Abstract
Urocortin is an endogenous vasodilator although the mechanism of vasorelaxation is not completely understood. The hypothesis that an alteration of smooth muscle calcium concentration is involved was tested using isometric tension recording and calcium fluorimetry. The relationship between contraction and intracellular calcium was also estimated.
Urocortin produced a concentration dependent relaxation (pD2 8.59±0.06, n=6) of vessels pre-contracted with a physiological salt solution containing 42 mM KCl (42 mM K-PSS).
Removal of the endothelium did not alter the effect of urocortin, pD2 was 8.49±0.11, n=5.
Corticotropin-releasing factor relaxed 42 mM K-PSS pre-contracted vessels with less potency compared to urocortin (pD2 6.99±0.28, n=5).
Urocortin at 100 nM relaxed vessels pre-contracted with 42 mM K-PSS by 59.6±4.6% (n=8) and vessels pre-contracted with 500 nM noradrenaline by 25.2±6.8% (n=6). Both effects were not accompanied by a change in the intracellular calcium concentration.
Urocortin at 100 nM produced a significant rightward shift of 0.33±0.07 units of normalized intracellular calcium (n=5) of the relationship between tension and intracellular calcium.
The urocortin-induced relaxation was considerably reduced in the presence of 0.3 mM Rp-8-CPT-cAMPS, a cyclic AMP-dependent protein kinase (PKA) inhibitor.
The PKA-activator Sp-5,6-DCl-cBIMPS relaxed 42 mM K-PSS pre-contracted vessels (pD2 4.98±0.07, n=6). Sp-5,6-DCl-cBIMPS at 0.1 mM relaxed vessels by 85.3±2.5% (n=5), but did not change the intracellular calcium concentration.
In conclusion, the data show that urocortin is a potent, endothelium-independent dilator of rat tail arteries and suggest that this effect is mediated by PKA causing a reduction of the sensitivity of the contractile apparatus for calcium.
Keywords: Arteries, calcium, calcium sensitivity, protein kinase, smooth muscle, urocortin
Introduction
Urocortin is a 40 amino acid peptide that displays a high degree of structural homology to human corticotropin-releasing factor (CRF) and to the peptides urotensin-I and sauvagine. It is an endogenous ligand for CRF receptors and binds with high affinity to all CRF receptor subtypes, i.e. CRF-1 and the two CRF-2 receptors, the neuronal splice variant 2α and the peripheral splice variant 2β. Noteworthy, urocortin is a more potent ligand for the CRF-2 receptor than CRF-1 (Vaughan et al., 1995; Eckart et al., 1999). The functional importance of CRF-2 receptor-mediated responses has recently been shown in mice lacking this receptor. Thus, alterations of urocortin-induced responses were observed in the central nervous system, as well as in the peripheral circulation, where the urocortin-evoked increase of cardiac performance and the reduction of blood pressure seen in wild-type mice were absent (Coste et al., 2000). The CRF-2 receptor-mediated effects of urocortin on the peripheral circulation seem to be caused by a direct action on blood vessels. CRF-2 receptors have been identified by molecular-biological techniques in rat cerebral and uterine arteries and aorta (Lovenberg et al., 1995; Jain et al., 2000). Furthermore, urocortin- or CRF-induced vasorelaxations have been observed in a variety of in vitro vessel preparations, e.g. rat mesenteric, cerebral and femoral arteries (Lei et al., 1993), rat aorta (Jain et al., 1997), rat basilar artery (Schilling et al., 1998), and rat uterine artery (Jain et al., 1999). The peripheral effect of urocortin on the circulation is additionally supported by the finding that locally produced urocortin regulates uteroplacental blood flow during human pregnancy (Clifton et al., 2000). Therefore, urocortin seems to be an endogenous regulator of blood pressure and blood flow, which produces its effects on the peripheral circulation by inducing vasorelaxation.
Some recent studies reported that a large part of CRF-induced CRF-2 receptor-mediated vasorelaxation in human foetal-placental circulation, rat aorta and rat uterine artery was endothelium-dependent. It has been suggested to be mediated by NO-release from the endothelium followed by an activation of the guanylyl cyclase signalling pathway (Clifton et al., 1995; Jain et al., 1997; 1999). By contrast, CRF-induced relaxations of rat mesenteric arteries and urocortin-induced relaxations of rat basilar arteries were found to be completely endothelium-independent (Lei et al., 1993; Schilling et al., 1998). All these effects were not affected by potassium channel blockers in rat mesenteric arteries (Lei et al., 1993) or by inhibitors of adenylate cyclase and potassium channels in endothelium-denuded rat uterine arteries (Jain et al., 1999). However, in rat basilar arteries adenylate cyclase and potassium channel inhibitors partly reduced the urocortin-induced vasorelaxation (Schilling et al., 1998). In addition, it has recently been shown that native and stably expressed CRF receptors can activate multiple G proteins, i.e. not only Gsα, but also Giα, Gqα and Gzα (for review Grammatopoulos et al., 2000) suggesting that CRF may influence several signalling pathways. The above cited data imply that the mechanism of endothelium-independent vasorelaxation mediated by the CRF-2 receptor and not involving potassium channel activation is still unknown.
