Abstract
Orexin A and B, recently identified in the rat hypothalamus are endogenous neuropeptide agonists for the G-protein coupled orexin-1 (OX1) and orexin-2 (OX2) receptors.
In the present study, we have examined the effects of orexin A, B and raised extracellular K+ on noradrenaline release from the rat cerebrocortical slice. We have compared this with other sleep – wake-related (excitatory) neurotransmitters; dopamine, glutamate, serotonin and histamine.
Neurotransmitter release studies were performed in rat cerebrocortical slices incubated in modified Krebs buffer (with and without Ca2++EGTA 1 mM) with various concentrations of orexin A, B and K+ for various times.
Orexin A and B-evoked (10−7 M) noradrenaline release was time-dependent reaching a maximum some 10 min after stimulation. K+ (40 mM) evoked release was also time dependent but reached a maximum after 6 min. Orexin A, B and K+ stimulation of release was concentration dependent with pEC50 and Emax (% of basal) values of 8.74±0.32 (1.8 nM) and 263±14% and 8.61±0.38 (2.4 nM) and 173±7% and 1.43±0.02 (37 mM) and 1430±70%, respectively. Orexin-evoked release was partially extracellular Ca2+ dependent.
Of the other transmitters studied there was a weak orexin A and B stimulation of glutamate release. In contrast K+ evoked dopamine, glutamate, histamine and serotonin release with pEC50 and Emax (% of basal) values of 1.47±0.05 (34 mM) and 3430±410%, 1.38±0.04 (42 mM) and 1240±50%, 1.47±0.02 (34 mM) and 480±10% and 1.40±0.05 (40 mM) and 560±60% respectively.
We conclude that the neuropeptides orexin A and B evoke noradrenaline release from rat cerebrocortical slices.
Keywords: Orexin, noradrenaline, rat cerebrocortical slices
Introduction
Orexin A and B, recently identified in the rat hypothalamus (Sakurai et al., 1998) are endogenous neuropeptide agonists for the G-protein coupled orexin-1 (OX1) and orexin-2 (OX2) receptors (Sakurai et al., 1998). Radioligand binding studies (Van den Pol et al., 1998) indicated that orexin A has equal affinity for OX1 and OX2, while orexin B has a higher affinity for OX2. In addition, Northern blot analysis and in situ hybridization studies suggest that orexins and their receptors are widely distributed in the brain (Trivedi et al., 1998; Peyron et al., 1998).
These neuropeptides and their receptors have been implicated in a range of physiological responses including control of the sleep – wake cycle and sympathetic tone (Peyron et al., 1998). Several reports (Hagan et al., 1999; Horvath et al., 1999) have demonstrated that orexins activate the locus coeruleus noradrenergic system and this activation may increase arousal and locomotor activity. Moreover, Chemelli et al. (1999) reported that orexin knockout mice exhibit a phenotype strikingly similar to human narcolepsy patients. In agreement with this observation Lin et al. (1999) found that canine narcolepsy results from a disruption of the orexin receptor 2 gene. Noradrenergic neurons have been suggested to contribute to sleep – wake cycle (Hagan et al., 1999) as neuronal activity increases and decreases during wake and sleep, respectively. Thus, orexin-modulation of noradrenergic neurons may contribute to sleep – wake cycle.
Cardiovascular effects of orexins have also been reported (Shirasaka et al., 1999; Samson et al., 1999; Chen et al., 2000). Shirasaka et al. (1999) showed that intracerebroventricular administration of orexin A and B increases arterial pressure, heart rate and renal sympathetic nerve activity with elevation of plasma noradrenaline in conscious rats. Chen et al. (2000) also reported that intracisternal injection of orexin A and B dose-dependently increased arterial pressure and heart rate in urethane-anaesthetized rats and similar data were reported by Samson et al. (1999). Collectively these data suggest orexin modulation of noradrenergic neurons.
