as appropriate. Determination of statistical significance was performed by a two-tailed unpaired Student's t-test; a P value <0.05 was considered significant. Synaptosomal and aortic re-uptake data were calculated as pmol mg protein−1 min−1 and nmol mg tissue−1 min−1 respectively and are graphed (Figure 3) as percentage of control values for comparative purposes.
Figure 3.
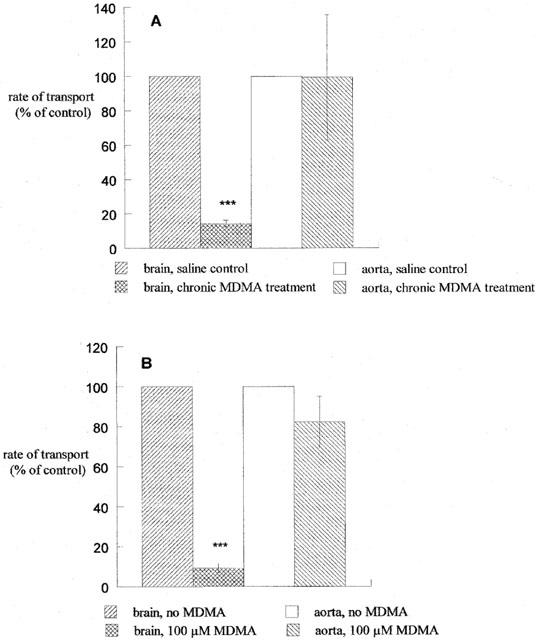
Measurement of [3H]5-HT re-uptake in cortical synaptosomes and aortic rings (A) isolated 1 day after chronic MDMA administration or (B) in the presence of 100 μM MDMA. Data are presented as the percentage of control transport (mean±s.e.mean) of 3 – 5 experiments, each performed in triplicate. ***P<0.001 vs brain, saline control (A) or vs brain, no MDMA (B).
Results
The effect of chronic MDMA treatment on synaptosomal 5-HT transport
Chronic MDMA administration significantly reduced the maximum rate (Vmax) of [3H]5-HT re-uptake into isolated whole brain synaptosomes for up to 21 days after treatment had ended. The Vmax in saline-treated controls, which did not change during the entire testing period, had a mean value of 26.2±1.4 pmol mg protein−1 min−1. Twenty-four hours after MDMA treatment, the Vmax had fallen to 12.9% of control (3.3±0.9 pmol mg protein−1 min−1), but showed gradual recovery to 10.0±2.7 pmol mg protein−1 min−1 (38.1% of control) at 7 days, and 20.0±2.3 pmol mg protein−1 min−1 (76.3% of control) at 21 days after the final injection of MDMA (Figure 1). There was no significant difference in the Km (substrate concentration required for half maximal rate of uptake) for 5-HT transport between MDMA- and saline-treated animals at any of the time points tested.
Figure 1.
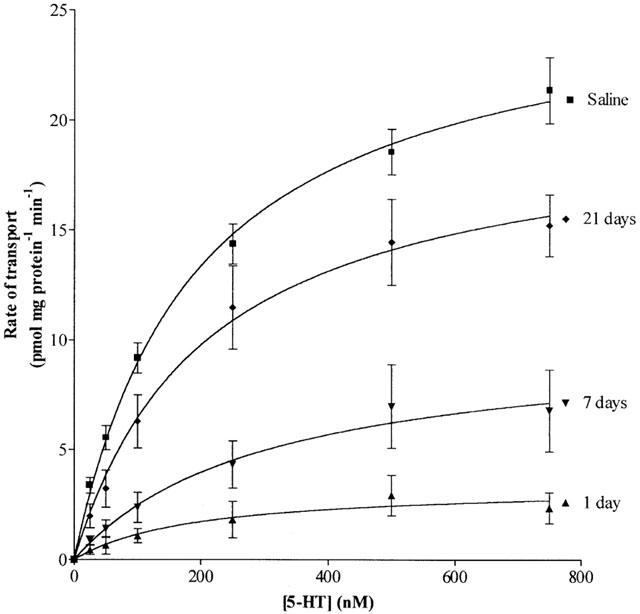
The effect of chronic MDMA treatment on the rate of specific high-affinity [3H]5-HT re-uptake into purified rat brain synaptosomes. Data are presented as the mean±s.e.mean of four individual experiments each performed in triplicate.
