Abstract
The role of a constitutively active population of α1D-adrenoceptors was analysed in arteries obtained from spontaneously hypertensive rats (SHR) and controls (WKY) divided into three groups: young prehypertensive, adult hypertensive, and adult animals chronically treated with captopril (50 mg kg−1 per day orally) in order to prevent the hypertensive state.
In adult SHR, a significant increase in BMY 7378 potency (not in prazosin potency) was observed in aorta, mesenteric artery, and the first and second branches of the small mesenteric arteries with respect to WKY rats. This difference was not observed in iliac and tail arteries, which suggests an increased functional role of α1D-adrenoceptors only in some vessels of SHR.
The increase in the resting tone (IRT) observed in absence of agonist, inhibited by BMY 7378, that represents the constitutively active population of α1D-adrenoceptors, was also significantly greater in aorta and mesenteric artery from adult SHR.
In young and captopril treated adult animals, no differences between strains with respect to BMY 7378 potency, or IRT were observed.
The increase in the functional role of α1D-adrenoceptors and their constitutive activity observed in hypertension is prevented by captopril treatment. The pathological consequence of this change is the slower rate of recovery of the basal tone after removal of an adrenergic stimulus, observed in vessels from hypertensive animals that had shown an increase in the functionality of constitutively active α1D-adrenoceptors. This change was not observed in prehypertensive or captopril treated animals.
Keywords: Hypertension, vessels, α1D-adrenoceptors, constitutive activity, BMY 7378
Introduction
Molecular cloning techniques and pharmacological studies have identified the existence of three subtypes of α1-adrenoceptors, α1A, α1B and α1D, all of which are expressed in vascular smooth muscle and play a vital role in the regulation of blood pressure. However, the functionality of each subtype has not been well defined, and the reason for the coexistence of different subtypes in the same tissue or for the different distribution of the subtypes between vessels remains unclear. In an attempt to clarify this question, we have shown in previous studies (Noguera et al., 1996; D'ocon et al., 2000; Gisbert et al., 2000) that in vessels where α1D-adrenoceptors have a functional role, this subtype exhibits constitutive activity, while the α1A and the α1B subtypes do not. Moreover, the α1D subtype seems to play a modulator role, because the contractile response of the tissue to an adrenergic stimulus can be sustained even when the agonist is removed, thus preventing abrupt changes in the vessel calibre and, consequently, sudden changes in blood flow in response to adrenergic stimulation. In contrast, the lack of a functional population of α1D-adrenoceptors in a vessel warrants a quick, fine adjustment of contractile tone and blood flow according to the adrenergic stimulus (D'ocon et al., 2000). An imbalance in this modulating mechanism could give rise to pathologies such as hypertension, in the pathogenesis and/or maintenance of which α1D-adrenoceptors could play a role, as has been postulated by different authors (Villalobos-Molina & Ibarra, 1996; 1999; Xu et al., 1998; Ibarra et al., 1998; 2000; Villalobos-Molina et al., 1999). In order to assess this hypothesis, we analysed the functional role of this subtype and its constitutive activity in spontaneously hypertensive rats. The study was done on three groups of animals: young rats (6 weeks old) in a prehypertensive state; adult rats (16 weeks old) with the hypertensive syndrome; and adult rats that had been treated from 6 to 16 weeks with captopril, an antihypertensive agent that without acting directly on adrenoceptors, prevents the hypertensive state.
Methods
Male normotensive (WKY) and spontaneously hypertensive (SHR) rats aged 6 weeks were used (Harlan Interfauna Ibérica, Barcelona, Spain) and housed under a 12-h light/dark cycle at 22°C and 60% humidity. Starting at 6 weeks, some of the rats received captopril (50 mg kg−1 per day in drinking water), until the age of 16 weeks, while others were given no drugs. Systolic blood pressure and heart rate were measured weekly from the tail of unanaesthetized rats using a plethysmographic method (LE 5650/6, Letica Scientific Instruments, Barcelona, Spain). An average of six readings was recorded for each animal. All experimental procedures were approved by the Institutional Animal Care and Use Committee.
Rats (6 weeks or 16 weeks old) were weighed, decapitated and the heart and selected vessels were removed and suspended in a 10 ml organ bath containing physiological solution, maintained at 37°C and gassed with 95% O2 and 5% CO2. An initial load of 1 g was applied to each preparation and maintained throughout a 75 – 90 min equilibration period. Tension was recorded isometrically by Grass FTO3 force-displacement transducers, and the data were recorded on a disc (MacLab).
Mesenteric arterial trees were dissected and a ring segment (2 mm in length) from each arterial tree (first or second branch) was mounted in a myograph (J.P. Trading, Aarhus, Denmark) with separate 6 ml organ baths. Following a 30 min stabilization period, the internal diameter of each vessel was set to a tension equivalent to 0.9 times the estimated diameter at 100 mmHg effective transmural pressure (l100=260 – 380 μm for the first branch and l100=180 – 370 μm for the second branch) according to the standard procedure (Mulvany & Halpern, 1977). Data were recorded on a disc (MacLab).
The composition of the physiological Ca2+-containing solution was (mM): NaCl 118, KCl 4.75, CaCl2 1.8, MgCl2 1.2, KH2PO4 1.2, NaHCO3 25 and glucose 11. In Ca2+-free solution CaCl2 was omitted and EDTA (0.1 mM) was added.
