Abstract
Contraction of smooth muscle is initiated, and to a lesser extent maintained, by a rise in the concentration of free calcium in the cell cytoplasm ([Ca2+]i). This activator calcium can originate from two intimately linked sources – the extracellular space and intracellular stores, most notably the sarcoplasmic reticulum. Smooth muscle contraction activated by excitatory neurotransmitters or hormones usually involves a combination of calcium release and calcium entry. The latter occurs through a variety of calcium permeable ion channels in the sarcolemma membrane. The best-characterized calcium entry pathway utilizes voltage-operated calcium channels (VOCCs). However, also present are several types of calcium-permeable channels which are non-voltage-gated, including the so-called receptor-operated calcium channels (ROCCs), activated by agonists acting on a range of G-protein-coupled receptors, and store-operated calcium channels (SOCCs), activated by depletion of the calcium stores within the sarcoplasmic reticulum. In this article we will review the electrophysiological, functional and pharmacological properties of ROCCs and SOCCs in smooth muscle and highlight emerging evidence that suggests that the two channel types may be closely related, being formed from proteins of the Transient Receptor Potential Channel (TRPC) family.
Keywords: Smooth muscle, receptor-operated calcium channels, store-operated calcium channels, transient receptor potential channels, capacitative calcium entry, excitation-contraction coupling, calcium
Introduction
Contraction of smooth muscle is initiated, and to a lesser extent maintained, by a rise in the concentration of free calcium in the cell cytoplasm ([Ca2+]i). As in other muscle types, this activator calcium can originate from two intimately linked sources – the extracellular space and intracellular stores, most notably the sarcoplasmic reticulum. Whilst almost without exception, smooth muscle contraction activated by excitatory neurotransmitters or hormones involves a combination of calcium entry and release, the various smooth muscle types can differ quite markedly in two main respects – first, in the relative contribution of the two calcium sources to excitation-contraction coupling and, secondly, in the ion channels through which extracellular calcium gains entry to the cell. The best-characterized calcium entry pathway utilizes voltage-operated calcium channels (VOCCs), particularly the dihydropyridine-sensitive ‘L-type' channels. However, also present in smooth muscle cells are several types of calcium-permeable channels which are non-voltage-gated, including the so-called receptor-operated calcium channels (ROCCs), activated by agonists acting on a range of G-protein-coupled receptors, and store-operated calcium channels (SOCCs), activated following depletion of the calcium stores within the sarcoplasmic reticulum. Our knowledge of the functional roles of ROCCs and, even more so, SOCCs in smooth muscle excitation-contraction coupling is much less advanced than our understanding of VOCCs. However, recent results obtained using a variety of experimental techniques, including those of pharmacology, electrophysiology and molecular biology, are beginning to shed light on the close functional inter-relationships amongst non-voltage-gated calcium channels. The purpose of the present article is to review the electrophysiological and pharmacological properties of ROCCs and SOCCs in smooth muscle, and to highlight emerging evidence that the two channel types may be closely related, being formed from proteins of the Transient Receptor Potential Channel (TRPC) family.
Electromechanical and pharmacomechanical coupling in smooth muscle
Excitation-contraction coupling in smooth muscle can be said to occur by two overlapping mechanisms – electromechanical and pharmacomechanical coupling. During the electromechanical coupling process, the primary drive for the rise in intracellular calcium (and thus contraction of the muscle) is membrane depolarization, with the consequential opening of VOCCs; neurotransmitters or hormones acting to depolarize the membrane will generally cause contraction whilst those producing membrane hyperpolarization will cause relaxation. Drugs which block calcium entry through VOCCs will inhibit electromechanical coupling and, indeed, this underlies the widespread use of calcium channel blocking agents (‘calcium antagonists') to relax vascular smooth muscle, thus producing vasodilatation and a decrease in blood pressure. Electromechanical coupling appears to play a predominant role in so-called phasic smooth muscle in which the membrane potential often displays marked oscillations upon which are superimposed calcium ‘spikes'. This oscillatory behaviour results from the synchronous gating of voltage- and calcium-dependent ion channels in the muscle membrane and acts to impose tight frequency- and amplitude-modulated control over [Ca2+]i.
