Abstract
The goal of this study was to investigate the effects of the delayed pharmacological preconditioning produced by an adenosine A1-receptor agonist (A1-DPC) against ventricular arrhythmias induced by ischaemia and reperfusion, compared to those of ischaemia-induced delayed preconditioning (I-DPC).
Eighty-nine instrumented conscious rabbits underwent a 2 consecutive days protocol. On day 1, rabbits were randomly divided into four groups: ‘Control' (saline, i.v.), ‘I-DPC' (six 4-min coronary artery occlusion/4-min reperfusion cycles), ‘A1-DPC100' (N6-cyclopentyladenosine, 100 μg kg−1, i.v.), and ‘A1-DPC400' (N6-cyclopentyladenosine, 400 μg kg−1, i.v.). On day 2, i.e., 24 h later, the incidence and severity of ventricular arrhythmias during a 30-min coronary artery occlusion and subsequent reperfusion were analysed in all animals, using an arrhythmia score.
I-DPC, A1-DPC100 and A1-DPC400 significantly reduced the infarct size (34±5, 42±3 and 43±7% of the area at risk, respectively) as compared to Control (55±3% of the area at risk).
During both ischaemia and reperfusion, neither the incidence nor the severity of ventricular arrhythmias were altered by A1-DPC100, A1-DPC400 or I-DPC as compared to Control.
Thus, despite reduction of infarct size induced by delayed preconditioning, A1-DPC as well as I-DPC failed to exert any anti-arrhythmic effect in the conscious rabbit model of ischaemia-reperfusion.
Keywords: Myocardial ischaemia, ventricular arrhythmias, delayed preconditioning, adenosine
Introduction
Brief periods of myocardial ischaemia are known to induce both an early and a delayed cardioprotection, i.e., preconditioning, against subsequent ischaemia (Murry et al., 1986; Marber et al., 1993). Ischaemia-induced delayed preconditioning (I-DPC) has been demonstrated to reduce infarct size (Marber et al., 1993; Yang et al., 1996), to limit post-ischaemia endothelial dysfunction (Kaeffer et al., 1997) and to protect against myocardial stunning (Sun et al., 1995; Shen & Vatner, 1996) but its effect against ischaemia- and reperfusion-induced arrhythmias remains controversial (Shiki & Hearse, 1987; Yamashita et al., 1998). Importantly, numerous drugs have been described to mimic this phenomenon, i.e., to induce a pharmacological delayed preconditioning. Adenosine A1- and A3-receptor agonists (Baxter et al., 1994; Baxter & Yellon, 1997; Takano et al.; 1999; Bernardo et al., 1999; Dana et al., 2000), monophosphoryl lipid A (Yao et al., 1993), NO-donors (Takano et al., 1998b) and an opioid δ1-receptor agonist (Fryer et al., 2000) exert delayed protection against myocardial infarction. Interestingly, stimulation of adenosine A1-receptor-induced delayed preconditioning (A1-DPC) can be maintained by repeated administration of an adenosine A1-receptor agonist, with no evidence of tachyphylaxis (Dana et al., 1998). Therefore, A1-DPC might represent a new approach for long-term cardioprotection. Although A1-DPC has been extensively investigated against myocardial infarction, its potential effect against another major consequence of myocardial ischaemia, i.e., ventricular arrhythmias, remains unknown.
Accordingly, the aim of the present study was to determine the effect of A1-DPC against ischaemia- and reperfusion-induced arrhythmias. We also re-evaluated the issue of I-DPC against arrhythmias. To avoid the confounding effect of numerous factors associated with the open-chest state, such as anaesthesia, hypothermia, trauma, and elevated catecholamines which interfere with arrhythmogenesis, we performed this study in a model of conscious chronically instrumented rabbits.
Methods
The animal instrumentation and the ensuing experiments were performed in accordance with the official regulations edicted by the French Ministry of Agriculture (Agreement # A94-043-12).
Animal surgery
Male New Zealand white rabbits (2 – 2.5 kg) were anaesthetized with a mixture of tiletamine (25 mg kg−1, i.v.) and zolazepam (25 mg kg−1, i.v.), intubated and mechanically ventilated with 100% oxygen via a positive pressure respirator. Anaesthesia was maintained with pentobarbitone sodium (20 to 30 mg kg−1, i.v.). An external electrocardiogram (ECG) was recorded during the surgery. The ventilation rate was 25 breaths per minute, and the tidal volume was approximately 25 ml. A left thoracotomy was performed in the fourth intercostal space under sterile conditions. A pneumatic occluder fashioned from 18-gauge Tygon tubing was implanted around a major branch of the left coronary artery according to a technique previously described by Cohen et al. (1994). Proper functioning of the occluder was confirmed by observing cyanosis of the distal myocardium and ST-segment deviation of the ECG after a brief inflation of the occluder. Conversely, hyperemia and normalization of the ECG were noticed after its deflation. The chest was closed in layers and a small tube was left in the thorax to evacuate air and fluids after surgery. Internal ECG leads were attached to intercostal muscles. The occluder and internal ECG wires were exteriorized between the scapulae. During the post-operative period, rabbits were treated for 3 days with buprenorphine (0.02 mg kg−1, s.c.) and flunixine meglumate (1 mg kg−1, i.m.) for analgesia. Gentamycin (0.5 mg kg−1, i.m.) was also administered during 5 consecutive days. Rabbits were allowed to recover for a minimum of 10 days after surgery.