Therefore, our studies have focused on cyclic AMP-dependent protein kinase (PKA) expected to be a significant factor of urocortin-induced vasorelaxation, as well as on the possible role of changes in the intracellular Ca2+ concentration in this process. Since preliminary patch clamp experiments have shown that urocortin does not affect potassium currents in smooth muscle cells freshly isolated from rat tail artery, this vessel was selected for the study.
Methods
Dissection and mounting of vessels
Male Wistar-Kyoto rats were killed by stunning and subsequent decapitation and the ventral tail artery was isolated. A 1.8 – 2.0 mm long piece of the artery was threaded on two 40 μm diameter stainless steel wires and mounted on a wire-myograph (model 300A, JP Trading, Denmark) containing physiological salt solution consisting of (in mM): 120 NaCl, 4.5 KCl, 1.2 NaH2PO4, 1 MgSO4, 1.6 CaCl2, 0.025 EDTA, 5.5 glucose, 26 NaHCO3, 5 HEPES at pH 7.4, which was continuously bubbled with carbogen. Isometric tension was recorded with the program Myodaq (JP Trading, Denmark). In all experiments, except those investigating the role of the endothelium in urocortin-induced responses, the endothelium was removed by gently moving a wire through the lumen of the vessel, a procedure that abolished acetylcholine induced relaxations. Probes for temperature and pH were placed in the experimental chamber and these experimental parameters were controlled throughout the experiment. After the temperature reached 37.0±0.5°C, the vessels were stretched radially to their optimal lumen diameter do corresponding to 90% of the passive diameter of the vessel at 100 mmHg and were allowed to stabilize for 15 min (Mulvany et al., 1977). Thereafter, the reactivity of the vessel was tested with two applications of a solution containing 10 μM noradrenaline. All drugs were applied directly into the experimental chamber. The potassium-rich solution was made by an equimollar replacement of sodium.
Measurement of intracellular calcium
Vessels were loaded with 5 μM FURA-2/AM, a calcium-sensitive dye, at 37°C for 80 min. During measurements the dye was excited with light at 340 and 380 nm with the use of a monochromator (PTI, Germany). The emission from the vessels was filtered at 520 nm with a long pass filter, detected by a photomultiplier and sent to a computer. There, during intracellular calcium measurements, the ratio of emission at both excitation wavelengths after subtraction of the background fluorescence was calculated. The acquisition rate was 1 Hz. Vessel background fluorescence was determined before dye loading. A calibration of the ratio in terms of calcium was not performed in view of the many uncertainties related to the binding properties of FURA-2 with calcium inside cells of intact vessel preparations. The effect of urocortin on calcium sensitivity of the contractile apparatus was estimated in vessels bathed in 42 mM K-PSS to facilitate the opening of voltage-operated calcium channels. This solution initially did not contain calcium. The latter was added afterwards in half-log increments from 0.03 mM to 3 mM either in the absence or in the continuous presence of 100 nM urocortin. In order to reduce vessel to vessel variability, all tension and calcium responses have been normalized to the tension and calcium response evoked by a single application of 42 mM K-PSS performed before the calcium-series.
Drugs
The salts for the solutions and CRF were obtained from Sigma (Deisenhofen, Germany). Rat urocortin and KT5823 were from Calbiochem (Bad Soden, Germany). FURA-2 was purchased from Molecular Probes (Leiden, The Netherlands) and Rp-8-CPT-cAMPS, 8-(4-Chlorophenylthio)adenosine-3′,5′-cyclic monophosphorothioate (Rp-8-CPT-cAMPS) as well as 5,6-Dichlorobenzimidazole riboside-3′,5′-cyclic monophosphorothioate (Sp-5,6-DCl-cBIMPS) from Biolog (Bremen, Germany).
Statistics
All data are means±s.e.; n is the number of vessels. Statistical analysis was performed using unpaired t-test and repeated measures ANOVA as appropriate (SPSS 9.0 for Windows). The pD2 values were obtained by fitting the curve
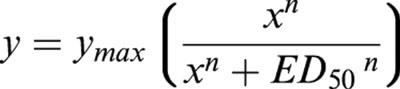 |
to the individual dose-response relationships and pD2-values were calculated as -log(ED50).
