Abstract
In rat small mesenteric arteries contracted with phenylephrine, 1-ethyl-2-benzimidazolinone (1-EBIO; 3 – 300 μM) evoked concentration-dependent relaxation that, above 100 μM, was associated with smooth muscle hyperpolarization.
1-EBIO-evoked hyperpolarization (maximum 22.1±3.6 mV with 300 μM, n=4) was endothelium-dependent and inhibited by charybdotoxin (ChTX 100 nM; n=4) but not iberiotoxin (IbTX 100 nM; n=4).
In endothelium-intact arteries, smooth muscle relaxation to 1-EBIO was not altered by either of the potassium channel blockers ChTX (100 nM; n=7), or IbTX (100 nM; n=4), or raised extracellular K+ (25 mM). Removal of the endothelium shifted the relaxation curve to the right but did not reduce the maximum relaxation.
In freshly isolated mesenteric endothelial cells, 1-EBIO (600 μM) evoked a ChTX-sensitive outward K-current. In contrast, 1-EBIO had no effect on smooth muscle cell conductance whereas NS 1619 (33 μM) stimulated an outward current while having no effect on the endothelial cells.
These data show that with concentrations greater than 100 μM, 1-EBIO selectively activates outward current in endothelial cells, which presumably underlies the smooth muscle hyperpolarization and a component of the relaxation. Sensitivity to block with charybdotoxin but not iberiotoxin indicates this current is due to activation of IKCa. However, 1-EBIO can also relax the smooth muscle by an undefined mechanism, independent of any change in membrane potential.
Keywords: 1-EBIO, endothelium, hyperpolarization, mesenteric artery, potassium channels, smooth muscle
Introduction
The putative IKCa channel activator, 1-EBIO has been employed recently to investigate the role of IKCa channels in the arterial endothelium-dependent hyperpolarizing factor (EDHF) pathway (Edwards et al., 1999). Interest in the involvement of IKCa channels has followed the demonstration that hyperpolarization sensitive to apamin and charybdotoxin could be evoked in endothelial cells (Edwards et al., 1998). This formed part of the evidence to support the suggestion that K+ can act as an EDHF. The suggestion was that an increase in endothelial cell calcium would stimulate K-channels, causing K+ efflux that could then hyperpolarize the adjacent smooth muscle cells causing relaxation (Edwards et al., 1998). Several types of K-channel have been found in endothelial cells, including small- and intermediate-conductance calcium-sensitive K-channels, which are sensitive to block with apamin and charybdotoxin, respectively (Groschner et al., 1992; Ling & O'neill, 1992; Vaca et al., 1992; van renterghem et al., 1995; Marchenko & Sage, 1996; Ishii et al., 1997). The ability to block EDHF responses with a combination of apamin and charybdotoxin but not apamin and iberiotoxin has become a defining pharmacological profile for the EDHF pathway in many tissues (Edwards & Weston, 2001), again emphasizing the importance of endothelial cell hyperpolarization in the pathway.
In this context, selective activators for SKCa and IKCa are of considerable potential use. Unfortunately, the number of selective activators for calcium-activated potassium channels is limited. Benzimidazolones such as NS 1619 activate the large-conductance KCa channel (BKCa; Olesen et al., 1994; Gribkoff et al., 1996) but not the intermediate-conductance KCa (IKCa; Cai et al., 1998). Unsurprisingly, these agents also have a variety of additional effects on other ion channels, such as inhibiting voltage-gated K-channels and voltage-dependent calcium channels in vascular smooth muscle (Edwards et al., 1994; Sheldon et al., 1997). Little is known about the mechanism of action of 1-EBIO. However, 1-EBIO does activate KCa channels in epithelial and endothelial cells (Devor et al., 1996; Cai et al., 1998, Edwards et al., 1999) and cloned IKCa channels in heterologous expression systems (Jensen et al., 1998). Recently, it has also been shown to activate cloned rSK2 channels, which is not really a surprise given the close structural homology between these channels (Syme et al., 2000). In arterial endothelial cells, sharp electrode recordings have shown that this agent can evoke a pronounced hyperpolarization that is sensitive to charybdotoxin but not iberiotoxin (Edwards et al., 1999). To what extent the activation of these channels with 1-EBIO links to smooth muscle relaxation, and the specificity of this action on IKCa channels are not known.
The aim of this study was to determine simultaneously the action of 1-EBIO on the tension and membrane potential in intact mesenteric arteries, to correlate these data with measurements of membrane conductance in smooth muscle and endothelial cells freshly isolated from the same vessel, in order to determine if 1-EBIO can selectively activate IKCa channels and cause smooth muscle relaxation as a consequence of the resulting hyperpolarization.