Experimental protocol
Throughout the experiment, rabbits were conscious and kept in a box in a quiet, dimly lit room. The internal ECG wires were connected to an amplifier (Gould Instruments Inc., Cleveland, OH, U.S.A.). An intra-arterial catheter was introduced into the ear artery and arterial pressure was measured using a Statham P23ID strain gauge transducer (Statham Instruments, Oxnard, CA, U.S.A.). ECG and arterial pressure were recorded on a multichannel oscillograph (DMS 1000, Graphtec, Vanderbilt, U.S.A.).
Eighty-nine rabbits were randomly assigned to one of four groups: Control, I-DPC, A1-DPC100 and A1-DPC400. The protocol was realized during 2 consecutive days, i.e., 24 h apart as illustrated in Figure 1. On day 1, the Control, A1-DPC100 and A1-DPC400 groups received an intravenous (ear vein) bolus injection of saline (5 ml), 100 and 400 μg kg−1 CPA (N6-cyclopentyladenosine, Sigma Aldrich, Steiheim, Germany), respectively. The doses of CPA were chosen in a preliminary study on the basis of the decrease in mean arterial pressure induced by the drug in eight rabbits investigated in the conscious state (data not shown). The dose of 100 μg kg−1 was the ED50 of CPA whereas the dose of 400 μg kg−1 was the ED80, i.e., the highest dose haemodynamically well tolerated. The I-DPC group underwent a sequence of six 4-min coronary artery occlusion (CAO)/4-min reperfusion cycles (Takano et al., 1998a). CAO was induced by manually inflating the balloon occluder and was confirmed by ST-segment deviation on the ECG. On day 2, all animals underwent a 30-min CAO followed by reperfusion. All animals received diazepam (1 mg kg−1 i.v.) 10 min before CAO. If ventricular fibrillation occurred, no defibrillation was attempted and rabbits were rapidly sacrificed.
Figure 1.
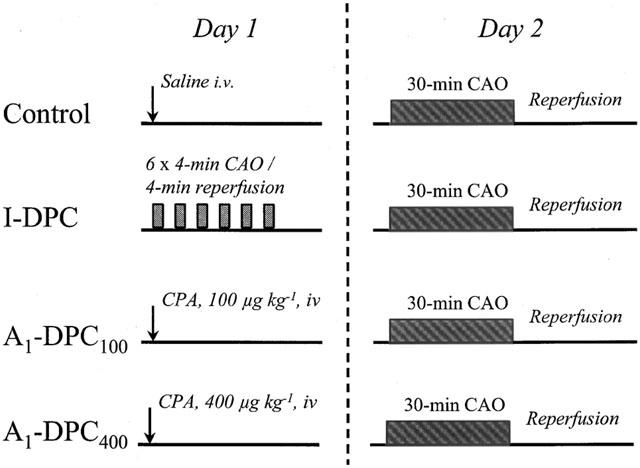
Experimental protocol. (I-DPC, ischaemia-induced delayed preconditioning; A1-DPC100, CPA-induced delayed preconditioning, 100 μg kg−1; A1-DPC400, CPA-induced delayed preconditioning, 400 μg kg−1; CAO, coronary artery occlusion; CPA, N6-cyclopentyladenosine).
Determination of arrhythmia scores
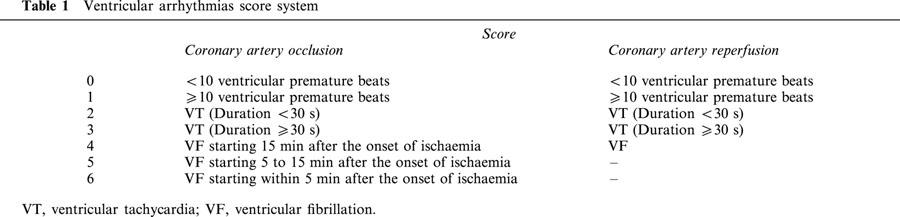
During the 30-min CAO, arrhythmias were quantitated by using a scoring system adapted from Curtis & Walker (1988) and Murphy & Murphy (1999). It assigned one score per rabbit representing the most severe type of arrhythmia observed during CAO. Ventricular premature beats were defined as identifiable premature QRS complexes. Ventricular tachycardia (VT) was defined as a run of four or more ventricular premature beats and ventricular fibrillation (VF) was defined as a signal for which individual QRS deflections can no longer be distinguished from one other and for which a rate can no longer be measured (Walker et al., 1988). Original ECG recordings are illustrated in Figure 2. Arrhythmia scores were assigned as summarized in Table 1. A similar scoring system was used to quantify arrhythmias during the first hour of reperfusion.
Figure 2.
