Abstract
The aim of this study was to establish how thromboxane receptors (TP) respond to the increase in levels of plasma thromboxane observed in both cardiac (cardiomyopathy, ischaemic heart disease and pulmonary hypertension) and vascular disease (atherosclerosis of coronary artery disease and accelerated atherosclerosis of saphenous vein grafts).
The agonist radioligand [125I]-BOP, bound rapidly to TP receptors in normal human cardiovascular tissue, displaying high affinity in left ventricle (KD 0.23±0.06 nM, Bmax 28.4±5.7 fmol mg−1 protein) and reversibility with a t1/2 of 10 min (n=five individuals±s.e.mean).
In the heart, TP receptor density in the right ventricle of primary pulmonary hypertensive patients were significantly increased (66.6±6 fmol mg−1 protein) compared to non-diseased right ventricle (37.9±4.1 fmol mg−1 protein, n=six individuals±s.e.mean, P<0.05).
In diseased vessels, TP receptor densities were significantly increased (3 fold in the intimal layer) in atherosclerotic coronary arteries, saphenous vein grafts with severe intimal thickening (n=8 – 12 individuals, P<0.05) and aortic tissue (n=5 – 6 individuals, P<0.05), compared with normal vessels.
Losartan, tested at therapeutic doses, competed for [125I]-BOP binding to human vascular tissue, suggesting that some of the anti-hypertensive effects of this AT1 receptor antagonist could also be mediated by blocking human TP receptors.
The differential distribution of TP receptors in the human cardiovascular system and the alteration of receptor density, accompanying the increase in endogenous thromboxane levels in cardiovascular disease, suggest that TP receptors represent a significant target for therapeutic interventions and highlights the importance for the development of novel selective antagonist for use in humans.
Keywords: Thromboxane, thromboxane receptor, human coronary artery, losartan, atherosclerosis, cardiomyopathy, pulmonary hypertension, saphenous vein graft, angiotensin, human heart
Introduction
The lipid mediator thromboxane A2 (Tx) is a biologically active metabolite of arachidonic acid, which is synthesized from prostaglandin endoperoxide (Narumiya et al., 1999) via thromboxane A synthase (Smith, 1987). Once formed, Tx which has a very short half life (t1/2=30 s), is rapidly broken down by hydrolysis to the inactive thromboxane B2 (Halushka & Lefer, 1987; Reilly & Fitzgerald, 1993; Sachinidis et al., 1995). Tx is produced locally by platelets, macrophages (Reilly & Fitzgerald, 1993), vascular smooth muscle cells of arteries and veins (Serneri et al., 1983), endothelial cells (Mehta & Roberts, 1983; Sung et al., 1989) and human cardiac atrial tissue (Mehta & Mehta, 1985). In addition to its major role as a powerful platelet aggregator (Halushka et al., 1989), Tx is a potent vasoconstrictor (Halushka et al., 1989), stimulator of vascular smooth muscle cell growth (Sachinidis et al., 1995) and is a positive inotropic mediator in the heart (Sakuma et al., 1989). Interestingly, increased production (approximately 10 ng ml−1, compared with 1 – 2 pg ml−1 in normal healthy plasma) of Tx has been implicated in cardiac pathology, including ischaemic heart disease (Serneri et al., 1981), pulmonary hypertension (Fuse & Kamiya, 1994) and heart failure (Gresele et al., 1991). Additionally, increased production of Tx is also associated in vascular pathology, particularly with atherosclerosis of coronary artery disease and accelerated atherosclerosis of saphenous vein graft (Mehta et al., 1988; Gresele et al., 1991).
The human thromboxane receptor (TP) is a G protein coupled receptor composed of 343 amino acids (Narumiya et al., 1999). Molecular biology and pharmacological studies have identified mRNA encoding the TP receptor and TP receptor protein in animal and human tissues in culture (Halushka et al., 1989; Coleman et al., 1994). Radioligand binding studies have characterized TP receptor protein in human kidney (Brown & Venuto, 1999) and human vascular smooth muscle cells in culture (Morinelli et al., 1990). However, TP receptor distribution and density in native human cardiovascular tissue has not been determined and it is not known if receptor density is altered with cardiovascular disease in native human tissue.
The aims of this study were to firstly, characterize the binding of [125I]-BOP (Morinelli et al., 1989) in human cardiovascular tissue as well as determining TP receptor density and the anatomical distribution. Secondly, to understand how TP receptors respond to increased levels of endogenous Tx as seen in cardiac disease (including cardiomyopathy, ischaemic heart disease, pulmonary hypertension) and in vascular pathology (atherosclerosis of coronary artery disease and accelerated atherosclerosis of saphenous vein graft). It is hypothezised that in response to an increase in endogenous ligand, a compensatory down regulation of receptors would be expected if normal homeostatic mechanisms are to be maintained.
Losartan is a potent, non peptide, angiotensin type 1 receptor antagonist that when orally administered produces inhibition of angiotensin II induced vasoconstriction in human forearm (Baan et al., 1996) and lowers blood pressure in human hypertension (Wong et al., 1990). However, evidence suggests additional mechanisms may be involved in the anti-hypertensive action of losartan. This is highlighted by the increased release of prostaglandin I2 in cultured vascular smooth muscle cells by 10 nM losartan (Jaiswal et al., 1991) and the inhibition of thromboxane A2 contraction in canine coronary artery (Li et al., 1997). Further studies have shown that losartan at its therapeutic concentration of 1 μM (Munafo et al., 1992), can bind to TP receptors in human platelets (Guerra-Cuesta et al., 1999; Jagroop & Mikhailidis, 2000; Levy et al., 2000). However, the possibility that losartan may bind to TP receptors in human non-diseased and diseased cardiac and vascular tissue has not been investigated. Therefore, the final aim of this study was to determine using radioligand binding if losartan competes for TP receptors in human coronary artery and by using in vitro receptor autoradiography to establish if 1 μM losartan, a concentration within the therapeutic range, competes for TP receptors in non-diseased and diseased human cardiovascular tissue. Preliminary data were presented to the British Pharmacological Society (Katugampola & Davenport, 2000; 2001).
