Abstract
Specific inhibitors of the sarcolemmal Na+/H+ exchanger (NHE) such as cariporide are being evaluated for cardioprotective therapy during cardiac surgery. We determined the effects of moderate hypothermia (25°C), as occurs during cardiac surgery, on (1) sarcolemmal NHE activity and (2) the NHE-inhibitory potency of cariporide, in isolated adult rat ventricular myocytes.
As the index of NHE activity, trans-sarcolemmal acid efflux rate (JH) was determined by microepifluorescence in single cells (n=8 to 11 per group), during recovery from intracellular acidosis in bicarbonate-free conditions.
Initially, myocytes were subjected to two consecutive acid pulses; these both occurred at 37°C in the normothermic control group but the second pulse was at 25°C in the moderate hypothermia group. JH values obtained after the first pulse were superimposed in both groups, indicating comparable cell populations. However, after the second pulse, JH values in the moderate hypothermia group were approximately 50% of those in the normothermic control group over the pHi range 6.80 – 7.10.
Similar results were obtained in cells subjected to a single acid pulse at 37 or 25°C, with JH values in the latter group measuring approximately 60% of those in the former over the pHi range 6.80 – 7.10.
Cariporide (0.01, 0.03, 0.1, 0.3, 1.0 or 3.0 μM), present during recovery from a single acid pulse, reduced JH in a concentration-dependent manner, with IC50 values of 150 and 130 nM at 37 and 25°C, respectively.
We conclude that moderate hypothermia produces (1) a significant, but partial, inhibition of sarcolemmal NHE activity, and (2) no significant effect on the NHE-inhibitory potency of cariporide.
Keywords: Na+/H+ exchange, cariporide, hypothermia, temperature, myocyte
Introduction
There is substantial pre-clinical evidence that recently-developed, specific Na+/H+ exchanger (NHE) inhibitors protect the myocardium during ischaemia and reperfusion (see reviews by Avkiran (1999b) and Karmazyn et al. (1999)). Indeed, the degree of protection afforded by NHE inhibition appears to be at least as good as that afforded by ischaemic preconditioning (Avkiran, 1999a; Gumina et al., 1999; Shipolini et al., 1997b). In the vast majority of pre-clinical studies with NHE inhibitors, hearts have been subjected to ischaemia and reperfusion under normothermic conditions, in an attempt to mimic the situation that occurs during spontaneous coronary occlusion and subsequent revascularization in patients with coronary artery disease. Nevertheless, a few studies (Kim et al., 1998a, 1998b; Myers & Karmazyn, 1996; Shipolini et al., 1997a; Yamauchi et al., 1997) have employed global hypothermic ischaemia, as encountered during cardiac surgery and transplantation, and have used NHE inhibitors in combination with established surgical cardioprotection techniques (such as hyperkalaemic cardioplegic arrest), with encouraging findings. For example, our laboratory was the first to show that the specific NHE inhibitor cariporide (HOE-642; 4-isopropyl-3-methylsulphonylbenzoyl-guanidine methanesulphonate), used as an adjunct or additive to crystalloid cardioplegia, provides additional cardioprotective benefit under conditions of both moderate hypothermia (28°C), as encountered during routine cardiac surgery, and severe hypothermia (7.5°C), as used for cardiac preservation for transplantation (Shipolini et al., 1997a). Interestingly, data from the GUARDIAN trial (Théroux et al., 2000) indicate that a subgroup of high-risk patients subjected to iatrogenic myocardial ischaemia during coronary artery bypass graft surgery uniquely benefited from treatment with cariporide.
Its common occurrence under hypothermic conditions is a potentially important factor that distinguishes iatrogenic myocardial ischaemia during cardiac surgery from myocardial ischaemia that manifests during spontaneous coronary events. Despite the experimental work that has been carried out to determine the cardioprotective efficacy of NHE inhibitors under conditions of normothermic and hypothermic ischaemia, however, the effects of reduced temperature per se on sarcolemmal NHE activity and on the potency of NHE inhibitors have not been fully characterized. Therefore, the objectives of the work described here were to determine, in adult rat ventricular myocytes, the effects of moderate hypothermia (25°C) on (1) sarcolemmal NHE activity and (2) the NHE-inhibitory potency of cariporide.
Methods
This investigation was performed in accordance with the Home Office ‘Guidance on the Operation of the Animals (Scientific Procedures) Act 1986', published by Her Majesty's Stationery Office, London.
Isolation of ventricular myocytes
Adult male Wistar rats (200 – 250 g) were anaesthetized with sodium pentobarbitone (60 mg kg−1 i.p.) and injected with heparin (50 u i.v.), and their hearts were excised for the isolation of ventricular myocytes by enzymatic digestion, as described previously (Yasutake et al., 1996).
Measurement of pHi
Intracellular pH (pHi) was monitored in single myocytes loaded with the pH-sensitive fluoroprobe carboxy-seminaphthorhodafluor-1 (cSNARF-1), using an established microepifluorescence technique (Avkiran & Yokoyama, 2000; Gunasegaram et al., 1999; Haworth et al., 1997; 1999; Snabaitis et al., 2000; Yasutake et al., 1996; Yokoyama et al., 1998; 2000). Calibration of the cSNARF-1 signal was carried out in situ at both 37°C (13 cells) and 25°C (10 cells) using nigericin-containing calibration solutions, as described in detail previously (Yasutake et al., 1996). Also as described previously (Yasutake et al., 1996), at the end of each experiment, myocytes were exposed to the pH 7.0 calibration solution and the normalized fluorescence emission ratios were converted to pHi values by reference to a calibration curve that was obtained by a nonlinear least-squares fit of data to the equation given below.
