Abstract
Recently, much attention has been paid to the relationship between the nervous and immune systems. The present study was conducted to clarify the role of neurotrophin low affinity receptor (p75N) in allergic airway inflammation and hyper-responsiveness (AHR) in mice by employing p75N gene deficient mice.
Mice were immunized twice by intraperitoneal injections of ovalbumin (OA) at intervals of 12 days. OA was inhaled 10 days after the secondary immunization and repeated three times at 4 days interval. Twenty-four hours after the last inhalation, airway responsiveness to acetylcholine was measured and bronchoalveolar lavage fluid (BALF) was obtained for examining the number of inflammatory cells and the level of cytokines. Serum immunoglobulin was measured as a marker of systemic immune response before the final inhalation.
In wild-type mice, repeated antigen provocation resulted in airway eosinophilia, AHR and elevations in serum IgE and interleukin (IL)-4 and -5 in BALF. In p75N gene deficient mice, none of the above parameters was observed after antigen provocation. The antigen-induced production of interferon (IFN)-γ and nerve growth factor (NGF) were not altered by depletion of p75N gene.
The present findings suggest that p75 gene deficiency disrupt an allergic airway inflammation and AHR in mice by interfering type 2 helper T (Th2) cell responses.
Keywords: Low affinity neurotrophin receptor p75, airway inflammation, airway hyper-responsiveness, mice
Introduction
Several recent studies have provided evidence that neuroimmune interaction participates in the onset and development of allergic diseases (Cockcroft & O'byrne, 1993; Corrigan & Kay, 1992; Djukanovic et al., 1990). As a mediator of the neuroimmune interaction, much attention has been paid to neurotrophins including nerve growth factor (NGF), brain derived nerve growth factor (BDNF) and neurotrophin 3 (NT-3) because neurotrophin widely acts on both neuron and immune systems (Barnes, 1996; Braun et al., 1999a; Cockcroft et al., 1977; Juniper et al., 1981; Killian et al., 1976; van oosterhout & Nijkamp, 1993).
Allergic bronchial asthma is characterized by airway eosinophilic inflammation and airway hyper-responsiveness (AHR) to various stimuli (Cockcroft & O'byrne, 1993; Djukanovic et al., 1990). AHR is thought to be positively correlated with the severity of the bronchial asthma (Cockcroft et al., 1997; Corrigan & Kay, 1992; Juniper et al., 1981; Killian et al., 1976; van Oosterhout et al., 1993). AHR is caused by complex pathophysiological changes in the airways including inflammation, nerve system abnormality and hormone inbalance (Barnes, 1996).
There are some reports to indicate the participation of neurotrophin on allergic airway obstruction. Virchow et al. (1998) reported the increase of BDNF and NT-3 in BALF from the patients with asthma after segmental allergen provocation. Bonini et al. (1996) reported an elevation of circulating NGF levels in allergic asthma patient. Similar findings were obtained in experiments using animal models (Braun et al., 1998; 1999b). This previous evidence suggests that neurotrophin is one of the candidates as a mediator of allergic bronchial asthma.
Contrary to neurotrophin itself, little is known about the role of neurotrophin receptor in allergic diseases. Four different kinds of neurotrophin receptor, TrkA, TrkB, TrkC and p75, were reported. p75 neurotrophin receptor (p75N) is known as a low-affinity receptor belonging to tumour necrosis factor (TNF) receptor family in addition to CD40 and Fas (Beutler & van Huffel, 1994). Structurally, they are 70- to 80-kDa membrane-associated proteins (Beutler & van Huffel, 1994) with four cysteine-rich structures in their extracellular domain (Casaccia-Bonnefil et al., 1996; Frade et al., 1996). Recently, many studies were carried out to clarify the existence and the function of p75N in the nervous system (Barbacid, 1995; Bothwell, 1996; Casaccia-Bonnefil et al., 1996; Frade et al., 1996; Hempstead et al., 1991; Klein et al., 1991; Singh et al., 1997) and inflammatory lesion (Brodie et al., 1996, Ehrhard et al., 1993; Horigome et al., 1993; Otten et al., 1989).
For the reasons given above, the present study was conducted to clarify the role of p75N in allergic airway inflammation and AHR using p75N gene deficient (knockout) mice.
Methods
Animals
Mice lacking low affinity neurotrophin receptor p75N gene and age-matched wild-type mice (BALB/cJ) were purchased from the Jackson Laboratory (Bar Harbor, ME, U.S.A.). p75N knockout mice were generated by disrupting the third exon of the p75N gene (Lee et al., 1992). During the experiment, the weight of knockout mice was lighter than that of wild-type mice. All animals were mated and maintained in our laboratory following the guidelines for the care and use of experimental animals from the Japanese Association for Laboratory Animals Science in 1987.