In the present study, we have examined the effects of orexin A and B on noradrenaline release from rat cerebrocortical slices. In order to determine whether any modulation of release is selective we have also measured a range of other excitatory neurotransmitters including dopamine, glutamate, serotonin and histamine and have used a K+ depolarizing stimulus as a positive control. We report that orexin A and B increase cerebrocortical noradrenaline release.
Methods
Materials
Orexin A (human: Pyr-Pro-Leu-Pro-Asp-Cys-Cys-Arg-Gln-Lys-Thr-Cys-Ser-Cys-Arg-Leu-Tyr-Glu-Leu-Leu-His-Gly-Ala-Gly-Asn-His-Ala-Ala-Gly-Ile-Leu-Thr-Leu-NH2) and B (rat: Arg-Pro-Gly-Pro-Pro-Gly-Leu-Gln-Gly-Arg-Leu-Gln-Arg-Leu-Leu-Gln-Ala-Asn-Gly-Asn-His-Ala-Ala-Gly-Ile-Leu-Thr-Met-NH2) were purchased from Peptide Institute Inc (Osaka, Japan). Pargyline and nomifensin were from Sigma, HEPES from Dojin Laboratories (Kumamoto, Japan). Glutamate dehydrogenase was from Oriental Yeast (Suita, Japan). All other chemicals used were of the highest quality available.
Cerebrocortical slice preparation
Male Wistar rats (250 – 300 g) were decapitated, the brains quickly removed and immersed in ice-cold Krebs-HEPES buffer solution (KRH) of the following composition (in mM): NaCl 133, KCl 4.8, KH2PO4 1.2, MgSO4 1.2, CaCl2 1.5, glucose 11.1, HEPES 10, pH 7.4 oxygenated with 95% O2. Cerebrocortical tissue was dissected from its internal structures and cross-chopped using a tissue chopper to produce slices of 350×350 μm. The slices were then washed three times in ice-cold KRH and transferred (1 ml aliquots of slices: equivalent to about 7 mg tissue) to polypropylene tubes. Cerebrocortical slices from one rat were used for one experiment (i.e., one concentration-response curve or time course for an agent was constructed).
Orexin-evoked neurotransmitter release
Noradrenaline and dopamine
After discarding the supernatant, the slices were resuspended in 1 ml of fresh KRH and incubated for 10 min at 37°C. This procedure was repeated to obtain a stable baseline. Immediately following this second incubation, the slices were resuspended (1 ml of KRH or Ca2+ free-KRH with EGTA 1 mM) and incubated for 0 – 14 min in the absence (basal release) and presence of 10−7 M orexin A or B (evoked release). In some experiments slices were incubated for a fixed time of 10 min with KRH containing 10−12 – 10−6 M orexin A or B in order to obtain an orexin A- or B-evoked release concentration response curve. All buffers used in release studies contained the monoamine oxidase inhibitor, pargyline (10 μM), and the reuptake inhibitor, nomifensin (10 μM). Monoamine contents in the release samples were determined directly by high-performance liquid chromatography with electrochemical detection (ESA Coulochem Model 5100A). Briefly, 20 μl aliquots of acidified (perchloric acid) release samples were injected onto a reverse-phase column (C18, 4.6×150 mm, MC Medical, Tokyo, Japan). Monoamines were separated using a mobile phase buffer consisting of 0.05 M NaH2PO4; 0.05 M CCl3COOH; 0.7 mM CH3(CH2)11OSO3Na; 0.02 mM EDTA2Na, 85; Acetonitrile 10, Methanol 5, pH 3.4 at a flow rate of 1 ml min−1 at 40°C and quantified using an electrochemical detector at 300 mV (optimum voltage for oxidation). Retention time under these conditions for noradrenaline and dopamine was 6.3 and 15.2 min respectively. The intra-assay maximal coefficient of variation was 3.3% for noradrenaline and 6.5% for dopamine.