Estimation of intracellular intrinsic buffering power
Intracellular intrinsic buffering power (βi) was estimated by stepwise removal of extracellular NH4Cl at both 37°C (10 cells) and 25°C (11 cells), as described in detail previously (Yasutake et al., 1996). At each step, calculated changes in [NH4+]i and measured changes in pHi were used to estimate βi, from the equation βi=[NH4+]i/pHi.
Determination of sarcolemmal NHE activity
The myocytes were maintained in bicarbonate-free Tyrode's solution throughout each experiment, thus enabling the rate of acid efflux (JH), which was calculated from the equation JH=βi·dpHi/dt (where dpHi/dt is the rate of recovery of pHi), to be used as an indicator of sarcolemmal NHE activity (Yasutake et al., 1996). JH values were determined either at pHi intervals of 0.05 throughout recovery from intracellular acidosis (when studying the effects of temperature) or during the first 60 s after the induction of intracellular acidosis (when studying the effects of cariporide).
Experimental protocols
In initial studies, myocytes (n=9 or 10 per group) were subjected to intracellular acidosis by transient exposure to 20 mM NH4Cl and its subsequent washout for 8 min (first acid pulse), which was repeated 10 – 12 min later (second acid pulse) (Avkiran & Yokoyama, 2000; Gunasegaram et al., 1999; Snabaitis et al., 2000; Yasutake et al., 1996; Yokoyama et al., 1998). In both normothermic control and moderate hypothermia groups, the first acid pulse occurred at 37°C and was induced by a 4 min exposure to NH4Cl. In the normothermic control group, cells were maintained at 37°C throughout the experiment and the second acid pulse was induced under identical conditions to the first. In contrast, in the moderate hypothermia group, cells were switched to superfusion at 25°C from 10 min before the second acid pulse and were maintained at this temperature thereafter; in this group, the second acid pulse was induced by a 6 min exposure to NH4Cl. In subsequent experiments, cells (n=8 to 11 per group) were subjected to a single acid pulse at either 37 or 25°C by transient (4 min at 37°C, 6 min at 25°C) exposure to 20 mM NH4Cl and its subsequent washout for 8 min; when used, cariporide (0.01, 0.03, 0.1, 0.3, 1.0 or 3.0 μM) was present throughout NH4Cl washout.
Data analysis
Data are expressed as mean±s.e.mean. Experiments within each protocol were randomized. For inter-group comparisons of data, either analysis of variance (for multi-group comparisons) or the unpaired t-test (for comparisons between normothermic control and moderate hypothermia groups) was used. P<0.05 was considered significant. Dose-response curves and IC50 values were obtained by nonlinear regression analysis, using GraphPad Prism software.
Results
Effects of moderate hypothermia on cSNARF-1 calibration
The in situ calibration curves obtained at 37 and 25°C are shown in Figure 1. As illustrated, moderate hypothermia altered the pHi-dependence of the fluorescence emission ratio of cSNARF-1, with estimated pK values for the fluoroprobe of approximately 7.00 at 37°C and 7.15 at 25°C. A comparable temperature-dependent pK change has been reported previously for another pH-sensitive fluoroprobe, 2 7-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (Graber et al., 1992). In subsequent experiments, fluorescence emission ratios were converted to pHi values by reference to the calibration curve obtained at the appropriate temperature (Figure 1).
Figure 1.
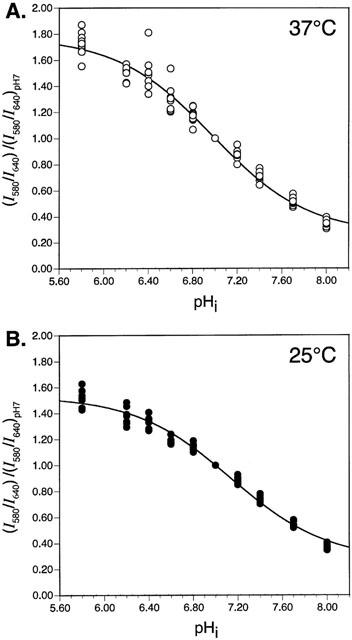
Calibration curves constructed using normalized I580/I640 ratio data obtained during exposure of adult rat ventricular myocytes to nigericin-containing calibration solutions at (A) 37°C (n=13 cells) or (B) 25°C (n=10 cells). See text for details.
Effects of moderate hypothermia on βi
As shown in Figure 2, moderate hypothermia produced a small change in the pHi-dependence of βi, such that βi tended to be greater at 25°C than at 37°C, particularly under non-acidic conditions. For the calculation of JH in subsequent experiments, βi was estimated by reference to the βi-versus-pHi relationship obtained at the appropriate temperature (Figure 2).
Figure 2.
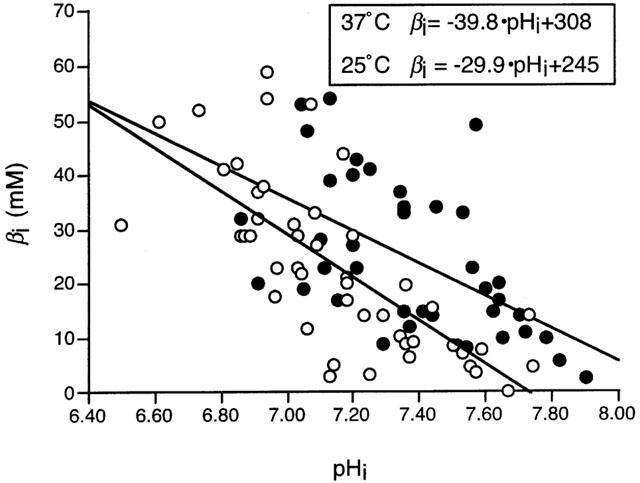
The relationship between intracellular pH (pHi) and intrinsic buffering power (βi) in adult rat ventricular myocytes at 37°C (n=10 cells, open symbols) and 25°C (n=11 cells, solid symbols). Inset shows the equations obtained by linear regression analysis of the data obtained at each temperature.