Conversely, pharmacomechanical coupling depends neither on changes in membrane potential (although changes may occur) nor, consequently, on calcium entry via VOCCs. Rather, the rise in intracellular calcium is brought about by a combination of calcium release from intracellular stores and calcium entry through non-voltage-operated channels, primarily ROCCs and SOCCs. The calcium signal produced following administration of an excitatory agonist to cells demonstrating pharmacomechanical coupling is often similar to that seen in many non-excitable cells, consisting of an initial rapid, but transient, rise in [Ca2+]i followed by a smaller, but sustained, increase dependent upon calcium entry from the extracellular space. This latter influx, allied to the process of ‘calcium sensitization' whereby the contractile apparatus can be activated by ‘near-resting' levels of [Ca2+]i, allows such muscles to maintain tone over prolonged periods in the presence of agonist. This process is energetically favourable and occurs in so-called tonic smooth muscles.
From a functional point of view, the relative importance of electromechanical or pharmacomechanical coupling for any given smooth muscle preparation can be estimated simply by determining the effects of inhibitors of VOCCs on the contraction to agonists. In some tissues, for example the guinea-pig ileum, dihydropyridines such as nifedipine will virtually abolish all contractions suggesting that electromechanical coupling predominates. However in others, such as the mouse anococcygeus (Gibson et al., 1994) and the gastric fundus of several species (Petkov & Boev, 1996a,1996b), nifedipine has relatively little effect, pointing to a role for pharmacomechanical mechanisms. It should be noted however that in these tissues nifedipine does produce some reduction in the response to contractile agonists suggesting that VOCCs are present in the tissue and that they can, and are, activated during contraction. Thus, the subdivision of muscles into those which uniquely utilize pharmacomechanical or electromechanical mechanisms, although convenient from a mechanistic point of view, is a gross oversimplification when considering functional responses, and in truth, both mechanisms probably occur to a greater or lesser extent in all smooth muscles. In the mouse anococcyeus for example, the transient rise in [Ca2+]i following the release of calcium from the sarcoplasmic reticulum activates a calcium-dependent chloride conductance to depolarize the muscle and transiently open VOCCs. Simultaneously, the depleted sarcoplasmic reticulum, by an as yet obscure mechanism, signals to the plasma membrane to open SOCCs and allow calcium entry for maintenance of the contraction (Gibson et al., 1998). This overlap between electromechanical and pharmacomechanical coupling also arises when one considers that the opening of ROCCs and SOCCs, in addition to allowing calcium entry, will also produce membrane depolarization. In some tissues, for example the guinea-pig ileum, the depolarization produced by the opening of ROCCs would appear to be, at least as far excitation-contraction coupling is concerned, more important than the entry of calcium through the channels. Thus, while this review will concentrate on ROCCs and SOCCs as voltage-independent pathways of calcium entry in smooth muscle, their interplay with voltage-dependent pathways must always be an important consideration.
Receptor operated calcium channels and receptor-operated currents
It is now over 20 years since the suggestion was made that receptor activation could lead to calcium entry into smooth muscle cells by mechanisms independent of membrane depolarization and the concept of ROCCs in smooth muscle was introduced (Somlyo & Somlyo, 1968; Bolton, 1979; van Breemen et al., 1979; Bolton & Large, 1986). Since then, receptor-operated cation currents have been described in a number of smooth muscle cell types following activation of a range of receptors, but it was the results from experiments on cells from three main preparations (the small intestine of the guinea-pig, rabbit ear artery and rabbit portal vein) that led to the development of our understanding of the electrophysiological properties of ROCCs. It is important to emphasize at this stage that the receptor-operated currents recorded in smooth muscle have all shown the properties of non-selective cation currents, with varying degrees of calcium selectivity, and therefore the abbreviation ROCC may be more accurately defined as ‘receptor-operated cation channel' rather than the original ‘receptor-operated calcium channel'.