The effect of chronic MDMA treatment on 5-HT- induced rat aortic ring contraction
Chronic MDMA treatment significantly reduced the maximum contraction induced by 5-HT (Emax) in aortae isolated 1 and 7 days after treatment (0.51±0.05 g; 1.18±0.07 g respectively), compared to saline-treated controls (0.78±0.03 g; 1.49±0.06 g, P<0.01 in each case). However the EC50 was not significantly altered, 1 and 7 days after treatment (3.51±1.69 μM; 3.61±0.99 μM respectively), compared to saline-treated controls (1.56±0.42 μM; 2.98±0.62 μM). At 14 and 21 days post treatment, neither Emax nor EC50 values for 5-HT were significantly different from saline-treated controls (Figure 2A – D).
Figure 2.
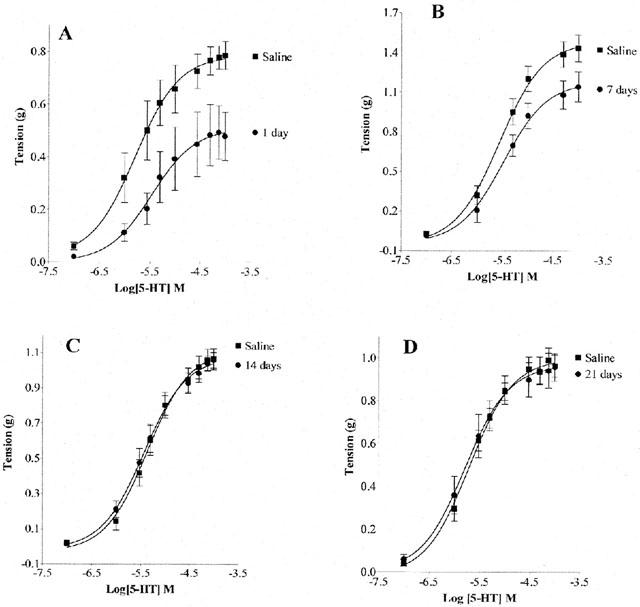
Chronic MDMA administration significantly reduced 5-HT-induced contraction of the rat thoracic aortic ring isolated at 1 – 7 days (A and B; P<0.01), but not 14 and 21 days post treatment (C and D). Data are presented as the mean±s.e.mean for five individual experiments each performed in duplicate.
Investigation of the effect of chronic MDMA treatment on transport of [3H]5-HT in aortic rings
Measurement of sodium-dependent transport of [3H]5-HT in aortic rings from rats that had received chronic MDMA treatment did not show any difference in the rate of transport 1 day after treatment when compared to saline-treated controls (113.8±41.6 nmol mg tissue−1 min−1; 100±37% of control; Figure 3A). The reduction in whole brain synaptosomal uptake of [3H]5-HT (100 nM) after chronic administration of MDMA is also shown in Figure 3B for comparative purposes (14±2% of saline-treated animals).
Investigation of the direct effect of 100 μM MDMA on transport of [3H]5-HT in synaptosomes and aortic rings
Addition of 100 μM MDMA to the incubation medium during measurement of uptake of 100 nM [3H]5-HT into synaptosomes prepared from untreated animals reduced the rate of transport from 10.38±1.76 pmol mg protein−1 min−1 in the absence of MDMA to 0.97±0.29 pmol mg protein−1 min−1 (9±2% of control). Addition of the same concentration of MDMA had no significant effect on the transport of 1 μM [3H]5-HT into aortic rings (88.1±23.5 nmol mg tissue−1 min−1 in the absence of MDMA, compared to 72.5±11.4 nmol mg tissue−1 min−1 (82±13% of control) in the presence of MDMA, Figure 3B).