Concentration-response curves to α1-agonists were performed by addition of cumulative concentrations of phenylephrine (1 nM – 100 μM) or noradrenaline (10 nM – 30 μM) to different vascular tissues. Contractions were expressed as a percentage of the maximal contraction to the agonist (Emax). The concentration (−log [M]) of agonist required to produce 50% of the maximal response (pEC50) was obtained from a non linear regression plot (Graph Pad Software; San Diego, California, U.S.A.)
Concentration-response curves of relaxation to selective α1-adrenoceptor antagonists were performed by addition of cumulative concentrations of prazosin (0.001 nM – 1 μM) or BMY 7378 (0.001 nM – 30 μM) to tissues in which sustained contractions had been induced by a maximal concentration of agonist. Relaxations were expressed as a percentage of the maximum increment in tension obtained by agonist addition. The concentration (−log [M]) needed to produce 50% relaxation (pIC50) and the pseudo-Hill slope were obtained from a non-linear regression plot (Graph Pad Software; San Diego, California, U.S.A.)
The experimental procedure that evidenced the constitutive activity of α1D-adrenoceptors is shown in Figure 1.
Figure 1.
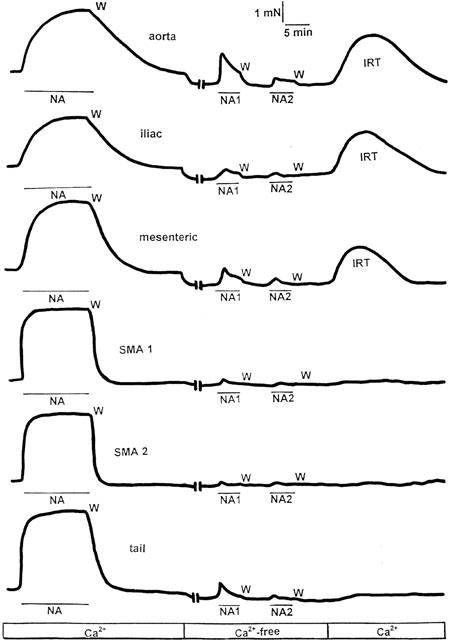
Experimental procedure designed to study the constitutive activity of α1D-adrenoceptors in rat aorta, iliac artery, mesenteric artery, small mesenteric artery first (SMA-1) or second branch (SMA-2), and tail artery. Noradrenaline (NA) was added in Ca2+-containing solution (Ca2+) and after washing (W) and recovery of the basal tone, the tissue was incubated for 20 min in Ca2+-free, EDTA-containing solution (Ca2+-free). After this time the agonist was applied (NA1, NA2) and washed until no contraction or a residual one was induced, indicating depletion of internal Ca2+ stores sensitive to noradrenaline. The tissue was then incubated for 20 min in physiological Ca2+-containing solution and a spontaneous increase in the resting tone (IRT) of aorta, iliac and mesenteric artery (not small mesenteric branches nor tail artery) was observed. In the experiments designed to assess the effect of BMY 7378 on IRT, the vessel was pretreated with 0.1 μM concentrations of the antagonist 10 min before the IRT and during (20 min) it was induced for a second time. The magnitude of the IRT was taken as an indicator of the constitutively active population of α1D-adrenoceptors present in a given vessel (Gisbert et al., 2000).
We have also analysed the kinetics of tissue relaxation after agonist removal from the tissue bath. The washing procedure was carried out by a total replacement of the bathing solution by three repeated washes within the first 30 s and by two other repeated washes every 5 min.
The results are presented as the mean±s.e.m. for n determinations obtained from different animals. Where ANOVA showed significant differences (P<0.05) the results were further analysed using the Student Newman-Keuls test, and differences were considered significant when P<0.05.
The following drugs were obtained from Sigma (St. Louis, MO, U.S.A.): phenylephrine, (−)-noradrenaline, prazosin, captopril or RBI (Natick, MA, U.S.A.): BMY 7378. Other reagents were of analytical grade. All compounds were dissolved in distilled water.
Results
Haemodynamic constants
Body weight, heart weight, heart/body weight ratios, systolic blood pressure (SBP) and heart rate of WKY and SHR animals are summarized in Table 1. We can observe that in the group of young animals (6 weeks old), significant differences were not found between SBP in WKY and SHR rats, and the same occurs in the group of adult animals (16 weeks old) that received captopril 50 mg kg−1 per day. However, in the adult animals without any antihypertensive treatment there were significant differences in the haemodynamic constants between WKY and SHR animals. Captopril pretreated WKY rats showed decreased values of SBP and heart rate with respect to untreated WKY animals (Table 1).
Table 1.
Mean body, heart weight and mean heart/body weight ratios, systolic blood pressure (SBP) and heart rate in young (6 weeks old), adult (16 weeks old) and adult rats pretreated with captopril
Functional role of α1D-adrenoceptors
First, concentration-response curves to agonist were performed using the α1-selective agonist phenylephrine in aorta, iliac, mesenteric and tail arteries, where α2-adrenoceptors could play a functional role, and noradrenaline in first (SMA-1) and second (SMA-2) small mesenteric branches, where the contractile role of α2-adrenoceptors is excluded (Phillips et al., 1998; Stam et al., 1999). The pEC50 and the maximal response to agonist obtained in different vessels from adult SHR and WKY rats were summarized in Table 2 and the mean concentration-response curves are illustrated in Figure 2. The results show that, in each of the vessels studied, the potency of the agonist does not change between SHR and WKY rats. The maximal response was not different between strains in most tissues and was obtained with phenylephrine 1 μM in aorta, 10 μM in mesenteric, iliac and tail arteries and with noradrenaline 30 μM in SMA-1 and SMA-2.