Effects of moderate hypothermia on sarcolemmal NHE activity
In the first set of experiments to determine the effect of moderate hypothermia on sarcolemmal NHE activity, two groups of cells were subjected to consecutive acid pulses, after each of which NHE activity was determined. The first pulse was at 37°C in both groups (to confirm comparable cell populations) while the second pulse was at either 37°C (normothermic control group, n=10) or 25°C (moderate hypothermia group, n=9). Basal pHi values at 37°C, obtained prior to the first acid pulse, were comparable in the normothermic control and moderate hypothermia groups, measuring 7.31±0.02 and 7.28±0.04, respectively (NS). Cells in the normothermic control and moderate hypothermia groups acidified to a similar extent during the first acid pulse at 37°C, with minimum pHi values of 6.62±0.03 and 6.64±0.07, respectively; during the second acid pulse, however, the minimum pHi was 6.66±0.03 in the normothermic control group but tended to be higher at 6.76±0.06 in the moderate hypothermia group. Figure 3 shows the JH-versus-pHi curves obtained after both acid pulses in the two study groups. NHE activity during recovery from the first acid pulse at 37°C was similar in both groups, with comparable JH values obtained throughout the pHi range 6.80 – 7.10 (Figure 3A). For example, JH at pHi 6.90 was 3.79±0.75 mM min−1 in the normothermic control group and 3.78±0.43 mM min−1 in the moderate hypothermia group (NS). In contrast, after the second acid pulse (which was carried out at 37°C in the normothermic control group but at 25°C in the moderate hypothermia group), JH in the moderate hypothermia group measured approximately 50% of that seen in the normothermic control group throughout the pHi range 6.80 – 7.10 (Figure 3B). This difference between the groups arose predominantly from an increase in NHE activity between the two acid pulses in the normothermic control group but not in the moderate hypothermia group. It appears therefore that consecutive acid pulses produce an increase in sarcolemmal NHE activity in adult rat ventricular myocytes under normothermic conditions and that this increase is inhibited by exposure of the cells to moderate hypothermia.
Figure 3.
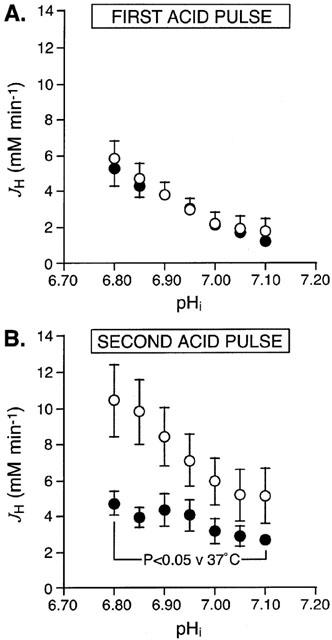
Effect of moderate hypothermia on sarcolemmal NHE activity in adult rat ventricular myocytes subjected to two consecutive acid pulses. Figure shows the JH-versus-pHi curves obtained during (A) the first acid pulse and (B) the second acid pulse. In the normothermic control group (n=10 cells, open symbols) both acid pulses occurred at 37°C, whereas in the moderate hypothermia group (n=9 cells, solid symbols) cells were switched from 37 to 25°C from 10 min before the second acid pulse. The curves were constructed by determining JH values at pHi intervals of 0.05 in each cell, throughout recovery from both acid pulses.
To determine the effect of moderate hypothermia on NHE activity in the absence of any changes arising from repeated episodes of intracellular acidosis, we next examined NHE activity in two groups of cells (n=8 or 9 per group) subjected to a single acid pulse, at either 37 or 25°C. In these experiments, the basal pHi value obtained prior to the acid pulse was 7.32±0.07 in the normothermic control group (n=9) but was significantly higher at 7.46±0.03 in the moderate hypothermia group (n=8). The minimum pHi achieved during the acid pulse was also significantly higher in the moderate hypothermia group (6.83±0.03) relative to the normothermic control group (6.67±0.04). Figure 4 shows the JH-versus-pHi curves obtained in both study groups; as can be seen, the curve was shifted to the left under conditions of moderate hypothermia, reflecting significantly lower NHE activity throughout the pHi range 6.80 – 7.10. For example, JH at pHi 6.90 was 3.91±0.55 mM min−1 in the normothermic control group but measured only 59% of that, at 2.32±0.24 mM min−1, in the moderate hypothermia group (P<0.05).
Figure 4.
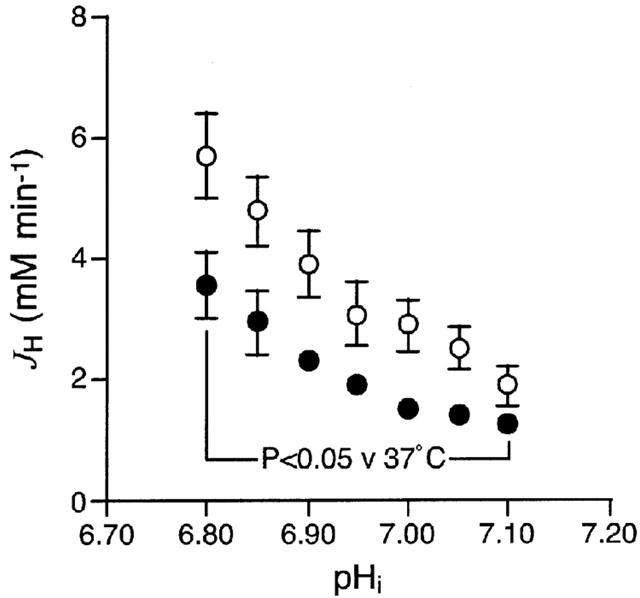
Effect of moderate hypothermia on sarcolemmal NHE activity in adult rat ventricular myocytes subjected to a single acid pulse. Figure shows the JH-versus-pHi curves for the normothermic control group (n=9 cells, open symbols), in which the acid pulse occurred at 37°C, and the moderate hypothermia group (n=8 cells, solid symbols), in which the acid pulse occurred at 25°C. The curves were constructed by determining JH values at pHi intervals of 0.05 in each cell, throughout recovery from the acid pulse.
