Abstract
There is evidence for interactions between mu and delta opioid systems both in vitro and in vivo. This work examines the hypothesis that interaction between these two receptors can occur intracellularly at the level of G protein in human neuroblastoma SH-SY5Y cells.
The [35S]GTPγS binding assay was used to measure G protein activation following agonist occupation of opioid receptors. The agonists DAMGO (EC50, 45 nM) and SNC80 (EC50, 32 nM) were found to be completely selective for stimulation of [35S]-GTPγS binding through mu and delta opioid receptors respectively. Maximal stimulation of [35S]-GTPγS binding produced by SNC80 was 57% of that seen with DAMGO. When combined with a maximally effective concentration of DAMGO, SNC80 caused no additional [35S]-GTPγS binding. This effect was also seen when measured at the level of adenylyl cyclase.
Receptor activation increased the dissociation of pre-bound [35S]-GTPγS. In addition, the delta agonist SNC80 promoted the dissociation of [35S]-GTPγS from G proteins initially labelled using the mu agonist DAMGO. Conversely, DAMGO promoted the dissociation of [35S]-GTPγS from G proteins initially labelled using SNC80.
Tolerance to DAMGO and SNC80 in membranes from cells exposed to agonist for 18 h was homologous and there was no evidence for alteration in G protein activity.
The findings support the hypothesis that mu- and delta-opioid receptors share a common G protein pool, possibly through a close organization of the two receptors and G protein at the plasma membrane.
Keywords: Mu-opioid receptor, delta-opioid receptor, G protein, [35S]-GTPγS binding, [35S]-GTPγS dissociation, DAMGO, SNC80, cross-talk, SH-SY5Y cell
Introduction
Opioid mu and delta receptors couple to pertussis toxin sensitive G proteins (Uhl et al., 1993), which inhibit adenylyl cyclase. Selective agonists at the mu and delta receptors have their own distinct characteristics and pharmacology, though there is evidence for cross-talk between the two receptor types. This comes from ligand binding assays and pharmacological assays including antinociception, bladder contraction, antitussive activity and inhibition of gut propulsion (for review see Traynor & Elliott, 1993), although cross-talk is not apparent in isolated tissue preparations (Elliott & Traynor, 1995; Matthes et al., 1998). In addition, there is evidence of a role for the delta opioid system in the development of tolerance to mu-opioid agonists (Abdelhamid et al., 1991; Kest et al., 1996; Hepburn et al., 1997).
More recently these interactions have been highlighted with the availability of opioid receptor knockout mice. Delta analgesia and respiratory depression are reported to be reduced in mu-receptor knockout mice (Matthes et al., 1998) and there is confirmation of a role for the delta system in mu-tolerance from studies with delta-receptor knockout mice (Zhu et al., 1999). Finally, there is evidence for the presence of mu-/ delta-receptor interactions when both receptors are co-expressed in GH3 cells (Martin & Prather, 2001) and evidence for hetero -dimers or -oligomers when both receptors are expressed in COS-7 (George et al., 2000) or HEK-293 (Gomes et al., 2000) cells, resulting in receptors with different ligand binding and functional properties.
Intracellular cross-talk mechanisms between receptors could occur at the level of G protein (Kenakin & Morgan, 1989). For example, in membranes from turkey erythrocytes and rat adipocytes different Gs-coupled receptors share a common G protein pool (Pike & Lefkowitz, 1981; Murayama & Ui, 1984), and in hamster adipocyctes different Gi-coupled receptors can access the same G proteins (Murayama & Ui, 1984). Both mu- and delta-opioid receptors couple to similar subtypes of Gi and Go proteins which inhibit adenylyl cyclase and are pertussis-toxin sensitive (Laugwitz et al., 1993; Chakrabarti et al., 1995; Prather et al., 1994a). This study was designed to test the hypothesis that mu and delta receptors activate the same individual G proteins in cells that express both receptor types and this provides a focus for interaction. Human neuroblastoma SH-SY5Y cells were chosen for this study because they endogenously express both mu- and delta-opioid receptors (Kazmi & Mishra, 1987) that couple to similar effectors. For example, in SH-SY5Y cells agonist occupation of both receptor types leads to inhibition of adenylyl cyclase and, in differentiated SH-SY5Y cells, inhibition of ω-conotoxin-sensitive Ca2+ channels (Toselli et al., 1997) and mobilization of Ca2+ from intracellular stores (Connor & Henderson, 1996).
Opioid-receptor-mediated activation of G proteins stimulates the binding of [35S]-GTPγS to G proteins in SH-SY5Y membranes (Traynor & Nahorski, 1995). Agonist stimulation of [35S]-GTPγS binding is dependent on the presence of GDP which binds to unoccupied G protein. Receptor activation leads to a conformational change in G protein that decreases its affinity for nucleotide, causing the dissociation of GDP which subsequently allows [35S]-GTPγS to bind, although the affinity of [35S]-GTPγS is also reduced. However, receptor stimulation by agonist also leads to an increased dissociation of [35S]-GTPγS which is already bound to the G protein, provided the receptor has access to the [35S]-GTPγS-bound Gα subunit. Such an effect has been demonstrated in both the muscarinic acetylcholine (Hilf et al., 1992) and cannabinoid (Breivogel et al., 1998) G protein-coupled receptor systems. Therefore, receptor-mediated G protein activation can facilitate both the binding of [35S]-GTPγS, and the dissociation of existing [35S]-GTPγS label from activated G proteins. Both of these effects are utilized in the current study to show that G protein stimulation through mu- and delta-receptor activation is non-additive and that [35S]-GTPγS binding to G protein as a result of mu- or delta-receptor activation can be caused to dissociate by either a mu- or a delta-agonist. Taken together these findings strongly support the hypothesis that mu- and delta-opioid receptors share a common G protein pool and this provides an intracellular mechanism for interaction between these two receptors.