Results
Effect of urocortin on rat tail arteries
Addition of urocortin to vessels pre-contracted with physiological salt solution containing 42 mM KCl (42 mM K-PSS) produced a concentration dependent relaxation (n=6; P<0.001) (Figure 1a). This effect was characterized by a maximum relaxation to 61.2±6.5% (n=6) of the full contraction and a pD2 of 8.59±0.06 (n=6). The removal of the endothelium did not alter the effect of urocortin (n=5; P=0.81) (Figure 1b). The pD2 after endothelium removal was 8.49±0.11, which was not different from the one in endothelium-intact vessels (n=5; P=0.51). Removal of the endothelium did not alter vessel contractile function; the tension produced by 42 mM K-PSS was 7.84±0.46 N/m (n=5) in intact vessels and 7.82±0.35 N/m (n=5; P=0.97) in endothelium-denuded vessels. CRF also dilated vessels pre-contracted with 42 mM K-PSS; however, its concentration-response curve was located to the right as compared to the concentration-response curve of urocortin (n=5; P<0.001) (Figure 1c). The pD2 for CRF was 6.99±0.28, significantly different from the urocortin effect (n=5; P<0.01).
Figure 1.
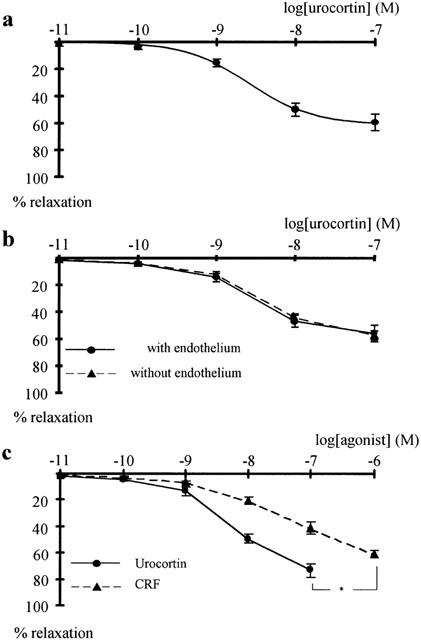
Effect of urocortin on rat tail arteries pre-contracted with 42 mM K-PSS. (a) Concentration-response curve of the effect of urocortin on vessels pre-contracted with 42 mM K-PSS. Significant effect of urocortin (n=6; P<0.001, repeated measures ANOVA). (b) Concentration-response curves of the effect of urocortin on vessels with and without intact endothelium; no significant difference was observed (n=5; P=0.81). (c) Effect of corticotropin-releasing factor (CRF) on rat tail arteries. Concentration-response curves of the effect of CRF and of urocortin on vessels pre-contracted with 42 mM K-PSS showing a significant difference between the effect of urocortin and CRF (n=5; *P<0.001).
Effect of urocortin on intracellular calcium
Urocortin at 100 nM, the concentration producing the maximum effect, relaxed vessels pre-contracted with 42 mM K-PSS by 59.6±4.6% as compared to the 2.2±2.3% relaxation recorded with time (n=8; P<0.001) (Figure 2a(i)). The urocortin-induced relaxation was accompanied by a reduction of intracellular calcium by 7.7±2.7%, which was not significantly different from the 7.4±3.7% reduction in intracellular calcium during time control (n=8; P=0.95) (Figure 2b(i)). In addition, urocortin at 100 nM relaxed vessels pre-contracted with 0.5 μM noradrenaline by 25.2±6.8% compared to the 0.2±3.3% relaxation during the time control (n=6; P<0.01) (Figure 2a(ii)). The urocortin-induced relaxation was accompanied by a reduction of intracellular calcium by 2.4±3.2%, which was not significantly different from the 5.7±2.4% reduction in intracellular calcium during the time control (n=6; P=0.48) (Figure 2b(ii)).
Figure 2.
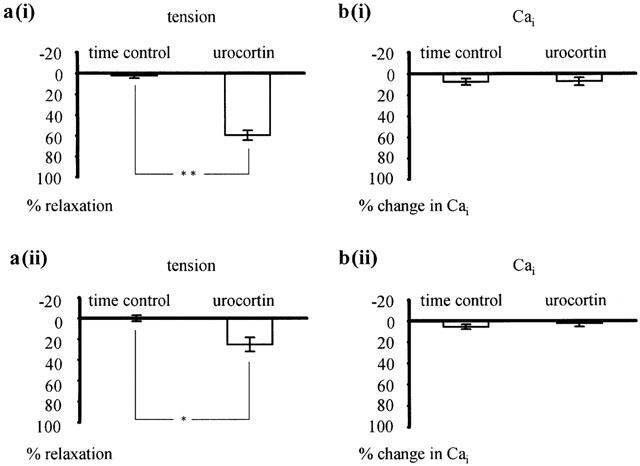
Effect of urocortin on intracellular calcium concentration. Effect of 100 nM urocortin on tension (a(i),(ii)) and intracellular calcium (b(i),(ii)) in vessels pre-contracted with 42 mM K-PSS (a(i), b(i)) and 0.5 μM noradrenaline (a(ii), b(ii)). Compared to the time control, urocortin produced significant effects on tension (in a(i) n=8; **P<0.001; in a(ii) n=6; *P<0.01) but no significant effect on intracellular calcium (in b(i) n=8; P=0.95; in b(ii) n=6; P=0.48).