Effects of moderate hypothermia on the NHE-inhibitory potency of cariporide
In this protocol, we sought to determine whether the NHE-inhibitory potency of cariporide is altered under conditions of moderate hypothermia. Twelve groups of cells (n=8 to 11 per group) were again subjected to a single acid pulse at either 37 or 25°C, with cariporide (0.01 – 3.0 μM) present in the superfusion solution throughout the recovery phase. Since cariporide inhibits recovery from intracellular acidosis, JH was determined only at the nadir of the acid pulse, as illustrated in Figure 5. The basal pHi value obtained prior to the acid pulse was 7.25±0.01 in the cells studied at 37°C (n=52) and again was significantly higher at 7.45±0.02 in the cells studied at 25°C (n=53). The minimum pHi value, obtained upon NH4Cl washout, was 6.64±0.02 in the cells studied at 37°C (n=52) and was also significantly higher at 6.72±0.02 in the cells studied at 25°C (n=53). At each temperature, however, there was no significant difference in either the basal pHi or the minimum pHi between the six groups that received the different concentrations of cariporide (Table 1).
Figure 5.
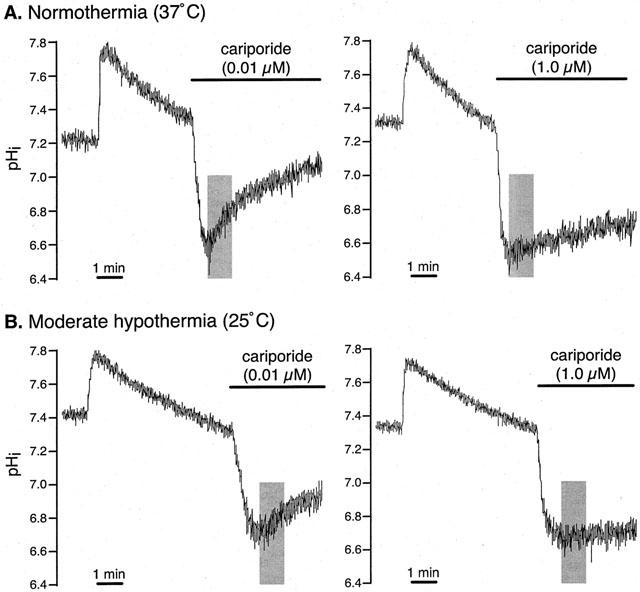
Effect of cariporide on recovery from intracellular acidosis in adult rat ventricular myocytes. Figure shows representative pHi recordings obtained in cells exposed to a low, non-inhibitory concentration (0.01 μM) or a high, inhibitory concentration (1.0 μM) of cariporide during acid pulses carried out under conditions of (A) normothermia (37°C) or (B) moderate hypothermia (25°C). Shaded areas indicate the period during which JH was estimated from the rate of recovery of pHi.
Table 1.
Basal and minimum pHi values in cells exposed to cariporide at 37 or 25°C.
Figure 6A shows the JH values obtained in the various study groups at 37 and 25°C. As can be seen, in the presence of each concentration of cariporide, the JH obtained at 25°C was approximately 50 – 60% of that obtained at 37°C, which is consistent with our observations above. At both temperatures, cariporide produced a concentration-dependent reduction in JH (Figure 6A). Notably, even in the presence of 3.0 μM cariporide, JH was not completely abolished, most likely due to residual Na+/HCO3− cotransport activity (Wu et al., 1994). Figure 6B illustrates the dose-response curves for NHE inhibition by cariporide, obtained after the correction of JH values for residual Na+/HCO3− cotransport activity, at both 37 and 25°C. As can be seen, moderate hypothermia did not have a significant effect on the NHE-inhibitory potency of cariporide, with IC50 values of approximately 150 nM at 37°C and 130 nM at 25°C.
Figure 6.
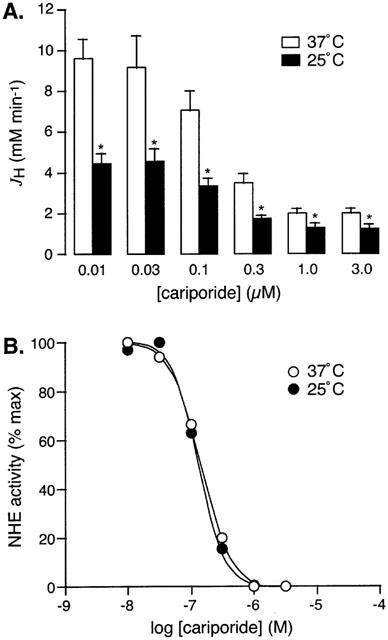
Effect of moderate hypothermia on the NHE-inhibitory potency of cariporide in adult rat ventricular myocytes. Figure shows (A) absolute JH values and (B) relative NHE activity, in cells subjected to a single acid pulse under conditions of normothermia (37°C, open bars and symbols) or moderate hypothermia (25°C, solid bars and symbols), in the presence of 0.01, 0.03, 0.1, 0.3, 1.0 or 3.0 μM cariporide (n=8 to 11 cells per group). *P<0.05 versus 37°C.