In the rabbit ear artery, externally applied ATP produced a rapid, transient depolarization of the muscle (Suzuki, 1985) later shown to result from activation of a non-selective cation conductance (Benham et al., 1987) with significant calcium permeability (Benham & Tsien, 1987). Similar responses have been reported to ATP in rat vas deferens (Nakazawa & Matsuki, 1987; Friel, 1988), rabbit portal vein (Xiong et al., 1991) and human saphenous vein (Loirand & Pacaud, 1995) and are produced following activation of P2X receptors (Khakh et al., 2001). These form true ligand-operated ion channels in which the ligand (ATP) binding site and the ion channel are contained within the same macromolecular complex. The importance of developing a full understanding of ROCC mechanisms, and identifying pharmacological agents which selectively modify ROCC function, has been highlighted by recent observations in P2X1 ‘knockout' mice. In these mice, the ATP-dependent component of nerve-induced contractions of the vas deferens is absent and male fertility is reduced by around 90%, raising the possibility of developing novel male contraceptive drugs which are directed towards inhibition of this particular ROCC (Mulryan et al., 2000). A full description of the pharmacological and electrophysiological properties of P2X receptors is beyond the scope of this review but interested readers are referred to a recent article by Khakh et al. (2001) and references therein.
In addition to ATP, noradrenaline, released as a co-transmitter from sympathetic nerves and acting on α-adrenoceptors, also produced depolarizations of the rabbit ear artery (Trapani et al., 1981) by activating a non-selective cation conductance (Amédée et al., 1990). The current produced by noradrenaline differed to that produced by ATP in both its time- and voltage- dependence but was similar to that activated by noradrenaline, again acting on α1 adrenoceptors, in cells isolated from the rabbit portal vein (Byrne & Large, 1988; Amédée & Large, 1989; Wang & Large, 1991). The channels underlying the current in portal vein were calcium permeable (Byrne & Large, 1988) as required for a ROCC. The steady-state current voltage-relationship for the noradrenaline response showed marked inward rectification, such that at membrane potentials positive to the reversal potential (around +10 mV in normal physiological salt solution) there was very little outward current recorded (Wang & Large, 1991; Helliwell & Large, 1996). Furthermore, the steady state conductance was also reduced at potentials negative to around −40 mV yielding a characteristic ‘S-shaped' current-voltage relationship (Helliwell & Large, 1996; Aromolaran & Large, 1999). A similarly complex relationship between the size of the receptor-operated current and membrane potential was reported for the current activated by acetylcholine, acting on M2 muscarinic receptors (Zholos & Bolton, 1997) in smooth muscle cells isolated from the guinea-pig ileum. In this case, rectification at negative membrane potentials is even more pronounced to the extent that there is a distinct region of negative slope conductance on the steady-state current voltage relationship below −50 mV (Inoue et al., 1987; Inoue & Isenberg, 1990a; Zholos & Bolton, 1995). This current in response to acetylcholine was first described in rabbit jejenum (Benham et al., 1985) and similar responses have since been recorded in cells isolated from elsewhere in the gastrointestinal tract, including the stomach (Vogalis & Sanders, 1990; Sims, 1992; Kim et al., 1995) and colon (Lee et al., 1993). Experiments in gastric myocytes have suggested that although the calcium permeability of the underlying channels appears to be small (the current carried by calcium ions amounting to 0.009 of the total current), it is sufficient to produce a significant rise in intracellular calcium on application of acetylcholine (Kim et al., 1998b).
In addition to the classical neurotransmitters acetylcholine and noradrenaline, several hormones have also been reported to activate non-selective cation conductances in smooth muscle cells (Table 1). In all cases, the response is initiated following activation of G-protein-coupled receptors and an essential role for the G-protein in the transduction pathway linking receptor and cation channel has been demonstrated in rabbit jejunum (Komori & Bolton, 1990), guinea-pig ileum (Komori et al., 1992; Zholos & Bolton, 1994; Kim et al., 1998a) and tracheal myocytes (Wang & Kotlikoff, 2000). The G-proteins involved generally appear to be members of the pertussis toxin-sensitive, Gi/Go sub-group (Komori et al., 1992; Kim et al., 1998a; Wang & Kotlikoff, 2000).