Glutamate, serotonin and histamine
After discarding the supernatant, slices were resuspended in 1 ml of fresh oxygenated KRH and incubated for 10 min at 37°C. This procedure was repeated to obtain a stable baseline. Immediately following this second incubation, the slices were resuspended (1 ml KRH) and incubated for 10 min in the absence (basal release) and presence of 10−10 – 10−6 M orexin A or B (evoked release) in order to obtain an orexin A or B-evoked release concentration response curve. Incubation times as for noradrenaline release above were used for all other neurotransmitters. Glutamate contents in the release samples were determined by a glutamate dehydrogenase-coupled assay. Five hundred μl aliquots of release sample were mixed with 50 μl of NADP (nicotinamide adenine nucleotide, final concentration: 1 mM) and 50 μl of glutamate dehydrogenase (final concentration: 30 U) then made up to 1600 μl with KRH. Glutamate dehydrogenase catalyses the conversion of glutamate released to 2-oxoglutarate, accompanied by the reduction of NAD+/NADP to NADH/NADPH. NADPH fluorescence was measured at 385 nm excitation and 450 nm emission using a fluorescence spectrophotometer (Hitachi 650-10 S, Tokyo, Japan). Intra-assay maximal coefficient of variation was 1.5%. Serotonin and histamine contents in the release sample were determined by enzyme-immunoassay (Serotonin ELIZA and Histamin(e)-ELIZA, IBL, Hamburg, Germany). Intra-assay maximal coefficient of variation was 10.9% for serotonin and 3.9% for histamine. Inter-assay maximal coefficient of variation was 13.4% for serotonin and 7.8% for histamine.
K+-evoked neurotransmitter release
After discarding the supernatant, the slices were resuspended in 1 ml of fresh KRH and incubated for 10 min at 37°C. This procedure was repeated to obtain a stable baseline. Immediately following this second incubation, the slices were resuspended (1 ml KRH) and incubated for a fixed time of 6 min (Hirota et al., 2000) with KRH containing 0 – 70 mM KCl in order to obtain a K+-evoked release concentration response curve. In some experiments the incubation time was varied with a fixed K+ (40 mM) concentration in order to obtain a K+-evoked release time course. All buffers used in K+-evoked release studies were as described above with an equal concentration of Na+ removed. Neurotransmitters were assayed as described above.
Data analysis
Orexin or K+-evoked neurotransmitter release was expressed as percentage of basal and all data are presented as mean±s.e.mean (n). The concentrations (EC50) of orexin A, B and K+ producing 50% of the maximal response (Emax) were estimated by non-linear regression analysis (GRAPHPAD-PRISM). Where appropriate, statistical analysis was by repeated measures ANOVA and unpaired t-test for intra- and inter-group comparison, respectively. P<0.05 was considered significant.
Results
Time course for Orexin A and B and K+-evoked noradrenaline release
Orexin A and B and K+-evoked noradrenaline release was time dependent (Figure 1). Following addition of orexin A or B there was a delay of some 6 min before noradrenaline release began and this release reached a maximum after 10 min. In contrast when K+ (40 mM) was applied there was a prompt increase in noradrenaline release which was some 6 fold above basal only 2 min (first sampling point) after addition, reaching a maximum at 6 min.
Figure 1.

Orexin A and B (100 nM at t=0) and K+ (40 mM at t=0)-evoked noradrenaline release from rat cerebrocortical slices are time-dependent. Maximal stimulation of orexin and K+ responses were observed at 10 and 6 – 8 min incubation respectively (mean±s.e.mean, n=6 for orexin and mean±range, n=2 for K+).