Methods
Tissue collection
With local ethical approval, human right atrial, right ventricular, left atrial and left ventricular tissues were obtained from patients with histologically normal hearts (Smith et al., 1996) not required for further transplantation (four female, two male, 24 – 53 years). Coronary arteries, left ventricle, aortic samples and pulmonary arteries were obtained from patients undergoing transplantation for dilated cardiomyopathy (DCM) or heart lung transplants (14 males, four females, 25 – 58 years). Right ventricular tissues were obtained from patients diagnosed with primary pulmonary hypertension (PPH; three males, three females, 35 – 52 years). Samples of epicardial coronary arteries containing advanced atheromatous lesions, occluded saphenous vein bypass grafts, aortic tissue samples, left ventricle and right atrial tissues were obtained from patients undergoing transplantation for ischaemic heart disease (IHD; 17 males, five females, 41 – 60 years). Non-diseased saphenous veins and left internal mammary arteries were obtained from patients undergoing coronary artery bypass graft surgery (seven females, five males, 54 – 80 years). Drug therapies included angiotensin converting enzyme inhibitors, beta blockers, diuretics, calcium channel blockers, vasodilators, lipid lowering agents, vitamin supplements and digoxin.
Kinetic studies
Kinetic studies were carried out as described for characterization of endothelin receptors in human tissue (Davenport et al., 1998). Briefly for association studies, 30 μm cryostat sections of non-diseased human left ventricle were incubated for 0 – 120 min with 0.1 nM [125I]-BOP. SQ29548 (1 μM) defined non-specific binding. For dissociation studies, sections of non-diseased human left ventricle were incubated with 0.1 nM [125I]-BOP, 30 min before sections were washed in an excess of buffer at room temperature for increasing time periods (0 – 240 min).
Saturation and competition binding studies
Following optimization of binding conditions, cryostat sections (30 μm) of human tissues were pre-incubated in 20 mM HEPES assay buffer, containing (mM): NaCl 140, KCl 5, MgCl2 5 (pH 7.4) for 10 min. Saturation binding curves with 12 points were constructed using increasing concentrations (0.01 – 1.5 nM) of [125I]-BOP for 30 min at 23°C. Non-specific binding was defined by incubating adjacent sections with 1 μM SQ29548. Protein concentration was determined using the Biorad DC 96-well microtiter plate system (Biorad Laboratories, Hertfordshire, U.K.).
Competition binding experiments were carried out under the same conditions described above. Sections of non-diseased human coronary artery were labelled with a fixed concentration (0.1 nM) of [125I]-BOP and in the presence of increasing concentrations of competing ligand (1 pM – 100 μM). Non-specific binding was defined by incubating adjacent sections with 1 μM SQ29548. Selectivity was further defined by incubating [125I]-BOP in the presence and absence of unlabelled prostaglandins and peptides at 1 μM concentration. The optimum conditions for separating tissue bound [125I]-BOP from free ligand was determined and found to be 2×5 s washes in 50 mM Tris buffer (pH 7.4, 4°C).
Quantitative autoradiography
Binding of [125I]-BOP was used to visualize TP receptors autoradiographically, with the method adopted for the endothelin receptors (Maguire & Davenport, 1999). Cryostat tissue sections were incubated with 0.1 nM [125I]-BOP in the absence or presence of SQ29548 (1 μM) under the conditions described above. Dried sections were apposed to radiation sensitive film (Hyperfilm βmax, Amersham Pharmacia Biotech., Bucks, U.K.) along with 125I miroscale standards for 4 days. Developed autoradiograms were analysed by computer assisted densitometry (Davenport et al., 1995). Binding densities were compared using Student's unpaired t-test. The significance level was set at 95% (P<0.05). Adjacent sections were stained with haematoxylin and eosin to facilitate histological identification.
Data analysis
The results from binding experiments were analysed as previously described (Maguire et al., 1996) using the iterative, non-linear curve fitting programmes EBDA and LIGAND in the KELL package (Biosoft, Cambridge, U.K.). All values are expressed as mean±s.e.mean. Kinetic analysis of [125I]-BOP binding provided estimates for the observed association (kobs) and dissociation rate constants. These were in turn used to derive values for the association rate constant and the kinetically determined KD. Individual saturation binding experiments were analysed with EBDA (McPherson, 1983) to obtain initial estimates. The resulting data files were co-analysed with LIGAND (Munson & Rodbard, 1980) to obtain values of ligand affinity (KD) and receptor density (Bmax expressed as fmol mg−1 protein). A two-site model was accepted only if it resulted in a significantly better fit as judged by an F-test. KD values were compared using the Mann-Whitney U-test with a significance of P<0.05 and Bmax values were compared using Student's unpaired t-test with a significance value of P<0.05.
Materials
[125I]-BOP ([1S-1 alpha, 2 beta (5Z), 3 alpha(1E,3R*),4 alpha)]-7-[3-(3-hydroxy-4′-iodophenoxy)-1-buteny) 7-oxabicyclo-[2.2.1]-heptan-2-yl]-5-heptanoic acid) (2000 Ci mmol−1, Cayman Chemicals, Ann Arbor, U.S.A), SQ29548 ([1S-[1α,2α(Z),3α,4α]] - 7 - [3 - 332 - [(Phenylamino)carbonyl]hydrazino]methyl] - 7 - oxabicyclo[2.2.1]hept - 2 - yl] - 5-heptenoic acid) (R.B.I., Natick, U.S.A.), Prostaglandin E2 and Prostaglandin I2 (Alexis bio-chemicals, Nottingham, U.K.), Other peptides (Peptide institute, Osaka, Japan). UK-147,535 (Dack et al., 1998) was a kind gift from Pfizer Pharmaceuticals (Sandwich, Kent, U.K.), losartan was kindly donated by Merck & Co., Inc (NJ, U.S.A.). All other chemicals and reagents were obtained from Sigma-Aldrich (Poole, Dorset, U.K.).
Results
Kinetic studies
At a concentration of 0.1 nM, the binding of [125I]-BOP was time dependent to sections of human left ventricle (Figure 1) with an association rate constant (kobs) of 0.143±0.011 min−1 and a half time for association (t1/2) of 5 min. The binding of [125I]-BOP to sections of human left ventricle was reversible at room temperature and analysis of the data indicated dissociation from a single site with a dissociation rate constant of 0.072±0.003 min−1. The half time for dissociation (t1/2) was 10 min (Figure 2). Kinetically derived KD of 0.10 nM was comparable to the value obtained from saturation studies (KD of 0.23±0.06 nM).
Figure 1.