Methods
Male Wistar rats (200 – 250 g) were killed by cervical dislocation and exsanguination following schedule 1 procedures (Animals Scientific Procedure Act 1986, U.K.). The mesentery was removed and placed in Krebs buffer.
Small artery tension and electrophysiology
A segment (2 mm in length) of a third order branch of the superior mesenteric artery was mounted in a Mulvany – Halpern myograph (model 400A, J.P. Trading, Denmark) at a tension equivalent to that generated at 0.9 times the diameter of the vessel at 100 mmHg. Endothelial cell functional viability was assessed as the ability to induce over 95% relaxation with 1 μM acetylcholine in arterial segments pre-constricted with a submaximal concentration of phenylephrine. All experiments were performed in the presence of the NO synthase inhibitor, Nω-nitro-L-arginine methyl ester (L-NAME, 100 μM), and where stated, in the presence of either ChTX or IbTx each applied for 10 – 15 min before the addition of phenylephrine. Endothelial cells were removed, where applicable, by gently rubbing with a human hair. The lack of a relaxation to acetylcholine (<5%) in the endothelium-denuded vessels was taken as evidence of the successful removal of the endothelium. This procedure does not modify relaxations to non-endothelium-dependent dilator agents (see Plane et al., 2001).
Smooth muscle membrane potential and tension were recorded simultaneously as previously described (Garland & Mcpherson, 1992). The artery was superfused with oxygenated and heated Krebs buffer at 3 – 4 ml/min. Individual smooth muscle cells were impaled with sharp glass electrodes (filled with 2 M KCl, tip resistances approximately 100 MΩ). After stopping the superfusion, phenylephrine was added to the bath (to evoke depolarization, PEEm and contraction), and mixed by gassing. Hyperpolarization and relaxation was then assessed to cumulative concentrations of 1-EBIO (10 – 300 μM final bath concentration). In artery segments with an intact endothelium, acetylcholine was then added to obtain a maximum hyperpolarization in the smooth muscle cell (AChEm). The maximum hyperpolarization was taken as the difference between PEEm and AChEm. In order to compare per cent hyperpolarization responses between endothelium intact and denuded arteries, the mean value for AChEm was used in denuded arteries.
Single cell electrophysiology
After removal, mesenteric arteries were cleaned of fat and connective tissue and were placed in a HEPES buffered low Ca2+ physiological saline solution (low Ca PSS) containing: 1.0 mg ml−1 dithiothreitol, 1.5 mg ml−1 papain and bovine serum albumin (1.5 mg ml−1) at 37°C for approximately 30 min. The tissue was then transferred to a collagenase (1.0 mg ml−1), elastase (1.0 mg ml−1) and bovine serum albumin (1.5 mg ml−1) containing low Ca PSS solution (37°C) for a further 15 min before several washes in physiological saline solution and trituration with a wide bore pipette to disperse the cells.
Recordings from mesenteric smooth muscle and endothelial cells were made at room temperature (22°C) using the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981). Pipettes were fabricated from borosilicate glass (Clarke, Electromedical Instruments) and had a resistance of 3 – 6 MΩ after being fire polished. Whole-cell currents were acquired using an Axopatch 1D amplifier (Axon Instruments) and digitised through a Digidata 1200 interface (Axon Instruments). Command paradigms and data analysis were performed using winwcp software (Strathclyde University) on a PC-compatible computer. To measure membrane current, voltage-step (from a holding potential of either −90 or −10 mV, a series of 500 ms voltage steps from −150 to +50 mV were applied) and voltage-ramp (from a holding potential of −60 or 0 mV followed by a step to +50 mV and a subsequent 1.5 s voltage ramp to −150 mV) protocols were employed.
Solutions and drugs
Arteries were maintained at 37°C in oxygenated Krebs buffer of the following composition (mM): NaCl 118.0, NaHCO3 25.0, KCl 3.6, MgSO4. 7H2 O 1.2, KH2PO4 1.2, glucose 11.0 and CaCl2 2.5 which was continuously aerated with 95% O2 and 5% CO2. For patch-clamp experiments, the external solution contained (mM): NaCl 140, KCl 5, MgCl2 1, H2PO42− 1, CaCl2 2, glucose 11, HEPES 10, (pH 7.4) and the low Ca2+ PSS (used with NS 1619) comprised (in mM): NaCl 136, KCl 5.6, MgCl2 1, NaHCO3 4.17, NaHPO4 0.4, Na2HPO4 0.4, HEPES 10, CaCl2 0.1. Pipette solution comprised (in mM): KCl 40, potassium aspartate 100, MgCl2 1, EGTA 0.5, Na2ATP 4, HEPES 10 (pH 7.2).