Methods
Chemicals and drugs
[3H]-[D-Ala2, NMePhe4, Gly5-ol]-enkephalin ([3H]-DAMGO; 54.5 Ci/mmol; 2.02 TBq/mmol), [3H]-[D-Pen2,D-Pen5]enkephalin; ([3H]-DPDPE: 30 Ci/mmol; 1.67 TBq/mmol) and [35S] - guanosine - 5′ - O - (3 - thio) triphosphate ([35S] - GTPγS; 1250 Ci/mmol; 46.25 TBq/mmol) were purchased from Du Pont NEN (Boston, MA, U.S.A.) The radioimmunoassay kit for cyclic AMP was from Diagnostic Products Corp. (Los Angeles, CA, U.S.A.) SNC80, (+)-4-[(R)-[(2S,5R)-2,5-dimethyl - 4 - (2 - propenyl) - 1 - piperazinyl] - (3 - methoxyphenyl)methyl]-N,N-diethyl-benzamide was a kind gift from Dr K.C. Rice, National Institutes of Health, Bethesda, MD, U.S.A. CTAP (D-Phe-c[Cys-Tyr-D-Trp-Asp-Thr-Pen]-Thr-NH2) and TIPP[ψ] (H-Tyr-Ticψ-[CH2NH]Phe-Phe-OH) were provided by the National Institute on Drug Abuse (Rockville, MD, U.S.A.) DAMGO, GTPγS, GDP, 3-isobutyl-1-methylxanthine (IBMX) and all other biochemicals were from the Sigma Chemical Co. (St. Louis, MO, U.S.A.) and were of analytical grade. Foetal bovine serum and all cell culture media and additives were purchased from Gibco Life Sciences (Gaithersberg, MD, U.S.A.)
Cell culture
SH-SY5Y cells and C6 rat glioma cells stably transfected with a rat mu (C6(μ)) or delta (C6(δ)) opioid receptor (Lee et al., 1999) were used. Cells were grown to confluence under 5% CO2 in either Minimum Essential Medium (SH-SY5Y cells) or Dulbecco's Modified Eagle's Medium (DMEM) (C6 cells) containing 10% foetal bovine serum. For subculture of stable transfected C6 cells one flask from each passage was grown in the presence of 1 mg ml−1 Geneticin. Cells used for experiments were grown in the absence of Geneticin without a significant loss in receptor density. For the study of tolerance, cells were grown in the presence of DAMGO (1 μM) or SNC80 (1 μM) or vehicle for 18 h prior to harvest. Compounds were added in a sterile water/DMSO vehicle such that the final DMSO concentration was 0.01%.
Membrane preparation
Cells were rinsed twice with ice-cold phosphate-buffered saline (0.9% NaCl, 0.61 mM Na2HPO4, 0.38 mM KH2PO4, pH 7.4), detached from dishes by incubation with lifting buffer (mM: glucose 5.6, KCl 5, HEPES 5, NaCl 137, EGTA 1, pH 7.4) and collected by centrifugation (500×g). The cells were resuspended in ice-cold lysis buffer (0.2 mM MgSO4, 0.38 mM KH2PO4, 0.61 mM Na2HPO4, pH 7.4) and homogenized using a glass-glass Dounce homogenizer. Crude membranes were isolated by centrifugation for 20 min at 20,000×g. The resulting membrane pellets were resuspended in 50 mM Tris-HCl buffer (pH 7.4) and stored at −80°C in 500 μl aliquots containing 0.5 mg protein (Bradford, 1976). All procedures were performed at 4°C.
Ligand-binding assays
SH-SY5Y cell membranes (75 μg) were incubated for 2 h in a shaking water bath at 25°C with varying concentrations (0.05 – 25 nM) of [3H]-DAMGO or [3H]-DPDPE in 2 ml 50 mM Tris-HCl buffer (pH 7.4) or GTPγS binding buffer (mM: Tris 50, NaCl 100, MgCl2 5, EDTA 1, dithiothreitol 1, pH 7.4) containing 30 μM GDP. The reactions were terminated by the addition of 2 ml ice-cold Tris-HCl buffer. The contents of the tubes were then rapidly vacuum-filtered through glass fibre filters (Schleicher & Schuell no.32, Keene, NH, U.S.A.) and the tubes and filters rinsed with ice-cold 3 ml Tris-HCl an additional three times. Radioactivity retained by the filters was determined by liquid scintillation counting. Non-specific binding was defined with 10 μM naloxone.
Cyclic AMP assay
SH-SY5Y cells were grown in 24-well plates for 24 h to confluency as described above. The culture medium was then replaced with DMEM without foetal bovine serum, followed by replacement of the media with DMEM at 37° containing 1.0 mM IBMX, 30 μM forskolin with or without appropriate opioid agonist. After 30 min at 37°, the assay was stopped by removing the assay medium and replacing with 1 ml ice cold 3% perchloric acid. After at least 30 min at 4°, a 400 μl aliquot was removed from each well, neutralized with 75 μl 2.5 M KHCO3 and centrifuged for 1 min at 15,000×g. A radioimmunoassay kit was used to quantify accumulated cyclic AMP in a 10 μl aliquot of the supernatant from each sample. Inhibition of cyclic AMP formation was determined as a per cent of forskolin-stimulated cyclic AMP accumulation in the absence of opioid agonist.
[35S]GTPγS binding
Membranes (30 μg protein) of SH-SY5Y, C6(μ) or C6(δ) cells were incubated with 50 pM [35S]GTPγS for 60 min at 25°C, in the absence or presence of varying concentrations of agonist, in GTPγS binding buffer (final concentration mM: Tris 50, NaCl 100, MgCl2 5, EDTA 1, dithiothreitol 1, GDP 50 μM, pH 7.4) in a final assay volume of 400 μl. Reactions were terminated by the addition of 2 ml ice-cold washing buffer (mM: Tris 50, NaCl 100, MgCl2 5, pH 7.4), followed by rapid filtration as above. The tubes and filters were rinsed three times with 3 ml ice-cold washing buffer and bound ligand determined by scintillation counting.
[35S]GTPγS dissociation
SH-SY5Y cell membranes (1 mg protein) were incubated for 80 min at 25°C with 80 pM [35S]-GTPγS in GTPγS binding buffer, in either the presence or absence of 1 μM DAMGO or 1 μM SNC80, in a total volume of 8 ml. An 800 μl sample of the membrane suspension was removed and filtered (as above) to determine maximal [35S]-GTPγS binding. Antagonist (TIPP[ψ], 3 μM or CTAP, 300 nM) or ddH2O in the presence or absence of appropriate agonist was added 5 min before 50 μM unlabelled GTPγS and eight×800 μl samples were then removed at 1 – 15 min intervals up to 58 min. Samples were filtered and counted as described for [35S]GTPγS association assays.
Data analysis
Graph Pad Prism (San Diego, CA, U.S.A.) was used to perform linear and nonlinear regression analysis of the data. Ligand saturation binding data were analysed using a one-site saturation binding equation. Concentration response curves for [35S]-GTPγS binding were fitted to a sigmoidal curve with a Hill coefficient of unity. [35S]-GTPγS dissociation experiments were fit to a one-phase exponential decay curve. Data are presented as mean±standard error of the mean or as 95% confidence intervals (C.I.) from at least three separate experiments each performed in duplicate.