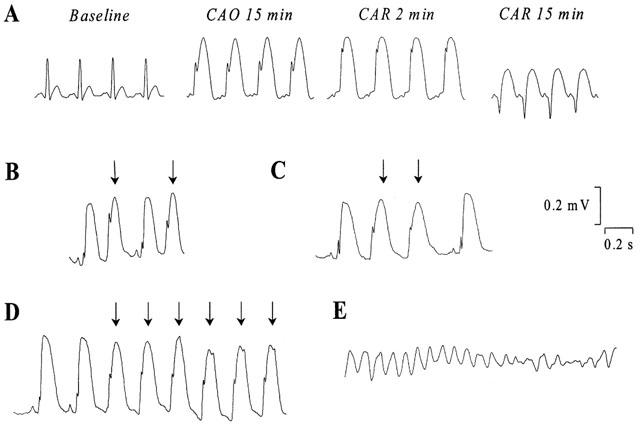
Representative electrocardiogram recordings (A) at baseline, during coronary artery occlusion and reperfusion in a rabbit with normal rhythm, (B) of ventricular premature beats (indicated by the arrows), (C) of salvos of two ventricular premature beats, (D) of ventricular tachycardia and (E) of ventricular fibrillation. (CAO, coronary artery occlusion; CAR, coronary artery reperfusion).
Table 1.
Ventricular arrhythmias score system
Determination of myocardial area at risk and infarct size
Thirty-two rabbits were randomly selected among the surviving animals of the four groups (11 out of 22 Control, six out of 12 I-DPC, seven out of 18 A1-DPC100 and seven out of nine A1-DPC400) to measure infarct size and to verify that I-DPC and A1-DPC were able to reduce infarct size as previously described (Baxter et al., 1994; Takano et al., 1998a). After completion of a 3 h-reperfusion, animals received an intravenous injection of heparin (5000 I.U.), were re-anaesthetized with sodium pentobarbitone (30 mg kg−1) and potassium chloride was administered to induce cardiac arrest. The hearts were excised and the ascending aorta was cannulated and perfused (120 mmHg) retrogradely with 50 ml saline followed by Evans blue (1%). The right ventricle was then removed and the left ventricle was cut into 3-mm slices. These slices were weighed and incubated in 1% triphenyltetrazolium chloride (TTC, Sigma, Poole, U.K.) in pH 7.4 buffer during 15 min at 37°C to identify the infarcted myocardium. Slices were overnight fixed in 10% formaldehyde and then photographed with a digital camera. Using a computerized planimetric program (Scion Image, Scion Corporation, Frederick, MD, U.S.A.), the area at risk and the infarcted zones were measured. The area at risk was identified as the non-blue region and was expressed as a percentage of the weight of the left ventricle. Infarcted area was identified as the TTC negative zone and infarct size was expressed as a percentage of the area at risk.
Data analysis
Data are reported as mean±s.e.mean. The effects of saline, CPA (100 μg kg−1 or 400 μg kg−1) and I-DPC on heart rate and mean arterial pressure were analysed on day 1 by a paired Student's t-test. On day 2, since the number of rabbits was not similar at the different times of the protocol, comparisons were made only between the four groups. Infarct size and area at risk were compared using a one-way ANOVA and post-hoc Fisher's PLSD test if necessary. The arrhythmia scores were analysed with Kruskall Wallis test. The incidences of VT and VF were compared with a Chi-square test. Significant differences were determined as P<0.05.
Results
Haemodynamics
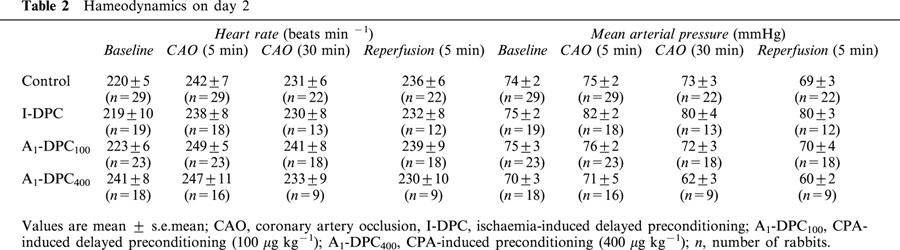
Baseline values of heart rate and mean arterial pressure were not significantly different between groups at day 1 (heart rate: 209±8, 210±9, 208±6 and 216±7 beats/min and mean arterial pressure: 73±3, 77±2, 75±2 and 70±4 mmHg for Control, I-DPC, A1-DPC100 and A1-DPC400, respectively). On day 1, intravenous injection of saline did not alter heart rate and mean arterial pressure in Control (data not shown). In I-DPC, the ischaemic preconditioning protocol induced a significant increase in heart rate during each CAO as compared to baseline (e.g., +13±3% during the last CAO) but mean arterial pressure did not change. In A1-DPC100 and A1-DPC400, CPA decreased both heart rate (−16±2 and −13±3%, respectively) and mean arterial pressure (−31±2 and −40±2%, respectively) as compared to baseline. On day 2 (Table 2), heart rate and mean arterial pressure were not significantly different between the four groups at baseline, during CAO and reperfusion.
Table 2.
Hameodynamics on day 2
Infarct size
Areas at risk measured in Control, I-DPC, A1-DPC100 and A1-DPC400 were similar (27±2, 28±5, 28±3 and 27±5% respectively). Both I-DPC, A1-DPC100 and A1-DPC400 reduced the infarct size (34±5, 42±3, 43±7% respectively) as compared to Control (55±3%, P<0.05).