Drugs used were all from Sigma except for synthetic charybdotoxin and iberiotoxin (Latoxan) and 1-ethyl-2-benzimidazolinone (Aldrich). Stock solutions of 1-EBIO (0.1 M) were dissolved in dimethylsulfoxide (DMSO); charybdotoxin was dissolved in 0.9% saline; and all other stock solutions of compounds were dissolved in distilled water. Control experiments indicated that DMSO had no direct action in the concentrations used.
Statistics
All results are expressed as means±s.e.mean of n animals, unless otherwise stated. Mann – Whitney test or ANOVA were used to assess the probability that differences between mean values had arisen by chance; P<0.05 was considered to be statistically significant.
Results
Effect of 1-EBIO on isometric tension
Under control conditions in the presence of L-NAME, 1-EBIO (3 – 300 μM) evoked a concentration dependent relaxation in phenylephrine (1 – 3 μM) pre-constricted arteries (Figures 1 and 2). Removal of the endothelium produced a rightward shift in the concentration-response curve for 1-EBIO which was statistically significant at 100 μM (P<0.01, n=6). In addition, in intact vessels neither ChTX (100 nM, n=7) nor IbTX (100 nM, n=4) had any effect on the relaxation induced by 1-EBIO.
Figure 1.
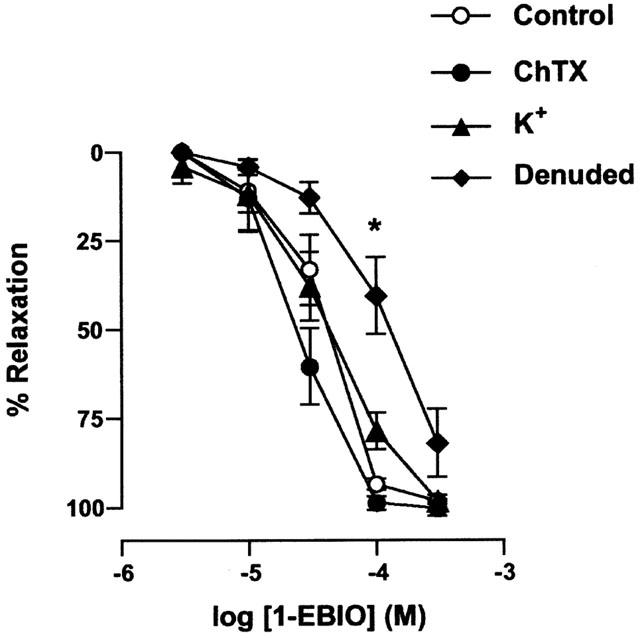
Concentration-response curves for 1-EBIO-induced relaxation in phenylephrine (PE) pre-contracted arteries. In endothelium-intact arteries in the presence of 100 μM L-NAME, 1-EBIO-evoked relaxation was unaffected by the additional presence of either ChTX (100 nM) or KCl (25 mM). Removing the endothelium shifted the 1-EBIO response curve to the right, without affecting the maximal relaxation. All values are means±s.e.mean from five to seven experiments, and were analysed non-parametrically with the Mann – Whitney test. *P<0.05 compared with control.
Figure 2.
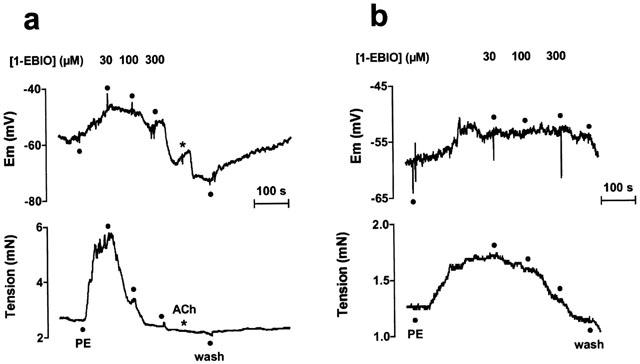
Representative traces showing simultaneous changes in membrane potential (upper panel) and tension (lower panel) elicited by 1-EBIO (30 – 300 μM) in isolated mesenteric artery smooth muscle pre-contracted with phenylephrine (0.1 μM, unpaired arteries). a Endothelium-intact artery (100 μM L-NAME present throughout); b Endothelium-denuded artery.