Discussion
This study confirms the view that 5-HT transport is one of the primary targets for MDMA action in the brain. After 21 days, the transport of 5-HT into synaptosomes prepared following chronic MDMA treatment remained significantly depressed, at 76% of control values. This is close to the level at which 5-HT transport remained 21 days after a single i.p. dose of the drug (20 mg kg−1; results not shown), which is an indication of the severity that even a ‘once-off' challenge with MDMA may have on 5-HT transport in the rat brain.
The dose of MDMA administered in the present study compares well with other administration protocols that have been shown to result in neurodegeneration together with a range of associated behavioural and physiological changes (Esteban et al., 2001; O'loinsigh et al., 2001). While we have not measured brain levels of MDMA after chronic administration, we have previously determined the concentration dependence of MDMA effects on cerebral 5-HT transport in vitro, and found the IC50 to be in the low micromolar range, with a maximal inhibition of uptake occurring at 100 μM MDMA (results not shown). This responsiveness to MDMA corresponds well with the estimate that a brain concentration of 11 – 20 μM MDMA would arise from a dose of 10 – 15 mg kg−1 i.p. (Esteban et al., 2001).
The mechanism by which chronic MDMA treatment results in reduced 5-HT responsiveness in vasculature is unclear. It does not appear that MDMA increases plasma levels of 5-HT, thereby exposing vascular 5-HT receptors to a chronic stimulus and receptor down-regulation, since no evidence was found for reduced [3H]5-HT re-uptake into aorta. Similarly, an MDMA-induced reduction in platelet uptake of 5-HT is thought unlikely, since rat platelets are reportedly much less sensitive than the cerebral cortex to the 5-HT-depleting effects of MDMA (Nash et al., 1991). Few studies have examined the long-term effect of MDMA treatment on the subsequent response to 5-HT agonist challenge. However, both Granoff & Ashby (2001) and Poland (1990) have shown altered 5-HT1A receptor-mediated responses in the peripheral nervous system after MDMA. This shows that such changes can occur in both the CNS and the periphery.
An obvious question is whether metabolism of chronically-administered MDMA generates compounds that target 5-HT-mediated responses in the vascular system. While this has not been tested in the present study, a number of novel amphetamine derivatives, structurally related to MDMA, have recently been found to reduce vascular responses to 5-HT in direct experiments in vitro (Murphy et al., 2001), suggesting the possibility that modification of the MDMA structure changes its bioactivity profile. It should also be noted that route of administration, as well as species, strain and sex differences (Chu et al., 1996) could potentially influence the mechanism and extent of MDMA metabolism.
Increasing evidence implicates MDMA-induced generation of free radicals and subsequent oxidative stress in MDMA-induced neurodegeneration (Bai et al., 2001; Colado et al., 1997). Formation of such species in the periphery could in principle contribute to compromised 5-HT receptor function in vasculature and 5-HT transporter dysfunction centrally.
Further studies will be required to demonstrate conclusively that MDMA modulates 5-HT responses in vasculature and brain by the same or different mechanisms. However, we can conclude that MDMA compromises vascular function, and although we have not presented definitive evidence for longer term damage, downstream events ensuing from those observed in the vasculature may well cause more lasting effects.
In summary, this study confirms that chronic exposure of rat brain to MDMA causes a severe and long-lasting depletion in the capacity of the 5-HT transporter to take up and therefore inactivate 5-HT. In addition, MDMA produces a reduced capacity of the aorta to contract in the presence of 5-HT. Thus, the long-term effects of MDMA on the 5-HT system extend far beyond its primary site of action. These results therefore serve to underline the potential dangers that may arise from abuse of MDMA in humans.