Results
Ligand binding
Saturation binding of the selective mu agonist [3H]-DAMGO and selective delta agonist [3H]-DPDPE was measured in membranes from SH-SY5Y cells. In Tris-HCl buffer [3H]-DAMGO bound to SH-SY5Y cell membranes with a Bmax of 600±60 fmol/mg protein and Kd of 0.4±0.1 nM and [3H]-DPDPE afforded a Bmax of 280±20 fmol/mg protein and Kd of 1.8±0.2 nM, indicating a 2 : 1 ratio of mu to delta receptors. In GTPγS binding buffer that contains NaCl (100 mM) and 30 μM GDP the level of binding of both ligands was considerably reduced. Thus, [3H]-DAMGO bound with Kd of 8.3 nM and Bmax of 85±9 fmols/mg protein and [3H]-DPDPE bound with Kd of 11.0±2.1 nM and Bmax of 91±27 fmols/mg protein. The presence of the delta antagonist TIPP[ψ] (10 nM) did not change the binding parameters for [3H]-DAMGO (Kd=8.0±1.9 nM; Bmax=90±4 fmol/mg protein).
Adenylyl cyclase
Cyclic AMP accumulation stimulated by forskolin was reduced to 65.3±3.2% by 1 μM DAMGO and to 86.2±4.8% by 1 μM SNC80. The addition of SNC80 (1 μM) and DAMGO (1 μM) together did not increase the level of cyclic AMP inhibited by DAMGO alone (65.4±3.3%).
[35S]-GTPγS binding
Basal [35S]-GTPγS binding to membranes from SH-SY5Y cells was 16.1±1.4 fmols/mg protein. DAMGO caused a doubling of [35S]-GTPγS binding with an EC50 of 45 nM (95% C.I., 30 – 67 nM). The selective delta full agonist SNC80 afforded an EC50 of 32 nM (95% C.I., 11 – 54 nM), but produced only 57±5% of the maximal [35S]-GTPγS binding stimulation seen with DAMGO (Figure 1).
Figure 1.
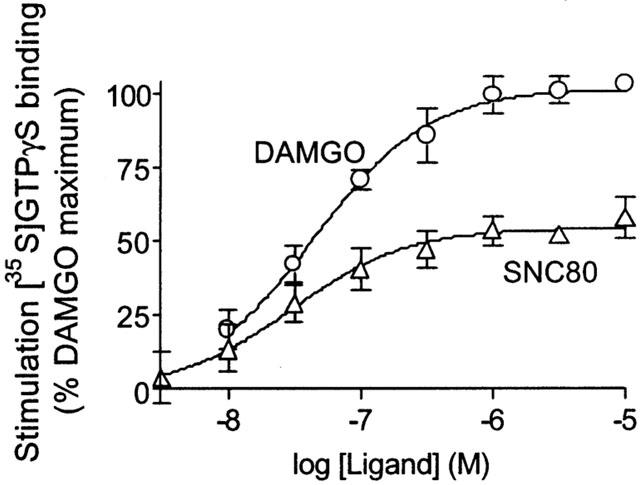
Stimulation of [35S]-GTPγS binding by DAMGO and SNC80. SH-SY5Y membrane homogenates were incubated with 50 pM [35S]-GTPγS in the presence of DAMGO or SNC80 as described in Methods. The data are expressed as [35S]-GTPγS binding stimulation relative to the maximum effect produced by DAMGO. Data represent means±s.e.mean from at least three experiments carried out in duplicate.
To verify that DAMGO and SNC80 produced their effects by selectively acting at mu and delta receptors respectively, concentration-response curves were determined in the presence of the mu-selective antagonist CTAP (Pelton et al., 1986) or the delta-selective antagonist TIPP[ψ] (Schiller et al., 1993). Addition of 300 nM CTAP shifted the DAMGO concentration-response curve 47 fold (Figure 2A), but produced only a statistically insignificant 1.5 fold shift in the SNC80 concentration-response curve (Figure 2B).
Figure 2.
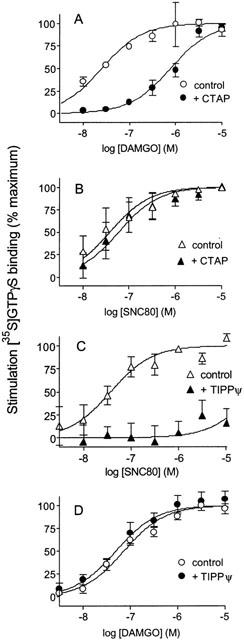
Effect of mu-(CTAP) and delta- (TIPPψ) selective antagonists on DAMGO and SNC80 concentration-response curves. Concentration-response curves for [35S]-GTPγS binding were determined for DAMGO and SNC80 in the absence and presence of antagonist, and expressed as % maximal stimulation. (A) 300 nM CTAP produces a 1.68±0.14 log rightward shift in the DAMGO concentration-response curve. (B) 300 nM CTAP has no significant effect (0.19±0.15 log rightward shift) on the SNC80 concentration-response curve. (C) 10 μM TIPP[ψ] blocks G protein stimulation by SNC80 at concentrations up to 10 μM. (D) 10 μM TIPP[ψ] has no significant effect (0.14±0.25 log leftward shift) on the DAMGO concentration-response curve. Shown are means±s.e.mean from at least three independent experiments carried out in duplicate.
Conversely, 10 μM TIPP[ψ] completely blocked the effect of SNC80 at concentrations up to 10 μM (Figure 2C) but had no significant effect (1.4 fold shift) on the concentration-response curve for DAMGO (Figure 2D). The selectivity of DAMGO and SNC80 for the mu and delta opioid receptors respectively was confirmed in membranes from C6 rat glioma cells stably expressing cloned mu or delta opioid receptors. In C6(μ) cell membranes, DAMGO stimulated [35S]-GTPγS binding with potency similar to that seen in SH-SY5Y membranes (EC50=32 nM, 95% C.I., 13 – 76 nM) but SNC80 at concentrations up to 10 μM had no significant effect (9±9% of maximal DAMGO stimulation). Conversely, in C6(δ) cell membranes, SNC80 stimulated [35S]-GTPγS binding with an EC50 of 18 nM (95% C.I., 6 – 56 nM), but DAMGO had no appreciable effect at concentrations up to 10 μM, producing only 4±1% of the maximal SNC80 stimulation.