Agents
Ovalbumin (OA, Seikagaku Kogyo, Tokyo, Japan), acetylcholine chloride (Ach, Nacalai Tesque, Kyoto, Japan), bovine serum albumin (BSA, Seikagaku Kogyo, Tokyo, Japan), Turk's solution (Wako Pure Chemical Industries, Ltd., Osaka, Japan), pancronium bromide (Sigma, St. Louis, MO, U.S.A.), pentobarbitone sodium (Abbott Lab., Chicago, IL, U.S.A.), disodium ethylendiaminetetraacetic acid (EDTA-2Na, Nacalai Tesque, Kyoto, Japan), Diff-Quick solution (International Reagent Corp., Kobe, Japan), monoclonal anti-mouse lgE antibody (LO-ME-3, Serotec Co., Ltd., Oxford, U.K.), and peroxidase-conjugated streptavidin (Dakopatts a/s, Glostrup, Denmark) were purchased commercially.
Sensitization and antigen challenge
Mice were actively sensitized by intraperitoneal injection of 50 μg OA with 1 mg alum on days 0 and 12. Starting on day 22, they were exposed to OA (1% w v−1 diluted in sterile saline) for 30 min, three times every 4th day according to a previously reported method (Nagai et al., 1997). Control animals were exposed to saline in a similar manner. The aerosol (particle size; 4.0 – 5.0 μm) was generated by a nebulizer (Ultrasonic nebulizer UN-701, Azwell, Osaka, Japan) driven by filling a perspex cylinder chamber with a nebulized solution.
Measurement of airway function
Bronchoconstriction was measured according to the overflow method described by Konzett & Rössler (1940). Briefly, to measure bronchial responsiveness to Ach, mice were anaesthetized with pentobarbitone sodium (80 mg kg−1, i.p.) and the jugular vein was cannulated for intravenous injection of Ach. Mice were injected with pancronium bromide (0.1 mg kg−1, i.v.) to suppress spontaneous respiration and animals were ventilated with a rodent ventilator (New England Medical Instruments Inc., Medway, MA, U.S.A.) with air supplemented with oxygen at 60 strokes min−1 at a stoke volume of 0.6 ml/animal. Bronchoconstriction was measured according to the overflow method, using a bronchospasm transducer (Ugo Basil 7020, Milano, Italy) connected to the tracheal cannula. To measure bronchial responsiveness to Ach, changes in respiratory overflow volume were measured using increasing doses of Ach. The increase in respiratory overflow volume provoked by Ach was represented as a percentage of the maximal overflow volume (100%) obtained by clamping the tracheal cannule. The area under the curve (AUC) was calculated from the dose-response curve for Ach (range: 31.25 – 2000 μg kg−1).
Bronchoalveolar lavage study
To evaluate airway inflammation, we studied the accumulation of inflammatory cells in bronchoalveolar lavage fluid (BALF). Experiments were carried out according to previously described methods (Nagai et al., 1997; Tanaka et al., 1998; Yamaguchi et al., 1994). Briefly, 24 h after the last inhalation of antigen (30 days after the first immunization), animals were killed by intraperitoneal injection of pentobarbitone sodium (100 mg kg−1). The trachea was cannulated and the air lumen was washed four times with 1 ml of calcium- and magnesium-free phosphate-buffered saline (PBS) containing 0.1 BSA and 0.05 mM EDTA-2Na and the procedure was repeated three times (total volumes; 3 ml, recovery >85%). BALF from each animal was pooled in a plastic tube, cooled in ice, and centrifuged (150×g) at 4°C for 10 min. Cell pellets were resuspended in the same buffer (1 ml). BALF was stained with Türk solution and the number of nucleated cells were counted in a Bürker chamber. A differential count was made on a smear prepared with a cytocentrifuge (Cytospin II, Shandon, U.K.) and stained with Diff-Quick solution (based on standard morphologic criteria) on at least 300 cells (magnification ×500).
Histological study
The mice were killed 24 h after the third antigen inhalation, and the whole lungs were distended by instillation of 10% buffered formalin via the trachea, removed, and immersed in the same fixative with the trachea clamped for 24 h. Tissue was sliced and embedded in paraffin, and 2 – 3 μm sections were stained with hematoxylin and eosin for light microscopic examination.
Cytokine assay
Interleukin (IL)-4, IL-5 and interferon (IFN)-γ were measured in cell-free BALF with commercial enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's instructions (Endogen, Cambridge, MA, U.S.A.). The detection limits were 5 pg ml−1 (IL-4 and IL-5) and 10 pg ml−1 (IFN-γ). In the preliminary experiments, non-specific luminescence was not detected in the system.
NGF assay
NGF was measured in cell-free BALF using a commercial ELISA kit according to the manufacturer's instructions (Promega, Madison, WI, U.S.A.). The detection limit was 4 pg ml−1. Non-specific background luminescence was negligible in the ELISA system.
Measurement of antigen-specific IgE levels
Immediately before the last antigen challenge, blood was collected, and each serum was obtained by centrifugation and stored at −80°C. Antigen-specific IgE in the mouse serum was measured using the enzyme-linked immunosorbent assay (ELISA) method as previously described (Nagai et al., 1997; Yamaguchi et al., 1994). Briefly, serum OA-specific IgE was measured by coating monoclonal rat anti-mouse IgE antibody (LO-ME-3) at a concentration of 5 μg ml−1. After blocking with 1% BSA, serum dilutions were incubated for 1 h followed by biotinylated-OA and peroxidase-conjugated streptavidin. Sequentially diluted monoclonal anti-OA IgE was used as a standard. Optical densities of the enzymatic reactions were read using an automatic ELISA plate reader (Titertek Multiscan MCC/340, Flow Laboratories, Inc., U.S.A.) at 450 nm (reference 690 nm). The detection limit was 10 ng ml−1.