Discussion
Although several studies have attempted to determine the effects of moderate hypothermia (20 – 30°C) on plasma membrane NHE activity, their findings have been somewhat contradictory, probably due to the variety of cell types that have been used and the manner in which NHE activity has been assessed. In guinea-pig erythrocytes, lowering the temperature from 37 to 20°C has been shown to produce an increase in Na+ influx that is sensitive to inhibition by the NHE inhibitor amiloride, which is indicative of a hypothermia-induced increase in NHE activity (Zhou & Willis, 1989). More recent work has shown that lowering the temperature from 37 to 27°C induces rapid swelling of rat glial cells in a manner that is inhibited by the amiloride analogue ethylisopropylamiloride, again suggesting increased NHE activity under conditions of moderate hypothermia (Plesnila et al., 2000). In contrast, the rate of swelling of rat lymphocytes following exposure to sodium propionate has been shown to be considerably slower at 22 and 27°C than at 37°C (and to be inhibited at each temperature by the NHE inhibitor FR168888), suggesting a hypothermia-induced decrease in NHE activity (Yamauchi et al., 1997). In the above studies (Plesnila et al., 2000; Yamauchi et al., 1997), NHE activity was surmized from the inhibitory effects of NHE inhibitors on the observed increase in cellular volume and direct measurements of NHE activity (i.e. the rate of NHE-mediated Na+ influx or H+ efflux) at known values of pHi, which is the principal regulator of NHE activity (Wakabayashi et al., 1997), are scarce. In this context, Graber et al. (1992) have measured the rate of recovery of pHi after the induction of an intracellular acid load in opossum kidney cells and shown this to be slower at 25°C than at 37°C. A similar observation has been reported in sheep cardiac Purkinje fibres, upon lowering of the ambient temperature from 37 to 22°C (Ellis & Macleod, 1985). Although these findings may indicate a reduction in NHE activity in the presence of moderate hypothermia, it is notable that, in both studies, the rate of recovery of pHi was measured at a different level of intracellular acidosis under conditions of normothermia versus moderate hypothermia.
The present study is the first detailed characterization of the effects of moderate hypothermia on sarcolemmal NHE activity in adult mammalian ventricular myocytes, and demonstrates a significant inhibition of such activity upon lowering of the ambient temperature from 37 to 25°C. Notably, this inhibition is not absolute, such that at 25°C sarcolemmal NHE activity is retained at approximately 50 – 60% of that observed at 37°C. A recent preliminary report indicates that moderate hypothermia (27°C) may produce a similar degree of inhibition of sarcolemmal NHE activity in guinea-pig ventricular myocytes also (Ch'en & Vaughan-Jones, 2000). Interestingly, other evidence in the literature suggests that pathophysiologically significant sarcolemmal NHE activity may be retained even under conditions of severe hypothermia (<20°C). Thus, Na+ has been shown to accumulate intracellularly during 6 h storage of embryonic chick cardiac myocytes at 10°C (Knerr & Lieberman, 1993) and 12 h storage of adult rat hearts at 4°C (Askenasy et al., 1996) in a manner that was significantly attenuated by the NHE inhibitor ethylisopropylamiloride.
In our experiments that employed two consecutive acid pulses, there was a marked increase in sarcolemmal NHE activity after the second acid pulse relative to the first, when both pulses occurred at 37°C (Figure 3). In contrast, no such increase in NHE activity was observed when the second pulse was at 25°C (Figure 3). In our previous studies that used similar 2-pulse protocols at 34°C (Avkiran & Yokoyama, 2000; Gunasegaram et al., 1999; Snabaitis et al., 2000; Yasutake et al., 1996; Yokoyama et al., 1998), there was only a small (<30%) increase in NHE activity after the second acid pulse (in the absence of any other intervention) and this increase was not statistically significant. It appears therefore that repeated episodes of intracellular acidosis can lead to increased sarcolemmal NHE activity, through a mechanism that is very sensitive to the ambient temperature. It would be of interest to determine the role of altered activity of NHE-regulatory signalling pathways (such as the protein kinase C and extracellular signal regulated kinase pathways (Moor & Fliegel, 1999; Snabaitis et al., 2000)) in such stimulation of sarcolemmal NHE activity by repeated episodes of acidosis. Regardless of the precise mechanisms underlying this interesting phenomenon, however, it is important to note that a similar reduction in sarcolemmal NHE activity by moderate hypothermia was evident also when cells were exposed to a single acid pulse (Figure 4). Therefore, it is likely that this negative effect of moderate hypothermia arose principally from the inhibition of sarcolemmal NHE activity per se rather than the attenuation of its stimulation by repeated episodes of intracellular acidosis.
The present work has also revealed that cariporide inhibits sarcolemmal NHE activity with comparable potency at 25 and 37°C, with an IC50 of 130 – 150 nM under each condition. Such an IC50 value is approximately 15 fold greater than that we have previously estimated for this drug in rat ventricular myocytes (Shipolini et al., 1997b). However, in our earlier study (Shipolini et al., 1997b), intracellular acidosis to activate the exchanger was induced in the absence of extracellular Na+, which was reintroduced concomitantly with cariporide. In contrast, in the present study, intracellular acidosis was induced by the washout of NH4Cl with Tyrode's solution, which contains Na+ at a concentration of 137 mM. Extracellular Na+ is known to antagonize competitively the binding of benzoylguanidine-based inhibitors such as cariporide to NHE (Baumgarth et al., 1998), which is likely to underlie the different IC50 values obtained in our studies. Indeed, in guinea-pig ventricular myocytes, the IC50 for HOE-694 (another benzoylguanidine-based NHE inhibitor that is closely related to cariporide structurally) has been estimated to be approximately 16 fold greater in the presence of an extracellular Na+ concentration of 150 mM, relative to the value obtained in the virtual absence of extracellular Na+ (Loh et al., 1996).