Effect of urocortin on the sensitivity of the contractile apparatus to calcium
The application of extracellular calcium produced an increase in tension, which was smaller in the presence of 100 nM urocortin as compared to its absence (n=5; P<0.001). The urocortin-induced reduction in tension was not reversed after 10 min washout. The increase in tension induced by the addition of calcium was accompanied by an increase in intracellular calcium concentration, which was not significantly different in the presence and absence of urocortin (n=5; P=0.64). The relationship between contractile tension and the intracellular calcium concentration was shifted to the right in the presence of urocortin (Figure 3a). Thus, the calcium concentration producing a normalized tension of 0.4 was shifted by 0.33±0.07 units of normalized calcium (n=5) after application of urocortin, which was significantly different from the change during time control (Figure 3b), where the calcium concentration producing a normalized tension of 0.4 was shifted only by 0.09±0.02 units of normalized calcium (n=5; P<0.05).
Figure 3.
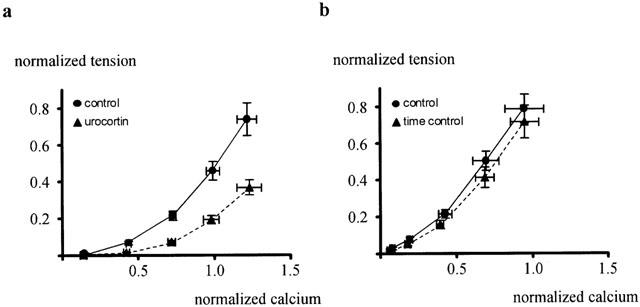
Effect of urocortin on the sensitivity of the contractile apparatus for calcium. The calcium concentration producing a normalized tension of 0.4 was shifted by 0.33±0.07 units of normalised calcium (n=5) after application of urocortin (a), which was significantly different from the change during time control (b), where the calcium concentration producing a normalized tension of 0.4 was shifted only by 0.09±0.02 units of normalized calcium (n=5; P<0.05).
Effect of urocortin in the presence of a PKA-inhibitor
The effect of urocortin was tested in vessels pretreated with 0.3 mM Rp-8-CPT-cAMPS, a potent and selective PKA-inhibitor (Schubert et al., 1997). In the presence of Rp-8-CPT-cAMPS the tension produced by 42 mM K-PSS was 6.09±1.02 N/m (n=5), which was not significantly different from 5.20±0.51 N/m (n=5; P=0.50), the tension produced by 42 mM K-PSS in the absence of the PKA-inhibitor. By contrast, the relaxation induced by urocortin was considerably smaller in the presence of the PKA-inhibitor compared to the results observed in its absence (n=5; P<0.001) (Figure 4). To validate these findings, the effect of urocortin was tested in the presence of 500 nM KT5832, a potent and selective inhibitor of cGMP-dependent protein kinase (PKG) (Adragna et al., 2000). In the presence of KT5832 the tension produced by 42 mM K-PSS was 6.44±0.91 N/m (n=5), which was not significantly different from 6.52±0.28 N/m (n=5; P=0.94), the tension produced by 42 mM K-PSS in the absence of the PKG-inhibitor. Urocortin at 1 nM produced a 6.0±0.6% relaxation in the absence of KT5832, which was not significantly different from the 4.2±1.0% relaxation in the presence of KT5832 (n=4; P=0.25). At 100 nM urocortin produced a 70.6±14.5% relaxation in the absence of KT5832, which was not significantly different from the 73.6±2.2% relaxation in the presence of the blocker (n=4; P=0.85).
Figure 4.
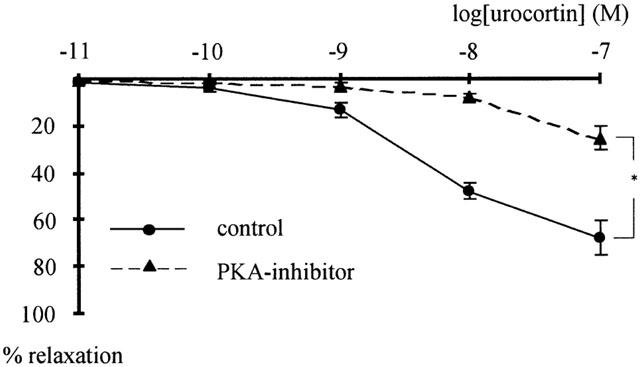
Effect of urocortin in the presence of a PKA-inhibitor. Concentration-response curves of the effect of urocortin on vessels pretreated with 0.3 mM of the PKA-inhibitor Rp-8-CPT-cAMPS (PKA-I) and in control showing a significant difference (n=5; *P<0.001).