To test the hypothesis that mu and delta opioid receptors share a common pool of G proteins, the additivity of G protein activation by DAMGO and SNC80 in SH-SY5Y cell membranes was measured. When maximally effective concentrations of DAMGO and SNC80 were combined, the level of [35S]-GTPγS binding was not significantly greater than that produced by DAMGO alone (Figure 3).
Figure 3.
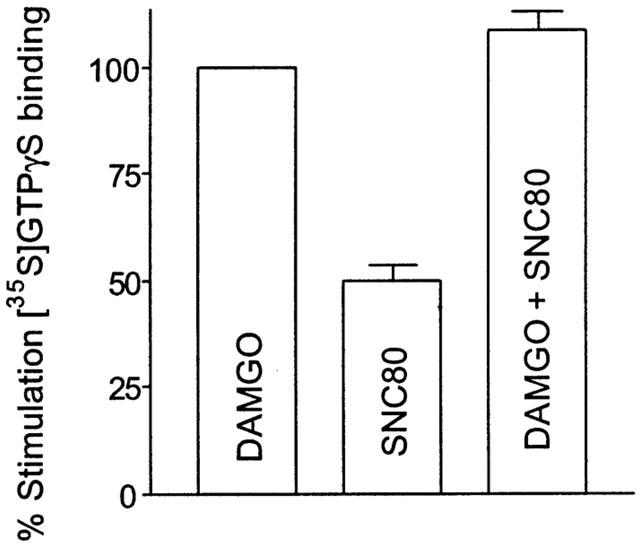
Non-additivity of mu- and delta- mediated G protein activation. Stimulation of [35S]-GTPγS binding by maximally effective (10 μM) concentrations of DAMGO and SNC80 was measured. SNC80 alone produced 50±4% of the effect afforded by 10 μM DAMGO. When 10 μM SNC80 was combined with 10 μM DAMGO, the total G protein activation totalled 108±4% of that produced by DAMGO alone. Values are means±s.e.mean from three independent experiments carried out in duplicate.
[35S]-GTPγS dissociation
To further test the hypothesis that mu and delta receptors access the same G proteins, the effect of receptor activation on [35S]-GTPγS dissociation was measured. Membranes from SH-SY5Y cells were incubated with [35S]-GTPγS and 1 μM DAMGO to label mu-receptor associated G proteins. Dissociation of the [35S]-GTPγS label was measured after addition of a large excess of unlabelled GTPγS (Figure 4A); the DAMGO was not removed. Continued mu receptor activation resulted in rapid [35S]-GTPγS dissociation with a t1/2 of 9±1 min, though only 58±6% of the DAMGO-induced [35S]-GTPγS binding was dissociable. The dissociation time was markedly extended (t1/2=37±18 min) in the presence of the mu antagonist CTAP (300 nM). When 1 μM SNC80 was added in addition to CTAP, an increase in the [35S]-GTPγS dissociation rate was seen to give a t1/2 of 17±2 min, though the dissociation observed was slower than that seen with DAMGO alone. Of the DAMGO-stimulated [35S]-GTPγS binding, 45±2% was found to dissociate in the presence of SNC80.
Figure 4.
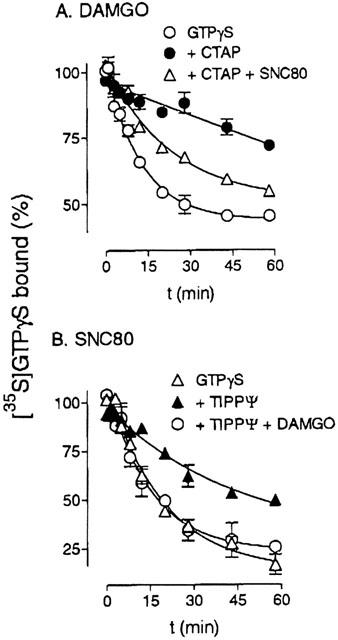
Agonist stimulation of [35S]-GTPγS dissociation. (A) SH-SY5Y membranes were incubated with 80 pM [35S]-GTPγS and 1 μM DAMGO to label mu-sensitive G proteins, as described in Methods. At time zero, dissociation was initiated by the addition of 50 μM unlabelled GTPγS in the absence or presence of 300 nM CTAP or 300 nM CTAP+1 μM SNC80. (B) Delta-sensitive G proteins were labelled using 1 μM SNC80. To start dissociation, 50 μM unlabelled GTPγS was added in the absence or presence of 3 μM TIPP[ψ], or 3 μM TIPP[ψ]+1 μM DAMGO. Shown are means±s.e.mean from three independent experiments carried out in duplicate.
Delta-receptor coupled G proteins in SH-SY5Y cell membranes were labelled with [35S]-GTPγS in the presence of 1 μM SNC80. Dissociation of [35S]-GTPγS was measured following addition of an excess of unlabelled GTPγS; the SNC80 was not removed (Figure 4B). Continued delta receptor activation resulted in rapid dissociation of [35S]-GTPγS (t1/2=14±2 min). In contrast to DAMGO-stimulated [35S]-GTPγS binding, almost all (88±7%) of SNC80-stimulated [35S]-GTPγS binding was found to be reversible. Blockade of the delta receptor with 3 μM TIPP[ψ] resulted in a decreased [35S]-GTPγS dissociation rate (t1/2=34±6 min). However, addition of 1 μM DAMGO increased the dissociation rate to a similar rate as seen in the presence of 1 μM SNC80 alone (t1/2=11±1 min), with a maximal dissociation of 77±5% of the bound [35S]-GTPγS. Neither DAMGO nor SNC80 produced dissociation of agonist-unstimulated (basal) [35S]-GTPγS binding (data not shown).
Tolerance
To determine whether cross-tolerance is exhibited between mu and delta agonists in this system, SH-SY5Y cells were treated for 18 h in the presence or absence of 1 μM DAMGO or 1 μM SNC80 prior to harvesting and membrane preparation. DAMGO-stimulated [35S]GTPγS binding in membranes from cells treated chronically with DAMGO was only 55±4% of that seen in control cells, but with no significant change in EC50. The EC50 value for DAMGO was 25 nM (95% C.I., 11 – 54 nM) in membranes from chronic DAMGO-treated cells versus 23 nM (95% C.I., 13 – 38 nM) in membranes from cells treated with vehicle only (Figure 5A). The SNC80 concentration-response curve was not significantly affected by 18 h DAMGO treatment. Thus, in membranes from DAMGO-treated cells maximal [35S]-GTPγS binding was equal to 97±5% of that seen in untreated cells and there was no change in the EC50 (10 nM, 95% C.I., 7 – 14 nM versus 9 nM, 95% C.I., 5 – 17 nM in control membranes; Figure 5B).