An interesting observation in the present study was the difference in basal pHi under conditions of normothermia versus moderate hypothermia, such that this was 0.15 – 0.20 pH unit greater at 25°C than at 37°C. To our knowledge, this is the first report of this phenomenon in isolated ventricular myocytes, although similar effects of moderate hypothermia have been reported previously in sheep Purkinje fibres (pHi increase of 0.21 (Ellis & Macleod, 1985) or 0.31 (Bright & Ellis, 1994) on reducing temperature from 35 to 21 – 22°C), isolated rat hearts (pHi increase of 0.16 on reducing temperature from 36 to 20°C (Gruwel et al., 1998)) and sheep myocardium in vivo (pHi increase of 0.19 on reducing temperature from 37 to 26°C (Swain et al., 1991)). Although the precise mechanism(s) underlying this increase in steady-state pHi have not been determined, hypothermia-induced changes in the pK of intracellular buffers, such as the imidazole moiety of histidine, are likely to play an important role (Roos & Boron, 1981). In this context, it is notable that the pK of imidazole has been estimated to be 6.75 at 37°C but to increase to 7.30 at 25°C (Durand et al., 1998). On the basis that a low level of sarcolemmal NHE activity appears to be retained under steady-state conditions in ventricular myocytes (Leem et al., 1999), our data suggest that the inhibition of such activity may also contribute to the increase in basal pHi during exposure to moderate hypothermia.
Previous data in sheep Purkinje fibres suggest that, under conditions of moderate hypothermia, the higher steady-state pHi is associated with an attenuated level of intracellular acidification in response to the NH4Cl pulse (Ellis & Macleod, 1985). Since pHi is a critical determinant of NHE activity (Wakabayashi et al., 1997), we attempted to compensate for this and obtain comparable levels of intracellular acidosis in the normothermic control and moderate hypothermia groups, by extending the duration of the NH4Cl pulse from 4 min at 37°C to 6 min at 25°C. This approach was only partially successful, however, in that the minimum pHi achieved at 25°C remained approximately 0.10 pH unit higher than that at 37°C. This difference is unlikely to contribute to the lower NHE activity observed at 25°C (Figures 3 and 4), since JH values were compared at identical pHi values in the two groups. Nevertheless, in the cariporide study, where JH was determined at the nadir of the acid pulse, a higher minimum pHi value may have contributed to the lower NHE activity at 25°C. Indeed, in the presence of a non-inhibitory concentration of cariporide (0.01 μM), JH at 25°C was only 45% of that at 37°C (Figure 6A). In contrast, when the comparison was made at identical values of pHi in a similar protocol in the absence of cariporide (Figure 4), hypothermia-induced inhibition of sarcolemmal NHE activity was somewhat attenuated, with JH values at 25°C measuring approximately 60% of those at 37°C.
The temperature-independence of the NHE-inhibitory potency of cariporide, at least within the temperature range that we have studied, suggests that this agent is likely to retain its cardioprotective efficacy under moderately hypothermic conditions. This is indeed borne out by our earlier work in isolated rat hearts (Shipolini et al., 1997a), which revealed that the use of cariporide as an additive to crystalloid cardioplegia improved the recovery of contractile function and reduced the leakage of creatine kinase following 120 min of global ischaemia at 28°C. This property is potentially important in relation to the application of cariporide for surgical myocardial protection and distinguishes this agent from other ion transport inhibitors, such as L-type calcium channel blockers. In this context, unlike cariporide (Shipolini et al., 1997a), verapamil (Hearse et al., 1984) and nifedipine (Fukunami & Hearse, 1985) have been shown to provide no significant cardioprotective benefit in isolated rat hearts when used as an additive to hyperkalaemic cardioplegia under conditions of moderate hypothermia, although both were effective at temperatures >30°C.
In conclusion, our work in isolated adult rat ventricular myocytes has shown that moderate hypothermia (25°C) produces a significant, but only partial, inhibition of sarcolemmal NHE activity. Furthermore, the NHE-inhibitory potency of cariporide is not affected by such a reduction in temperature. These findings may help provide a mechanistic basis for the previously demonstrated ability of cariporide to protect ischaemic myocardium under conditions of moderate hypothermia and suggest that this effect is likely to arise from the inhibition of retained NHE activity.
Acknowledgments
M. Avkiran is the holder of a Basic Science Award (BS/93002) from the British Heart Foundation and K. Hoshino is an International Research Fellow from the Nippon Medical School, Tokyo. The authors thank Aventis Pharma for the gift of cariporide and support of this project, and Drs Robert Haworth and Andrew Snabaitis for critical reading of this manuscript.