Effect of a PKA-activator on rat tail arteries
Addition of Sp-5,6-DCl-cBIMPS, a potent and selective PKA-activator (Schubert et al., 1999), to vessels pre-contracted with 42 mM K-PSS produced a concentration dependent relaxation (n=6; P<0.001) (Figure 5a). This effect was characterized by a maximum relaxation to 93.7±0.8% (n=6) of the full contraction and a pD2 of 4.98±0.07 (n=6). Sp-5,6-DCl-cBIMPS at 100 μM, the maximum concentration used, relaxed vessels pre-contracted with 42 mM K-PSS by 85.3±2.5% as compared to the 1.4±2.6% relaxation recorded with time (n=5; P<0.001) (Figure 5b(i)). The Sp-5,6-DCl-cBIMPS-induced relaxation was accompanied by a reduction of intracellular calcium by 4.7±2.5%, which was not significantly different from the 5.7±2.3% reduction in intracellular calcium during time control (n=5; P=0.79) (Figure 5b(ii)).
Figure 5.
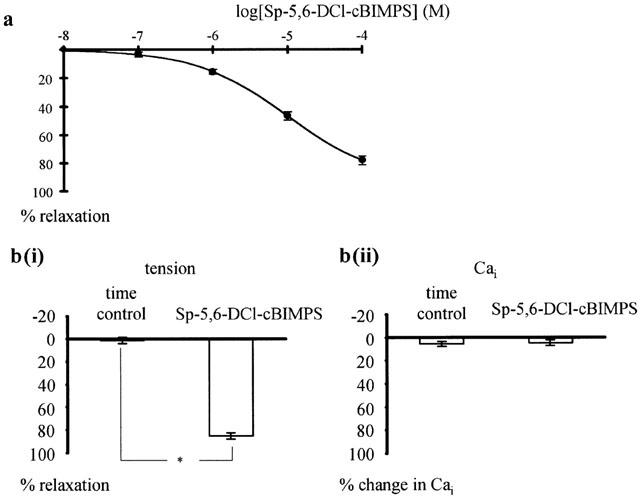
Effect of the PKA-activator Sp-5,6-DCl-cBIMPS on rat tail arteries pre-contracted with 42 mM K-PSS. (a) Concentration-response curve of the effect of Sp-5,6-DCl-cBIMPS. Significant effect of Sp-5,6-DCl-cBIMPS (n=6; P<0.001, repeated measures ANOVA). (b) Effect of Sp-5,6-DCl-cBIMPS on intracellular calcium concentration. Effect of 100 μM Sp-5,6-DCl-cBIMPS on tension (b(i)) and intracellular calcium (b(ii)) in vessels pre-contracted with 42 mM K-PSS. Compared to the time control, Sp-5,6-DCl-cBIMPS produced a significant effect on tension (in b(i) n=5; *P<0.001) but no significant effect on intracellular calcium (in b(ii) n=5; P=0.79).
Discussion
The present study shows that urocortin relaxes in vitro preparations of rat tail arteries. An effect of urocortin or CRF on this vessel has not been previously reported. The present study also shows that urocortin is a more potent dilator of rat tail arteries than CRF. A similar finding has been reported for rat basilar arteries and, together with the known affinity of these hormones to cloned CRF receptors, suggests an activation of CRF-2 receptors (for a more detailed discussion see Schilling et al., 1998). Thus, our data show that urocortin is a potent, endothelium-independent dilator of rat tail arteries.
The results show that urocortin-induced relaxation of rat tail arteries is not accompanied by an alteration of the intracellular calcium concentration and that the relationship of contractile tension to intracellular Ca2+ concentration is markedly shifted to the right after pretreatment with urocortin. Relaxation without a change in calcium concentration was observed under both potassium-induced and noradrenaline-induced pre-contractions. Both types of pre-contractions were always accompanied by a marked increase in the intracellular calcium concentration – a widely observed phenomenon, usually explained by a calcium influx via voltage-operated and receptor-operated calcium channels, as well as by a calcium release from intracellular stores (Ganitkevich & Isenberg, 1992). Thus, the employed precontraction induced a functional state of the vessel, where mechanisms producing vasorelaxation by a decrease of the intracellular calcium concentration could have been identified. Vasorelaxations not accompanied by a change of intracellular calcium concentration have been described, in particular in rat tail artery after the application of nitroglycerin (Chen & Rembold, 1996), forskolin (Rembold & Chen, 1998) or NO (Tran et al., 1998). These relaxations have been attributed to a reduction of the sensitivity of the contractile apparatus to calcium as a consequence of down-regulation of myosin light chain kinase and/or up-regulation of myosin light chain phosphatase (SMPP-1M), i.e. desensitization to calcium (for review see Somlyo et al., 1999). Hence, our data show that urocortin-induced relaxation of the rat tail arteries is produced by a reduction of the sensitivity of the contractile apparatus to calcium.