Statistical analysis
Values were represented as the means±s.e.mean. Statistical significance between saline-inhaled and OA-inhaled animals, or between wild-type and knockout mice, was estimated using the two-tailed Student's t-test or the Mann – Whitney U-test after checking normal distribution by F-test. Values at P<0.05 were considered statistically significant.
Results
Airway responsiveness to Ach
To clarify the role of p75N in airway function, airway responsiveness to Ach was examined after sensitization and airway challenge with antigen. Figure 1 shows the AUC of the dose-response to Ach in all groups. In wild-type mice, repeated antigen provocation caused a significant increase in the airway responsiveness to Ach. In contrast, no AHR was observed in p75N knockout mice. This is confirmed by calculation of the provocative dose of Ach to cause 50% bronchoconstriction (PD50). The average PD50 in saline treated wild-type mice is 552 μg kg−1 Ach and that in OA treated wild-type mice is 62.5 μg kg−1 Ach. The average PD50 in saline treated KO mice is 580 μg kg−1 Ach and that in OA treated KO mice was 586 μg kg−1 Ach.
Figure 1.
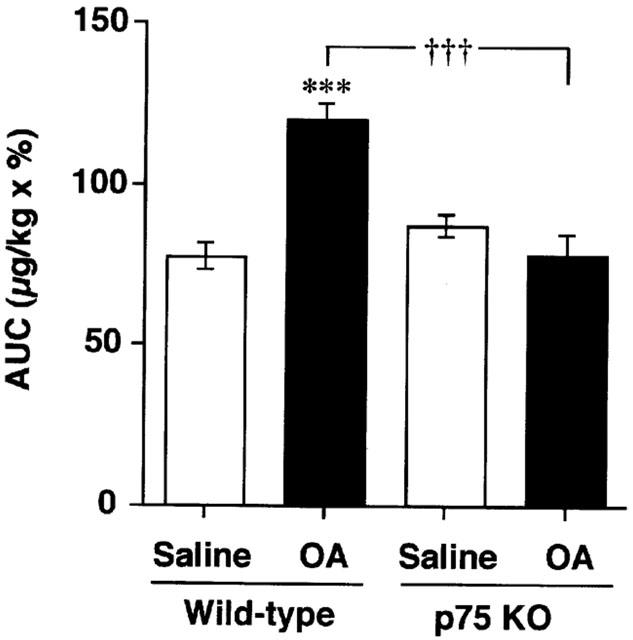
Airway responsiveness to acetylcholine (Ach) after repeated antigen provocation in BALB/c (wild-type: WT) or p75N knockout (KO) mice. Values represent the means±s.e.mean of 5 – 7 animals. The number of animals in each group was as follows: saline treated wild-type mice (5); OA treated wild-type mice (6) and others (7). AUC: area under the curve (range 31.25 – 2,000 μg kg−1 acetylcholine). OA: ovalbumin. ***P<0.001 (vs saline: Student's t-test). †††P<0.001 (vs wild-type: Student's t-test).
Cellular composition of BALF
To clarify the airway inflammation, BALF cells were collected 24 h after the final antigen inhalation. Figure 2 shows the cellular composition of BALF in all groups. In wild-type mice, repeated antigen provocation caused a significant increase in the number of total leukocytes, eosinophils and lymphocytes. Eosinophils were the predominant cell type. The numbers of total cells and lymphocytes increased two or three times more by the inhalation of OA in wild-type mice. In contrast, in p75N knockout mice, the number of total leukocytes, eosinophils and lymphocytes were not increased at all after antigen provocation.
Figure 2.
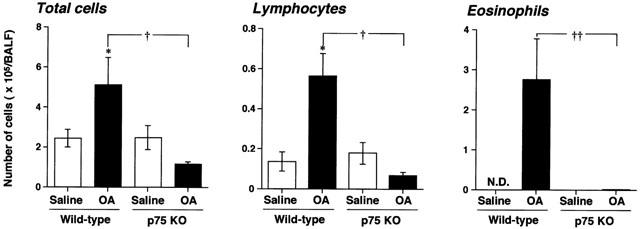
Antigen-induced leukocyte accumulation in bronchoalveolar lavage fluid (BALF) obtained from BALB/c (wild-type: WT) or p75N knockout (KO) mice. Values represent the means±s.e.mean of 5 – 7 animals. The number of animals are as follows: saline treated wild-type mice (5); OA treated wild-type mice (6); Others (7). ND: not detected. OA: ovalbumin. *P<0.05 (vs saline: Student's t-test). †, ††P<0.05 and 0.01 respectively (vs wild-type: Mann – Whitney U-test).