Concentration-response relationships for Orexin A and B and K+-evoked noradrenaline release
The release of noradrenaline in response to Orexin A and B was concentration dependent with pEC50 and Emax values of 8.74±0.32 (1.8 nM) and 263±14% and 8.61±0.38 (2.4 nM) and 173±7%, respectively (Figure 2A and B). Orexin B-evoked release was lower than that evoked by orexin A. In addition orexin A and B evoked release was significantly decreased (whole curves P<0.05) but not abolished by removal of extracellular calcium from the buffer (Figure 2A and B). As a positive control K+ produced a concentration dependent and saturable release of noradrenaline with pEC50 and Emax of 1.43±0.02 (37 mM) and 1430±70% respectively (n=4, Figure 3A)
Figure 2.
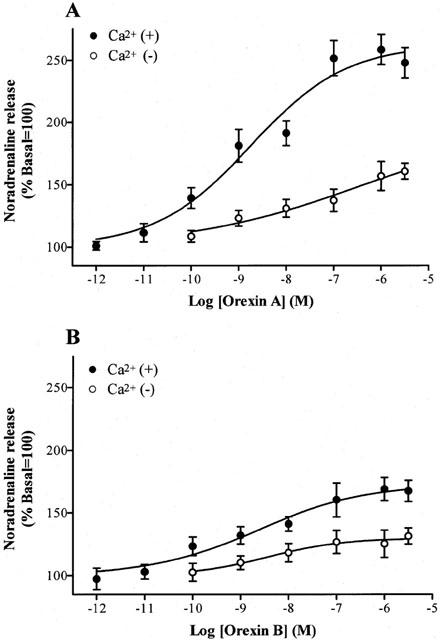
Effects of orexin A and B on the release of noradrenaline from rat cerebrocortical slices in the absence and presence of extracellular calcium. Incubations were for a fixed time of 10 min. All data are expressed as mean±s.e.mean, n=6.
Figure 3.

K+ (40 mM) stimulated the release of (A) catecholamines (noradrenaline and dopamine) and (B) glutamate, histamine and serotonin from rat cerebrocortical slices. Incubations were for a fixed time of 6 min. All data are expressed as mean±s.e.mean, n=4.
Glutamate, histamine, dopamine and serotonin release
There was a significant release of glutamate in response to orexin A at 1 μM added peptide (Table 1). Orexin A and B did not stimulate the release of histamine, dopamine or serotonin (Table 1). In marked contrast K+ produced a concentration dependent stimulation of release for all transmitters studied with EC50 values of 34 – 42 mM (Figure 3 and Table 2). There was marked variation in the degree of stimulation observed with a rank order Emax of dopamine>glutamate>serotonin>histamine.
Table 1.
Effects of orexins on dopamine, glutamate, serotonin and histamine release

Table 2.
pEC50 (mean EC50) and Emax of K+-evoked neurotransmitter release

Discussion
In the present study orexin A and B significantly evoked noradrenaline but not dopamine, glutamate, histamine or serotonin release from rat cerebrocortical slices. In marked contrast, high K+ stimulated the release of all transmitters studied. Whilst there were no differences in the EC50 there were marked differences in the degree of stimulation with evoked-dopamine release being the greatest and evoked-histamine level the lowest. Collectively our data suggest that orexins may predominantly evoke noradrenaline release from the rat cerebrocortex. However, due to the differences in the strength of stimulus (orexins compared with K+) and hence release, care should be taken in comparing these data sets.
Noradrenaline is a major neurotransmitter in the central nervous system and noradrenergic neurons play an important role in physiological responses such as sleep, attention and learning (Hagan et al., 1999). In addition, all noradrenergic projections to the cerebrocortex originate from the locus coeruleus (Nutt et al., 1997), which regulates sleep – wake cycle and is densely innervated with orexinergic neurones (Peyron et al., 1998; Hagan et al., 1999; Date et al., 1999). Hagan et al. (1999) reported that orexin A stimulates locus coeruleus cell firing and increases arousal in rats. In addition, orexinergic neurons also project to the cerebrocortex (Peyron et al., 1998; Date et al., 1999). Our data suggest that orexins may activate noradrenergic neurons predominantly to affect sleep – wake cycle.