The present study shows that the urocortin-induced relaxation of rat tail arteries is markedly reduced by a PKA-inhibitor while an inhibitor of PKG is without effect. The PKA-inhibitor used, Rp-8-CPT-cAMPS, at a concentration of 0.3 mM, is a potent and selective blocker of this kinase (see discussions in Schubert et al., 1997; 1999). The pretreatment of vessels with the PKA-inhibitor did not block the effect of urocortin completely probably due to the incomplete blockade of PKA. Unfortunately, higher concentrations of the PKA-inhibitor are not useful because of possible side effects on PKG. In addition, the conclusion about the involvement of PKA in the urocortin-induced relaxation was strengthened by the observation that a PKA-activator produced a marked relaxation without any change of the intracellular calcium concentration, i.e. evoked the same effect on tension and intracellular calcium as urocortin. The PKA-activator used, Sp-5,6-DCl-cBIMPS, is a potent and selective activator of this kinase (see discussions in Schubert et al., 1997; 1999). A PKA-independent mechanism contributing to the effect of urocortin could not be excluded, either. The observation of a PKA-mediated effect of urocotin in tail artery is supported by the findings that urocortin increased intracellular cyclic AMP levels in cells transfected with CRF receptors 1, 2α, and 2β, i.e. suggesting that these receptors are coupled to the adenylate cyclase-PKA pathway (Donaldson et al., 1996). Additionally, it has been shown that the urocortin-induced relaxation of rat basilar artery was reduced by an inhibitor of adenylate cyclase (Schilling et al., 1998). However, in the latter study the adenylate cyclase-PKA pathway was suggested to mediate the effect of urocortin via calcium-activated potassium channels. Potassium channel activation leads to membrane hyperpolarization, a closure of voltage-operated calcium channels and subsequently, to a decrease of the intracellular calcium concentration. Since such a change of the intracellular calcium concentration was not observed in the present study, differences in the adenylate cyclase – PKA signalling pathways in rat basilar and tail arteries should be considered. To date multiple differently regulated adenylate cyclase isoforms have been found (Sunahara et al., 1996). Such a variety may explain the above mentioned tissue-dependent diversity of PKA-dependent signalling by creating spatial and functional compartmentalization of cyclic AMP-PKA effects (Vegesna & Diamond, 1983; Duridanova et al., 1999). Despite the intensive studies focused on the cyclic nucleotide-induced desensitization, its molecular mechanism remains unknown (MacDonald et al., 2000; Carvajal et al., 2000). Several hypotheses have been proposed to explain PKA- and PKG-induced desensitization: (i) stimulation of SMPP-1M either by a direct activation of its regulatory subunit, or by phosphorylation and inactivation of an inhibitory protein (Wu et al., 1996); (ii) inhibition of protein kinase C-induced sensitization (for review Carvajal et al., 2000); (iii) phosphorylation of telokin and other still unidentified low molecular weight proteins (MacDonald et al., 2000) including a small heat-shock related protein (for review Carvajal et al., 2000). In addition, PKG was found to inhibit RhoA-induced calcium sensitization of rat aorta contraction (Sauzeau et al., 2000). The possible in vivo cross-activation of PKA and PKG regulatory systems additionally complicates the picture. Hence, the mechanism of PKA-induced, calcium-independent relaxation of smooth muscles remains to be elucidated.
In conclusion, the data presented here show that urocortin is a potent, endothelium-independent dilator of rat tail arteries and suggest that its effect is mediated by PKA causing a reduction of the sensitivity of the contractile apparatus to calcium.
Acknowledgments
This research was supported by Deutsche Forschungsgemeinschaft Grant 436 BUL 17/2/00, SCHU 805/5-3 (to R. Schubert), and K-606/1996 of the National Fund ‘Scientific Research' of Bulgaria.