Histopathology of the lung
Histopathological examinations were performed to confirm the existence of airway eosinophilic inflammation. Figure 3 shows the histological analysis of lung sections taken from all groups. In both wild-type and p75N knockout mice without antigen challenge, histological analysis of lung sections showed no inflammation and normal lung histology (Figure 3A,C). However, the lung sections taken from wild-type mice after sensitization and airway challenge showed a pulmonary eosinophilic inflammation mainly seen in the peribronchial and perivascular regions of the lungs (Figure 3B). In contrast, p75N knockout mice showed no pulmonary eosinophilic inflammation after antigen challenge (Figure 3D).
Figure 3.
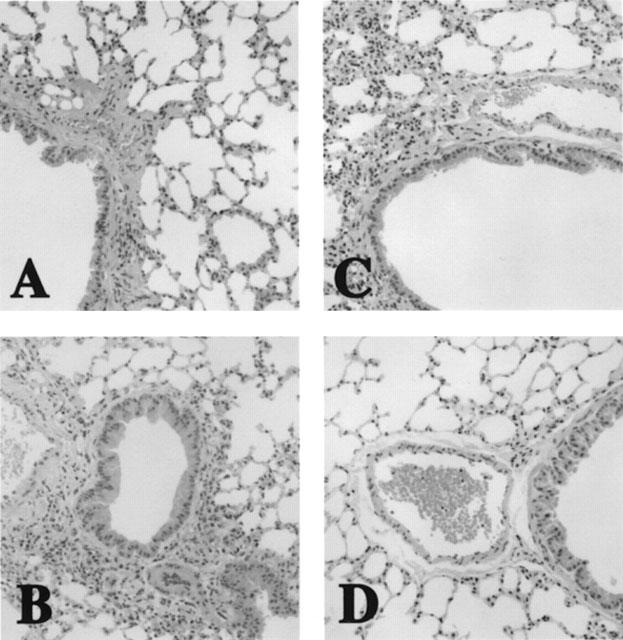
Histopathological picture of lungs of mice. (A) saline treated wild-type mice. (B) OA treated wild-type mice. (C) saline treated p75N knockout mice. (D) OA treated p75N knockout mice. (HE, ×170).
Cytokine production in BALF
To investigate the role of cytokines in the induction of inflammatory cell accumulation into the airway after antigen challenge, we measured the production of IL-4, IL-5 and IFN-γ in BALF. Figure 4 shows the cytokine production in BALF in all groups. The production of IL-4 and IL-5 was not detected in BALF obtained from both wild-type and p75N knockout mice without antigen challenge. However, IL-4 and IL-5 levels were increased in BALF obtained from wild-type mice after airway antigen-challenge. In contrast, no increase in IL-4 and IL-5 levels was observed in p75N knockout mice after antigen challenge, and the levels were below the limit of detection (<5 pg ml−1). However, there was no significant difference in the IFN-γ levels in BALF between wild-type and p75N knockout mice.
Figure 4.
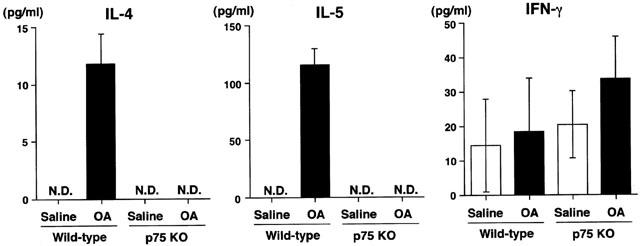
Antigen-induced IL-4, IL-5 and IFN-γ production in bronchoalveolar lavage fluid obtained from BALB/c (wild-type: WT) or p75N knockout (KO) mice. Values represent the means±s.e.mean of 4 – 5 animals. The number of animals are as follows: saline treated wild-type mice (4), others (5). ND: not detected (<5 pg ml−1). OA: ovalbumin.
NGF production in BALF
Since p75N is thought to be a low-affinity receptor of neurotrophin, which is known as an important molecule in allergic inflammation, the production of one of neutrophins, NGF, in BALF was measured. Figure 5 shows the NGF production in BALF in all groups. NGF production in BALF was increased after sensitization and airway challenge in both p75N knockout and wild-type mice. In p75N knockout mice, there were higher NGF levels in BALF than those of wild-type mice.
Figure 5.
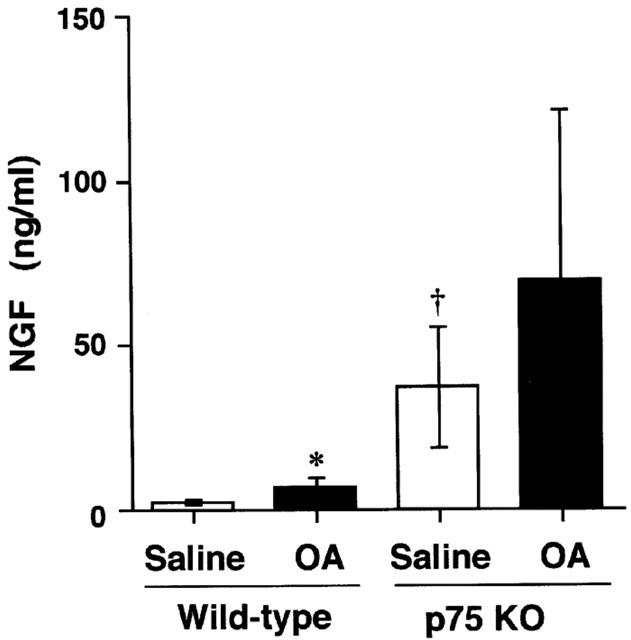
Antigen-induced NGF production in bronchoalveolar lavage fluid obtained from BALB/c (wild-type: WT) or p75N knockout (KO) mice. Values represent the mean±s.e.mean of 4 – 5 animals. The number of animals are as follows: saline treated wild-type mice (4); others (5). OA: ovalbumin. *P<0.05 (vs saline: Mann – Whitney U-test). †P<0.05 (vs wild-type: Mann – Whitney U-test).