Arrhythmias during coronary artery occlusion
Table 3 shows the incidence of VT and VF during CAO. No overall significant differences were found between the four groups. Isolated VT, i.e. not followed by VF, was observed in only two rabbits in Control (one of them had haemodynamic intolerance leading to coma) and two rabbits in A1-DPC400. One Control rabbit died from bradycardia and cardiac arrest (without any preliminar ventricular premature beats). VF occurred in 25 out of the 89 rabbits and its incidence tended to increase with A1-DPC400. All VF were preceded by VT.
Table 3.
Ventricular arrhythmias during coronary artery occlusion
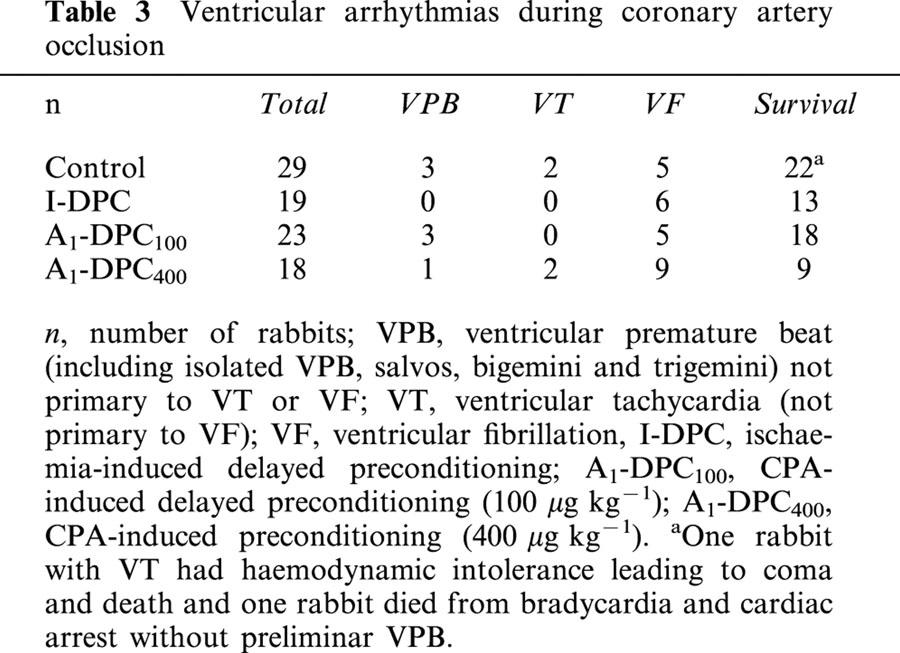
Figure 2 illustrates the distribution of arrhythmia scores for Control, I-DPC, A1-DPC100 and A1-DPC400 during CAO. No overall significant difference in the average arrhythmia scores were found between the four groups (1.1±0.3, 1.5±0.5, 1.1±0.4 and 2.9±0.6 for Control, I-DPC, A1-DPC100 and A1-DPC400, respectively) although it tended to increase in A1-DPC400.
Arrhythmias during coronary artery reperfusion
Table 4 shows the incidence of VT and VF during coronary artery reperfusion. No significant differences were found between the four groups. In contrast to CAO, more than 70% of rabbits underwent ventricular arrhythmias during reperfusion. Isolated VT, i.e. not followed by VF, was observed in 11 out of 22 rabbits in Control, six out of 13 in I-DPC, eight out of 18 in A1-DPC100 and five out of nine in A1-DPC400. Only one rabbit underwent VF in the I-DPC group. Most of the arrhythmias started during the first 5 min of reperfusion.
Table 4.
Ventricular arrhythmias during reperfusion
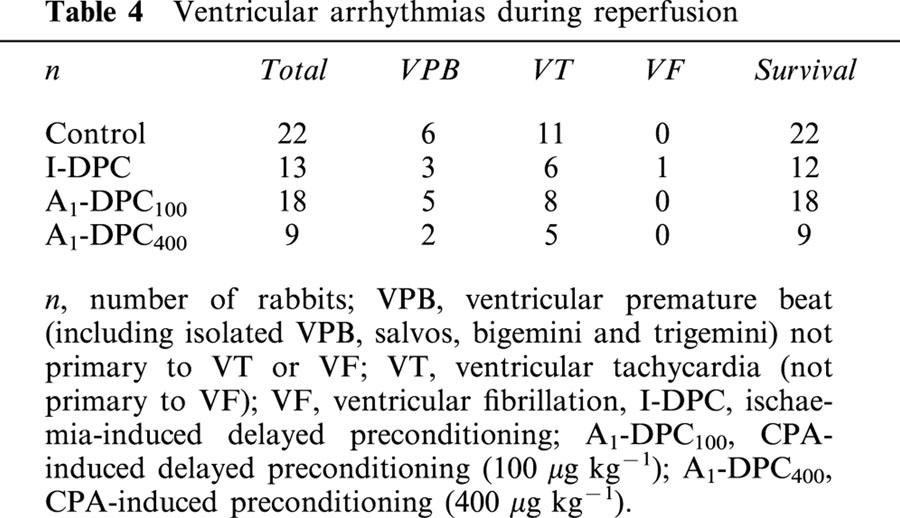
Figure 3 illustrates the distribution of arrhythmia scores for Control, I-DPC, A1-DPC100 and A1-DPC400 during reperfusion. No significant differences were observed between the four groups and the average arrhythmia scores were similar (1.5±0.2, 1.8±0.4, 1.4±0.3 and 1.4±0.3 for Control, I-DPC, A1-DPC100 and A1-DPC400, respectively).