Table 2.
Emax and pEC50 values for phenylephrine or noradrenaline in the arterial vasculature of adult (16 weeks old) WKY or SHR animals
Figure 2.

Cumulative concentration-response curves to phenylephrine or noradrenaline in thoracic aorta (a) mesenteric artery (b), first (c) or second (d) branch of the small mesenteric arteries (SMA), iliac artery (e) and tail artery (f) obtained from adult SHR or WKY rats. Data are means±s.e.mean of 5 – 6 experiments and are expressed as a percentage of the maximal contraction to the agonist. The Emax and the pEC50 values are shown in Table 2.
In order to examine the functional role of the α1D-adrenoceptor subtype in different vessels from young and adult (treated and untreated with captopril) WKY and SHR animals, concentration-response curves of relaxation to the selective α1D-adrenoceptor antagonist BMY 7378 (0.001 nM – 30 μM) were obtained by addition of cumulative concentrations of the antagonist to arteries in which sustained contractions had been induced by maximal concentrations of agonist.
The potency (pIC50) of this antagonist obtained in each tissue from young and adult rats is summarized in Table 3 and is interpreted as an indicator of the functionality of the α1D subtype in these tissues. The affinities for BMY 7378 at α1A-, α1B-adrenoceptor are about 6.2 – 6.7 and α1D-adrenoceptor about 8.2 – 8.3 (Kenny et al., 1995).
Table 3.
pIC50 values for the selective α1D-adrenoceptor antagonist BMY 7378 in the arterial vasculature of young rats (6 weeks old), adult (16 weeks old) and adult rats pretreated with captopril 50 mg kg-1 per day from age 6 to 16 weeks, WKY or SHR animals

In young prehypertensive animals, the higher potency shown by BMY 7378 in aorta, iliac and mesenteric arteries from WKY and SHR animals suggests a major functional role of α1D-adrenoceptors in the adrenergic response in these tissues. The lower potency of BMY 7378 in tail artery and small mesenteric arteries, first and second branch, indicates that the participation of α1D-adrenoceptors in the functional response of these vessels in WKY and SHR animals is not relevant. No significant differences in BMY 7378 potency were found between 6-week-old SHR and WKY rats (Table 3).
In adult rats (16 weeks old), the potency shown by BMY 7378 in the different arteries of WKY animals was similar to that obtained in tissues from young animals (Table 3). However, if we compare the pIC50 of BMY 7378 in SHR and WKY adult animals, we can observe an increase in the potency of BMY 7378 in all the vessels of the SHR group. This increase was especially marked in the mesenteric vessels, and statistically significant higher potency of BMY 7378 was found in aorta, mesenteric artery, and SMA-1 and SMA-2 of SHR with respect to WKY rats. Moreover, a significant decrease in the pseudo Hill slope in aorta and mesenteric artery of the SHR group with respect to WKY rats was observed (Table 3, Figure 3).
Figure 3.

Cumulative concentration-response curves of relaxation for BMY 7378 on phenylephrine induced contraction in aorta (a), mesenteric artery (b), and on noradrenaline-induced contraction in first (c) or second (d) branch of the small mesenteric arteries (SMA), obtained from adult SHR or WKY rats pretreated or not with captopril (50 mg kg−1 per day orally) from age 6 – 16 weeks. Data are means±s.e.mean of 5 – 9 experiments. The pIC50 values are shown in Table 3.
However, in SHR animals treated with captopril (50 mg kg−1 per day during 10 weeks) the potency of BMY 7378 in all the vessels is similar to that observed in the same vascular tissue obtained from captopril-treated WKY rats (Table 3, Figure 3). Moreover, if we compare the pIC50 of BMY 7378 in vessels from treated and untreated WKY animals, we can observe that after captopril treatment, the potency of BMY 7378 is lower in aorta, iliac and mesenteric artery from treated WKY rats. In addition, we observed a marked steeping of the concentration-response curves (Figure 3).
In order to clarify that these changes in the potency of BMY 7378 are directly related to α1D-adrenoceptor subtype and were not a consequence of differences in α1-adrenoceptors in general, we tested prazosin, an α1-adrenoceptor antagonist that does not discriminate between the three subtypes of α1-adrenoceptors. No significant differences were found in the potency (Table 4) or in the pseudo-Hill slopes (results not shown) of prazosin in the different vessels obtained from SHR and WKY animals.
Table 4.
pIC50 values for the selective α1-adrenoceptor antagonist prazosin in the arterial vasculature of young (6 weeks old) and adult (16 weeks old) WKY or SHR animals

Constitutive activity of α1D-adrenoceptors
Another set of experiments was performed to analyse the constitutive activity of the population of α1D-adrenoceptors present in each vessel. Figure 1 shows the experimental procedure designed to study this constitutive activity which is easily quantified by the increase in the resting tone (IRT) observed in absence of agonist, after depletion of intracellular Ca2+ stores sensitive to noradrenaline and subsequent exposure to Ca2+-containing physiological solution (Noguera et al., 1996; Gisbert et al., 2000; D'ocon et al., 2000). Noradrenaline 1 μM in aorta, 10 μM in iliac, tail and mesenteric arteries or 30 μM in small mesenteric arteries (first and second branches) evoked a sustained contraction, that was used as a control of the maximal response obtained with this agonist in each preparation.