Antigen-specific IgE levels in serum
To investigate the role of p75N in antigen-induced IgE production, antigen-specific IgE levels in serum was measured. Figure 6 shows the antigen-specific IgE levels in all groups. The antigen-specific IgE in both wild-type and p75N knockout mice was not detected without antigen challenge. However, repeated antigen provocation caused a significant increase in the levels of antigen-specific IgE in wild-type mice. In contrast, in p75 knockout mice, antigen-specific IgE levels in serum were increased after sensitization and airway challenge, but were significantly lower levels than those of wild-type mice.
Figure 6.
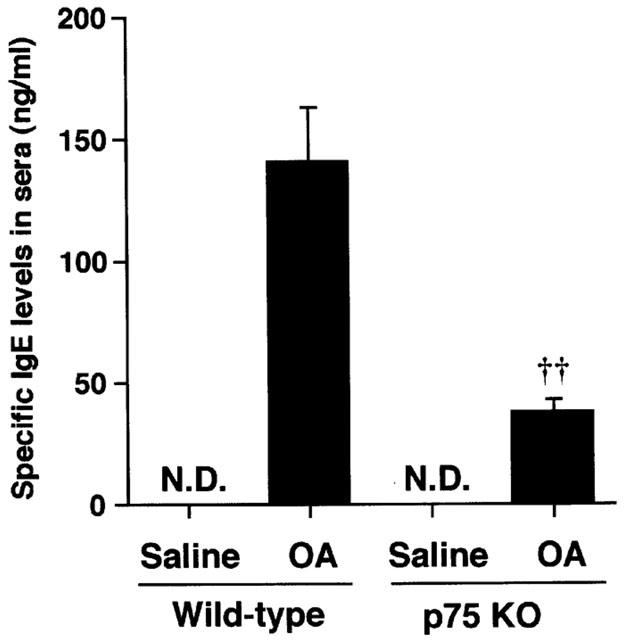
The increase in antigen-specific IgE levels of BALB/c (wild-type WT) or p75N knockout (KO) mice. Values represent the mean±s.e.mean of seven animals. ND: not detected (<1 ng ml−1). OA: ovalbumin. *P<0.01 (vs saline: Student's t-test). ††P<0.00 (vs wild-type: Mann – Whitney U-test).
Discussion
In the present study, we investigated the role of p75N in antigen-induced airway inflammation and AHR in mice, using p75N knockout mice. The repeated antigen provocation caused an airway inflammation and AHR in wild-type mice but not in the p75N knockout mice. Moreover, the serum antigen-specific IgE levels and Th2 cytokines levels in BALF were significantly lowered by the depletion of the p75N gene. On the other hand, the increase in NGF and Th1 cytokine (IFN-γ) production in BALF after provocation of the antigen was similar in p75N knockout and wild-type mice.
The present findings suggest the suppression of antigen-induced AHR and airway eosinophilia due to the selective inhibition of Th2 response in p75N gene deficient mice. There is no information about the existence of p75N on mice T cells. However, Ehrhard et al. (1994) and Lambiase et al. (1997) reported the existence of high affinity NGF receptor, TrkA, on human T cells. They described that TrkA was of functional importance in T cell activation and cytokine production. In addition to their studies, many investigators reported the cooperation of p75N and TrkA for expressing NGF function in many kinds of cells and tissues (Bull et al., 1998; Frade & Barde, 1998; Pincelli, 2000). In the present study, an antigen-induced Th2 cytokine and antigen-specific IgE production are not caused in p75N deficient mice. These findings suggest the p75N may play a role in Th2 cell function in terms of the production of cytokines. Simultaneously, the present data suggest the low positive participation of TrkA in the activation of mice Th2 cells because NGF is produced by antigen in p75N gene deficient mice. Unfortunately, the role of TrkA was not investigated in the present study. To elucidate the role of TrkA might improve the understanding of the role of p75N. The precise reason of lowered Th2 cell function in p75N gene deficient mice is not clear in the present study. Further experiments examining the role of p75N in whole processes of antigen-induced Th2 cytokine production will be necessary.