Figure 5.
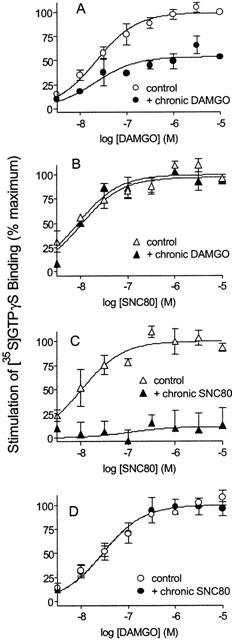
Effect of chronic opioid treatment on [35S]-GTPγS binding. SH-SY5Y cells were incubated for 18 h in the absence or presence of 1 μM DAMGO (A and B) or 1 μM SNC80 (C and D) prior to harvesting. Stimulation of [35S]-GTPγS binding in response to subsequent DAMGO or SNC80 was then measured. Data are presented as means±s.e.mean from three independent experiments carried out in duplicate.
Chronic treatment of cells with SNC80 resulted in a complete insensitivity of membranes to subsequent SNC80 administration at concentrations up to 10 μM (Figure 5C), with no effect on the DAMGO concentration-response curve (Figure 5D). Maximal DAMGO stimulation of [35S]-GTPγS binding in membranes from chronic SNC80-treated cells was equal to 99±8% of that seen in control cells, with EC50 of 28 nM (95% C.I., 17 – 45 nM) versus 29 nM (95% C.I., 18 – 46 nM) in membranes from cells treated with vehicle alone.
Discussion
Mu and delta opioid receptors can activate the same inhibitory G protein subtypes (Prather et al., 1994a; Chakrabarti et al., 1995) although in SH-SY5Y human neuroblastoma cells mu and delta receptors do show a different preference for Gα subunits (Laugwitz et al., 1993). The current study was designed to test the hypothesis that mu and delta receptors share a common pool of G proteins and that at full receptor occupancy the receptors compete for G protein.
The mu-selective agonist DAMGO and the delta-selective agonist SNC80 stimulated [35S]-GTPγS binding in a concentration-dependent manner in SH-SY5Y cells. SNC80 produced 57±5% of the maximum effect seen with DAMGO, consistent with the 1 : 2 ratio of total delta to mu receptors as measured in Tris buffer. In the presence of the more complex buffer containing Na+ ions and GDP the measured number of mu and delta receptors was equivalent, but much reduced, together with a reduction in ligand affinity. This suggests the total receptor number, rather than the number of receptors in a particular affinity state, governs the maximal level of [35S]-GTPγS binding.
When maximally effective concentrations of DAMGO and SNC80 were combined, the effect produced was not significantly greater than that produced by DAMGO alone determined either by stimulation of [35S]-GTPγS binding or by the inhibition of cyclic AMP accumulation, indicating that agonist-occupied mu and delta receptors activate common G proteins. This conclusion was confirmed by the ability of agonists specific for either receptor to afford dissociation of [35S]-GTPγS that had been caused to bind to G protein α-subunits by either a mu or a delta agonist. Indeed, the ability of both mu and delta agonists to cause dissociation suggests the [35S]-GTPγS-occupied Gα subunit remains accessible to both mu and delta receptors, such that both receptors can access the C-terminus of the Gα subunit that is important for receptor-G protein coupling (Conklin et al., 1993). This conclusion is supported by studies demonstrating a persistent membrane localization of Gα1 throughout the cycle of G protein activation (Huang et al., 1999) and the ability of receptor-G protein fusion proteins to interact with adenylyl cyclase (Bertin et al., 1994; Milligan, 2000). Alternatively the dissociation of bound [35S]-GTPγS could be through an indirect mechanism. Gβγ is known to promote the dissociation of [35S]-GTPγS from purified Gαo and Gαi subunits and this process is inhibited by Mg2+ (Sternweis & Robishaw, 1984; Higashijima et al., 1987). It is feasible that in native membranes [35S]-GTPγS dissociation from Gα subunits can be induced by Gβγ, even in the presence of the level of Mg2+ (5 mM) used in the present assays. Thus, by agonist action in the presence of a large excess of unlabelled GTPγS, GDP-bound Gαβγ will be induced to bind unlabelled GTPγS, releasing Gβγ subunit that promotes [35S]-GTPγS dissociation from Gα-[35S]-GTPγS labelled subunits (Breivogel et al., 1998).
The simplest explanation for the ability of DAMGO and SNC80 to activate the same G proteins would be if either or both ligands lack receptor selectivity. However, this explanation can be ruled out based on the effects of the selective antagonists CTAP and TIPP[ψ] on agonist-induced [35S]-GTPγS binding, and on the highly mu- selective action of DAMGO and delta-selective action of SNC80 in membranes from C6 cells expressing a single receptor type.
The finding that mu and delta receptors share G proteins in SH-SY5Y cell membranes could indicate access of the receptors to the complete inhibitory G protein pool, as for example with Gi-coupled receptors in hamster adipocyte membranes (Murayama & Ui, 1984). However, there is evidence for compartmentalization of signalling in SH-SY5Y cells with each receptor able to activate approximately four G proteins (Remmers et al., 2000) and that the number of G protein activated depends upon the receptor concentration. The observation that maximal delta-agonist mediated G protein activation was only half of that produced by agonist occupation of the mu receptors indicates either that delta receptors cannot access the entire G protein pool available to mu receptors or that one or more species of mu receptor-sensitive G proteins exist which are relatively insensitive to delta receptor activation.