After removal of the agonist by repeated washings, the return to the baseline was slower (Figure 4) in aorta, iliac, mesenteric and tail artery than in small mesenteric artery (first and second branch) from normotensive rats; this confirms previous results obtained in Wistar rats (Gisbert et al., 2000; D'ocon et al., 2000). In vessels from SHR adult animals, that showed an increased potency of BMY 7378 as compared with WKY rats, the return to the baseline was significantly slower (aorta, mesenteric artery and first and second small mesenteric branches) than the decay observed in the same vessels obtained from WKY animals (Figure 4a – d). This difference was not detected when the vessels were obtained from rats pretreated with captopril. In this case, the return to the baseline after washing the agonist was as fast as in WKY animals (Figure 4a – d).
Figure 4.
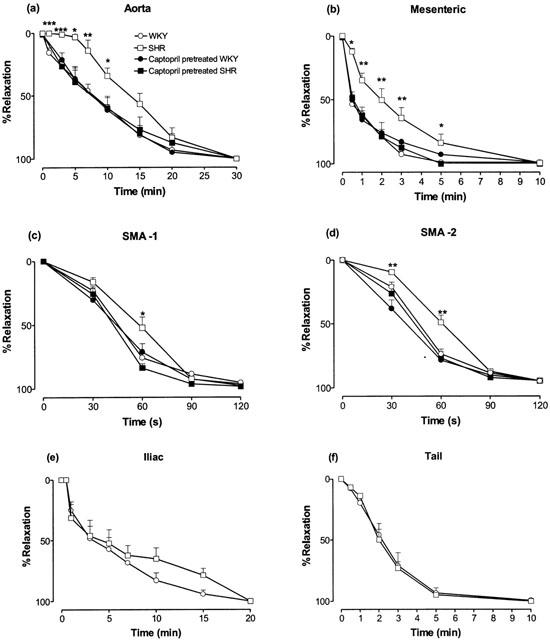
Time course of the decay in the maximal contractile response to noradrenaline after removal of the agonist in thoracic aorta (a), mesenteric artery (b), and first (c) or second (d) branch of the small mesenteric arteries (SMA), iliac (e), and tail arteries (f), obtained from adult WKY or SHR animals pretreated or not with captopril (50 mg kg−1 per day orally) from age 6 – 16 weeks. Data are means±s.e.mean of 5 – 9 experiments. *P<0.05, **P<0.01, ***P<0.001 vs untreated WKY rats.
In iliac and tail artery, where the potency of BMY 7378 was similar in WKY and SHR animals, no differences were observed in the decay of noradrenaline-induced contraction after removal of the agonist (Figure 4e,f).
We then changed to a Ca2+-free solution and a slight decrease in the baseline (10 – 20% of the control response to noradrenaline) was obtained in aorta, iliac, mesenteric and tail arteries but not in SMA (Figure 1). After 20 min in this medium, the addition of noradrenaline also induced a contraction in all the vessels (NA1 in Figure 1). Either no contraction or a residual one was evoked upon a second application of the agonist (NA2 in Figure 1) in the same solution. The tissue was then incubated for 20 min in Ca2+-containing solution and a spontaneous increase in the resting tone (IRT) was observed in aorta, iliac and mesenteric artery, but not in SMA-1 and SMA-2 or tail artery (Figure 1). The magnitude of the IRT was expressed as a percentage of the noradrenaline-induced contraction obtained in Ca2+-containing solution after subtracting the decrease in the baseline when we change to a Ca2+-free solution.
Comparative analysis of the IRT, obtained in different vessels of each group of animals gives evidence that it is significantly higher in aorta and mesenteric arteries from adult SHR than from respective WKY rats (Figure 5), but this difference is not appreciable when the SHR animals had been pretreated with captopril. In this respect, no differences was detectable in young rats. Moreover, the IRT observed in pretreated WKY animals is lower than that obtained in non captopril treated WKY rats, which means that it follows the same pattern as the potency of BMY 7378 does (Figure 5, Table 3).
Figure 5.

Increase in the resting tone (IRT) observed in different vessels obtained from young (a), adult (b), and captopril pretreated adult animals (c), after depletion of noradrenaline-sensitive internal calcium stores and posterior loading in calcium containing solution in absence of the agonist (see experimental procedure in Figure 1). Captopril was administered orally in a dose of 50 mg kg−1 per day from age 6 to 16 weeks. Data are means±s.e.mean of 5 – 9 experiments. *P<0.05, **P<0.01, ***P<0.001 vs untreated adult WKY rats.
Inhibition of the IRT was obtained by addition of 0.1 μM BMY 7378, 10 min before and during the loading period in Ca2+ containing solution. In all cases, the IRT observed in aorta, iliac and mesenteric artery from SHR and Wistar rats during this period in presence of BMY 7378 was almost completely inhibited (Figure 6) as compared with the IRT observed in absence of the antagonist. This result relates the IRT to a constitutively active population of α1D-adrenoceptors, as has been previously shown (Gisbert et al., 2000; D'ocon et al., 2000).
Figure 6.