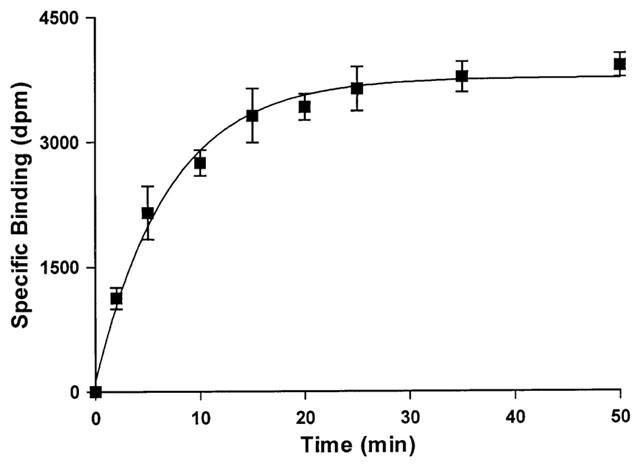
Time dependent association of [125I]-BOP binding to sections of human non-diseased left ventricle. Tissue sections were incubated for 120 min at room temperature. Data points are mean±s.e.mean from three individuals.
Figure 2.
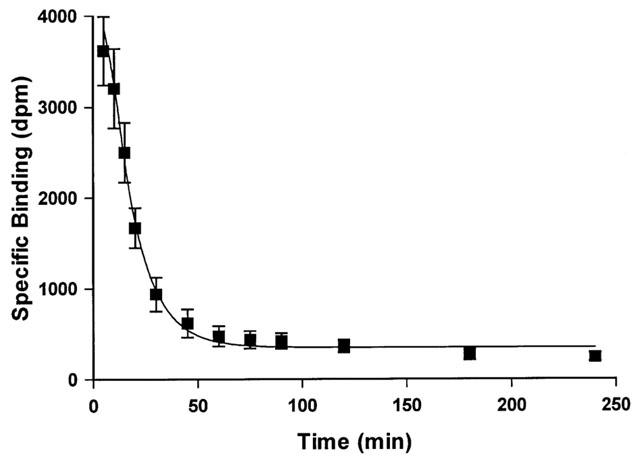
Time course for dissociation of 0.1 nM [125I]-BOP from sections of non-diseased human left ventricle. Tissue sections were incubated for 30 min and washed in excess of Tris buffer for differing time points up to 4 h. Data points are mean±s.e.mean from three individuals.
Saturation binding studies in normal and diseased cardiovascular tissue
Human heart
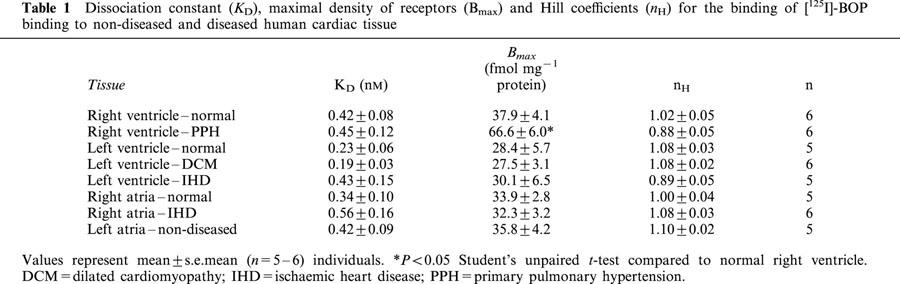
Over the concentration range tested (0.01 – 1.5 nM) binding of [125I]-BOP revealed evidence of saturable and specific binding (75 – 80% specific binding) (Figure 3) to all human cardiac tissues examined. [125I]-BOP bound with sub-nanomolar affinity to sections of human non-diseased and diseased cardiac tissue with no significant difference in ligand affinity (Table 1). For each tissue, a one site fit was preferred to a two site model and the Hill coefficients were close to unity. There was no significant difference in receptor density comparing the four chambers from non-diseased tissue (Table 1). No change in receptor density was associated with DCM or IHD in the human left ventricle, compared with non-diseased tissue. Similarly, no change in receptor density was highlighted in right atrial tissue obtained from IHD subjects, compared to non-diseased tissue. Interestingly, TP receptor density was increased in the right ventricle of PPH subjects, compared to non-diseased right ventricle (P<0.05).
Figure 3.
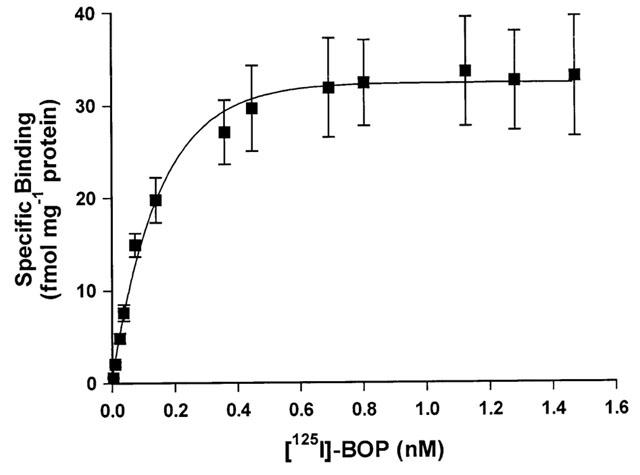
Saturation binding curve for [125I]-BOP. Increasing concentrations of radioligand (0.01 – 1.5 nM) were incubated with sections of non-diseased human left ventricle for 30 min at room temperature. Data points are mean±s.e.mean from five individuals with a dissociation constant (KD) of 0.23±0.10 nM and maximal receptor density (Bmax) of 28.4±5.7 fmol mg−1 protein.
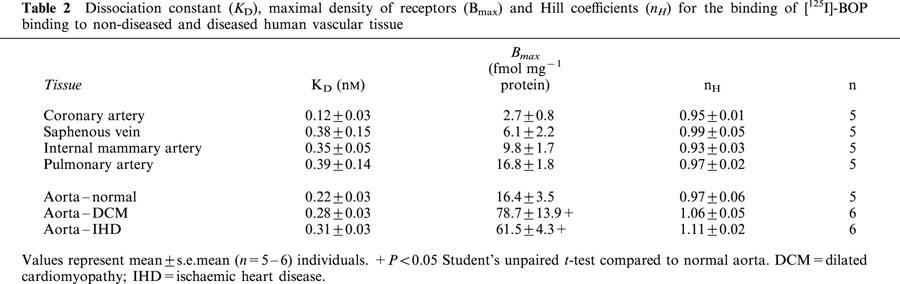
Table 1.