The findings are also consistent with a model in which mu and delta receptors are associated in a mu-delta complex (Vaught et al., 1982) although the evidence for such a complex has remained indirect (Traynor & Elliott, 1993). More recently, opioid receptors have been shown to form homo- (Cvejic & Devi, 1997), and hetero-oligomers (Jordan & Devi, 1999; George et al., 2000; Gomes et al., 2000). The ability of mu and delta receptors to share individual G proteins in the current study may indicate that the receptors are in close physical proximity to each other, and it is tempting to speculate that these opioid receptors exist as heterooligomers in SH-SY5Y cell membranes. Certainly, mu and delta receptors form heterooligomers when co-expressed in COS-7 cells and such heterooligomers have unique properties (George et al., 2000). However, there is no evidence for a new signalling entity composed of mu and delta receptors in the SH-SY5Y cell membranes employed in this study. Firstly, the EC50 for stimulation of [35S]-GTPγS binding by DAMGO or SNC80 is the same in these cells as in C6 cells expressing the mu and delta opioid receptor separately. Secondly, the delta antagonist TIPP[ψ] does not alter the activation of [35S]-GTPγS binding via mu receptor activation and the mu antagonist CTAP does not effect activation of [35S]GTPγS binding via the delta receptor. This contrasts with findings that TIPP[ψ] increases the potency and efficacy of DAMGO, and the mu antagonist CTOP increases the potency and efficacy of the delta-agonist Deltorphin II, to induce phosphorylation of p-42/44 MAP kinase in SK-N-SH cells (Gomes et al., 2000). Finally, the delta antagonist TIPP[ψ] does not alter the binding of the mu agonist [3H]-DAMGO to membranes from SH-SY5Y cells, an effect reported in both SK-N-SH cells and HEK-393 cells expressing mu and delta opioid receptors and believed to be due to the delta antagonist releasing the mu binding pocket by disruption of the heterodimer (Gomes et al., 2000). Thus, our results suggest that in human neuroblastoma SH-SY5Y cells mu and delta receptors access the same G proteins without necessarily forming hetero-complexes or a new signalling entity.
The ability of mu and delta opioid receptors to activate the same pool of G proteins could explain reported mu delta interactions. For example, in certain in vivo systems the potency and efficacy of morphine, but not higher efficacy agonists such as DAMGO, is increased by sub-effective concentrations of DPDPE or Leu-enkephalin (Vaught et al., 1982; Heyman et al., 1989a; Sheldon et al., 1989; Jiang et al., 1990). Since some cells do co-express both mu and delta receptors (Ji et al., 1995) this may be caused by addition of the stimulatory effects of mu and delta agonists on G protein or by a ‘priming' of G protein by the delta agonists. Presumably such interaction would also occur in the opposite direction and so could contribute to the modulation of spinal analgesia and the lack of delta-mediated respiratory depression in mu-receptor knock-out mice. However, the observation that the delta agonists Met-enkephalin and Met-enkephalinamide inhibit morphine antinociception (Vaught & Takemori, 1979; Vaught et al., 1982; Heyman et al., 1989b; Jiang et al., 1990) is difficult to explain with this model.
Prolonged exposure of opioid receptors to agonist is known to produce a state of tolerance which includes uncoupling of receptor and G protein, receptor down-regulation and compensatory changes in downstream effectors. Although mu and delta receptors share G proteins in SH-SY5Y membranes, tolerance was seen to be homologous and therefore occurred at the receptor, rather than at the G protein. Chronic DAMGO treatment of SH-SY5Y cells results in a reduction in receptor number (Elliott et al., 1997) but it can be inferred that the majority of G proteins remained fully functional, since SNC80 still produced its full G protein activation in the face of tolerance to DAMGO and vice-versa. This is consistent with findings that functional coupling of the delta opioid receptor is not altered in mu-receptor knockout mice (Matthes et al., 1998). Furthermore the findings agree with previous reports that opioid tolerance is homologous in SH-SY5Y cells, both in terms of receptor down-regulation (Zadina et al., 1994), and desensitization of the adenylyl cyclase response (Prather et al., 1994b).
In SK-N-SH, the parent cell line of SH-SY5Y cells, which express mu and delta receptors, mu and delta opioid receptor down-regulation is also homologous (Baumhaker et al., 1993). However, in SK-N-SH cells there is no evidence for cross-talk at the level of G protein and each receptor appears to activate a separate pool of G proteins (Shapiro et al., 2000). When transfected into COS-7 cells mu and delta receptors do share a common pool of G proteins and so Shapiro et al. (2000) suggest the findings are due to differences between transfected cell lines, where receptors show promiscuous coupling, and cells which natively express mu and delta opioid receptors. Our results with mu and delta receptors endogenously expressed in SH-SY5Y cells suggest differences between this cell and its parent SK-N-SH and thus do not support this conclusion. The results, however, do support the broader concept that cellular organization is important in governing which signal transduction pathways are activated by a particular receptor and so varies across cell types.
In conclusion, the data presented confirm that although mu and delta receptors may prefer particular Gi/Go subtypes there is no absolute specificity governed by receptor structure. The findings provide strong evidence for a common activation of G protein by mu and delta opioid receptors in SH-SY5Y human neuroblastoma cells that may play a role in the pharmacology of mu and delta opioid receptors. Moreover the results are consistent with a compartmental organization of mu receptors, delta receptors and G protein for the control of receptor signalling.
Acknowledgments
This work was supported by United States Public Health Service Grant DA00254. We thank Drs Huda Akil and Alfred Mansour for the mu- and delta-receptor clones.