Figure 3.
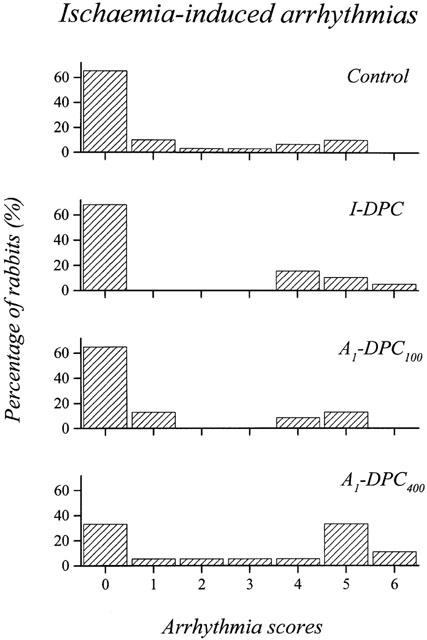
Distribution of the arrhythmia scores during the 30-min coronary artery occlusion. (I-DPC, ischaemia-induced delayed preconditioning; A1-DPC100, CPA-induced delayed preconditioning, 100 μg kg−1; A1-DPC400, CPA-induced delayed preconditioning, 400 μg kg−1).
Discussion
Arrhythmias are one of the major deleterious consequences of prolonged myocardial ischaemia and subsequent reperfusion. Regarding the fact that A1-DPC and I-DPC significantly reduced infarct size, we expected that both interventions would also be able to elicit anti-arrhythmic properties, but surprisingly none of these exerted such a protection. To our knowledge, this study is the first to extensively investigate the relationship between A1-DPC and ventricular arrhythmias in conscious rabbits. Events that occurred during both CAO and subsequent reperfusion were described and quantified using an arrhythmia score adapted from Curtis & Walker (1988) according to the Lambeth conventions (Walker et al., 1988). This approach, extensively used to investigate cardioprotective agents (Murphy & Murphy, 1999; Fryer et al., 2000), offers the advantage of improving the sensitivity of the global analysis, mainly by taking into account critical components of the severity of arrhythmias, i.e., the duration of VT and the time to onset of CAO-induced VF.
Arrhythmias occurred during the two periods of the protocol, i.e., CAO and reperfusion. During CAO, the main arrhythmia was VF. The present study conducted in conscious chronically instrumented rabbits clearly demonstrates that VF incidence was not significantly decreased by A1-DPC (5/23 and 9/18 for A1-DPC100 and A1-DPC400, respectively) as compared to Control (5/29). Furthermore, both VF incidence and arrhythmia scores, although the overall difference was not significant, tended to increase in A1-DPC400 (the highest dose usable in our experimental conditions) suggesting that the lack of delayed cardioprotection against arrhythmias observed with CPA in our study was not related to an insufficient dose. Previous studies investigating A1-DPC focused mainly on infarct-limiting effects (Baxter et al., 1994; Baxter & Yellon, 1997) but anti-arrhythmic properties were not in their scopes. In these studies, only less than 5% of severe and persistent VF were reported. Under these conditions, the incidence of ischaemia-induced reported VF was not high enough to allow any precise comparison between A1-DPC and Control rabbits. Similarly, our results demonstrate that I-DPC also fails to reduce the occurrence of arrhythmias during CAO. Although the difference did not reach statistical significance, Yang et al. (1996) demonstrated that the incidence of VF tended to be lower in I-DPC conscious rabbits (0/7) as compared to control animals (3/7). This result was not confirmed by the same group in a further study involving a larger number of rabbits (Miki et al., 1999). Using similar experimental conditions, Takano et al. (2000) demonstrated that the incidence of VF during a 30-min CAO was not significantly reduced by I-DPC. In contrast, I-DPC has been demonstrated to be protective in rats (Yamashita et al., 1998) but species differences could explain such a discrepancy as previously suggested in another study (Huang et al., 1999).
The reperfusion-induced arrhythmias were also investigated in the present study. In contrast to ischaemia-induced arrhythmias, the incidence of VF was very low (1/62 rabbits) but that of other events such as ventricular premature beats or VT was high (6 and 11/22 rabbits in Control, respectively). A1-DPC as well as I-DPC were not able to significantly induce any qualitative or quantitative cardioprotection against these arrhythmias during early reperfusion. Shiki & Hearse (1987) and Qiu et al. (1997) previously reported similar negative results in anaesthetized rats and conscious pigs, respectively.