Abbreviations
- βi
intrinsic buffering power
- cSNARF-1
carboxy-seminaphthorhodafluor-1
- GUARDIAN
Guard During Ischaemia Against Necrosis trial
- HOE-642
4-isopropyl-3-methylsulphonylbenzoyl-guanidine methanesulphonate
- JH
rate of acid efflux
- NHE
Na+/H+ exchange
- pHi
intracellular pH
References
- ASKENASY N., VIVI A., TASSINI M., NAVON G. The relation between cellular sodium, pH and volumes and the activity of Na/H antiport during hypothermic ischemia: multinuclear NMR studies of rat hearts. J. Mol. Cell. Cardiol. 1996;28:589–601. doi: 10.1006/jmcc.1996.0055. [DOI] [PubMed] [Google Scholar]
- AVKIRAN M. Protection of the myocardium during ischemia and reperfusion: Na+/H+ exchange inhibition versus ischemic preconditioning. Circulation. 1999a;100:2469–2472. doi: 10.1161/01.cir.100.25.2469. [DOI] [PubMed] [Google Scholar]
- AVKIRAN M. Rational basis for use of sodium-hydrogen exchange inhibitors in myocardial ischemia. Am. J. Cardiol. 1999b;83:10G–18G. doi: 10.1016/s0002-9149(99)00215-5. [DOI] [PubMed] [Google Scholar]
- AVKIRAN M., YOKOYAMA H. Adenosine A1 receptor stimulation inhibits α1-adrenergic activation of the cardiac sarcolemmal Na+/H+ exchanger. Brit. J. Pharmacol. 2000;131:659–662. doi: 10.1038/sj.bjp.0703647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUMGARTH M., BEIER N., GERICKE R. Bicyclic acylguanidine Na+/H+ antiporter inhibitors. J. Med. Chem. 1998;41:3736–3747. doi: 10.1021/jm981031w. [DOI] [PubMed] [Google Scholar]
- BRIGHT C.M., ELLIS D. Hypoxia-induced intracellular acidification in isolated sheep heart Purkinje fibres and the effects of temperature. J. Mol. Cell. Cardiol. 1994;26:463–469. doi: 10.1006/jmcc.1994.1057. [DOI] [PubMed] [Google Scholar]
- CH'EN F.F.-T., VAUGHAN-JONES R.D. Temperature dependence of cardiac Na+-H+ exchange and Na+-HCO3− cotransport. J. Physiol. 2000;527.P:66P. [Google Scholar]
- DURAND T., VIDAL G., CANIONI P., GALLIS J.L. Cytosolic pH variations in perfused rat liver at 4 C: role of intracellular buffering power. Cryobiology. 1998;36:269–278. doi: 10.1006/cryo.1998.2086. [DOI] [PubMed] [Google Scholar]
- ELLIS D., MACLEOD K.T. Sodium-dependent control of intracellular pH in Purkinje fibres of sheep heart. J. Physiol. 1985;359:81–105. doi: 10.1113/jphysiol.1985.sp015576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUNAMI M., HEARSE D.J. Temperature-dependency of nifedipine as a protective agent during cardioplegia in the rat. Cardio. Res. 1985;19:95–103. doi: 10.1093/cvr/19.2.95. [DOI] [PubMed] [Google Scholar]
- GRABER M., BARRY C., DIPAOLA J., HASAGAWA A. Intracellular pH in OK cells: effects of temperature on cell pH. Am. J. Physiol. 1992;262:F723–F730. doi: 10.1152/ajprenal.1992.262.5.F723. [DOI] [PubMed] [Google Scholar]
- GRUWEL M.L., KUZIO B., DESLAURIERS R., KUPRIYANOV V.V. Observation of two inorganic phosphate NMR resonances in the perfused hypothermic rat heart. Cryobiology. 1998;37:355–361. doi: 10.1006/cryo.1998.2131. [DOI] [PubMed] [Google Scholar]
- GUMINA R.J., BUERGER E., EICKMEIER C., MOORE J., DAEMMGEN J., GROSS G.J. Inhibition of the Na+/H+ exchanger confers greater cardioprotection against 90 minutes of myocardial ischemia than ischemic preconditioning in dogs. Circulation. 1999;100:2519–2526. doi: 10.1161/01.cir.100.25.2519. [DOI] [PubMed] [Google Scholar]
- GUNASEGARAM S., HAWORTH R.S., HEARSE D.J., AVKIRAN M. Regulation of sarcolemmal Na+/H+ exchanger activity by angiotensin II in adult rat ventricular myocytes: opposing actions via AT1 versus AT2 receptors. Circul. Res. 1999;85:919–930. doi: 10.1161/01.res.85.10.919. [DOI] [PubMed] [Google Scholar]
- HAWORTH R.S., SINNETT-SMITH J., ROZENGURT E., AVKIRAN M. Protein kinase D inhibits plasma membrane Na+/H+ exchanger activity. Am. J. Physiol. Cell. Physiol. 1999;277:C1202–C1209. doi: 10.1152/ajpcell.1999.277.6.C1202. [DOI] [PubMed] [Google Scholar]
- HAWORTH R.S., YASUTAKE M., BROOKS G., AVKIRAN M. Cardiac Na+/H+ exchanger during postnatal development in the rat: changes in mRNA expression and sarcolemmal activity. J. Mol. Cell. Cardiol. 1997;29:321–332. doi: 10.1006/jmcc.1996.0277. [DOI] [PubMed] [Google Scholar]
- HEARSE D.J., YAMAMOTO F., SHATTOCK M.J. Calcium antagonists and hypothermia: the temperature dependency of the negative inotropic and anti-ischemic properties of verapamil in the isolated rat heart. Circulation. 1984;70:I54–I64. [PubMed] [Google Scholar]
- KARMAZYN M., GAN X.T., HUMPHREYS R.A., YOSHIDA H., KUSUMOTO K. The myocardial Na+-H+ exchange: structure, regulation, and its role in heart disease. Circul. Res. 1999;85:777–786. doi: 10.1161/01.res.85.9.777. [DOI] [PubMed] [Google Scholar]
- KIM Y.L., HERIJGERS P., LAYCOCK S.K., VAN LOMMEL A., VERBEKEN E., FLAMENG W. Na+/H+ exchange inhibition improves long-term myocardial preservation. Ann. Thor. Surg. 1998a;66:436–442. doi: 10.1016/s0003-4975(98)00464-0. [DOI] [PubMed] [Google Scholar]
- KIM Y.L., HERIJGERS P., VAN LOMMEL A., VERBEKEN E., FLAMENG W. Na+/H+ exchange inhibition improves post-transplant myocardial compliance in 4-hour stored donor hearts. Cardio. Surg. 1998b;6:67–75. doi: 10.1016/s0967-2109(97)00080-x. [DOI] [PubMed] [Google Scholar]