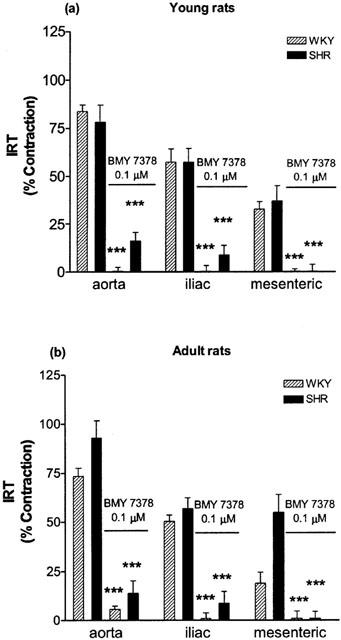
Inhibition by BMY 7378 (0.1 μM) of the increase in the resting tone (IRT) observed in different vessels obtained from young (a) and adult animals (b), after depletion of noradrenaline-sensitive internal calcium stores and posterior loading in calcium containing solution in absence of the agonist (see experimental procedure in Figure 1). Data are means±s.e.mean of 5 – 9 experiments. ***P<0.001 vs IRT obtained in absence of BMY 7378.
Discussion
The aims of the present work were: first, to analyse the functional role of α1D-adrenoceptors in arterial vessels obtained from spontaneously hypertensive rats (SHR); second, to determine whether this subtype exhibits constitutive activity in these vessels; and third, to clarify the role of the α1D-adrenoceptor in hypertension. For this purpose we designed our study using different arterial vessels (aorta, iliac, mesenteric, first and second branches of small mesenteric bed, and tail) obtained from two different groups of animals depending on their age. The younger group (6 weeks old) included controls (WKY) and SHR animals in a pre-hypertensive state as indicated by blood pressure. The group of adults (16 weeks old) included WKY rats with normal blood pressure values, and SHR animals with the higher blood pressure values typical of a hypertensive state. The adult group also included WKY and SHR animals treated from 6 to 16 weeks of age with captopril at a dose that prevents the hypertensive state. The weekly measurement of blood pressure confirmed this point.
In order to analyse the functional role of α1D-adrenoceptors we used BMY 7378, a selective α1D-adrenoceptor antagonist (Goetz et al., 1995), as a relaxant of phenylephrine or noradrenaline-induced maximal contraction in each vessel, and in these conditions the potency of BMY 7378 as a relaxant can be interpreted as an indicator of the functional role of the α1D-subtype in each tissue, as we have previously shown in Wistar rats (Gisbert et al., 2000).
If we compare the potency of BMY 7378 in vessels from WKY animals of different ages, the results obtained show that α1D-adrenoceptors play a significant role in adrenergic responses of aorta, iliac and proximal mesenteric artery, but not in the first and second branches of the mesenteric bed or in tail artery. These results corroborate previous data describing the same functional role for α1D-adrenoceptors in these vessels (Kenny et al., 1995; Piascik et al., 1995; Graham et al., 1996; Hussain & Marshall, 1997; Lachnit et al., 1997; Hrometz et al., 1999; Gisbert et al., 2000).
If we compare the results obtained in the different strains, and taking account that the potency of the agonist does not change between SHR and WKY rats, the higher potency and lower pseudo-Hill slope shown by BMY 7378 in some arterial vessels of adult SHR animals with respect to WKY, together with the fact that prazosin showed the same potency and pseudo-Hill slope in these vessels, demonstrates the increased functionality of the α1D-adrenoceptor respect to the other subtypes in some vessels from adult SHR animals, especially in aorta and the mesenteric arterial tree. This observation, together with the fact that in prehypertensive animals (young rats) no differences were found between the potency and pseudo-Hill slope of BMY 7378 in vessels from WKY and SHR rats, suggests that either the α1D-adrenoceptor is involved in the genesis and/or maintenance of high blood pressure or that an increase in the functional role of the α1D-adrenoceptors is a consequence of the hypertensive state. These suggestions are strongly supported by the fact that in captopril treated adult SHR animals, where a hypertensive state is avoided, the potency shown by BMY 7378 in the different vessels was similar to that observed in the group of captopril treated WKY rats. These results corroborate previous data showing higher mRNA levels for the α1D-subtype in aortas of SHR than in WKY rats (Xu et al., 1998) and also demonstrating an increased functionality of α1D-adrenoceptors in SHR animals (Villalobos-Molina & Ibarra, 1996; 1999; Villalobos-Molina et al., 1999). Until now, however, the exact role of this subtype in hypertension was not clear.
After showing the existence of an increased functionality of α1D-adrenoceptors in some vessels from hypertensive rats, our second aim was to determine whether this increased functionality was accompanied by an increased population of constitutively active α1D-adrenoceptors because, as has been pointed out above, this subtype has a peculiar behaviour that differentiates it from the other two subtypes: it shows constitutive activity in physiological conditions (Noguera et al., 1996; García Sainz & Torres Padilla, 1999; Gisbert et al., 2000; McCune et al., 2000).