Dissociation constant (KD), maximal density of receptors (Bmax) and Hill coefficients (nH) for the binding of [125I]-BOP binding to non-diseased and diseased human cardiac tissue
Human vasculature
[125I]-BOP bound with sub-nanomolar affinity to human muscular arteries, elastic arteries and veins of both non-diseased and diseased vascular tissues examined, with no significant difference in ligand affinity (Table 2). For each tissue, a one site fit was preferred to a two site model and the Hill coefficients were close to unity. The binding density of the two non-diseased elastic arteries (aorta and pulmonary artery) was significantly greater than non-diseased muscular epicardial coronary artery (P<0.05). TP receptors were significantly increased (P<0.05) in aortic tissue containing advanced atherosclerotic lesions obtained from both DCM and IHD patients, compared to non-diseased vessels (Table 2).
Table 2.
Dissociation constant (KD), maximal density of receptors (Bmax) and Hill coefficients (nH) for the binding of [125I]-BOP binding to non-diseased and diseased human vascular tissue
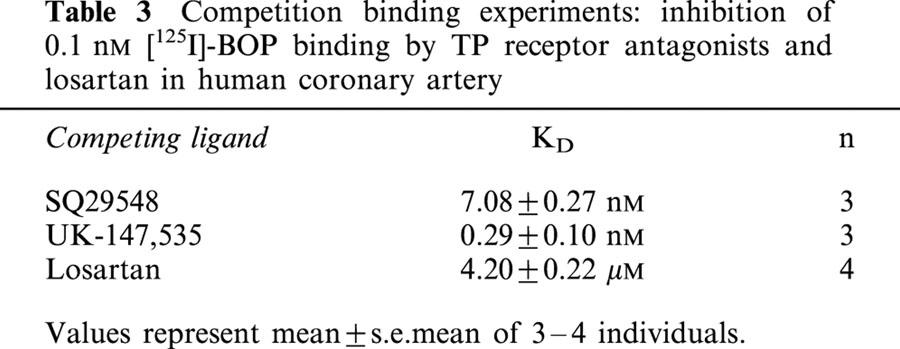
Competition binding experiments
TP receptor antagonist SQ29548 competed monophasically, with nanomolar affinity for the binding of a fixed concentration of [125I]-BOP in human coronary artery (Table 3). Within the concentration range investigated, UK-147,535 competed with high sub-nanomolar affinity for the TP receptor in human coronary artery and a one site fit was preferred over a two site model (Table 3). Selectivity was defined further by incubating 0.1 nM [125I]-BOP with a series of peptides (endothelin-1, angiotensin II, calcitonin gene-related peptide, atrial natriuretic peptide, ghrelin, apelin-13 and angiotensin IV) and prostaglandins (prostaglandin E2 and prostaglandin I2). At 1 μM concentration, these compounds did not compete for the [125I]-BOP binding site in human coronary artery (data not shown).
Table 3.
Competition binding experiments: inhibition of 0.1 nM [125I]-BOP binding by TP receptor antagonists and losartan in human coronary artery
Surprisingly, the angiotensin type 1 receptor antagonist losartan, did compete for the [125I]-BOP binding site in human coronary artery (Table 3). Autoradiography revealed that 0.1 μM losartan, a concentration even lower than achieved following oral dosing, competed for thromboxane receptors in cardiac tissue obtained from hearts of patients transplanted for both dilated cardiomyopathy and ischaemic heart disease (Figure 4) (n=eight individuals), with binding localized to cardiac myocytes. Losartan also competed for thromboxane receptors in human saphenous vein graft and atherosclerotic coronary artery with binding to both the thinned media and proliferated intimal smooth muscle layers (n=eight individuals). The angiotensin converting enzyme inhibitor captopril, the non-selective angiotensin receptor antagonist saralasin, and the angiotensin type II receptor agonist CGP42112 did not compete for [125I]-BOP binding (data not shown).
Figure 4.
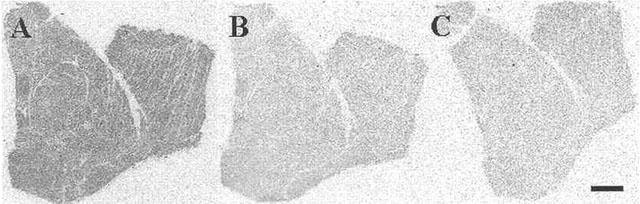
Losartan the angiotensin type 1 receptor antagonist, at a concentration lower than its therapeutic dosage competing for the thromboxane receptor binding site in human left ventricle obtained from a dilated cardiomyopathic patient using 0.1 nM [125I]-BOP (A) total binding in the absence of SQ29548 (B) non-specific binding in the presence of 1 μM SQ29548 (C) binding in the presence of 0.1 μM losartan (a concentration lower than its therapeutic dosage), which has competed for the radioligand binding site. Scale bar=1 mm.
Quantitative in vitro receptor autoradiography to determine relative TP receptor density and anatomical localization
Using a fixed concentration of radioligand to label approximately 35 – 40% of the total [125I]-BOP binding sites, the relative TP receptor density was measured in the smooth muscle layers of human blood vessels with quantitative in vitro receptor autoradiography. TP receptors were present on the endothelial cell layer of all vessels examined and on both the media and the intimal smooth muscle layers (when detectable) of non-diseased and diseased vessels. TP receptor density was significantly increased (P<0.05) on both muscle layers of atherosclerosis of coronary artery disease and accelerated atherosclerosis of as saphenous vein grafts. (Figure 5a,b), compared to non-diseased vessels. However, in diseased vessels, fewer TP receptors were localized to the intimal layer compared to the medial smooth muscle layer (P<0.05) (Figure 5a,b). Within the human heart, TP receptors were predominantly localized to cardiac myocytes (Figure 6). Binding was also detected on the medial layer of human small intra-myocardial coronary arteries (Figure 7) with no significant difference in relative receptor density comparing DCM and IHD patients (data not shown).
Figure 5.