There was a lag of some 6 min prior to orexin stimulation of noradrenaline release. In contrast K+-evoked release was evident some 2 min after stimulation (the first sampling point). Whilst this comparison does not give much insight into mechanisms it provides two pieces of information; (a) that the release can be evoked over a more ‘conventional' time frame and (b) something ‘more complex' may be occurring. Whilst we cannot give a firm explanation for this lag phase it is unlikely to result from penetration of the relatively large peptides into the slice preparation as Hagan et al. (1999) showed that the brain slice electrophysiological response to orexin A started within 30 s and reached a peak at 2 min. Moreover delayed activation of components in the signal transduction cascade are also unlikely as orexins produce rapid (Van den Pol et al., 1998; Lund et al., 2000) increases in intracellular Ca2+ and these are likely to have immediate effects on releasable pools of neurotransmitter.
Previous reports demonstrated that not only noradrenaline (Crochet & Sakai, 1999) but also dopamine (Crochet & Sakai, 1999), glutamate (Kodama & Honda, 1999), serotonin (Park et al., 1999) and histamine (Crochet & Sakai, 1999; Monti, 1993) regulate sleep – wake cycle. Date et al. (1999) suggested that orexins may function in serotonin-mediated behaviour as orexin projections and Fos expression in raphe nuclei and central grey are areas where the major serotoninergic neurons are located. Van den Pol et al. (1998) showed that orexins increased the release of GABA and glutamate from hypothalamic slices and Nakamura et al. (2000) suggest that orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Although we cannot reconcile the discrepancy between the present data (no effect on dopamine release) and these previous reports, differences in brain region and experimental protocol used may explain these discrepancies.
Several reports (Smart et al., 1999; Nakamura et al., 2000; Lund et al., 2000) indicate that orexins induce Ca2+ influx via OX receptor activation. Smart et al. (1999) reported that orexin A and B increased intracellular Ca2+ in Chinese hamster ovary cells expressing OX1 or OX2 (CHO-OX1 or CHO-OX2, respectively) receptors. The pEC50 of orexin A and B for OX1 were 8.03±0.08 (9.3 nM) and 7.30±0.08 (50.1 nM), and those for OX2 were 8.18±0.10 (6.6 nM) and 8.43±0.09 (3.7 nM), respectively. These pEC50 values are very close to those for orexin A and B stimulated noradrenaline release in the present study of 8.74±0.32 (1.8 nM) and 8.61±0.38 (2.4 nM), respectively. Thus, orexin stimulated noradrenaline release likely results from increased Ca2+ influx. However, we have demonstrated that orexins are also capable of stimulating release of noradrenaline in Ca2+ free buffer to which EGTA has been added in excess, although higher concentrations of orexins are required. These data may indicate a role for released intracellular Ca2+ stores. Although it is possibly due to incomplete chelation of extracellular Ca2+ in this slice preparation, in a preliminary study we confirmed that this procedure reduced K+-evoked noradrenaline release by >90%. Using CHO-OX1 Lund et al. (2000) also reported that OX1 activation leads to Ca2+ influx and direct stimulation of phospholipase C. In addition, increases in intracellular Ca2+ were not primarily due to IP3 activated Ca2+ influx as both Ca2+ and IP3 responses required extracellular Ca2+ at low concentrations of orexin A although high concentrations of orexin A increased IP3 production without extracellular Ca2+.
Orexin receptors have been identified not only in the brain but also the adrenal glands (López et al., 1999), ganglioneuroblastoma and neuroblastoma (Arihara et al., 2000). These tissues are known to secrete noradrenaline. In addition, orexins activate the sympathetic nervous system to increase blood pressure and heart rate with an elevation in plasma noradrenaline. Therefore, orexinergic neurones may be found in many noradrenaline secreting tissues.