In order to confirm this point, we used the experimental procedure that permits us to easily quantify this constitutive activity (Noguera & D'ocon, 1993; Noguera et al., 1996; 1997; 1998; Gisbert et al., 2000; D'ocon et al., 2000). In aorta, iliac and proximal mesenteric artery from Wistar rats (Noguera et al., 1996; Gisbert et al., 2000; D'ocon et al., 2000), noradrenaline releases Ca2+ from internal stores by activating α1-adrenoceptors, and during the posterior loading in Ca2+-containing solution in the absence of the agonist, an increase in the resting tone (IRT) and inositol phosphate accumulation were observed (Gisbert et al., 2000). As has been analysed and discussed in previous papers (Noguera & D'ocon, 1993; Noguera et al., 1996; 1997; 1998; Gisbert et al., 2000), endogenous or exogenous agonists are not present. Therefore, the fact that the IRT and the inositol phosphate accumulation related to it were inhibited by prazosin and BMY 7378, the selective antagonist of the α1D-subtype, suggests the existence of a population of α1D-adrenoceptors that temporarily remains in a constitutively active state, and the IRT provides the functional evidence of a constitutively active population of native α1D-adrenoceptors. That this IRT is closely related to α1-adrenoceptors and not just to the emptying of intracellular Ca2+ pools is demonstrated by the fact that depletion of internal Ca2+-stores by methoxamine and phenylephrine also elicits an IRT, whereas clonidine, serotonin, caffeine, ryanodine, thapsigargine and cyclopiazonic acid, which also depleted internal Ca2+ stores, did not elicit any IRT (Noguera & D'ocon, 1993; Noguera et al., 1996; 1998). Moreover, the fact that this IRT was not observed in tail, distal mesenteric and small mesenteric branches (Gisbert et al., 2000; D'ocon et al., 2000), where a population of α1A- and/or α1B-adrenoceptors but not α1D-adrenoceptors has been described (Hussain & Marshall, 1997; Lachnit et al., 1997; Hrometz et al., 1999; Gisbert et al., 2000), suggests that these subtypes do not show constitutive activity.
Following the same experimental procedure as that described in Wistar rats, an IRT was observed (see Figure 1) only in vessels where α1D-adrenoceptors play a functional role such as aorta, iliac and proximal mesenteric arteries from WKY and SHR animals, but not in small mesenteric branches (first or second) or tail artery, where α1D-adrenoceptor does not intervene in the response to adrenergic stimulation. The fact that the IRT observed can be inhibited by BMY 7378 (Figure 6) in conditions in which the presence of exogenous or endogenous agonists can be ruled out confirms the existence of a population of α1D-adrenoceptors with constitutive activity in these vessels, as has been previously shown in Wistar rats (Noguera & D'ocon, 1993; Noguera et al., 1996; Gisbert et al., 2000). The present results also show that the magnitude of the IRT, which represents the population of constitutively active α1D-adrenoceptors, is significantly increased in aorta and mesenteric artery from adult SHR with respect to WKY animals, but no significant changes were observed between the two strains in young animals or captopril pretreated adult animals.
On the basis of these results we can conclude that in some vessels from hypertensive animals, there is an increased functionality of the α1D-subtype, accompanied by an increased population of constitutively active receptors. In vessels from prehypertensive animals or pretreated animals where the hypertensive state was avoided, no significant differences were found in the functionality of α1D adrenoceptors nor in their constitutively active population. However, these results are only a pharmacological curiosity if they are not related to the pathogenesis or maintenance of hypertension. Our third proposal was to find the possible relationship between the increased α1D-adrenoceptor population and constitutive activity on the one hand and the genesis and/or maintenance of the hypertensive state on the other.
It is well known that hypertension is accompanied by an elevated sympatho-adrenal tone and recent findings (Ibarra et al., 1998; Xu et al., 1998; Villalobos-Molina & Ibarra, 1999; Villalobos-Molina et al., 1999) point to an important role for the α1D-subtype in this pathology and suggest that the receptor appears first in the vasculature followed by the increase in blood pressure (Villalobos-Molina et al., 1999). However, the pathophysiological significance of this observation is still unclear. Our hypothesis is that an increased functional expression of α1D-adrenoceptors could be the reason behind this elevated sympatho-adrenal tone observed in SHR animals and other models of hypertension (Marín, 1993, Villalobos-Molina & Ibarra, 1999).
According to our previous work (Gisbert et al., 2000; D'ocon et al., 2000), in physiological conditions, after noradrenaline-induced contraction and removal of the agonist, in vessels where the α1D-subtype exhibits a functional role such as aorta, iliac and proximal mesenteric artery, the population of α1D-adrenoceptors that remains temporarily in a constitutively active state is responsible for the slow disappearance of the contractile response to the agonist. This mechanism is not observed in vessels where α1D-adrenoceptors do not seem to play a functional role such as distal mesenteric artery, small mesenteric branches and tail artery. Therefore, the constitutively active population of α1D-adrenoceptors plays a modulator role in the vasculature preventing abrupt changes in the vessel calibre when the adrenergic stimulus disappears. We propose that an imbalance in this modulatory mechanism, due to the increased constitutively active population of α1D-adrenoceptors observed in our conditions, would maintain the contractile tone pathologically elevated when the stimulus disappears and that this phenomenon could be involved in the genesis and/or maintenance of the hypertension.
In order to confirm this, we have compared the response to noradrenaline in different arterial vessels, and analysed the kinetics of disappearance of the contractile tone when the agonist was removed. The results obtained show significant differences between certain vessels from adult SHR and WKY animals in the time course of the decay of the maximal response to noradrenaline after removal of the agonist. The vessels that gave significant differences were aorta, mesenteric artery and, to a lesser extent, small mesenteric branches (Figure 4), all of which also showed an increased population of functionally active α1D-adrenoceptors (Table 3). Differences were not observed in iliac or tail artery (Figure 4), vessels in which no statistically significant differences between α1D-functionality in WKY and SHR animals were found. In addition, animals pretreated with captopril in order to avoid the hypertensive state did not show any significant differences between the two strains in the decay of contraction after agonist removal, nor significant differences in the constitutively active population of α1D-adrenoceptors.