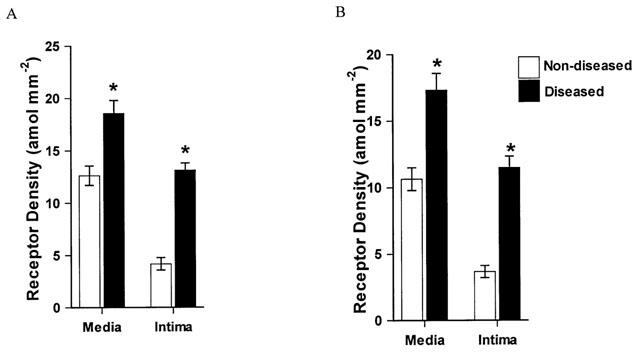
Relative thromboxane receptor densities determined autoradiographically in (A) human non-diseased saphenous vein and saphenous vein grafts. (B) human non-diseased and atherosclerotic coronary artery. In saphenous vein grafts, the thromboxane receptor density in both the media and intima were significantly increased, compared to non-diseased saphenous veins of the corresponding smooth muscle cell layer. Compared to non-diseased coronary artery, TP receptor density was significantly increased in human atherosclerotic coronary artery, on both the media and intimal smooth muscle layer. In diseased vessels significantly fewer thromboxane receptors were localised to the intimal layer, compared to the medial smooth muscle layer. *P<0.05 Student's unpaired t-test, compared to non-diseased from respective smooth muscle layer. Values represent mean±s.e.mean (n=8 – 12 individuals).
Figure 6.
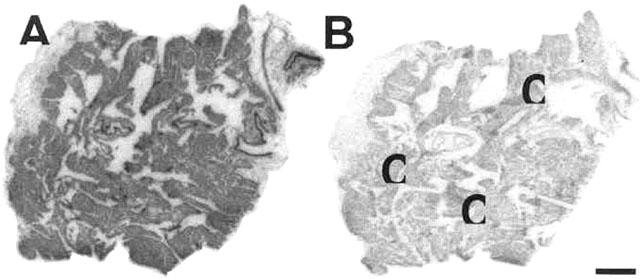
Autoradiographical localization of thromboxane receptors to human right atrial tissue sections obtained from an ischaemic heart diseased patient using 0.1 nM [125I]-BOP (A) total binding to the cardiac muscle, in the absence of 1 μM SQ29548 and (B) non-specific binding in the presence of 1 μM SQ29548, displaying the high degree of specific binding obtained by using [125I]-BOP in human tissue. C=cardiac myocytes. Scale bar=1 mm.
Figure 7.
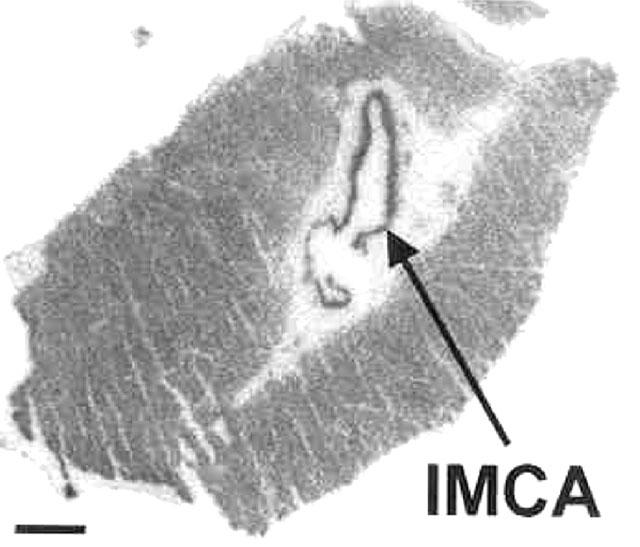
Autoradiographical localization of thromboxane receptors to human small intra-myocardial coronary artery section obtained from a dilated cardiomyopathic patient, using 0.1 nM [125I]-BOP to define total binding in the absence of 1 μM SQ29548. Binding being localized to the medial vascular smooth muscle layer of intra-myocardial coronary arteries and to the cardiac muscle in the left ventricular section. IMCA=Intramyocardial coronary artery. Scale bar=1 mm.
Discussion
This study has shown TP receptor distribution in the human cardiovascular system and regional specific alteration of receptor density associated with cardiovascular disease. We have also documented how TP receptors respond to increased levels of endogenous ligand in cardiovascular disease such as atherosclerosis and pulmonary hypertension. Finally, we have provided evidence that the angiotensin II type 1 receptor antagonist losartan, surprisingly below its therapeutic concentration, competes for the TP receptor in both non-diseased and diseased human cardiovascular tissue.
[125I]-BOP bound to TP receptors expressed in human cardiovascular tissue with high affinity, saturable, reversible and specifically, characteristic of a receptor-ligand interaction and similar to the properties of TP receptors in human platelets (Morinelli et al., 1989). Within the concentration range tested for saturation experiments, the radioligand bound with a single high sub-nanomolar affinity to all tissues examined and a one-site fit was preferred. Hill slopes were close to unity confirming that [125I]-BOP bound with a single affinity, with no evidence for receptor subtypes. In agreement, in kinetic studies the radioligand bound rapidly at room temperature to a single site. Binding was reversible with the ligand also dissociating from a single site. There was no significant difference in TP receptor density comparing the four chambers of non-diseased human hearts, unlike in the guinea-pig where the atria contains a significantly greater density of TP receptors than the ventricle (Bowling et al., 1994). This highlights the discrepancies that is reported to exist between species with TP receptors (Dorn, 1991; Ogletree & Allen, 1992), thus establishing the importance of studies on native human tissue. Whilst several receptors have been proposed to exist for which Tx have differing affinities in vascular smooth muscle (Becker et al., 1999), SQ29548 and UK-147,535 have at least one binding site in common, since we were able to show that these compounds completely inhibited specifically bound [125I]-BOP (0.1 nM) in both vascular smooth muscle and human heart in a monophasic manner, in agreement with a single gene encoding a single human TP receptor protein in vascular smooth muscle cells (Hirata et al., 1991; Nüsing et al., 1993).
TP receptor density in the left ventricle was not altered with cardiac disease (DCM or IHD). Similarly in the right atria TP receptor density was not altered with IHD. Primary pulmonary hypertension is characterized by right ventricular hypertrophy and by an increase in Tx production (Christman et al., 1992). Interestingly, the expected compensatory down-regulation of TP receptors in response to the increased Tx production in PPH was not observed, but the apparent high levels of ligand accompanying an increase in receptor density may lead to a possible detrimental positive feedback loop. This increase in TP receptor density provides a useful target for the development of novel therapeutics for the treatment of right ventricular hypertrophy, characteristic of pulmonary hypertension.