If the action of 1-EBIO were solely due to the activation of K-channels, then precontraction of the arteries with KCl (25 mM) would be expected to prevent or inhibit the relaxation recorded in the presence of 1-EBIO. However, under such conditions there was no significant difference between the concentration-relaxation curve for 1-EBIO with phenylephrine-preconstriction in the presence and absence of raised K+ (n=5, Figure 1). In the presence of 25 mM KCl, 300 μM 1-EBIO did not alter the smooth muscle cell membrane potential, at −34.2±2.1 mV without and −36.6±5.1 mV with 1-EBIO present (n=4).
Effect of 1-EBIO on smooth muscle membrane potential in arteries
Resting membrane potential in endothelium-intact arteries was −56.4±0.6 mV (n=5). Phenylephrine depolarized the arteries to −41.9±3.0 mV and increased tension to 3.9±0.5 mN (n=5). 1-EBIO (300 μM) hyperpolarized the smooth muscle by 22.1±4 mV to a mean potential of −64.0±2.4 mV (n=5, Figure 2a). The hyperpolarization to 1-EBIO was not modified by the presence of IbTX (15.9±3.9 mV, n=4), yet was significantly reduced by ChTX when the change was only 6.3±4.3 mV (n=4). Figures 2 and 3 show that at concentrations <100 μM, 1-EBIO produced marked relaxation of the artery without any measurable change in the membrane potential. The effect of either ChTX or IbTX on 1-EBIO induced hyperpolarization and relaxation is summarised in Figure 3. The tension measurements indicate that the action of 1-EBIO is both ChTX and IbTX-insensitive, whereas 1-EBIO-induced hyperpolarization was significantly blocked in the presence of ChTX (P<0.05).
Figure 3.
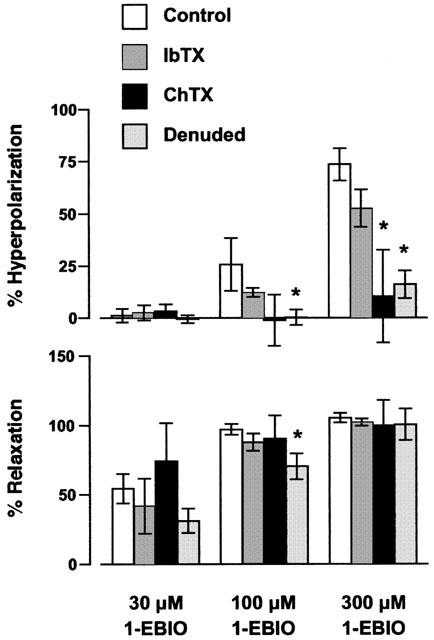
Summary data representing the 1-EBIO-induced hyperpolarization and relaxation in phenylephrine-stimulated arteries. In endothelium-intact arteries (in the presence of 100 μM L-NAME) 1-EBIO-evoked hyperpolarization was markedly reduced in the presence of ChTX (100 nM), but not IbTX (100 nM). The hyperpolarization to 1-EBIO was dependent on an intact endothelium. All values are means±s.e.mean from four to eight experiments (compared with the Mann – Whitney test, *P<0.05 compared with control). Hyperpolarization to 1-EBIO was calculated as a per cent of the Achmax.
Removal of the endothelium slightly depolarized the resting membrane potential (−51.9±1.2 mV, n=8) and did not alter the resting tension (1.62±0.28 compared to 1.81±0.15 mN after removal, n=5 and 8, respectively). Low concentrations of phenylephrine (50 – 300 nM) were chosen to depolarize the smooth muscle to a similar level as in endothelium-intact arteries (−40.5±2.1 mV), and gave a mean increase in tension to 2.3±0.2 mN (n=8). Removal of the endothelium effectively abolished membrane potential changes to 300 μM 1-EBIO, with a mean residual response of only 5.8±2.4 mV, while relaxation was unaffected (Figures 2b and 3).
Effect of 1-EBIO on endothelial and smooth muscle whole-cell K-currents
The action of 1-EBIO on endothelial and smooth muscle cells freshly isolated from rat small mesenteric arteries was investigated using the whole-cell patch-clamp technique. In endothelial cells, a holding potential of 0 mV followed by a step to +50 mV and a subsequent 1.5 s voltage ramp to −150 mV generated an inwardly rectifying K-current (IK(IR), Figure 4a). The presence of 1-EBIO (600 μM) induced a voltage-insensitive outward K-current with a mean amplitude of 42.8±3.4 pA at +50 mV (n=6, Figure 4b). The current induced by 1-EBIO was completely blocked by the addition of ChTX (250 nM, n=4). In addition, the amplitude of IK(IR) was significantly reduced in the presence of both 1-EBIO and ChTX. The BKCa current activator, NS 1619 (33 μM) did not modify the conductance of mesenteric artery endothelial cells (n=4, Figure 4d).