- KNERR S.M.M., LIEBERMAN M. Ion transport during hypothermia in cultured heart cells: implications for protection of the immature myocardium. J. Mol. Cell. Cardiol. 1993;25:277–288. doi: 10.1006/jmcc.1993.1034. [DOI] [PubMed] [Google Scholar]
- LEEM C.H., LAGADIC-GOSSMANN D., VAUGHAN-JONES R.D. Characterization of intracellular pH regulation in the guinea-pig ventricular myocyte. J. Physiol. 1999;517:159–180. doi: 10.1111/j.1469-7793.1999.0159z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOH S.-H., SUN B., VAUGHAN-JONES R.D. Effect of Hoe 694, a novel Na+-H+ exchange inhibitor, on intracellular pH regulation in the guinea-pig ventricular myocyte. Brit. J. Pharmacol. 1996;118:1905–1912. doi: 10.1111/j.1476-5381.1996.tb15623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOOR A.N., FLIEGEL L. Protein kinase-mediated regulation of the Na+/H+exchanger in the rat myocardium by mitogen-activated protein kinase-dependent pathways. J. Biol. Chem. 1999;274:22985–22992. doi: 10.1074/jbc.274.33.22985. [DOI] [PubMed] [Google Scholar]
- MYERS M.L., KARMAZYN M. Improved cardiac function after prolonged hypothermic ischemia with the Na+/H+ exchange inhibitor HOE 694. Ann. Thor. Surg. 1996;61:1400–1406. doi: 10.1016/0003-4975(96)00088-4. [DOI] [PubMed] [Google Scholar]
- PLESNILA N., MÜLLER E., GURETZKI S., RINGEL F., STAUB F., BAETHMANN A. Effect of hypothermia on the volume of rat glial cells. J. Physiol. 2000;523.1:155–162. doi: 10.1111/j.1469-7793.2000.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROOS A., BORON W.F. Intracellular pH. Physiol. Rev. 1981;61:296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- SHIPOLINI A.R., GALIÑANES M., EDMONDSON S.J., HEARSE D.J., AVKIRAN M. Na+/H+ exchanger inhibitor HOE-642 improves cardioplegic myocardial preservation under both normothermic and hypothermic conditions. Circulation. 1997a;96:II266–II273. [PubMed] [Google Scholar]
- SHIPOLINI A.R., YOKOYAMA H., GALIÑANES M., EDMONDSON S.J., HEARSE D.J., AVKIRAN M. Na+/H+ exchanger activity does not contribute to protection by ischemic preconditioning in the isolated rat heart. Circulation. 1997b;96:3617–3625. doi: 10.1161/01.cir.96.10.3617. [DOI] [PubMed] [Google Scholar]
- SNABAITIS A.K., YOKOYAMA H., AVKIRAN M. Roles of mitogen-activated protein kinases and protein kinase C in α1A-adrenoceptor-mediated stimulation of the sarcolemmal Na+/H+ exchanger. Circul. Res. 2000;86:214–220. doi: 10.1161/01.res.86.2.214. [DOI] [PubMed] [Google Scholar]
- SWAIN J.A., MCDONALD T.J., ROBBINS R.C., BALABAN R.S. Relationship of cerebral and myocardial intracellular pH to blood pH during hypothermia. Am. J. Physiol. Heart. Circ. Physiol. 1991;260:H1640–H1644. doi: 10.1152/ajpheart.1991.260.5.H1640. [DOI] [PubMed] [Google Scholar]
- THÉROUX P., CHAITMAN B.R., DANCHIN N., ERHARDT L.R.W., MEINERTZ T., SCHROEDER J.S., TOGNONI G., WHITE H.D., WILLERSON J.T., JESSEL A. Inhibition of the sodium-hydrogen exchanger with cariporide to prevent myocardial infarction in high-risk ischemic situations: main results of the GUARDIAN trial. Circulation. 2000;102:3032–3038. doi: 10.1161/01.cir.102.25.3032. [DOI] [PubMed] [Google Scholar]
- WAKABAYASHI S., SHIGEKAWA M., POUYSSÉGUR J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiol. Rev. 1997;77:51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- WU M.L., TSAI M.L., TSENG Y.Z. DIDS-sensitive pHi regulation in single rat cardiac myocytes in nominally HCO3-free conditions. Circul. Res. 1994;75:123–132. doi: 10.1161/01.res.75.1.123. [DOI] [PubMed] [Google Scholar]
- YAMAUCHI T., ICHIKAWA H., SAWA Y., FUKUSHIMA N., KAGISAKI K., MAEDA K., MATSUDA H., SHIRAKURA R. The contribution of Na+/H+ exchange to ischemia-reperfusion injury after hypothermic cardioplegic arrest. Ann. Thor. Surg. 1997;63:1107–1112. doi: 10.1016/s0003-4975(96)01390-2. [DOI] [PubMed] [Google Scholar]
- YASUTAKE M., HAWORTH R.S., KING A., AVKIRAN M. Thrombin activates the sarcolemmal Na+/H+ exchanger: evidence for a receptor-mediated mechanism involving protein kinase C. Circul. Res. 1996;79:705–715. doi: 10.1161/01.res.79.4.705. [DOI] [PubMed] [Google Scholar]
- YOKOYAMA H., GUNASEGARAM S., HARDING S.E., AVKIRAN M. Sarcolemmal Na+/H+ exchanger activity and expression in human ventricular myocardium. J. Am. Coll. Cardiol. 2000;36:534–540. doi: 10.1016/s0735-1097(00)00730-0. [DOI] [PubMed] [Google Scholar]
- YOKOYAMA H., YASUTAKE M., AVKIRAN M. α1-Adrenergic stimulation of sarcolemmal Na+/H+ exchanger activity in rat ventricular myocytes: evidence for selective mediation by the α1A-adrenoceptor subtype. Circul. Res. 1998;82:1078–1085. doi: 10.1161/01.res.82.10.1078. [DOI] [PubMed] [Google Scholar]
- ZHOU Z., WILLIS J.S. Differential effects of cooling in hibernator and nonhibernator cells: Na permeation. Am. J. Physiol. 1989;256:R49–R55. doi: 10.1152/ajpregu.1989.256.1.R49. [DOI] [PubMed] [Google Scholar]