Abbreviations
- 42 mM K-PSS
physiological salt solution containing 42 mM KCl
- CRF
corticotropin-releasing factor
- PKA
cyclic AMP-dependent protein kinase
- PKA-I
cyclic AMP-dependent protein kinase inhibitor
- PKG
cyclic GMP-dependent protein kinase
- Rp-8-CPT-cAMPS
8-(4-Chlorophenylthio)adenosine-3′,5′-cyclic monophosphorothioate
- SMPP-1M
smooth muscle myosin light chain phosphatase
- Sp-5,6-DCl-cBIMPS
5,6-dichlorobenzimidazole riboside-3′,5′-cyclic monophosphorothioate
References
- ADRAGNA N.C., WHITE R.E., ORLOV S.N., LAUF P.K. K-Cl cotransport in vascular smooth muscle and erythrocytes: possible implication in vasodilation. Am. J. Physiol. 2000;278:C381–C390. doi: 10.1152/ajpcell.2000.278.2.C381. [DOI] [PubMed] [Google Scholar]
- CARVAJAL J.A., GERMAIN A.M., HUIDOBRO-TORO J.P., WEINER C.P. Molecular mechanism of cGMP-mediated smooth muscle relaxation. J. Cell. Physiol. 2000;184:409–420. doi: 10.1002/1097-4652(200009)184:3<409::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- CHEN X.L., REMBOLD C.M. Nitroglycerin relaxes rat tail artery primarily by lowering Ca2+ sensitivity and partially by repolarization. Am. J. Physiol. 1996;271:H962–H968. doi: 10.1152/ajpheart.1996.271.3.H962. [DOI] [PubMed] [Google Scholar]
- CLIFTON V.L., QING G., MURPHY V.E., SCHWARTZ J., MADSEN G., SMITH R. Localization and characterization of urocortin during human pregnancy. Placenta. 2000;21:782–788. doi: 10.1053/plac.2000.0570. [DOI] [PubMed] [Google Scholar]
- CLIFTON V.L., READ M.A., LEITCH I.M., GILES W.B., BOURA A.L., ROBINSON P.J., SMITH R. Corticotropin-releasing hormone-induced vasodilatation in the human fetal-placental circulation: involvement of the nitric oxide-cyclic guanosine 3′,5′-monophosphate-mediated pathway. J. Clin. Endocrinol. Metab. 1995;80:2888–2893. doi: 10.1210/jcem.80.10.7559870. [DOI] [PubMed] [Google Scholar]
- COSTE S.C., KESTERSON R.A., HELDWEIN K.A., STEVENS S.L., HEARD A.D., HOLLIS J.H., MURRAY S.E., HILL J.K., PANTELY G.A., HOHIMER A.R., HATTON D.C., PHILLIPS T.J., FINN D.A., LOW M.J., RITTENBERG M.B., STENZEL P., STENZEL-POORE M.P. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat. Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- DONALDSON C.J., SUTTON S.W., PERRIN M.H., CORRIGAN A.Z., LEWIS K.A., RIVIER J.E., VAUGHAN J.M., VALE W.W. Cloning and characterization of human urocortin. Endocrinology. 1996;137:2167–2170. doi: 10.1210/endo.137.5.8612563. [DOI] [PubMed] [Google Scholar]
- DURIDANOVA D.B., PETKOVA-KIROVA P.S., LUBOMIROV L.T., GAGOV H.S., BOEV K.K. Corticotropin-releasing hormone acts on guinea pig ileal smooth muscle via protein kinase A. Pflügers Arch. 1999;438:205–212. doi: 10.1007/s004240050899. [DOI] [PubMed] [Google Scholar]
- ECKART K., RADULOVIC J., RADULOVIC M., JAHN O., BLANK T., STIEDL O., SPIESS J. Actions of CRF and its analogs. Curr. Med. Chem. 1999;6:1035–1053. [PubMed] [Google Scholar]
- GANITKEVICH V.Y., ISENBERG G. Contribution of Ca2+-induced Ca2+ release to the [Ca2+]i transients in myocytes from guinea-pig urinary bladder. J. Physiol. 1992;458:119–137. doi: 10.1113/jphysiol.1992.sp019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAMMATOPOULOS D.K., RANDEVA H.S., LEVINE M.A., KATSANOU E.S., HILLHOUSE E.W. Urocortin, but not corticotropin-releasing hormone (CRH), activates the mitogen-activated protein kinase signal transduction pathway in human pregnant myometrium: an effect mediated via R11α and R2β CRH receptor subtypes and stimulation of Gq-proteins. Mol. Endocrinol. 2000;14:2076–2091. doi: 10.1210/mend.14.12.0574. [DOI] [PubMed] [Google Scholar]
- JAIN V., LONGO M., ALI M., SAADE G.R., CHWALISZ K., GARFIELD R.E. Expression of receptors for corticotropin-releasing factor in the vasculature of pregnant rats. J. Soc. Gynecol. Investig. 2000;7:153–160. doi: 10.1016/s1071-5576(00)00044-7. [DOI] [PubMed] [Google Scholar]