Figure 4.
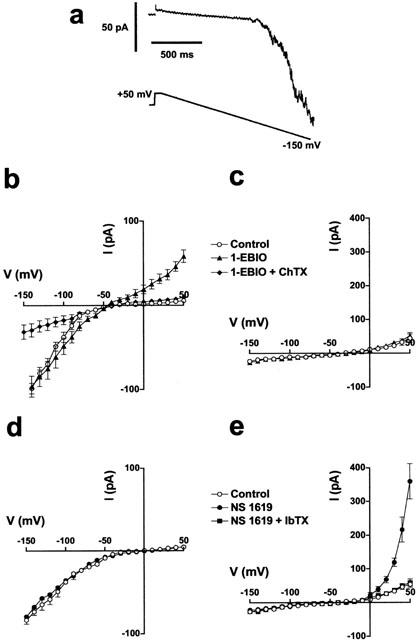
Effects of 1-EBIO and NS 1619 on whole-cell currents. (a) Typical whole-cell current recording from a freshly isolated endothelial cell under control conditions. (b,d) Current (I)-voltage (V) relationship for K-current in endothelial cells generated from a holding potential of −60 mV followed by a step to +50 mV and a subsequent 1.5 s voltage ramp to −150 mV, in the absence and presence of 1-EBIO (600 μM) and with the addition of ChTX (250 nM, b); or NS 1619 (33 μM, d). (c,e) I – V relationship for K-current in smooth muscle cells from a holding potential of −10 mV to test potentials of −150 to +50 mV (500 mS voltage steps) in the absence and presence of 1-EBIO (c); or from a holding potential of −10 mV to test potentials of −150 to +50 mV with either NS 1619 or NS 1619 and IbTX (250 nM, e). Each point represents the mean±s.e.mean (n=4).
In smooth muscle cells held at −10 mV and then subjected to a series of voltage steps between −150 and +50 mV, 600 μM 1-EBIO failed to induce any measurable K-current (n=4, Figure 4c), whereas NS 1619 (33 μM) activated a large K-current (mean amplitude of 360±52 pA at +50 mV, n=4) with characteristics similar to BKCa. This current was sensitive to block with IbTX (Figure 4e).
Discussion
The present study addressed two main questions: whether 1-EBIO caused smooth muscle relaxation by selectively activating IKCa channels, and whether these channels were restricted to the endothelium. In answering these questions, the importance of simultaneous measurements of tension and membrane potential is apparent, as relaxation to 1-EBIO was found to occur at lower concentrations than the effect of this agent on K-channels.
The relaxation with 1-EBIO, at concentrations below 100 μM, occurred in the absence of any apparent hyperpolarization. This indicates an action that is independent of potassium channel activation. This finding was confirmed in experiments where both smooth muscle and endothelial cell hyperpolarization were prevented by raising extracellular [K+] to 25 mM, which has previously been shown to abolish hyperpolarization and relaxation of mesenteric arteries to the release of EDHF by acetylcholine (Waldron & Garland, 1994). This manoeuvre did not affect the relaxation to 1-EBIO at any concentration. Furthermore, the ability of 1-EBIO to evoke hyperpolarization appeared to be dependent entirely on the endothelium (Edwards et al., 1999). So the fact that the concentration-response curve for smooth muscle relaxation with 1-EBIO was shifted, but not depressed, in endothelium-denuded mesenteric arteries also indicates a direct action on the smooth muscle cells, an effect that was independent of any change in membrane potential.
How 1-EBIO evokes relaxation without modifying the membrane potential is not clear. The structurally related benzimidazolone, NS 1619, which activates BKCa is known to inhibit both KV channels and plasmalemma calcium currents (Edwards et al., 1994; Sheldon et al., 1997). However, similar effects seem unlikely to explain the relaxation with 1-EBIO. Block of KV channels would, if anything, reduce smooth muscle relaxation. With calcium channels, we were unable to modify relaxation to 1-EBIO with nifedipine (data not shown), so it may be that calcium currents are unaffected by this agent but this remains to be determined.