The slower decay in the contractile tone of the aorta and mesenteric vessels from SHR animals after agonist removal implies that when the agonist disappears, the tone of the vessel remains significantly elevated for more than 10 min in a poorly innervated vessel such as aorta (Stassen et al., 1998), which is rich in the α1D-subtype, or for more than 1 min in a densely innervated vessel such as small mesenteric artery (Stassen et al., 1998), which is rich in the α1A-subtype and where the functional role of the α1D-subtype is inappreciable in WKY but increased in SHR animals. This significantly slower decay could be the reason for the pathological increase in the adrenergic vascular tone, previously described as characteristic of hypertension (Marín, 1993; Villalobos-Molina & Ibarra, 1999). This situation was prevented by the chronic treatment with the anti-hypertensive agent captopril.
In conclusion, our present results suggest a relationship between the slower decay after an adrenergic stimulus and the increased constitutively active population of α1D adrenoceptors present in these vessels. Analysing the results obtained in rats pretreated with captopril, we find that in SHR animals, a significant decrease in the potency of BMY 7378 with respect to non-treated animals, was accompanied by a concomitant decrease in the IRT and a faster decay of the contractile tone after agonist removal, thus confirming once again the close relationship between the three parameters.
In summary, present work demonstrates the existence of an increased functionality of α1D-adrenoceptors in some vessels of hypertensive animals that is accompanied by an increase in the population of these constitutively active receptors. In addition, the contractile tone of these vessels remained increased as compared with WKY animals when the agonist was removed and this change is evidenced only when the hypertensive state appears since it is not observed in prehypertensive animals or captopril-treated animals. Therefore, it could be directly related to hypertension, and we suggest that it could be involved in the genesis and/or maintenance of this pathology. Future analysis of the mechanisms controlling the functional expression of each subtype of α1-adrenoceptors in a given vessel could provide the clue to an understanding of the mechanism that triggers the hypertensive state.
Acknowledgments
This work was supported by research grants from the Spanish Comisión Interministerial de Ciencia y Tecnología (SAF98-0123, FEDER 1FD97-1029), and the Conselleria de Cultura de la Generalitat Valenciana (GR006).
Abbreviations
- BMY 7378
8-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-8-azaspiro[4,5]decane-7,9-dione dihydrochloride
- IRT
increase in the resting tone
- NA
noradrenaline
- SHR
spontaneously hypertensive rats
- SMA-1 and SMA-2
first and second small mesenteric arterial branches
- WKY
Wistar Kyoto rats
References
- D'OCON P., ZIANI K., GISBERT R., BOLUFER N., NOGUERA M.A., IVORRA M.D. Modulatory role of a constitutively active population of α1D-adrenoceptors in conductance arteries. Methods Finds Exp. Pharmacol. 2000;22:441. [Google Scholar]
- GARCIA SAINZ J.A., TORRES PADILLA M.E. Modulation of basal intracellular calcium by inverse agonists and phorbol myristate acetate in rat-1 fibroblasts stably expressing α1d-adrenoceptors. FEBS Lett. 1999;443:277–281. doi: 10.1016/s0014-5793(98)01738-4. [DOI] [PubMed] [Google Scholar]
- GISBERT R., NOGUERA M.A., IVORRA M.D., D'OCON P. Functional evidence of a constitutively active population of α1D-adrenoceptors in rat aorta. J. Pharmacol. Exp. Ther. 2000;295:810–817. [PubMed] [Google Scholar]
- GOETZ A.S., KING H.K., WARD S.D., TRUE T.A., RIMELE T.J., SAUSSY D.L. BMY 7378 is a selective antagonist of the D subtype of α1-adrenoceptors. Eur. J. Pharmacol. 1995;272:R5–R6. doi: 10.1016/0014-2999(94)00751-r. [DOI] [PubMed] [Google Scholar]
- GRAHAM R.M., PEREZ D.M., HWA J., PIASCIK T. α1-Adrenergic receptor subtypes. Molecular structure, function and signalling. Circ. Res. 1996;78:737–749. doi: 10.1161/01.res.78.5.737. [DOI] [PubMed] [Google Scholar]
- HROMETZ S.L., EDELMANN S.E., MCCUNE D.F., OLGES J.R., HADLEY R.W., PEREZ D.M., PIASCIK M.T. Expression of multiple α1-adrenoceptors on vascular smooth muscle: correlation with the regulation of contraction. J. Pharmacol. Exp. Ther. 1999;290:452–463. [PubMed] [Google Scholar]
- HUSSAIN M.B., MARSHALL I. Characterization of α1-adrenoceptor subtypes mediating contractions to phenylephrine in rat thoracic aorta, mesenteric artery and pulmonary artery. Br. J. Pharmacol. 1997;122:849–858. doi: 10.1038/sj.bjp.0701461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBARRA M., LÓPEZ-GUERRERO J.J., VILLALOBOS-MOLINA R. Further evidence for the predominance of α1D-adrenoceptors in arteries of normotensive and spontaneously hypertensive rats. Pharmacol. Rev. Comm. 1998;10:135–142. [Google Scholar]