In summary, collectively, our data may indicate that orexins selectively evoke noradrenaline release in the rat cerebrocortex but before a firm conclusion can be made further detailed studies will need to be performed.
Acknowledgments
Supported in part by grant-in-aid for scientific research (No 09470323 and 13671560) from the Ministry of Education, Science and Culture in Japan.
Abbreviations
- CHO
Chinese hamster ovary cells
- EC50
concentrations producing 50% of the maximal response
- Emax
maximal response
- KRH
Krebs-HEPES buffer solution
- OX1
orexin-1
- OX2
orexin-2
References
- ARIHARA Z., TAKAHASHI K., MURAKAMI O., TOTSUNE K., SPNE M., SATOH F., ITO S., HAYASHI Y., SASANO H., MOURI T. Orexin-A in the human brain and tumor tissues of ganglioneuroblastoma and neuroblastoma. Peptides. 2000;21:565–570. doi: 10.1016/s0196-9781(00)00184-4. [DOI] [PubMed] [Google Scholar]
- CHEMELLI R.M., WILLIE J.T., SINTON C.M., ELMQUIST J.K., SCAMMELL T., LEE C., RICHARDSON J.A., WILLIAMS S.C., XIONG Y., KISANUKI Y., FITCH T.E., NAKAZATO M., HAMMER R.E., SAPER C.B., YANAGISAWA M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- CHEN C.T., HWANG L.L., CHANG J.K., DUN N.J. Pressor effects of orexins injected intracisternally and to rostral ventrolateral medulla of anesthetized rats. Am. J. Physiol. 2000;278:R692–R697. doi: 10.1152/ajpregu.2000.278.3.R692. [DOI] [PubMed] [Google Scholar]
- CROCHET S., SAKAI K. Effects of microdialysis application of monoamines on the EEG and behavioural states in the cat mesopontine tegmentum. Eur. J. Neurosci. 1999;11:3738–3752. doi: 10.1046/j.1460-9568.1999.00760.x. [DOI] [PubMed] [Google Scholar]
- DATE Y., UETA Y., YAMASHITA H., YAMAGUCHI H., MATSUKURA S., KANGAWA K., SAKURAI T., YANAGISAWA M., NAKAZATO M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc. Natl. Acad. Sci. U.S.A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGAN J.J., LESLIE R.A., PATEL S., EVANS M.L., WATTAM T.A., HOLMES S., BENHAM C.D., TAYLOR S.G., ROUTLEDGE C., HEMMATI P., MUNTON R.P., ASHMEADE T.E., SHAH A.S., HATCHER J.P., HATCHER P.D., JONES D.N., SMITH M.I., PIPER D.C., HUNTER A.J., PORTER R.A., UPTON N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc. Natl. Acad. Sci. U.S.A. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIROTA K., KUDO M., KUDO T., MATSUKI A., LAMBERT D.G. Inhibitory effects of intravenous anaesthetic agents on K+-evoked noradrenaline and dopamine release from rat striatal slices.-Possible involvement of P/Q type voltage sensitive Ca2+ channels. Br. J. Anaesth. 2000;85:874–880. doi: 10.1093/bja/85.6.874. [DOI] [PubMed] [Google Scholar]
- HORVATH T.L., PEYRON C., DIANO S., IVANOV A., ASTON JONES G., KILDUFF T.S., VAN DEN POL A.N. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J. Comp. Neurol. 1999;415:145–159. [PubMed] [Google Scholar]
- KODAMA T., HONDA Y. Acetylcholine and glutamate release during sleep–wakefulness in the pedunculopontine tegmental nucleus and norepinephrine changes regulated by nitric oxide. Psychiatry Clin. Neurosci. 1999;53:109–111. doi: 10.1046/j.1440-1819.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- LIN L., FARACO J., LI R., KADOTANI H., ROGERS W., LIN X., QIU X., DE JONG P.J., NISHINO S., MIGNOT E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- LÓPEZ M., SEÑARÍS R., GALLEGO R., GARCÍA-CABALLERO T., LAGO F., SEOANE L., CASANUEVA F., DIÉGUEZ C. Orexin receptors are expressed in the adrenal medulla of the rat. Endocrinology. 1999;140:5991–5994. doi: 10.1210/endo.140.12.7287. [DOI] [PubMed] [Google Scholar]