The finding that 1-EBIO can act to cause relaxation independently of potassium channel activation is supported by the lack of effect of either IbTX or ChTX. These observations appear to contrast somewhat with a previous report in the rat isolated and perfused mesenteric bed (Adeagbo, 1999). In this study, 1-EBIO evoked endothelium-dependent dilatation (0.1 – 30 nmol, bolus additions) during constrictor stimulation with the α-agonist, cirazoline. The dilator action of 1-EBIO appeared to reflect an action of both nitric oxide and EDHF, confirming the role of both agents in endothelium-dependent responses in this perfused vascular bed (Parsons et al., 1994). The nitric oxide-independent relaxant effect could be blocked with an inhibitor of BKCa penitrem A, and as a consequence it was concluded that the vasodilator action of 1-EBIO involved the opening of BKCa (Adeagbo, 1999). It may be the case that a component of the relaxation to 1-EBIO does involve the activation of BKCa in the mesenteric artery arcade. If so, this effect is not in the same mesenteric artery branches that we studied, as the selective blocker of BKCa, iberiotoxin, did not block relaxation or hyperpolarization to 1-EBIO. Further, from the present study it seems extremely unlikely that BKCa channels are present on endothelial cells in the mesenteric artery. This is also the case in other vessels, as several reports using freshly isolated endothelial cells also report an absence of BKCa channels (Kestler et al., 1998; Jow et al., 1999). It remains a possibility that penitrem A may block IKCa as well as BKCa, which would then explain the attenuation reported by Adeagbo (1999).
With concentrations of 100 μM and above, 1-EBIO evoked endothelium-dependent smooth muscle hyperpolarization as well as relaxation. This effect was consistent with the hyperpolarization recorded to 600 μM 1-EBIO in the rat hepatic artery (bolus addition, Edwards et al., 1999). In the hepatic artery, this concentration of 1-EBIO was shown to stimulate around 20 and 12 mV hyperpolarization in endothelial and smooth muscle cells, respectively. The hyperpolarizations were abolished with ChTX but not IbTX. This profile was similar to the current study with the mesenteric artery, consistent with an action of 1-EBIO on IKCa. The finding that the hyperpolarization was endothelium dependent, indicates the channels are selectively located on these cells, a conclusion supported by the patch clamp studies with freshly isolated cells from the mesenteric artery (see below). The fact that removing the endothelium did significantly reduce relaxation, at least with 100 μM, shows that 1-EBIO induced hyperpolarization contributes to the functional response. As removal of the endothelium was associated with a greater shift in the relaxation-response curve to 1-EBIO than in the presence of ChTX, this may also reflect an activation of endothelial cell SKCa channels with this agent (Syme et al., 2000). With the higher concentration of 1-EBIO, or in the presence of either ChTX or high K+, failure to detect any significant reduction in relaxation after endothelium-removal may simply reflect the intensity of the membrane-independent drive to relaxation. It may also be that in part these voltage-independent effects are also exerted through the endothelium, so endothelium removal has a more significant impact than blocking the voltage-dependent component with ChTX or high K+.
Patch clamp studies with freshly isolated endothelial and smooth muscle cells from the mesenteric artery showed that 1-EBIO could activate outward current only in the endothelial cells. This current was sensitive to block with charybdotoxin. In addition, together, but not individually, 1-EBIO and ChTX were also associated with an inhibition of the inward K-current recorded at negative voltages in the endothelial cells. These observations are very similar to data obtained from bovine aortic endothelial cells in culture (Edwards et al., 1999). We are unable to explain this blocking effect at present. In mesenteric artery smooth muscle cells, 1-EBIO did not induce any outward K-current indicating that IKCa channels are not located on these cells. These data do indicate, however, that 1-EBIO will not activate BKCa channels, so that in activating calcium-sensitive potassium channels the effect of this agent is sub-type selective. BKCa channels are commonly found in smooth muscle cells (Edwards et al., 1994; Holland et al., 1996; Mistry & Garland, 1998; Walker et al., 1996) and were activated with NS 1619 in the mesenteric smooth muscle but not endothelial cells in this study. The latter observation is consistent with previous studies on freshly-isolated endothelial cells, where no evidence for BKCa channels could be found in human umbilical vein endothelial cells and human capillary endothelial cells (Kestler et al., 1998; Jow et al., 1999).