Importantly, single intravenous administration of a selective A1-receptor agonist at the two investigated doses produced a delayed reduction in infarct size in the present model of conscious rabbits, in agreement with previous investigations performed in open-chest rabbits (Baxter et al., 1994; Baxter & Yellon, 1997). Interestingly, the two doses of CPA used in this study similarly decreased infarct size after 3 h of reperfusion, suggesting that 100 μg kg−1 i.v. induces the maximal delayed cardioprotective effect against infarction in our model. This protection against infarction was rather mild in our experimental conditions as compared to that reported in previous studies using another adenosine A1-receptor agonist but differences in rabbits' strains might account for such a difference as previously demonstrated in mice (Bao et al., 2000). Furthermore, it is reasonable to consider that both 100 and 400 μg kg−1 CPA infusions did not induce transient global ischaemia (31±2 and 40±2% decrease in blood pressure, respectively, with a decrease in heart rate) that might have served as a trigger for a delayed preconditioning response. Our I-DPC protocol (six consecutive 4-min CAO/4-min reperfusion cycles) was effective at inducing a delayed protection against myocardial infarction, as previously described (Ping et al., 1999; Takano et al., 2000). This suggests that the protection against arrhythmias by A1-DPC and I-DPC either does not exist or, if present, is weaker than that against myocardial infarction. Such a discrepancy between a protection against infarction and a lack of effect against arrhythmias has already been described with both ischaemic and adenosine A1-receptor agonist-induced early preconditionings (Huang et al., 1999). However, our results do not exclude the possibility that other pharmacologically-induced delayed preconditioning procedures already known for reducing infarct size (Yao et al., 1993; Fryer et al., 1999) might exert cardioprotective effects against arrhythmias. Indeed, opioid δ1-receptor agonists (Fryer et al., 2000) significantly reduced ischaemia- and reperfusion-induced arrhythmias. The monophosphoryl lipid A demonstrated similar beneficial effects (György et al., 1999; Vegh et al., 1999) by inducing a prolongation of the ventricular refractoriness (Szilvassy et al., 1998). These apparent discrepancies might be explained by the fact that different mechanisms are involved in the different types of preconditioning (Bolli, 2000), e.g., ATP-sensitive potassium channels play an obligatory role in I-DPC against infarction but not against stunning (Takano et al., 2000). These results suggest that in order to avoid inappropriate generalization of a concept, any delayed pharmacological preconditioning approach should be specifically investigated for its potential anti-arrhythmic, anti-infarct or anti-stunning effects.
In conclusion, the present study demonstrates that A1-DPC failed to protect the heart against both ischaemia- and reperfusion-induced arrhythmias in chronically instrumented conscious rabbits.
Figure 4.
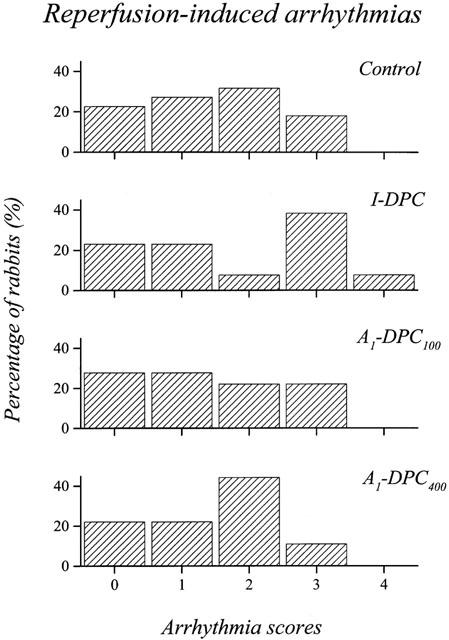
Distribution of the arrhythmia scores during the first hour of reperfusion. (I-DPC, ischaemia-induced delayed preconditioning; A1-DPC100, CPA-induced delayed preconditioning, 100 μg kg−1; A1-DPC400, CPA-induced delayed preconditioning, 400 μg kg−1).
Acknowledgments
Renaud Tissier was a recipient from the Académie Française de Médecine. This study was supported by a grant from the Fondation de l'Avenir (ETO-293). The authors are greatly indebted to Alain Bizé and Dominique Caillaud for their excellent technical support as well as Stéphane Bloquet for his cautious animal care.