Acknowledgments
This work was supported by the ‘Technology against Drugs' Initiative of Enterprise Ireland [DC:SD/96/010; CB:SD/96/013].
Abbreviations
- 5-HIAA
5-hydroxyindoleacetic acid
- 5-HT
5-hydroxytryptamine
- MDMA
3,4-methylenedioxymethamphetamine
- TPH
tryptophan hydroxylase
References
- BAI F., JONES D.C., LAU S.S., MONKS T.J. Serotonergic neurotoxicity of 3,4-(+/−)-methylenedioxyamphetamine and 3,4-(+/−)-methylenedioxymethamphetamine (Ecstasy) is potentiated by inhibition of gamma-glutamyl transpeptidase. Chem. Res. Toxicol. 2001;14:863–870. doi: 10.1021/tx010011l. [DOI] [PubMed] [Google Scholar]
- BENGEL D., MURPHY D.L., ANDREWS A.M., WICHEMS C.H., FELTNER D., HEILS A., MOSSNER R., WESTPHAL H., LESCH K.P. Altered brain serotonin homeostasis and locomotor insensitivity to 3,4-methylenedioxymethamphetamine (‘Ecstasy') in serotonin transporter-deficient mice. Mol. Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- BRAUN U., SHULGIN A.T., BRAUN G. Centrally acting N-substituted analogs of 3,4-methylenedioxyphenylisopropylamine (3,4-methylenedioxyamphetamine) J. Pharm. Sci. 1980;69:192–195. doi: 10.1002/jps.2600690220. [DOI] [PubMed] [Google Scholar]
- BRODY S., KRAUSE C., VEIT R., RAU H. Cardiovascular antonomic dysregulation in users of MDMA (‘Ecstasy') Psychopharmacol. 1998;136:390–393. doi: 10.1007/s002130050582. [DOI] [PubMed] [Google Scholar]
- CHU T., KUMAGAI Y., DISTEFANO E.W., CHO A.K. Disposition of methylenedioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochem. Pharmacol. 1996;22:789–796. doi: 10.1016/0006-2952(95)02397-6. [DOI] [PubMed] [Google Scholar]
- COLADO M.I., O'SHEA E., GRANADOS R., MURRAY T.K., GREEN A.R. In vivo evidence for free radical involvement in the degeneration of rat brain 5-HT following administration of MDMA (Ecstasy) and p-chloroamphetamine but not following fenfluramine. Br. J. Pharmacol. 1997;121:889–900. doi: 10.1038/sj.bjp.0701213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUMMINS D.L. Biochemical and histological evidence that MDMA is toxic to neurons in the rat brain. J. Pharm. Exp. Ther. 1986;241:338–345. [PubMed] [Google Scholar]
- DOWLING G.P., MCDONOUGH E.T., BOST R.O. ‘Eve' and ‘Ecstasy'. A report of five deaths associated with the use of MDEA and MDMA. JAMA. 1987;257:1615–1617. doi: 10.1001/jama.257.12.1615. [DOI] [PubMed] [Google Scholar]
- DUNKLEY P.R., HEATH J.W., HARRISON S.M., JARVIE P.E., GLENFIELD P.J., ROSTAS J.A.P. A rapid Percoll gradient procedure for isolation of synaptosomes directly from an S1 fraction; homogeneity and morphology of subcellular fractions. Brain Res. 1988;441:59–71. doi: 10.1016/0006-8993(88)91383-2. [DOI] [PubMed] [Google Scholar]
- ESTEBAN B., O'SHEA E., CAMARERO J., SANCHEZ V., GREEN A.R., COLADO M.I. 3,4-methylenedioxymethamphetamine induces monoamine release, but not toxicity, when administered centrally at a concentration occurring following a peripherally injected neurotoxic dose. Psychopharmacol. 2001;154:251–260. doi: 10.1007/s002130000645. [DOI] [PubMed] [Google Scholar]