Abbreviations
- CTAP
D-Phe-c [Cys-Tyr-D-Trp-Arg-Thr-Pen]-Thr-NH2
- DAMGO
Tyr-D-Ala-Gly-N-Me-Phe-Gly5-ol
- DPDPE
Tyr-D-Pen-Gly-Phe-D-Pen
- GTPγS
guanosine-5′-O-(3-thio)triphosphate
- SNC80
(+)-4-[(R)-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-methoxyphenyl)methyl]-N,N-diethyl-benzamide
- TIPP[ψ]
H-Tyr-Ticψ-[CH2NH]Phe-Phe-OH
References
- ABDELHAMID E.E., SULTANA M., PORTOGHESE P.S., TAKEMORI A.E. Selective blockage of delta opioid receptors prevents the development of morphine tolerance and dependence in mice. J. Pharmacol. Exp. Ther. 1991;258:299–303. [PubMed] [Google Scholar]
- BAUMHAKER Y., GAFNI M., KEREN O., SARNE Y. Selective and interactive down-regulation of μ- and δ- opioid receptors in human neuroblastoma SK-N-SH cells. Mol. Pharmacol. 1993;44:461–467. [PubMed] [Google Scholar]
- BERTIN B., FREISSMUTH M., JOCKERS R., STROSBERG A.D., MARULLO S. Cellular signaling by an agonist-activated receptor/Gsα fusion protein. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8827–8831. doi: 10.1073/pnas.91.19.8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principal of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BREIVOGEL C.S., SELLEY D.E., CHILDERS S.R. Cannabinoid receptor agonist efficacy for stimulating [35S]GTPγS binding to rat cerebellar membranes correlates with agonist-induced decreases in GDP affinity. J. Biol. Chem. 1998;273:16865–16873. doi: 10.1074/jbc.273.27.16865. [DOI] [PubMed] [Google Scholar]
- CHAKRABARTI S., PRATHER P.L., YU L., LAW P.Y., LOH H.H. Expression of mu-opioid receptor in CHO cells: ability of mu-ligands to promote α-azidoanilide[32P]GTP labeling of multiple G protein subunits. J. Neurochem. 1995;64:2534–2543. doi: 10.1046/j.1471-4159.1995.64062534.x. [DOI] [PubMed] [Google Scholar]
- CONKLIN B.R., FARFEL Z., LUSTIG K.D., JULIUS D., BOURNE H.R. Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature. 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- CONNOR M., HENDERSON G. δ- and μ-opioid receptor mobilization of intracellular calcium in SH-SY5Y human neuroblastoma cells. Br.J. Pharmacol. 1996;117:333–340. doi: 10.1111/j.1476-5381.1996.tb15195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CVEJIC S., DEVI L.A. Dimerization of the δ opioid receptor: implication for a role in receptor internalization. J. Biol. Chem. 1997;272:26959–26964. doi: 10.1074/jbc.272.43.26959. [DOI] [PubMed] [Google Scholar]
- ELLIOTT J., GUO L., TRAYNOR J.R. Tolerance to μ-opioid agonists in human neuroblastoma SH-SY5Y cells as determined by changes in guanosine-5′-O-(3-[35S]-thio)triphosphate binding. Br. J. Pharmacol. 1997;121:1422–1428. doi: 10.1038/sj.bjp.0701253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT J., TRAYNOR J.R. Evidence for lack of modulation of μ-opioid agonist action by δ-opioid agonists in the mouse vas deferens and guinea-pig ileum. Br. J. Pharmacol. 1995;114:1064–1068. doi: 10.1111/j.1476-5381.1995.tb13314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE S.R., FAN T., XIE T., TSE R., TAM V., VARGHESE G., O'DOWD B.F. Oligomerization of μ and δ opioid receptors: generation of novel functional properties. J. Biol. Chem. 2000;275:26128–26135. doi: 10.1074/jbc.M000345200. [DOI] [PubMed] [Google Scholar]
- GOMES I., JORDAN B.B., GUPTA A., TRAPAIDZE N., NAGY V., DEVI L.A. Heterodimerization of μ and opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110 (1–5). doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPBURN M.J., LITTLE P.J., GINGRAS J., KUHN C.M. Differential effects of naltrindole on morphine-induced tolerance and physical dependence in rats. J. Pharmacol. Exp. Ther. 1997;281:1350–1356. [PubMed] [Google Scholar]
- HEYMAN J.S., JIANG Q., ROTHMAN R.B., MOSBERG H.I., PORRECA F. Modulation of mu-mediated antinociception by delta agonists: characterization with antagonists. Eur. J. Pharmacol. 1989a;169:43–52. doi: 10.1016/0014-2999(89)90815-7. [DOI] [PubMed] [Google Scholar]
- HEYMAN J.S., VAUGHT J.L., MOSBERG H.I., HAASETH R.C., PORRECA F. Modulation of mu-mediated antinociception by delta agonists in the mouse: selective potentiation of morphine and normorphine by [D-Pen2,D-Pen5]enkephalin. Eur. J., Pharmacol. 1989b;165:1–10. doi: 10.1016/0014-2999(89)90764-4. [DOI] [PubMed] [Google Scholar]
- HIGASHIJIMA T., FERGUSON K.M., STERNWEIS P.C., SMIGEL M.D. and , GILMAN A.G. Effects of Mg2+ and the βγ-subunit complex on the interactions of guanine nucleotides with G proteins. J. Biol. Chem. 1987;262:762–766. [PubMed] [Google Scholar]
- HILF G., KUPPRION C., WIELAND T., JAKOBS K.H. Dissociation of guanosine 5′-[γ-thio]triphosphate from guanine-nucleotide-binding regulatory proteins in native cardiac membranes. Eur. J. Biochem. 1992;204:725–731. doi: 10.1111/j.1432-1033.1992.tb16687.x. [DOI] [PubMed] [Google Scholar]
- HUANG C., DUNCAN J.A., GILMAN A.G., MUMBY S.M. Persistent membrane association of activated and depalmitoylated G protein α subunits. Proc. Natl. Acad. Sci. U.S.A. 1999;96:412–417. doi: 10.1073/pnas.96.2.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JI R.R., ZHANG Q., LAW P.Y., LOH H.H., ELDE R., HOKFELT T. Expression of μ- δ- and κ-opioid receptor-like immunoreactivities in rat dorsal root ganglia after carrageenan-induced inflammation. J. Neurosci. 1995;15:8156–8166. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIANG Q., MOSBERG H.I., PORRECA F. Modulation of the potency and efficacy of mu-mediated antinociception by delta agonists in the mouse. J. Pharmacol. Exp. Ther. 1990;254:683–689. [PubMed] [Google Scholar]
- JORDAN B.A., DEVI L.A. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAZMI S.M.I., MISHRA R.K. Comparative pharmacological properties and functional coupling of μ and δ opioid receptor sites in human neuroblastoma SH-SY5Y cells. Mol. Pharmacol. 1987;32:109–118. [PubMed] [Google Scholar]
- KENAKIN T.P., MORGAN P.H. Theoretical effects of single and multiple transducer receptor coupling proteins on estimates of the relative potency of agonists. Mol. Pharmacol. 1989;35:214–222. [PubMed] [Google Scholar]