Abbreviations
- A1-DPC
adenosine A1-receptor-induced delayed preconditioning (A1-DPC100 and A1-DPC400: CPA was administered at 100 and 400 μg kg−1, respectively)
- CAO
coronary artery occlusion
- CPA
N6-cyclopentyladenosine
- ECG
electrocardiogram
- I-DPC
ischaemia-induced delayed preconditioning
- VF
ventricular fibrillation
- VT
ventricular tachycardia
References
- BAO W., GUO Y., WU W.J., TANG X.L. Both ischemic injury and preconditioning in mice are strain dependent. Circulation. 2000;102:77. [Google Scholar]
- BAXTER G.F., YELLON D.M. Time course of delayed myocardial protection after transient adenosine A1-receptor activation in the rabbit. J. Cardiovasc. Pharmacol. 1997;29:631–638. doi: 10.1097/00005344-199705000-00011. [DOI] [PubMed] [Google Scholar]
- BAXTER G.F., MARBER M.S., PATEL V.C., YELLON D.M. Adenosine receptor involvement in a delayed phase of myocardial protection 24 hours after ischemic preconditioning. Circulation. 1994;90:2993–3000. doi: 10.1161/01.cir.90.6.2993. [DOI] [PubMed] [Google Scholar]
- BERNARDO N.L., OKUBO S., MAAIEH M.M., WOOD M.A., KUKREJA R.C. Delayed preconditioning with adenosine is mediated by opening of ATP-sensitive K+ channels in rabbit heart. Am. J. Physiol. 1999;277:H128–H135. doi: 10.1152/ajpheart.1999.277.1.H128. [DOI] [PubMed] [Google Scholar]
- BOLLI R. The late phase of preconditioning. Circ. Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- COHEN M.V., YANG X.M., LIU Y., SNELL K.S., DOWNEY D.M. A new animal model of controlled coronary artery occlusion in conscious rabbits. Cardiovasc. Res. 1994;28:62–65. doi: 10.1093/cvr/28.1.61. [DOI] [PubMed] [Google Scholar]
- CURTIS M.J., WALKER M.J. Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc. Res. 1988;22:656–665. doi: 10.1093/cvr/22.9.656. [DOI] [PubMed] [Google Scholar]
- DANA A., BAXTER G.F., WALKER M., YELLON D.M. Prolonging the delayed phase of myocardial protection: repetitive adenosine A1 receptor activation maintains rabbit myocardium in a preconditioned state. J. Am. Coll. Cardiol. 1998;31:1142–1149. doi: 10.1016/s0735-1097(98)00054-0. [DOI] [PubMed] [Google Scholar]
- DANA A., SKARLI M., PAPAKRIVOPOULOU J., YELLON D.M. Adenosine A1 receptor induced delayed preconditioning in rabbits. Induction of p38 mitogen-activated protein kinase activation and Hsp27 phosphorylation via a tyrosine kinase- and protein kinase C-dependent mechanism. Circ. Res. 2000;86:989–997. doi: 10.1161/01.res.86.9.989. [DOI] [PubMed] [Google Scholar]
- FRYER R.M., HSU A.K., EELLS J.T., NAGASE H., GROSS G.J. Opioid-induced second window of cardioprotection: potential role of mitochondrial KATP channels. Circ. Res. 1999;84:846–851. doi: 10.1161/01.res.84.7.846. [DOI] [PubMed] [Google Scholar]
- FRYER R.M., HSU A.K., NAGASE H., GROSS G.J. Opioid-induced cardioprotection against myocardial infarction and arrhythmias: mitochondrial versus sarcolemmal ATP-sensitive potassium channels. J. Pharmacol. Exp. Ther. 2000;294:451–457. [PubMed] [Google Scholar]
- GYÖRGY K., MULLER B., VEGH A., KLESCHOYV A.L., STOCLET J.C. Triggering role of nitric oxide in the delayed protective effect of monophosphoryl lipid A in rat heart. Br. J. Pharmacol. 1999;127:1892–1898. doi: 10.1038/sj.bjp.0702725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG C.H., KIM S.J., GHALEH B., KUDEJ R.K., SHEN Y.T., BISHOP S.P., VATNER S.F. An adenosine agonist and preconditioning shift the distribution of myocardial blood flow in conscious pigs. Am. J. Physiol. 1999;276:H358–H375. doi: 10.1152/ajpheart.1999.276.2.H368. [DOI] [PubMed] [Google Scholar]
- KAEFFER N., RICHARD V., THUILLEZ C. Delayed coronary endothelial protection 24 hours after preconditioning: role of free radicals. Circulation. 1997;96:2311–2316. doi: 10.1161/01.cir.96.7.2311. [DOI] [PubMed] [Google Scholar]
- MARBER M.S., LATCHMAN D.S., WALKER J.M., YELLON D.M. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- MIKI T., SWAFFORD A.N., COHEN M.V., DOWNEY J.M. Second window of protection against infarction in conscious rabbits: real or artifactual. J. Mol. Cell. Cardiol. 1999;314:809–816. doi: 10.1006/jmcc.1998.0917. [DOI] [PubMed] [Google Scholar]
- MURPHY D.B., MURPHY M.B. Opioid antagonist modulation of ischemia-induced ventricular arrhythmias: a peripheral mechanism. J. Cardiovasc. Pharmacol. 1999;33:122–125. doi: 10.1097/00005344-199901000-00018. [DOI] [PubMed] [Google Scholar]