- FITZGERALD J.L., REID J.J. Sympathomimetic actions of methylenedioxymethamphetamine in rat and rabbit isolated cardiovascular tissue. J. Pharm. Pharmacol. 1994;46:826–832. doi: 10.1111/j.2042-7158.1994.tb03738.x. [DOI] [PubMed] [Google Scholar]
- FUKUDA S., SU C., LEE J.-F. Mechanisms of extraneuronal serotonin uptake in the rat aorta. J. Pharmacol. Exp. Ther. 1986;239:264–269. [PubMed] [Google Scholar]
- GORDON C.J., WATKINSON W.P., O'CALLAGHAN J.P., MILLER D.B. Effects of 3,4-methylenedioxymethamphetamine on autonomic thermoregulatory responses of the rat. Pharmacol. Biochem. Behav. 1991;38:339–344. doi: 10.1016/0091-3057(91)90288-d. [DOI] [PubMed] [Google Scholar]
- GRANOFF M.I., ASHBY C.R. Effect of the repeated administration of (+/−)-3,4-methylenedioxymethamphetamine on the behavioural response of rats to the 5-HT1A receptor agonist (+/−)-8-hydroxy-(di-n-proplyamino)tetralin. Neuropsychobiol. 2001;43:42–48. doi: 10.1159/000054864. [DOI] [PubMed] [Google Scholar]
- HAYNER G.N., MCKINNEY H. MDMA. The dark side of ecstasy. J. Psychoactive Drugs. 1986;18:341–347. doi: 10.1080/02791072.1986.10472367. [DOI] [PubMed] [Google Scholar]
- HEKMATPANAH C.R., PEROUTKA S.J. 5-Hydroxytryptamine blockers attenuate the 5-Hydroxytryptamine-releasing effect of 3,4-methylenedioxymethamphetamine and related agents. Eur. J. Pharmacol. 1990;177:95–98. doi: 10.1016/0014-2999(90)90555-k. [DOI] [PubMed] [Google Scholar]
- LAVELLE A., HONNER V., DOCHERTY J.R. Investigations of the prejunctional α2-adrenoceptor mediated actions of MDMA in rat atrium and vas deferens. Brit. J. Pharmacol. 1999;128:975–980. doi: 10.1038/sj.bjp.0702875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARKWELL M., HAAS S.M., BIEBER L., TOLBERT N. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- MAS M., FARRÉ M., DELATORRE R., ROSET P.N., ORTUÑO J., SEGURA J., CAMÍ J. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4-methylenedioxymethamphetamine in humans. J. Pharm. Exp. Ther. 1999;290:136–145. [PubMed] [Google Scholar]
- MCDAID J., DOCHERTY J.R. Vascular actions of MDMA involve α1 and α2-adrenoceptors in the anaesthetised rat. Br. J. Pharmacol. 2001;133:429–437. doi: 10.1038/sj.bjp.0704094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCKENNA D.J., GUAN X.M., SHULGIN A.T. 3,4-methylenedioxyamphetamine analogues exhibit different effects on synthesis and release of [3H]-dopamine and [3H]-serotonin. Pharmacol. Biochem. Behav. 1991;38:505–512. doi: 10.1016/0091-3057(91)90005-m. [DOI] [PubMed] [Google Scholar]
- MURPHY J.E.J., CANNON D.M., GUIRY P., MCCORMACK P., BAIRD A.W., MCBEAN G.J., KEENAN A.K.A comparison of the in vitro effects of (+)-4-methylthioamphetamine and (+)-MDMA on 5-HT re-uptake in the brain and on vascular responses to 5-HT Br. J. Pharmacol. 2001(in press)