In addition to T cells, Brodie et al. (1996) reported the existence of p75N and TrkA on human B cells. They indicated that NGF inhibited the induction of IgE by IL-4 in human B cells. They reported that the action of NGF receptor was different from CD40 that belongs to the same receptor superfamily and exerts similar proliferative effects on B cells. In the present studies, the lack of IgE production in p75N gene deficient mice was demonstrated. Both findings appear to be different, however, the present observations are mainly owing to the suppression of the production of Th2 cytokines. The reduction of IgE antibody is, probably due to the inhibition of Th2 cytokine by p75N gene depletion. Moreover, Braun et al. (1999a) reported NGF plays an important role for the production of IgE in mice. Species differences in the role of NGF in the production of IgE is suggested.
In addition to the immune system, an important role of p75N in a certain kind of inflammation was reported by Nataf et al. (1998), who demonstrated the role of p75N in an experimental allergic encephalomyelitis (EAE) model in rats. Bull et al. (1998), Conti et al. (1998), Vaidyanathan et al. (1998) and Pincelli (2000) also reported the increase of p75N expression in cutaneous inflammation and neuropathic bladder. In addition, the role of p75N in inflammatory cells including basophils (Bischoff & Dahinden, 1992), eosinophils (Hamada et al., 1996), and mast cells (Leon et al., 1994) was reported. These findings suggest the direct role of p75 in inflammation. In the present study, the antigen-induced increase in inflammatory cells in airway was significantly lowered by p75N gene depletion. This is probably due to both the direct inhibition of inflammatory cells recruitment and down regulation of Th2 cytokine production.
In addition to p75N, the present findings also suggest the role of neurotrophin itself in allergic airway inflammation. In 1996, Bonini et al. (1996) reported the elevation of NGF levels in serum from patients with allergic disease. Moreover, Virchow et al. (1998) reported the elevation of neurotrophin concentrations in the BALF patients with asthma after segmental allergen provocation. They indicated that the NGF levels after allergen provocation were negatively correlated with baseline FEV1. In addition to above clinical studies, the role of NGF in allergic reaction was confirmed by the experiments employing mice and guinea pigs (Braun et al., 1998; 1999a). The elevation in the NGF level in BALF was also demonstrated in the present experiments. The level of NGF in p75N deficient mice was higher than that in wild-type mice. These findings suggest that p75N has some role in the feedback regulation of NGF production after antigen stimulation. But the details are still obscure, further experiments will be necessary to elucidate the role of p75N in the production of NGF.
Regarding the role of NGF in the onset of AHR, Braun et al. (1998) reported the blocking of NGF activity with anti-NGF antibodies almost entirely prevented development of AHR in mice. In their experiments, anti-NGF antibody treatment had no effect on the recruitment of inflammatory cells into the airways, but significantly reduced the production of IL-4 that is elevated during airway inflammation. Moreover, they demonstrated that NGF augments the production of IL-4, IL-5, and IgE in in vitro experiments. They proposed that NGF augmented the ongoing Th2 response. In the present study, the decrease in IL-4 and IL-5 production in BALF and serum IgE was demonstrated in p75N knockout mice. The present findings support their evidence about the role of NGF in AHR and antigen-induced immune response in mice. Regarding the role of other neurotrophin, BDNF and NT-3, Virchow et al. (1998) and Braun et al. (1999a) indicated the increase of BDNF and NT-3 in BALF and serum after antigen-challenge. Whereas data suggest the participation of BDNF and NT-3 in allergic airway obstruction, further investigation will be necessary to know the role of these neurotrophins in allergic inflammation.
In the present study, we demonstrated the importance of p75 in allergic airway inflammation mainly from the immunopharmacological aspects. The present findings suggest that blockade of p75 or NGF may provide a novel therapeutic approach to the treatment of allergic airway disorders.