- KEST B., LEE C.E., MCLEMORE G.L., INTURRISI C.E. An antisense oligodeoxynucleotide to the delta opioid receptor (DOR-1) inhibits morphine tolerance and acute dependence in mice. Brain Research Bull. 1996;39:185–188. doi: 10.1016/0361-9230(95)02092-6. [DOI] [PubMed] [Google Scholar]
- LAUGWITZ K.-L., OFFERMANS S., SPICHER K., SCHULTZ G. μ and δ opioid receptors differentially couple to G protein subtypes in membranes of human neuroblastoma SH-SY5Y cells. Neuron. 1993;10:233–242. doi: 10.1016/0896-6273(93)90314-h. [DOI] [PubMed] [Google Scholar]
- LEE K.O., AKIL H., WOODS J.H., TRAYNOR J.R. Differential binding properties of oripavines at cloned μ- and δ-opioid receptors. Eur. J. Pharmacol. 1999;278:323–330. doi: 10.1016/s0014-2999(99)00460-4. [DOI] [PubMed] [Google Scholar]
- MARTIN N.A., PRATHER P.L. Interaction of co-expressed μ- and δ-opioid receptors in transfected rat pituitary GH3 cells. Mol. Pharmacol. 2001;59:774–783. doi: 10.1124/mol.59.4.774. [DOI] [PubMed] [Google Scholar]
- MATTHES H.W., SMADJA C., VALVERDE O., VONESCH J.L., FOUTZ A.S., BOUDINOT E., DENAVIT-SAUBIE M., SEVERINI C., NEGRI L., ROQUES B.P., MOLDONADO R., KEIFFER B.L. Activity of the δ-opioid receptor is partially reduced, whereas activity of the κ-receptor is maintained in mice lacking the μ-receptor. J. Neurosci. 1998;18:7285–7295. doi: 10.1523/JNEUROSCI.18-18-07285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLIGAN G. Insights into ligand pharmacology using receptor-G protein fusion proteins. Trends Pharmacol. Sci. 2000;21:24–28. doi: 10.1016/s0165-6147(99)01404-2. [DOI] [PubMed] [Google Scholar]
- MURAYAMA T., UI M. [3H]GDP release from rat and hamster adipocyte membranes independently linked to receptors involved in activation or inhibition of adenylate cyclase. J. Biol. Chem. 1984;259:761–769. [PubMed] [Google Scholar]
- PELTON J.T., KAZMIERSKI W., GULYA K., YAMAMURA H.I., HRUBY V.J. Design and synthesis of conformationally constrained somatostatin analogues with high potency and specificity for mu opioid receptors. J. Med. Chem. 1986;29:2370–2375. doi: 10.1021/jm00161a037. [DOI] [PubMed] [Google Scholar]
- PIKE L.J., LEFKOWITZ R.J. Correlation of β-adrenergic receptor-stimulated [3H]GDP release and adenylate cyclase activation. J. Biol. Chem. 1981;256:2207–2212. [PubMed] [Google Scholar]
- PRATHER P.L., LOH H.H., LAW P.Y. Interaction of delta-opioid receptors with multiple G proteins: a non-relationship between agonist potency to inhibit adenylyl cyclase and to activate G proteins. Mol. Pharmacol. 1994a;45:997–1003. [PubMed] [Google Scholar]
- PRATHER P.L., TSAI A.W., LAW P.Y. Mu and delta opioid receptor desensitization in undifferentiated human neuroblastoma SH-SY5Y cells. J. Pharmacol. Exp. Ther. 1994b;270:177–184. [PubMed] [Google Scholar]
- REMMERS A.E., CLARK M.J., ALT A., MEDZIHRADSKY F., WOODS J.H., TRAYNOR J.R. Activation of G protein by opioid receptors: role of receptor number and G protein concentration. Eur. J. Pharmacol. 2000;396:67–75. doi: 10.1016/s0014-2999(00)00212-0. [DOI] [PubMed] [Google Scholar]
- SCHILLER P.W., WELTROWSKA G., NGUYEN T.M.D., WILKES B.C., CHUNG N.N., LEMIEUX C. TIPP[ψ]: a highly potent and stable pseudopeptide δ opioid receptor antagonist with extraordinary δ selectivity. J. Med. Chem. 1993;36:3182–3187. doi: 10.1021/jm00073a020. [DOI] [PubMed] [Google Scholar]
- SHAPIRO M., VOGEL Z., SARNE Y. Opioid and cannabinoid receptors share a common pool of GTP-binding proteins in cotransfected cells, but not in cells which endogenously coexpress the receptors. Cell Mol. Neurobiol. 2000;20:291–304. doi: 10.1023/a:1007058008477. [DOI] [PubMed] [Google Scholar]
- SHELDON R.J., NUNAN L., PORRECA F. Differential modulation by [D-Pen2, D-Pen5]enkephalin and dynorphin A-(1-17) of the inhibitory bladder motility effects of selected mu agonists in vivo. J. Pharmacol. Exp. Ther. 1989;249:462–469. [PubMed] [Google Scholar]
- STERNWEIS P.C., ROBISHAW J.D. Isolation of two proteins with high affinity for guanine nucleotides from membranes of bovine brain. J. Biol. Chem. 1984;259:13806–13813. [PubMed] [Google Scholar]
- TOSELLI M., TOSETTI P., TAGLIETTI V. μ and δ opioid receptor activation inhibits ω-conotoxin-sensitive calcium channels in a voltage- and time-dependent mode in the human neuroblastoma cell line SH-SY5Y. Pflugers Arch-Eur. J. Physiol. 1997;433:587–596. doi: 10.1007/s004240050318. [DOI] [PubMed] [Google Scholar]
- TRAYNOR J.R., ELLIOTT J. δ-Opioid receptor subtypes and cross-talk with μ-receptors. Trends Pharmacol. Sci. 1993;14:84–86. doi: 10.1016/0165-6147(93)90068-u. [DOI] [PubMed] [Google Scholar]
- TRAYNOR J.R., NAHORSKI S.R. Modulation by μ-opioid agonists of guanosine-5′-O-(3-[35S]thio)triphosphate binding to membranes from human neuroblastoma SH-SY5Y cells. Mol. Pharmacol. 1995;47:848–854. [PubMed] [Google Scholar]
- UHL G.R., CHILDERS S.R., PASTERNAK G.W. An opiate-receptor gene family reunion. Trends Pharmacol. Sci. 1993;17:848–854. doi: 10.1016/0166-2236(94)90110-4. [DOI] [PubMed] [Google Scholar]
- VAUGHT J.L., ROTHMAN R.B., WESTFALL T.C. Mu and delta receptors: their role in analgesia and in the differential effects of opioid peptides on analgesia. Life Sci. 1982;30:1443–1455. doi: 10.1016/0024-3205(82)90558-6. [DOI] [PubMed] [Google Scholar]
- VAUGHT J.L., TAKEMORI A.E. Differential effects of leucine -enkephalin and methionine enkephalin on morphine-induced analgesia, acute tolerance and dependence. J. Pharmacol. Exp. Ther. 1979;208:86–90. [PubMed] [Google Scholar]
- ZADINA J.E., HARRISON L.M., GE L.-J., KASTIN A.J., CHANG S.L. Differential regulation of μ and delta opiate receptors by morphine, selective agonists and antagonists and differentiating agents in SH-SY5Y human neuroblastoma cells. J. Pharmacol. Exp. Ther. 1994;270:1086–1096. [PubMed] [Google Scholar]
- ZHU Y., KING M.A., SCHULLER A.G.P., NITSCHE J.F., REIDL M., ELDE R.P., UNTERWALD E., PASTERNAK G.W., PINTAR J.E. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in δ opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]