- MILROY C.M., CLARK J.C., FORREST A.R.W. Pathology of deaths associated with ‘ecstasy' and ‘eve' misuse. J. Clin. Pathol. 1996;49:149–153. doi: 10.1136/jcp.49.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASH J.F., ARORA R.C., SCHREIBER M.A., MELTZER H.Y. Effect of 3,4-methylenedioxymethamphetamine on [3H]paroxetine binding in the frontal cortex and blood platelets of rats. Biochem. Pharmacol. 1991;41:79–84. doi: 10.1016/0006-2952(91)90013-u. [DOI] [PubMed] [Google Scholar]
- NICHOLS D.E., LLOYD D.H., HOFFMAN A.J., NICHOLS M.D., YIM G.K.W. Effects of certain hallucinogenic amphetamine analogues on the release of [3H]-serotonin from rat brain synaptosomes. J. Med. Chem. 1982;25:530–535. doi: 10.1021/jm00347a010. [DOI] [PubMed] [Google Scholar]
- O'LOINSIGH E.D., BOLAND G., KELLY J.P., O'BOYLE K.M. Behavioural, hyperthermic and neurotoxic effects of 3,4-methylenedioxymethamphetamine analogues in the Wistar rat. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25:621–638. doi: 10.1016/s0278-5846(00)00179-2. [DOI] [PubMed] [Google Scholar]
- POLAND R.E. Diminished corticotropin and enhanced prolactin responses to 8-hydroxy-2-(di-n-propylamino) tetralin in methylenedioxymethamphetamine pretreated rats. Neuropharmacol. 1990;29:1099–1101. doi: 10.1016/0028-3908(90)90120-g. [DOI] [PubMed] [Google Scholar]
- RICAURTE G.A., FINEGAN K.F., IRWIN I., LANSTON J.W. Aminergic metabolites in cerebrospinal fluid of humans previously exposed to MDMA: preliminary observations. Ann. NY Acad. Sci. 1990;600:699–708. doi: 10.1111/j.1749-6632.1990.tb16919.x. [DOI] [PubMed] [Google Scholar]
- RUDNICK G., WALL S.C. The molecular mechanism of ‘ecstasy' [3,4-methylenedioxymethamphetamine(MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc. Natl. Acad. Sci. U.S.A. 1992;89:1817–1821. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABOL K.E., LEW R., RICHARDS J.B., VOSMER G.L., SEIDEN L.S. Methylenedioxymethamphetamine-induced serotonin deficits are followed by partial recovery over a 52-week period. Part I: synaptosomal uptake and tissue concentrations. J. Pharm. Exp. Ther. 1996;276:846–854. [PubMed] [Google Scholar]
- SCHMIDT C.J., TAYLOR V.L. Depression of rat brain tryptophan hydroxylase activity following the acute administration of methylenedioxymethamphetamine. Biochem. Pharmacol. 1987;36:4095–4102. doi: 10.1016/0006-2952(87)90566-1. [DOI] [PubMed] [Google Scholar]
- SCHMIDT C.J., LEVIN J.A., LOVENBERG W. In vitro and in vivo neurochemical effects of methylenedioxymethamphetamine on striatal monoaminergic systems in the rat brain. Biochem. Pharmacol. 1987;36:747–755. doi: 10.1016/0006-2952(87)90729-5. [DOI] [PubMed] [Google Scholar]
- SUAREZ R.V., RIEMERSMA R. ‘Ecstasy' and sudden cardiac death. Am. J. Forens. Med. Pathol. 1988;9:339–341. doi: 10.1097/00000433-198812000-00015. [DOI] [PubMed] [Google Scholar]
- VOLLENWEIDER F.X., GAMMA A., LIECHTI M., HUBER T. Psychological and cardiovascular effects and short-term sequelae of MDMA (‘Ecstasy') in MDMA-naïve healthy volunteers. Neuropsychopharmacol. 1998;19:241–251. doi: 10.1016/S0893-133X(98)00013-X. [DOI] [PubMed] [Google Scholar]