TP receptors were localized, as described, to the endothelial cell layer (Sung et al., 1989; Kent et al., 1993) and the medial smooth muscle layer of all human blood vessels examined. Intriguingly, with the reported rise in Tx production by ischaemic arteries (Mehta et al., 1988), we saw a significant increase in TP receptors in both the aorta and epicardial coronary arteries obtained from ischaemic atherosclerotic vessels, possibly predisposing these vessels to an even greater vasoconstriction in disease. It is not known weather the endogenous ligand production is increased or decreased with DCM in the aorta. Therefore, the up-regulation of TP receptor density in DCM cannot be correlated with the levels of endogenous ligand. In agreement with reports that patients with DCM have normal epicardial coronary arteries (Dec & Fuster, 1994), we observed no difference in TP receptor density in epicardial coronary arteries obtained from non-diseased patients compared with those from DCM. Furthermore, human small intra-myocardial coronary arteries, which are located down stream to large epicardial coronary arteries, did not display any significant difference in TP receptor density between DCM and IHD. This suggests that in ischaemic conditions, intra-myocardial coronary arteries, which are not prone to atherosclerosis, respond to enhanced levels of endogenous ligand with no significant alteration in receptor density.
Thromboxane is one of the major spasmogens of both arterial and venous graft conduits during harvesting and following grafting to the coronary circulation (Teoh et al., 1987). Furthermore, elevated levels of Tx have been found in coronary artery bypass grafts (Teoh et al., 1987). The increase in TP receptor density in SVG in response to the increase in Tx production would lead to even greater vasoconstriction and hypertrophy of the vessel, worsening the condition of the graft vessel. Thromboxane has been implicated as a strong stimulant for coronary artery smooth muscle cell proliferation in animal tissue (Morinelli et al., 1994). The significantly greater TP receptor density in the thickened intimal smooth muscle layer of atherosclerotic coronary arteries and saphenous vein grafts compared with non-diseased intima suggest that, phenotypic change of vascular smooth muscle cells leading to vascular narrowing is accompanied by a notable increase of TP receptors in diseased vessels.
In this study we have shown that losartan at concentrations lower than its therapeutic dosage, completely inhibited [125I]-BOP binding to human diseased cardiac tissue and bound to TP receptors in human non-diseased coronary artery with a KD of 4.2 μM. Furthermore, [125I]-BOP binding was also displaced by losartan in occluded saphenous vein grafts in both the media and the thickened intimal smooth muscle layers. Although losartan has been reported to inhibit human platelet aggregation (Guerra-Cuesta et al., 1999; Jagroop & Mikhailidis, 2000; Levy et al., 2000) and functional studies have established the antagonist properties in animal tissues (Li et al., 1997), the binding characteristics of losartan to TP receptors in native human non-diseased and diseased cardiovascular tissue has not been documented. Since species differences exists in TP receptor binding characteristics, our findings, in native human cardiovascular tissue, will prove vital to further understand the added beneficial effects of losartan and to extend these findings to rest of the ‘sartan' (e.g. irbesartan, candersartan) family. We believe that losartan, by binding to TP receptors in human cardiovascular system would contribute to the overall beneficial effects seen in the treatment of cardiac disease as well as vascular pathology.
In conclusion, we have shown the distribution of TP receptors in the human heart and the selective increase of this receptor in the right ventricle of primary pulmonary hypertension, highlighting the importance of this receptor in cardiac disease. Additionally, the increase in TP receptors in atherosclerosis of coronary artery disease and accelerated atherosclerosis of saphenous vein grafts with severe intimal thickening emphasises the need for developing novel therapeutics for the treatment of vascular disease. Following oral dosing, some of the beneficial effects of the angiotensin type 1 receptor antagonist losartan, maybe mediated by binding to TP receptors in the human cardiovascular tissue, further strengthening the dual receptor binding properties of losartan.
Acknowledgments
We would like to thank Merck & Co., Inc for supplying losartan and Pfizer pharmaceuticals for supplying UK-147,535. This work was supported by grants from the British Heart Foundation and The Royal Society.
Abbreviations
- DCM
dilated cardiomyopathy
- IHD
ischaemic heart disease
- PPH
primary pulmonary hypertension
- Tx
thromboxane
References
- BAAN J., CHANG P.C., VERMEIJ P., PFAFFENDORF M., VAN ZWIETEN P.A. Effects of losartan on vasoconstrictor responses to angiotensin II in the forearm vascular bed of healthy volunteers. Cardiovasc. Res. 1996;32:973–979. [PubMed] [Google Scholar]
- BECKER K.P., GARNOVSKAYA M., GETTYS T., HALUSHKA P.V. Coupling of thromboxane A2 receptor isoforms to Galpha13: effects on ligand binding and signalling. Biochim. Biophys. Acta. 1999;1450:288–296. doi: 10.1016/s0167-4889(99)00068-3. [DOI] [PubMed] [Google Scholar]