In summary, the benzimidazolinone 1-EBIO has been shown to stimulate ChTX-sensitive but IbTX-insensitive smooth muscle hyperpolarization, which is dependent on the endothelium. Patch clamp recordings from cells freshly isolated from the same artery, demonstrate a ChTX-sensitive K-current induced by 1-EBIO in endothelial but not smooth muscle cells. Together these findings suggest that 1-EBIO can activate K channels, probably IKCa channels, on the endothelium. The simultaneous measurement of membrane potential and tension change, has revealed that at <100 μM, smooth muscle relaxation to 1-EBIO occurred without any change in membrane potential. Our data also show that BKCa channels are present in rat mesenteric artery smooth muscle cells but not endothelial cells.
Acknowledgments
This work was supported by the Wellcome Trust, U.K.
Abbreviations
- BKCa
large-conductance calcium-activated potassium channels
- 1-EBIO
1-ethyl-2-benzimidazolinone
- EDHF
endothelium derived hyperpolarizing factor
- IKCa
intermediate-conductance calcium-activated potassium channels
- L-NAME
Nω-nitro-L-arginine methyl ester
References
- ADEAGBO A.S.O. 1-Ethyl-2-benzidazolinone stimulates endothelial KCa channels and nitric oxide formation in rat mesenteric vessels. Eur. J. Pharmacol. 1999;379:151–159. doi: 10.1016/s0014-2999(99)00489-6. [DOI] [PubMed] [Google Scholar]
- CAI S., GARNEAU L., SAUVE R. Single-channel characterization of the pharmacological properties of the K(Ca2+) channel of intermediate conductance in bovine aortic endothelial cells. J. Membr. Biol. 1998;163:147–158. doi: 10.1007/s002329900379. [DOI] [PubMed] [Google Scholar]
- DEVOR D.C., SINGH A.K., FRIZZELL R.A., BRIDGES R.J. Modulation of Cl− secretion by benzimidazolones. 1. Direct activation of a Ca2+-dependent K+ channel. Am. J. Physiol. 1996;271:L775–L784. doi: 10.1152/ajplung.1996.271.5.L775. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., GARDENER M.J., FELETOU M., BRADY G., VANHOUTTE P.M., WESTON A.H. Further investigation of endothelium-derived hyperpolarizing factor in rat hepatic artery: studies using 1-EBIO and ouabain. Br. J. Pharmacol. 1999;128:1064–1070. doi: 10.1038/sj.bjp.0702916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., NIEDERSTE-HOLLENBERG A., SCHNEIDER J., NOACK T.H., WESTON A.H. Ion channel modulation by NS1619, the putative BKCa channel opener, in vascular smooth muscle. Br. J. Pharmacol. 1994;113:1538–1547. doi: 10.1111/j.1476-5381.1994.tb17171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., WESTON A.H. EDHF–are there any gaps in the pathway. J. Physiol. 2001;531:299. doi: 10.1111/j.1469-7793.2001.0299i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND C.J., MCPHERSON G.A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br. J. Pharmacol. 1992;105:429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIBKOFF V.K., LUM-RAGAN J.T., BOISSARD C.G., POST-MUNSON D.J., MEANWELL N.A., STARRETT J.E., KOZLOWSKI E.S., ROMINE J.L., TROJNACKI J.T., MCKAY M.C., ZHONG J., DWORETSKY S.I. Effects of channel modulators on cloned large-conductance calcium-activated potassium channels. Mol. Pharmacol. 1996;50:206–217. [PubMed] [Google Scholar]
- GROSCHNER K., GRAIER W.F., KUKOVETZ W.R. Activation of a small-conductance Ca2+-dependent K+ channel contributes to bradykinin-induced stimulation of nitric oxide synthesis in pig aortic endothelial cells. Biochim. Biophys. Acta. 1992;1137:162–170. doi: 10.1016/0167-4889(92)90198-k. [DOI] [PubMed] [Google Scholar]
- HAMILL O.P., MARTY A., NEHER E., SAKMANN B., SIGWORTH F.J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pfuglers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- HOLLAND M., LANGTON P.D., STANDEN N.B., BOYLE J.P. Effects of the BKCa channel activator, NS1619, on rat cerebral artery smooth muscle. Br. J. Pharmacol. 1996;117:119–129. doi: 10.1111/j.1476-5381.1996.tb15163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHII T.M., SILVIA C., HIRSCHBERG B., BOND C.T., ADELMAN J.P., MAYLIE J. A human intermediate conductance calcium-activated potassium channel. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN B.S., STROBAEK D., CHRISTOPHERSEN P., JORGENSEN T.D., HANSEN C., SILAHTAROGLU A., OLESEN S.P., AHRING P.K. Characterization of the cloned human intermediate-conductance Ca2+-activated K+ channel. Am. J. Physiol. 1998;275:C848–C856. doi: 10.1152/ajpcell.1998.275.3.C848. [DOI] [PubMed] [Google Scholar]