- MURRY C.E., JENNINGS R.B., REIMER K.A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- PING P., TAKANO H., ZHANG J., TANG X.L., QIU Y., LI R.C., BANERJEE S., DAWN B., BALAFONOVA Z., BOLLI R. Isoform-selective activation of protein kinase C by nitric oxide in the heart of conscious rabbits: a signaling mechanism for both nitric oxide-induced and ischemia-induced preconditioning. Circ. Res. 1999;84:587–604. doi: 10.1161/01.res.84.5.587. [DOI] [PubMed] [Google Scholar]
- QIU Y., TANG X.L., PARK S.W., SUN J.Z., KALYA A., BOLLI R. The early and late phases of ischemic preconditioning: a comparative analysis of their effects on infarct size, myocardial stunning, and arrhythmias in conscious pigs undergoing a 40-minute coronary occlusion. Circ. Res. 1997;80:730–742. doi: 10.1161/01.res.80.5.730. [DOI] [PubMed] [Google Scholar]
- SHEN Y.T., VATNER S.F. Differences in myocardial stunning following coronary artery occlusion in conscious dogs, pigs, and baboons. Am. J. Physiol. 1996;270:H1312–H1322. doi: 10.1152/ajpheart.1996.270.4.H1312. [DOI] [PubMed] [Google Scholar]
- SHIKI K., HEARSE D.J. Preconditioning of ischemic myocardium: reperfusion-induced arrhythmias. Am. J. Physiol. 1987;253:H1470–H1476. doi: 10.1152/ajpheart.1987.253.6.H1470. [DOI] [PubMed] [Google Scholar]
- SZILVASSY Z., FERDINANDY P., CLUFF C.W., ELLIOTT G.T. Antiischemic effects of monophosphoryl lipid A in conscious rabbits with hypercholesterolemia and atherosclerosis. J. Cardiovasc. Pharmacol. 1998;32:206–212. doi: 10.1097/00005344-199808000-00006. [DOI] [PubMed] [Google Scholar]
- SUN J.Z., TANG X.L., KNOWLTON A.A., PARK S.W., QIU Y., BOLLI R. Late preconditioning against myocardial stunning. An endogenous protective mechanism that confers resistance to postischemic dysfunction 24 h after brief ischemia in conscious pigs. J. Clin. Invest. 1995;95:388–403. doi: 10.1172/JCI117667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKANO H., BOLLI R., TANG X.L., YANG Z., BLACK R., AUCHAMPACH J.A. Activation of A1 or A3 receptors produces delayed protection against infarction in conscious rabbits by a mechanism involving KATP channels. Circulation. 1999;100:156. [Google Scholar]
- TAKANO H., MANCHIKALAPUDI S., TANG X.L., QIU Y., RIZVI A., JADOON A.K., ZHANG Q., BOLLI R. Nitric oxide synthase is the mediator of late preconditioning against myocardial infarction in conscious rabbits. Circulation. 1998a;98:441–449. doi: 10.1161/01.cir.98.5.441. [DOI] [PubMed] [Google Scholar]
- TAKANO H., TANG X.L., BOLLI R. Differential role of K(ATP) channels in late preconditioning against myocardial stunning and infarction in rabbits. Am. J. Physiol. 2000;279:H2350–H2359. doi: 10.1152/ajpheart.2000.279.5.H2350. [DOI] [PubMed] [Google Scholar]
- TAKANO H., TANG X.L., QIU Y., GUO Y., FRENCH B.A., BOLLI R. Nitric oxide donors induce late preconditioning against myocardial stunning and infarction in conscious rabbits via an antioxidant-sensitive mechanism. Circ. Res. 1998b;83:73–84. doi: 10.1161/01.res.83.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEGH A., GYORGY K., RASTEGAR M.A., PAPP J.G., PARRATT J.R. Delayed protection against ventricular arrhythmias by monophosphoryl lipid-A in a canine model of ischaemia and reperfusion. Eur. J. Pharmacol. 1999;382:81–90. doi: 10.1016/s0014-2999(99)00557-9. [DOI] [PubMed] [Google Scholar]
- WALKER M.J., CURTIS M.J., HEARSE D.J., CAMPBELL R.W., JANSE M.J., YELLON D.M., COBBE S.M., COKER S.J., HARNESS J.B., HARRON D.W., HIGGINS A.J., JULIAN D.G., LAB M.J., MANNING A.S., NORTHOVER B.J., PARRATT J.R., RIEMERSMA R.A., RIVA E., RUSSEL D.C., SHERIDAN D.J., WINSLOW B., WOODWARD B. The Lambeth conventions: guidelines for the study of arrhythmias in ischemia, infarction, and reperfusion. Cardiovasc. Res. 1988;22:447–455. doi: 10.1093/cvr/22.7.447. [DOI] [PubMed] [Google Scholar]
- YAMASHITA N., HOSHIDA S., TANIGUCHI N., KUZUYA T., HORI M. A ‘second window of protection' occurs 24 h after ischemic preconditioning in the rat heart. J. Mol. Cell. Cardiol. 1998;30:1181–1189. doi: 10.1006/jmcc.1998.0682. [DOI] [PubMed] [Google Scholar]
- YANG X.M., BAXTER G.F., HEADS R.J., YELLON D.M., DOWNEY J.M., COHEN M.V. Infarct limitation of the second window of protection in a conscious rabbit model. Cardiovasc. Res. 1996;31:777–783. doi: 10.1016/0008-6363(96)00026-0. [DOI] [PubMed] [Google Scholar]
- YAO Z., RASMUSSEN J.L., HIRT J.L., MEI D.A., PIEPER G.M., GROSS G.J. Effects of monophosphoryl lipid A on myocardial ischemia/reperfusion injury in dogs. J. Cardiovasc. Pharmacol. 1993;22:653–663. doi: 10.1097/00005344-199310000-00021. [DOI] [PubMed] [Google Scholar]