- BOWLING N., DUBE G.P., KURTZ W.L., BRUNE K.A., SAUSSY D.L., JR, DORN G.W., MAIS D.E. Characterisation of thromboxane A2/prostaglandin H2 binding sites in guinea pig cardiac membrane preparations. J. Mol. Cell. Cardiol. 1994;26:915–923. doi: 10.1006/jmcc.1994.1109. [DOI] [PubMed] [Google Scholar]
- BROWN G.P., VENUTO R.C. Thromboxane receptors in human kidney tissue. Prostaglandins Other Lipid Mediat. 1999;57:179–188. doi: 10.1016/s0090-6980(99)00002-7. [DOI] [PubMed] [Google Scholar]
- CHRISTMAN B.W., MCPHERSON C.D., NEWMAN J.H., KING G.A., BERNARD G.R., GROVES B.M., LOYD J.E. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N. Engl. J. Med. 1992;327:70–75. doi: 10.1056/NEJM199207093270202. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. International Union of Pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- DACK K.N., DICKINSON R.P., LONG C.J., STEELE J. Thromboxane modulating agents. 4. Design and synthesis of 3-(2 - [[(4 - chlorophenyl)sulfonyl] - amino]ethyl) benzenepropanoic acid derivatives as potent thromboxane receptor antagonists. Bioorg. Med. Chem. Lett. 1998;8:2061–2066. doi: 10.1016/s0960-894x(98)00242-x. [DOI] [PubMed] [Google Scholar]
- DAVENPORT A.P., O'REILLY G., KUC R.E. Endothelin ETA and ETB mRNA and receptors expressed by smooth muscle in the human vasculature: majority of the ETA sub-type. Br. J. Pharmacol. 1995;114:1110–1116. doi: 10.1111/j.1476-5381.1995.tb13322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVENPORT A.P., KUC R.E., ASHBY M.J., PATT W.C., DOHERTY A.M. Characterisation of [125I]-PD164333, an ETA selective non-peptide radiolabelled antagonist, in normal and diseased human tissue. Br. J. Pharmacol. 1998;123:223–230. doi: 10.1038/sj.bjp.0701597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEC G.W., FUSTER V. Idiopathic dilated cardiomyopathy. N. Engl. J. Med. 1994;331:1564–1575. doi: 10.1056/NEJM199412083312307. [DOI] [PubMed] [Google Scholar]
- DORN G.W. Tissue and species-specific differences in ligand binding to thromboxane A2 receptors. Am. J. Physiol. 1991;261:R145–R153. doi: 10.1152/ajpregu.1991.261.1.R145. [DOI] [PubMed] [Google Scholar]
- FUSE S., KAMIYA T. Plasma thromboxane B2 concentrations in pulmonary hypertension associated with congenital heart disease. Circulation. 1994;90:2952–2955. doi: 10.1161/01.cir.90.6.2952. [DOI] [PubMed] [Google Scholar]
- GRESELE P., DECKMYN H., NENCI G.G., VERMYLEN J. Thromboxane synthase inhibitors, thromboxane receptor antagonist and dual blockers in thrombotic disorders. Trends. Pharmacol. Sci. 1991;12:158–163. doi: 10.1016/0165-6147(91)90533-x. [DOI] [PubMed] [Google Scholar]
- GUERRA-CUESTA J.I., MONTON M., RODRIGUEZ-FEO J.A., JIMENEZ A.M., GONZALEZ-FERNANDEZ F., RICO L.A., GARCIA R., GOMEZ J., FARRE J., CASADO S., LOPEZ-FARRE A. Effect of losartan on human platelet activation. J. Hypertens. 1999;17:447–452. doi: 10.1097/00004872-199917030-00019. [DOI] [PubMed] [Google Scholar]
- HALUSHKA P.V., LEFER A.M. Thromboxane A2 in health and disease. Fed. Proc. 1987;46:131–132. [PubMed] [Google Scholar]
- HALUSHKA P.V., MAIS D.E., MAYEUX P.R., MORINELLI T.A. Thromboxane, prostaglandin and leukotriene receptors. Annu. Rev. Pharmacol. Toxicol. 1989;29:213–239. doi: 10.1146/annurev.pa.29.040189.001241. [DOI] [PubMed] [Google Scholar]
- HIRATA M., HAYASHI Y., USHIKUBI F., YOKOTA Y., KAGEYAMA R., NAKANISHI S., NARUMIYA S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991;349:617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- JAGROOP I.A., MIKHAILIDIS D.P. Angiotensin II can induce and potentiate shape change in platelets: effect of losartan. J. Hum. Hyperten. 2000;14:581–585. doi: 10.1038/sj.jhh.1001102. [DOI] [PubMed] [Google Scholar]
- JAISWAL N., DIZ D.I., TALLANT E.A., KHOSLA M.C., FERRARIO C.M. The nonpeptide angiotensin II antagonist DuP 753 is a potent stimulus for prostacyclin synthesis. Am. J. Hypertens. 1991;4:228–233. doi: 10.1093/ajh/4.3.228. [DOI] [PubMed] [Google Scholar]
- KATUGAMPOLA S.D., DAVENPORT A.P. Human internal mammary artery possesses a greater density of thromboxane A2 receptors than the coronary artery: differential distribution in human vasculature. Br. J. Pharmacol. 2000;131:84P. [Google Scholar]
- KATUGAMPOLA S.D., DAVENPORT A.P. Distribution of thromboxane receptors in the normal human heart and its alteration with disease. Br. J. Pharmacol. 2001;133:114P. [Google Scholar]
- KENT K.C., COLLINS L.J., SCHWERIN F.T., RAYCHOWDHURY M.K., WARE J.A. Identification of functional PGH2/TxA2 receptors on human endothelial cells. Circ. Res. 1993;72:958–965. doi: 10.1161/01.res.72.5.958. [DOI] [PubMed] [Google Scholar]
- LEVY P.J., YUNIS C., OWEN J., BROSNIHAN K.B., SMITH R., FERRERIO C.M. Inhibition of platelet aggregability by losartan in essential hypertension. Am. J. Cardiol. 2000;86:1188–1192. doi: 10.1016/s0002-9149(00)01200-5. [DOI] [PubMed] [Google Scholar]
- LI P., FERRARIO C.M., BROSNIHAN B. Non peptide angiotensin II antagonist losartan inhibits thromboxane A2 induced contractions in canine coronary arteries. J. Pharmacol. Exp. Ther. 1997;281:1065–1070. [PubMed] [Google Scholar]
- MAGUIRE J.J., KUC R.E., ROUS B.A., DAVENPORT A.P. Failure of BQ123, a more potent antagonist of sarafotoxin 6b than of endotehlin-1, to distinguish between these agonists in binding experiments. Br. J. Pharmacol. 1996;118:335–342. doi: 10.1111/j.1476-5381.1996.tb15407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGUIRE J.J., DAVENPORT A.P. Endothelin receptor expression and pharmacology in human saphenous vein grafts. Br. J. Pharmacol. 1999;126:443–450. doi: 10.1038/sj.bjp.0702326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCPHERSON G. A practical computer-based approach to the analysis of radioligand binding experiments. Comput. Prog. Biomed. 1983;17:107–114. doi: 10.1016/0010-468x(83)90031-4. [DOI] [PubMed] [Google Scholar]