- IBARRA M., PARDO J.P., LOPEZ-GUERRERO J.J., VILLALOBOS-MOLINA R. Differential response to chloroethylclonidine in blood vessels of normotensive and spontaneously hypertensive rats: role of α1D and α1A adrenoceptors in contraction. Br. J. Pharmacol. 2000;129:653–660. doi: 10.1038/sj.bjp.0703097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNY B.A., CHALMERS D.H., PHILPOTT P.C., NAYLOR A.M. Characterization of an α1D-adrenoceptor mediating the contractile response of rat aorta to noradrenaline. Br. J. Pharmacol. 1995;115:981–986. doi: 10.1111/j.1476-5381.1995.tb15907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACHNIT W.G., TRAN A.M., CLARKE D.E., FORD A.P. Pharmacological characterization of an α1A-adrenoceptor mediating contractile responses to noradrenaline in isolated caudal artery of the rat. Br. J. Pharmacol. 1997;120:819–826. doi: 10.1038/sj.bjp.0700983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARÍN J. Mechanisms involved in the increased vascular resistance in hypertension. J. Auton. Pharmacol. 1993;13:127–132. doi: 10.1111/j.1474-8673.1993.tb00264.x. [DOI] [PubMed] [Google Scholar]
- MCCUNE D.F., EDELMANN S.E., OLGES J.R., POST G.R., WALDROP B.A., WAUGH D.J.J., PEREZ D.M., PIASCIK M.T. Regulation of the cellular localization and signaling properties of the α1B and α1D-adrenoceptors by agonists and inverse agonists. Mol. Pharmacol. 2000;57:659–666. doi: 10.1124/mol.57.4.659. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NOGUERA M.A., D'OCON M.P. Evidence that depletion of internal calcium stores sensitive to noradrenaline elicits a contractile response dependent on extracellular calcium in rat aorta. Br. J. Pharmacol. 1993;110:861–867. doi: 10.1111/j.1476-5381.1993.tb13892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOGUERA M.A., IVORRA M.D., CHULIA S., D'OCON P. Capacitative Ca2+ entry associated with α1-adrenoceptors in rat aorta. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:83–89. doi: 10.1007/pl00005033. [DOI] [PubMed] [Google Scholar]
- NOGUERA M.A., IVORRA M.D., D'OCON P. Functional evidence of inverse agonism in vascular smooth muscle. Br. J. Pharmacol. 1996;119:158–164. doi: 10.1111/j.1476-5381.1996.tb15689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOGUERA M.A., MADRERO Y., IVORRA M.D., D'OCON P. Characterization of two different Ca2+ entry pathways dependent on depletion of internal Ca2+ pools in rat aorta. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;357:92–99. doi: 10.1007/pl00005156. [DOI] [PubMed] [Google Scholar]
- PHILLIPS J.K., MCLEAN A.J., HILL C.E. Receptors involved in nerve-mediated vasoconstriction in small arteries of the rat hepatic mesentery. Br. J. Pharmacol. 1998;124:1403–1412. doi: 10.1038/sj.bjp.0701976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIASCIK M.T., GUARINO R.D., SMITH M.S., SOLTIS E.E., SAUSSY D.L., Jr, PEREZ D.M. The specific contribution of the novel α1D adrenoceptor to the contraction of vascular smooth muscle. J. Pharmacol. Exp. Ther. 1995;275:1583–1589. [PubMed] [Google Scholar]
- STAM W.B., VAN DER GRAAF P.H., SAXENA P.R. Analysis of α1L-adrenoceptor pharmacology in rat small mesenteric artery. Br. J. Pharmacol. 1999;127:661–670. doi: 10.1038/sj.bjp.0702598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STASSEN F.R.M., MAAS R.G.H.T., SCHIFFERS P.M.H., JANSSEN G.M.J., DE MEY J.G.R. A positive and reversible relationship between adrenergic nerves and alpha-1A adrenoceptors in rat arteries. J. Pharmacol. Exp. Ther. 1998;284:399–405. [PubMed] [Google Scholar]
- VILLALOBOS-MOLINA R., IBARRA M. α1-adrenoceptors mediating contraction in arteries of normotensive and spontaneously hypertensive rats are of the α1D or α1A subtypes. Eur. J. Pharmacol. 1996;298:257–263. doi: 10.1016/0014-2999(95)00781-4. [DOI] [PubMed] [Google Scholar]
- VILLALOBOS-MOLINA R., IBARRA M. Vascular α1D-adrenoceptors: are they related to hypertension. Arch. Med. Res. 1999;30:347–352. doi: 10.1016/s0188-0128(99)00047-0. [DOI] [PubMed] [Google Scholar]
- VILLALOBOS-MOLINA R., LÓPEZ-GUERRERO J.J., IBARRA M. Functional evidence of α1D-adrenoceptors in the vasculature of young and adult spontaneously hypertensive rats. Br. J. Pharmacol. 1999;126:1534–1536. doi: 10.1038/sj.bjp.0702468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU K., LU Z., WE I.H., ZHANG Y., HAN C. Alteration of α1-adrenoceptor subtypes in aortas of 12-month-old spontaneously hypertensive rats. Eur. J. Pharmacol. 1998;344:31–36. doi: 10.1016/s0014-2999(97)01559-8. [DOI] [PubMed] [Google Scholar]