- JOW F., SULLIVAN K., SOKOL P., NUMANN R. Induction of Ca2+-activated K+ current and transient outward currents in human capillary endothelial cells. J. Memb. Biol. 1999;167:53–64. doi: 10.1007/s002329900471. [DOI] [PubMed] [Google Scholar]
- KESTLER H.A., JANKO S., HAUSSLER U., MUCHE R., HOMBACH V., HOHER M., WIECHA J. A remark on the high-conductance calcium-activated potassium channel in human endothelial cells. Res. Exp. Med. (Berl.) 1998;198:133–143. doi: 10.1007/s004330050097. [DOI] [PubMed] [Google Scholar]
- LING B.N., O'NEILL W.C. Ca2+-dependent and Ca2+-permeable ion channels in aortic endothelial cells. Am. J. Physiol. 1992;263:H1827–H1838. doi: 10.1152/ajpheart.1992.263.6.H1827. [DOI] [PubMed] [Google Scholar]
- MARCHENKO S.M., SAGE S.O. Calcium-activated potassium channels in the endothelium of intact rat aorta. J. Physiol. 1996;492:53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISTRY D.K., GARLAND C.J. Characteristics of single, large-conductance calcium-dependent potassium channels (BKCa) from smooth muscle cells isolated from the rabbit mesenteric artery. J. Memb. Biol. 1998;164:125–138. doi: 10.1007/s002329900399. [DOI] [PubMed] [Google Scholar]
- OLESEN S.P., MUNCH E., MOLDT P., DREJER J. Selective activation of Ca2+-dependent K+ channels by novel benzimidazolone. Eur. J. Pharmacol. 1994;251:53–59. doi: 10.1016/0014-2999(94)90442-1. [DOI] [PubMed] [Google Scholar]
- PARSONS S.J., HILL A., WALDRON G.J., PLANE F., GARLAND C.J. The relative importance of nitric oxide and nitric oxide- independent mechanisms in acetylcholine-evoked dilatation of the rat mesenteric bed. Br. J. Pharmacol. 1994;113:1275–1280. doi: 10.1111/j.1476-5381.1994.tb17136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLANE F., SAMPSON L.J., SMITH J.J., GARLAND C.J. Relaxation to authentic nitric oxide and SIN-1 in rat isolated mesenteric arteries: variable role for smooth muscle hyperpolarization. Br. J. Pharmacol. 2001;133:665–672. doi: 10.1038/sj.bjp.0704127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHELDON J.H., NORTON N.W., ARGENTIERI T.M. Inhibition of guinea pig detrusor contraction by NS-1619 is associated with activation of BKCa and inhibition of calcium currents. J. Pharmacol. Exp. Ther. 1997;283:1193–2000. [PubMed] [Google Scholar]
- SYME C.L., GERLACH A.C., SINGH A.K., DEVOR D.D. Pharmacological activation of cloned intermediate- and small-conductance Ca2+-activated K+ channels. Am. J. Physiol. 2000;278:C570–C581. doi: 10.1152/ajpcell.2000.278.3.C570. [DOI] [PubMed] [Google Scholar]
- VACA L., SCHILLING W.P., KUNZE D.L. G-protein-mediated regulation of a Ca2+-dependent K+ channel in cultured vascular endothelial cells. Pflugers Archiv. 1992;422:66–74. doi: 10.1007/BF00381515. [DOI] [PubMed] [Google Scholar]
- VAN RENTERGHEM C., VIGNE P., FRELIN C. A charybdotoxin-sensitive, Ca2+-activated K+ channel with inward rectifying properties in brain microvascular endothelial cells: properties and activation by endothelins. J. Neurochem. 1995;65:1274–1281. doi: 10.1046/j.1471-4159.1995.65031274.x. [DOI] [PubMed] [Google Scholar]
- WALDRON G.J., GARLAND C.J. Contribution of both nitric oxide and a change in membrane potential to acetylcholine-induced relaxation in the rat small mesenteric artery. Br. J. Pharmacol. 1994;112:831–836. doi: 10.1111/j.1476-5381.1994.tb13154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER S.D., GREEN M.E., EDWARDS G.E., WESTON A.H. Characterisation of potassium currents in rat pulmonary arterial smooth muscle cells. Br. J. Pharmacol. 1996;119:80P. doi: 10.1111/j.1476-5381.1996.tb16066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