- MEHTA J., ROBERTS A. Human vascular tissues produce thromboxane as well as prostacyclin. Am. J. Physiol. 1983;244:R839–R444. doi: 10.1152/ajpregu.1983.244.6.R839. [DOI] [PubMed] [Google Scholar]
- MEHTA J.L., MEHTA P. Prostacyclin and thromboxane A2 production by human cardiac atrial tissues. Am. Heart. J. 1985;109:1–3. doi: 10.1016/0002-8703(85)90407-7. [DOI] [PubMed] [Google Scholar]
- MEHTA J.L., LAWSON D., MEHTA P., SALDEN T. Increased prostacyclin and thromboxane biosynthesis in atherosclerosis. Proc. Natl. Acad. Sci. U.S.A. 1988;85:4511–4515. doi: 10.1073/pnas.85.12.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORINELLI T.A., OATIS J.E., JR, OKWU A.K., MAIS D.E., MAYEUX P.R., MASUDA A., KNAPP D.R., HALUSHKA P.V. Characterisation of an 125I-labeled thromboxane A2/prostaglandin H2 receptor agonist. J. Pharmacol. Exp. Ther. 1989;251:557–562. [PubMed] [Google Scholar]
- MORINELLI T.A., MAIS D.E., OATIS J.E., JR, CRUMBLEY III A.J., HALUSHKA P.V. Characterisation of thromboxane A2/prostaglandin H2 receptors in human vascular smooth muscle cells. Life. Sci. 1990;46:1765–1772. doi: 10.1016/0024-3205(90)90140-m. [DOI] [PubMed] [Google Scholar]
- MORINELLI T.A., ZHANG L.M., NEWMAN W.H., MEIER K.E. Thromboxane A2/prostaglandin H2–stimulated mitogenesis of coronary artery smooth muscle cells involves activation of mitogen-activated protein kinase and S6 kinase. J. Biol. Chem. 1994;269:5693–5698. [PubMed] [Google Scholar]
- MUNAFO A., CHRISTEN Y., NUSSBERGER J., SHUM L.Y., BORLAND R.M., LEE R.J., WAEBER B., BIOLLAZ J., BRUNNER H.R. Drug concentration response relationships in normal volunteers after oral administration of losartan, an angiotensin II receptor antagonist. Clin. Pharmacol. Ther. 1992;51:513–521. doi: 10.1038/clpt.1992.56. [DOI] [PubMed] [Google Scholar]
- MUNSON P.J., RODBARD D. LIGAND: a versatile computerised approach for the characterisation of ligand binding systems. Anal. Biochem. 1980;107:220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- NARUMIYA S., SUGIMOTO Y., USHIKUBI F. Prostanoid receptors: structure, properties and functions. Physiol. Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- NÜSING R.M., HIRATA M., KAKIZUKA A., EKI T., OZAWA K., NARUMIYA S. Characterisation and chromosomal mapping of the human thromboxane A2 receptor gene. J. Biol. Chem. 1993;268:25253–25259. [PubMed] [Google Scholar]
- OGLETREE M.L., ALLEN G.T. Interspecies differences in thromboxane receptors–studies with thromboxane receptor antagonists in rat and guinea pig smooth muscles. J. Pharmacol. Exp. Ther. 1992;260:789–795. [PubMed] [Google Scholar]
- REILLY M., FITZGERALD G.A. Cellular activation by thromboxane A2 and other eicosanoids. Eur. Heart. J. 1993;14:88–93. [PubMed] [Google Scholar]
- SACHINIDIS A., FLESCH M., KO Y., SCHROR K., BOHM M., DUSING R., VETTER H. Thromboxane A2 and vascular smooth muscle cell proliferation. Hypertension. 1995;26:771–780. doi: 10.1161/01.hyp.26.5.771. [DOI] [PubMed] [Google Scholar]
- SAKUMA I., GROSS S.S., LEVI R. Positive inotropic effect of the thromboxane analogue U-46619 on guinea pig left atrium: mediation by specific receptors and association with increased phosphoinositide turnover. Can. J. Physiol. Pharmacol. 1989;67:943–949. doi: 10.1139/y89-148. [DOI] [PubMed] [Google Scholar]
- SERNERI N., GENSINI G.F., ABBATE R., MUGNAINI C., FAVILLA S., BRUNELLI C., CHIERCHIA S., PARODI O. Increased fibrinopeptide A formation and thromboxane A2 production in patients with ischemic heart disease: relationships to coronary pathoanatomy, risk factors and clinical manifestations. Am. Heart. J. 1981;101:185–194. doi: 10.1016/0002-8703(81)90665-7. [DOI] [PubMed] [Google Scholar]
- SERNERI N., ABBATE R., GENSINI G.F., PANETTA A., CASOLO G.C., CARINI M. TxA2 production by human arteries and veins. Prostaglandins. 1983;25:753–766. doi: 10.1016/0090-6980(83)90001-1. [DOI] [PubMed] [Google Scholar]
- SMITH J.B. Pharmacology of thromboxane synthetase inhibitors. Fed. Proc. 1987;46:139–143. [PubMed] [Google Scholar]
- SMITH J.A., ROBERTS M., MCNEIL K., SHARPLES L.D., SCHOFIELD P.M., LARGE S.R., NASHEF S.A., WELLS F.C., WALLWORK J. Excellent outcome of cardiac transplantation using domino donor hearts. Eur. J. Cardiothorac. Surg. 1996;10:628–633. doi: 10.1016/s1010-7940(96)80377-0. [DOI] [PubMed] [Google Scholar]
- SUNG C., ARLETH A.J., BERKOWITZ B.A. Endothelial thromboxane receptors: biochemical characterisation and functional implications. Biochem. Biophys. Res. Commun. 1989;158:326–333. doi: 10.1016/s0006-291x(89)80216-5. [DOI] [PubMed] [Google Scholar]
- TEOH K.H., FREMES S.E., WEISEL R.D., CHRISTAKIS G.T., TEASDALE S.J., MADONIK M.M., IVANOV J., MEE A.V., WONG P.Y. Cardiac release of prostacyclin and thromboxane A2 during coronary revascularisation. J. Thorac. Cardiovasc. Surg. 1987;93:120–126. [PubMed] [Google Scholar]
- WONG P.C., PRICE W.A., JR, CHIU A.T., DUNCIA J.V., CARINI D.J., WEXLER R.R., JOHNSON A.L., TIMMERMANS P.B. Nonpeptide angiotensin II receptor antagonists. XI. Pharmacology of EXP3174: an active metabolite of DuP 753, an orally active antihypertensive agent. J. Pharmacol. Exp. Ther. 1990;252:719–725. [PubMed] [Google Scholar]