Table 1.
Neurotransmitters and hormones reported to activate a receptor-operated cation current in smooth muscle

Many of the agonists which activate the cation current also liberate IP3 and thus release calcium from the sarcoplasmic reticulum, though these responses are not always mediated by the same receptor subtype. Whilst the IP3-mediated release of calcium from intracellular stores does not appear to be necessary for activation of the cation conductance (Amédée et al., 1990; Komori & Bolton, 1990) it does act to augment the current (Inoue & Isenberg, 1990b; Pacaud & Bolton, 1991) to the extent that, in ileal smooth muscle cells, intracellular calcium oscillations initiated by muscarinic receptor activation (Komori et al., 1993; Kohda et al., 1996) are mirrored by oscillations in the amplitude of the cation current (Komori et al., 1993). As mentioned above, activation of the cation current in the guinea-pig ileum occurs as a result of agonists acting on M2 muscarinic receptors which do not activate phospholipase C to liberate IP3. However, the simultaneous activation of M3 receptors, which does lead to the release of calcium from the sarcoplasmic reticulum, potentiates the M2-mediated response (Zholos & Bolton, 1997; Kotlikoff et al., 1999). Similarly, in tracheal myocytes, activation of H1 receptors has been proposed to release intracellular calcium (via a pertussis-toxin insensitive pathway) while simultaneously activating the cation conductance via a pertussis-toxin sensitive pathway (Wang & Kotlikoff, 2000). Thus, whilst it would appear that intracellular calcium store depletion does not play a permissive role in activation of receptor-operated calcium channels, the calcium released from the sarcoplasmic reticulum acts to increase the cation current and thus calcium entry. Similarly, the receptor-operated current can be increased by calcium entering through voltage-operated channels (Pacaud & Bolton, 1991) emphasizing the degree of interaction between not only calcium stores and ROCCs but also the different calcium entry pathways.
Further details of the transduction pathway coupling receptors to activation of the receptor-operated cation current have remained elusive. This is partly because neither the single channel properties nor the molecular identity of the underlying channels have been elucidated. Emerging evidence points towards a role for TRPC family members in forming ROCCs (see later), and whilst single channel recordings from ROCCs have been reported on a number of occasions (Inoue et al., 1987; Vogalis & Sanders, 1990; Inoue & Kuriyama, 1993; Nakajima et al., 1996; Kim et al., 1998b) these have not proved stable enough, in either excised or cell-attached patches, to obtain information on receptor-channel coupling mechanisms. The limited information available suggests a single channel conductance of around 25 pS at near-resting membrane potentials in physiological ionic gradients. However, whole-cell clamp experiments have shown that current through ROCCs can be increased by a range of intracellular signalling pathways including calcium-calmodulin (Kim et al., 1995), myosin-light chain kinase (Kim et al., 1997; Aromolaran et al., 2000), and tyrosine kinase (Albert et al., 2000). Protein kinase C has been variously reported to activate (Oike et al., 1993) or inhibit (Ahn et al., 1997; Kim et al., 1998c) ROCCs. More recently, an atypical form of protein kinase C, activated by phosphatidylinositol 3,4,5-trisphosphate produced in response to Gβγ-dependent activation of phosphoinositide 3-kinase, has been reported to increase non-selective cation current in equine trachealis muscle (Wang et al., 1999).
In addition to changes in intracellular calcium, the extracellular concentration of calcium, or more correctly divalent cations, has profound effects on receptor-operated cation currents. This has been most extensively studied by Large and co-workers on the current activated by noradrenaline in the rabbit portal vein (Helliwell & Large, 1996; 1998; Aromolaran & Large, 1999). These workers have suggested that, on raising the extracellular calcium concentration from zero, there is an initial facilitatory action of the cation on the noradrenaline-evoked current (EC50 6 μM) followed by an inhibitory effect (IC50 400 μM). Even when calcium is absent from the extracellular solution noradrenaline is able to activate the current suggesting a regulatory, rather than a permissive, role for extracellular calcium (Helliwell & Large, 1996). The facilitatory effects of calcium can be mimicked by strontium and barium (Aromolaran & Large, 1999) but neither effect can be reproduced by magnesium (Helliwell & Large, 1996). Membrane current noise analysis suggests that the effects of calcium are complex and influence the gating of the channel (Helliwell & Large, 1998; Aromolaran & Large, 1999). In contrast, whilst an inhibitory action of extracellular calcium and magnesium has been reported for the cation current activated by acetylcholine in guinea-pig ileum (Zholos & Bolton, 1995), this effect seemed to reflect a rather non-specific shift in the voltage-dependence of the current due to charge screening.