Abbreviations
- Ach
acetylcholine chloride
- AHR
airway hyper-responsiveness
- AUC
area under the curve
- BALF
bronchoalveolar lavage fluid
- BDNF
brain derived nerve growth factor
- BSA
bovine serum albumin
- EAE
experimental allergic encephalomyelitis
- ELISA
enzyme-linked immunosorbent assay
- IFN
interferon
- IL
interleukin
- NGF
nerve growth factor
- NT
neurotrophin
- OA
ovalbumin
- PBS
phosphate-buffered saline
- Th
T helper
- TNF
tumour necrosis factor
References
- BARBACID M. Neurotrophic factors and their receptors. Curr. Opin. Cell. Biol. 1995;7:148–155. doi: 10.1016/0955-0674(95)80022-0. [DOI] [PubMed] [Google Scholar]
- BARNES P.J. Neuroeffector mechanisms: the interface between inflammation and neuronal responses. J. Allergy Clin. Immunol. 1996;98:S73–S81. [PubMed] [Google Scholar]
- BEUTLER B., VAN HUFFEL C. Unraveling function in the TNF ligand and receptor families. Science. 1994;264:667–668. doi: 10.1126/science.8171316. [DOI] [PubMed] [Google Scholar]
- BISCHOFF S.C., DAHINDEN C.A. Effect of nerve growth factor on the release of inflammatory mediators by mature human basophils. Blood. 1992;79:2662–2669. [PubMed] [Google Scholar]
- BONINI S., LAMBIASE A., BONINI S., ANGELUCCI F., MAGRINI L., MANNI L., ALOE L. Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma. Proc. Natl. Acad. Sci. U.S.A. 1996;93:10955–10960. doi: 10.1073/pnas.93.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOTHWELL M. P75NTR: a receptor after all. Science. 1996;272:506–507. doi: 10.1126/science.272.5261.506. [DOI] [PubMed] [Google Scholar]
- BRAUN A., APPLE E., BARUCH R., HERZ U., BOTCHKAREV V., PAUS R., BRODIE C., RENZ H. Role of nerve growth factor in a mouse model of allergic airway inflammation and asthma. Eur. J. Immunol. 1998;28:3240–3251. doi: 10.1002/(SICI)1521-4141(199810)28:10<3240::AID-IMMU3240>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- BRAUN A., LOMMATZSCH M., LEWIN G.R., VIRCHOW J.C., RENZ H. Neurotrophins: a link between airway inflammation and airway smooth muscle contractility in asthma. Int. Arch. Allergy Immunol. 1999b;118:163–165. doi: 10.1159/000024056. [DOI] [PubMed] [Google Scholar]
- BRAUN A., LOMMATZSCH M., MANNSFELDT A., NEUHAUS-STEINMATZ U., FISCHER A., SCHNOY N., LEWIN G.R., RENZ H. Cellular sources of enhanced brain-derived neurotrophic factor production in a mouse model of allergic inflammation. Am. J. Respir. Cell. Mol. Biol. 1999a;21:537–546. doi: 10.1165/ajrcmb.21.4.3670. [DOI] [PubMed] [Google Scholar]
- BRODIE C., OSHIBA A., RENZ H., BRADLEY K., GELFAND E.W. Nerve growth-factor and anti-CD40 provide opposite signals for the production of IgE in interleukin-4-treated lymphocytes. Eur. J. Immunol. 1996;26:171–178. doi: 10.1002/eji.1830260127. [DOI] [PubMed] [Google Scholar]
- BULL H.A., LESLIE T.A., CHOPRA S., DOWD P.M. Expression of nerve growth factor receptors in cutaneous inflammation. Br. J. Dermatol. 1998;139:776–783. doi: 10.1046/j.1365-2133.1998.02500.x. [DOI] [PubMed] [Google Scholar]
- CASACCIA-BONNEFIL P., CARTER B.D., DOBROWSKY R.T., CHAO M.V. Death of oligodendrocytes mediated by the interaction of nerve growth factor with its receptor p75. Nature. 1996;383:716–719. doi: 10.1038/383716a0. [DOI] [PubMed] [Google Scholar]
- COCKCROFT D.W., KILLIAN D.N., MELLON J.J., HARGREAVE F.E. Bronchial reactivity to inhaled histamine: a method and clinical survey. Clin. Allergy. 1977;7:235–243. doi: 10.1111/j.1365-2222.1977.tb01448.x. [DOI] [PubMed] [Google Scholar]
- COCKCROFT D.W., O'BYRNE P.M.Mechanisims of airway hyperresponsiveness Bronchial Athma: Mechanisms and Therapeutics 1993Boston: Little Brown; 32–42.3rd edned. Weiss, E.B. & Stein, M. pp [Google Scholar]
- CONTI G., SCARPINI E., ROSTAMI A., LIVRAGHI S., BARON P.L., PLEASURE D., SCARLATO G. Schwann cell undergoes apoptosis during experimental allergic neuritis (EAN) J. Neurol. Sci. 1998;161:29–35. doi: 10.1016/s0022-510x(98)00260-3. [DOI] [PubMed] [Google Scholar]
- CORRIGAN C.J., KAY A.B. T cells and eosinophils in the pathogenesis of asthma. Immunol. Today. 1992;13:501–507. doi: 10.1016/0167-5699(92)90026-4. [DOI] [PubMed] [Google Scholar]
- DJUKANOVIC R., ROCHE W.R., WILSON J.W., BEASLEY C.R., TWENTYMAN O.P., HOWARTH R.H., HOLGATE S.T. Mucosal inflammation in asthma. Am. Rev. Respir. Dis. 1990;142:434–457. doi: 10.1164/ajrccm/142.2.434. [DOI] [PubMed] [Google Scholar]
- EHRHARD P.B., ERB P., GRAUMANN U., OTTEN U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10984–10988. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EHRHARD P.B., ERB P., GRAUMANN U., SCHMUTZ B., OTTEN U. Expression of functional trk tyrosine kinase receptors after T cell activation. J. Immunol. 1994;152:2705–2709. [PubMed] [Google Scholar]