- JAIN V., VEDERNIKOV Y.P., SAADE G.R., CHWALISZ K., GARFIELD R.E. The relaxation responses to corticotropin-releasing factor in rat aorta are endothelium dependent and gestationally regulated. Am. J. Obstet.Gynecol. 1997;176:234–240. doi: 10.1016/s0002-9378(97)80042-7. [DOI] [PubMed] [Google Scholar]
- JAIN V., VEDERNIKOV Y.P., SAADE G.R., CHWALISZ K., GARFIELD R.E. Endothelium-dependent and -independent mechanisms of vasorelaxation by corticotropin-releasing factor in pregnant rat uterine artery. J. Pharmac. Exp. Ther. 1999;288:407–413. [PubMed] [Google Scholar]
- LEI S., RICHTER R., BIENERT M., MULVANY M.J. Relaxing actions of corticotropin-releasing factor on rat resistance arteries. Br. J. Pharmacol. 1993;108:941–947. doi: 10.1111/j.1476-5381.1993.tb13490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVENBERG T.W., CHALMERS D.T., LIU C., DE SOUZA E.B. CRF2α and CRF2b receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- MACDONALD J.A., WALKER L.A., NAKAMOTO R.K., GORENNE I., SOMLYO A.V., SOMLYO A.P., HAYSTEAD T.A. Phosphorylation of telokin by cyclic nucleotide kinases and the identification of in vivo phosphorylation sites in smooth muscle. FEBS Lett. 2000;479:83–88. doi: 10.1016/s0014-5793(00)01884-6. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- REMBOLD C.M., CHEN X.-L. Mechanisms responsible for forskolin-induced relaxation of rat tail artery. Hypertension. 1998;31:872–877. doi: 10.1161/01.hyp.31.3.872. [DOI] [PubMed] [Google Scholar]
- SAUZEAU V., LE JEUNE H., CARIO-TOUMANIANTZ C., SMOLENSKI A., LOHMANN S.M., BERTOGLIO J., CHARDIN P., PACAUD P., LOIRAND G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J. Biol. Chem. 2000;275:21722–21729. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- SCHILLING L., KANCLER C., SCHMIEDEK P., EHRENREICH H. Characterization of the relaxant action of urocortin, a new peptide related to corticotropin-releasing factor in rat isolated basilar artery. Br. J. Pharmacol. 1998;125:1164–1171. doi: 10.1038/sj.bjp.0702182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUBERT R., LEHMANN G., SEREBRYAKOV V.N., MEWES H., HOPP H.H. cAMP-dependent protein kinase is in an active state in rat small arteries possessing a myogenic tone. Am. J. Physiol. 1999;277:H1145–1155. doi: 10.1152/ajpheart.1999.277.3.H1145. [DOI] [PubMed] [Google Scholar]
- SCHUBERT R., SEREBRYAKOV V.N., MEWES H., HOPP H.H. Iloprost dilates rat small arteries: role of KATP- and KCa-channel activation by cAMP-dependent protein kinase. Am. J. Physiol. 1997;272:H1147–1156. doi: 10.1152/ajpheart.1997.272.3.H1147. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., WU X., WALKER L.A., SOMLYO A.V. Pharmacomechanical coupling: the role of calcium, G-proteins, kinases and phosphatases. Rev. Physiol. Biochem. Pharmacol. 1999;134:201–234. doi: 10.1007/3-540-64753-8_5. [DOI] [PubMed] [Google Scholar]
- SUNAHARA R.K., DESSAUER C.W., GILMAN A.G. Complexity and diversity of mammalian adenylyl cyclases. Annu. Rev. Pharmacol. Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- TRAN N.N., SPITZBARTH E., ROBERT A., GIUMMELLY P., ATKINSON J., CAPDEVILLE-ATKINSON C. Nitric oxide lowers the calcium sensitivity of tension in the rat tail artery. J. Physiol. 1998;507:163–174. doi: 10.1111/j.1469-7793.1998.163bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHAN J., DONALDSON C., BUTTENCOURT J., PERRINM H., LEWIS K., SUTTON S., CHAN R., TURNBULL A.V., LOVEJOY D., RIVIER C., RIVIER J., SAWCHENKO P.E., VALE W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- VEGESNA R.V., DIAMOND J. Comparison of the effects of forskolin and isoproterenol on cyclic AMP levels and tension in bovine coronary artery. Can. J. Physiol. Pharmacol. 1983;61:1202–1205. doi: 10.1139/y83-179. [DOI] [PubMed] [Google Scholar]
- WU X., SOMLYO A.V., SOMLYO A.P. Cyclic GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphate. Biochem. Biophys. Res. Commun. 1996;220:658–663. doi: 10.1006/bbrc.1996.0460. [DOI] [PubMed] [Google Scholar]