Store-operated calcium channels and store-operated currents
In the late 1980's, Putney proposed his model for ‘capacitative calcium entry' in which intracellular calcium store depletion stimulated calcium influx across the plasma membrane to maintain a raised [Ca2+]i in the face of prolonged agonist application and to aid in refilling of the stores on agonist withdrawal (Putney, 1986; 1990; Berridge, 1995; Parekh & Penner, 1997; Putney et al., 2001). Some of the earliest evidence suggesting an interaction between the state of filling of the sarcoplasmic reticulum and the rate of calcium influx across the plasma membrane arose from experiments in smooth muscle (Casteels & Droogmans, 1981). Unfortunately, the widespread acceptance of the view that receptor-operated calcium influx was of primary importance during pharmacomechanical coupling, allied to the fact that capacitative calcium entry could be studied more easily in a variety of non-excitable cell types, meant that the process was not widely investigated in smooth muscle. More recently, evidence has again been accumulating to suggest that store-operated calcium entry may play a role in determining the contractile state of some smooth muscles. Before reviewing this evidence it is necessary to emphasise two essential features of capacitative calcium entry that are crucial in developing an understanding of how it operates in smooth muscle and, perhaps more importantly in the present context, allowing a distinction to be drawn between store-operated and receptor-operated channels or mechanisms.
The first important point of note is that it is not the calcium released from the stores that activates SOCCs (ie they are not ‘calcium-operated'). Thus, if the rise in [Ca2+]i occurring as a consequence of store depletion is prevented, for example by the inclusion of a calcium buffer in the micropipette-filling solution during whole-cell patch-clamp experiments, then the store-operated current should still be present. That is not to say that store-operated currents (like their receptor-operated counterparts), do not show some degree of calcium dependence. Evidence from experiments in other cell types would predict that store-operated channels in smooth muscle are likely to be inhibited by a rise, and activated by a fall, in [Ca2+]i especially in the vicinity of the SOCCs themselves (Barritt, 1999; Putney et al., 2001). Rather, it is the fact that the stores are empty of calcium that drives the response, by an as yet unknown mechanism. This accounts for the second defining characteristic of the process; that store-depletion, regardless of how it is brought about, activates the store-operated current. Experimentally, this can be demonstrated using a variety of drugs all of which share the ability to deplete intracellular calcium stores. Such drugs include the sarco-endoplasmic reticulum Ca-ATPase (SERCA) pump inhibitors thapsigargin and cyclopiazonic acid (CPA) which, by inhibiting the active uptake of calcium into the sarcoplasmic reticulum of smooth muscle, allow the stores to deplete ‘passively' leading to receptor-independent activation of store-operated calcium entry. These drugs have proved invaluable in elucidating a role for SOCCs in smooth muscle as in other tissues and, more importantly, distinguishing between store- and receptor-operated mechanisms. This need arises because, as mentioned above, many of the neurotransmitters which activate ROCCs simultaneously activate phospholipase C, liberating IP3. Consequently, these same neurotransmitters might also be expected to activate store-operated calcium entry as a result of IP3-mediated depletion of the sarcoplasmic reticulum. Thus, the introduction of the concept of SOCCs raised some important questions regarding non-voltage-gated calcium entry in smooth muscle since it was possible that neurotransmitter responses previously reported to utilize receptor-operated calcium entry mechanisms might also have involved, at least in part, store-operated calcium entry (i.e. were some responses reported to be mediated by ROCCs actually mediated by SOCCs, or are there two distinct channel types with differing activation mechanisms?). This highlights not only the importance of distinguishing between these two pathways experimentally but also of identifying any functional overlap between them. In the following discussion, we have concentrated on studies wherein SERCA pump inhibitors have been used to confirm a response occurs as a result of store depletion.