- FRADE J.M., BARDE Y.A. Nerve growth factor: two receptors, multiple functions. Bioessays. 1998;20:137–145. doi: 10.1002/(SICI)1521-1878(199802)20:2<137::AID-BIES6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- FRADE J.M., RODRIGUEZ-TEBAR A., BARDE Y.A. Induction of cell death by endogenous nerve growth factor through its p75 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- HAMADA A., WATANABE N., OHTOMO H., MATSUDA H. Nerve growth factor enhances survival and cytotoxic activity of human eosinophils. Br. J. Haematol. 1996;93:299–302. doi: 10.1046/j.1365-2141.1996.5151055.x. [DOI] [PubMed] [Google Scholar]
- HEMPSTEAD B.L., MARTIN-ZANCA D., KAPLAN D.R., PARADA L.F., CHAO M.V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991;350:678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- HORIGOME K., PRYOR J.C., BULLOCK E.D., JOHNSON E.M., JR Mediator release from mast cells by nerve growth factor. Neurotrophin specificity and receptor mediation. J. Biol. Chem. 1993;268:14881–14887. [PubMed] [Google Scholar]
- JUNIPER E.F., FRITH P.A., HARGREAVE F.E. Airway responsiveness to histamine and methacholine: relationship to minimum treatment to control symptoms of asthma. Thorax. 1981;36:575–579. doi: 10.1136/thx.36.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILLIAN D., COCKCROFT D.W., HARGREAVE F.E., DOLOVICH J. Factors in allergen-induced asthma: relevance of the intensity of the airways allergic reaction and non-specific bronchial reactivity. Clin. Allergy. 1976;6:219–225. doi: 10.1111/j.1365-2222.1976.tb01900.x. [DOI] [PubMed] [Google Scholar]
- KLEIN R., JING S.Q., NANDURI V., O'ROURKE E., BARBACID M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991;65:189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- KONZETT H., RÖSSLER R. Versuchsanordhung zu Untersuchungen an der Bronchialmuskultur. Arch. Exp. Path. Pharmacol. 1940;195:71–77. [Google Scholar]
- LAMBIASE A., BRACCI LAUDIERO L., BONINI S., BONINI S., STARACE G., D'ELIOS M.M., DE CARLI M., ALOE L. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J. Allergy Clin. Immunol. 1997;100:408–414. doi: 10.1016/s0091-6749(97)70256-2. [DOI] [PubMed] [Google Scholar]
- LEE K.F., LI E., HUBER L.J., LANDIS S.C., SHARPE A.H., CHAO M.V., JAENISCH R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- LEON A., BURIANI A., DAL TOSO R., FABRIS M., ROMANELLO S., ALOE L., LEVI-MONTALCINI R. Mast cells synthesize, sotre, and release nerve growth factor. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGAI H., MAEDA Y., TANAKA H. The effect of anti-IL-4 monoclonal antibody, rapamycin and interferon-γ on airway hyperreactivity to acetylcholine in mice. Clin. Exp. Allergy. 1997;27:218–224. [PubMed] [Google Scholar]
- NATAF S., NAVEILHAN P., SINDJI L., DARCY F., BRACHET P., MONTERO MENEI C.N. Low affinity NGF receptor expression in the central nervous system during experimental allergic encephalomyelitis. J. Neurosci. Res. 1998;52:83–92. doi: 10.1002/(SICI)1097-4547(19980401)52:1<83::AID-JNR8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- OTTEN U., EHRHARD P., PECK R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 1989;86:10059–10063. doi: 10.1073/pnas.86.24.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINCELLI C. Nerve growth factor and keratinocytes: a role in psoriasis. Eur. J. Dermatol. 2000;10:85–90. [PubMed] [Google Scholar]
- SINGH N., BIRDI T.J., ANTIA N.H. Nerve growth factor production and expression of p75 by Schwann cells and neurofibroblasts in response to M.leprae infection and macrophage secretory products. Neuropathol. Appl. Neurobiol. 1997;23:59–67. [PubMed] [Google Scholar]
- TANAKA H., NAGAI H., MAEDA Y. Effect of anti-IL-4 and anti-IL-5 antibodies on allergic airway hyperresponsiveness in mice. Life Sci. 1998;62:PL169–PL174. doi: 10.1016/s0024-3205(98)00047-2. [DOI] [PubMed] [Google Scholar]
- VAIDYANATHAN S., KRISHNAN K.R., MANSOUR P., SONI B.M., MCDICKEN I. p75 nerve growth factor receptor in the vesical urothelium of patients with neuropathic bladder: an immunohistochemical study. Spinal Cord. 1998;36:541–547. doi: 10.1038/sj.sc.3100589. [DOI] [PubMed] [Google Scholar]
- VAN OOSTERHOUT A.J., NIJKAMP F.P. Role of cytokines in bronchial hyperresponsiveness. Pulm. Pharmacol. 1993;6:225–236. doi: 10.1006/pulp.1993.1030. [DOI] [PubMed] [Google Scholar]
- VIRCHOW J.C., JULIUS P., LOMMATZSCH M., LUTTMANN W., RENZ H., BRAUN A. Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am. J. Respir. Crit. Care Med. 1998;158:2002–2005. doi: 10.1164/ajrccm.158.6.9803023. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI S., NAGAI H., TANAKA H., TSUJIMOTO M., TSURUOKA N. Time course study for antigen-induced airway hyperreactivity and the effect of soluble IL-5 receptor. Life Sci. 1994;54:PL471–PL475. doi: 10.1016/0024-3205(94)90139-2. [DOI] [PubMed] [Google Scholar]