- LUND P.E., SHARIATMADARI R., UUSTARE A., DETHEUX M., PARMENTIER M., KUKKONEN J.P., ÅKERMAN K.E. The orexin OX1 receptor activates a novel Ca2+ influx pathway necessary for coupling to phospholipase C. J. Biol. Chem. 2000;275:30806–30812. doi: 10.1074/jbc.M002603200. [DOI] [PubMed] [Google Scholar]
- MONTI J.M. Involvement of histamine in the control of the waking state. Life Sci. 1993;53:1331–1338. doi: 10.1016/0024-3205(93)90592-q. [DOI] [PubMed] [Google Scholar]
- NAKAMURA T., URAMURA K., NAMBU T., YADA T., GOTO K., YANAGISAWA M., SAKURAI T. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–187. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- NUTT D.J., LALIES M.D., LION L.A., HUDSON A.L. Noradrenergic mechanisms in the prefrontal cortex. J. Psychopharmacol. 1997;11:163–168. doi: 10.1177/026988119701100209. [DOI] [PubMed] [Google Scholar]
- PARK S.P., LOPEZ-RODRIGUEZ F., WILSON C.L., MAIDMENT N., MATSUMOTO Y., ENGEL J., JR In vivo microdialysis measures of extracellular serotonin in the rat hippocampus during sleep-wakefulness. Brain Res. 1999;833:291–296. doi: 10.1016/s0006-8993(99)01511-5. [DOI] [PubMed] [Google Scholar]
- PEYRON C., TIGHE D.K., VAN DEN POL A.N., DE LECES L., HELLER H.C., SUTCLIFFE J.G., KILDUFF T.S. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J. Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAKURAI T., AMEMIYA A., ISHII M., MATSUZAKI I., CHEMELLI R.M., TANAKA H., WILLIAMS S.C., RICHARDSON J.A., KOZLOWSKI G.P., WILSON S., ARCH J.R., BUCKINGHAM R.E., HAYNES A.C., CARR S.A., ANNAN R.S., MCNULTY D.E., LIU W.S., TERRETT J.A., ELSHOURBAGY N.A., BERGSMA D.J., YANAGISAWA M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behaviour. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- SAMSON W.K., GOSNELL B., CHANG J.K., RESCH Z.T., MURPHY T.C. Cardiovascular regulatory actions of the hypocretins in brain. Brain Res. 1999;831:248–253. doi: 10.1016/s0006-8993(99)01457-2. [DOI] [PubMed] [Google Scholar]
- SHIRASAKA T., NAKAZATO M., MATSUKURA S., TAKASAKI M., KANNAN H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am. J. Physiol. 1999;277:R1780–R1785. doi: 10.1152/ajpregu.1999.277.6.R1780. [DOI] [PubMed] [Google Scholar]
- SMART D., JERMAN J.C., BROUGH S.J., RUSHTON S.L., MURDOCK P.R., JEWITT F., ELSHOURBAGY N.A., ELLIS C.E., MIDDLEMEISS D.N., BROWN F. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br. J. Pharmacol. 1999;128:1–3. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRIVEDI P., YU H., MACNEIL D.J., VAN DER PLOEG L.H., GUAN X.M. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 1998;438:71–75. doi: 10.1016/s0014-5793(98)01266-6. [DOI] [PubMed] [Google Scholar]
- VAN DEN POL A.N., GAO X.B., OBRIETAN K., KILDUFF T.S., BELOUSOV A.B. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J. Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]