SERCA pump inhibitors have been shown to stimulate calcium influx, and consequently raise [Ca2+]i, in a range of smooth muscles (Table 2). In addition, these drugs have also been reported to produce contractions in a number of smooth muscles, suggesting that store-operated calcium entry may be important in regulating the contractile state of the tissues (Table 2). An alternative interpretation is that the SERCA pump inhibitors are not directly initiating calcium entry, but rather, by disrupting the superficial buffer barrier function of the sarcoplasmic reticulum (van Breemen et al., 1995) are allowing calcium entering by other routes, including poorly defined ‘leak' pathways, to gain access to the cell interior and the contractile proteins. The main argument against this possibility comes from ‘manganese quench' studies wherein the rate of manganese influx into the cell, monitored by its ability to quench Fura-2 fluorescence, is used as an indirect measure of calcium influx. A number of studies have shown that SERCA pump inhibitors increase the rate of manganese influx (Byron & Taylor, 1995; Ohta et al., 1995; Wallace et al., 1999). More directly, 45Ca2+ uptake has been shown to be increased in the presence of thapsigargin (Xuan et al., 1992). Thus, whilst disruption of the buffering capacity of the SR might contribute to the overall rise in calcium produced by SERCA pump inhibitors in many smooth muscles, available evidence strongly suggests that the drugs also stimulate calcium influx. One exception to this would appear to be the inferior vena cava of the rabbit wherein thapsigargin, though able to produce an increase in [Ca2+]i, did not stimulate calcium entry as measured by manganese quench (Chen & van Breemen, 1993); this was despite noradrenaline activating calcium entry via both ROCCs and VOCCs in the same tissue. Presumably, under physiological circumstances, calcium entering by these pathways is able to refill a depleted store, negating the need for store-operated calcium entry. It is therefore important to note that a rise in intracellular calcium (or an associated contraction) in response to a SERCA pump inhibitor is not in itself indicative of store-operated calcium entry being important in the contractile process.
Table 2.
Smooth muscles in which SERCA pump inhibitors have to be reported to raise intracellular calcium and/or produce contraction

Only in a very few cases have the membrane currents underlying store-operated calcium entry in smooth muscle been recorded. In single cells isolated from the mouse anococcygeus, CPA activated a sustained, non-selective cation conductance. The current-voltage relationship for the CPA-induced current was linear with a reversal potential of approximately +30 mV in near physiological cation gradients. The reversal potential was moved in the negative direction on removal of extracellular calcium indicating a significant proportion of the current was carried by calcium (Wayman et al., 1996). This was supported by simultaneous recordings of changes in current and intracellular calcium which showed that activation or inhibition of the current was mirrored by increases and decreases in [Ca2+]i respectively (Wayman et al., 1999). A store-operated current has recently been reported in rabbit choroidal arteriolar smooth muscle (Curtis & Scholfield, 2001). Here again, store-depletion activated a non-selective cation current, but in this case the current voltage-relationship displayed marked inward rectification at membrane potentials negative to −60 mV. This current also had a unique pharmacology, being blocked by the L-type VOCC blocking agent nifedipine. Single channel currents through SOCCs have been recorded in single cells isolated from the aortae of mice and rabbits (Trepakova et al., 2001). In these tissues, the underlying channels were stable in excised inside-out patches, showed little selectivity for calcium over monovalent cations, and had a single channel conductance of approximately 3 pS. The single channel conductance showed little voltage-dependence, although the open probability of the channels appeared to increase significantly at very positive membrane potentials, producing marked outward rectification of the whole-cell current. Overall, existing evidence suggests that, in smooth muscle, store-operated calcium influx occurs via a family of non-selective cation channels. This is in contrast to the channels underlying the first store-operated current recorded in mast cells (Hoth & Penner, 1992; 1993), termed ICRAC, which was highly calcium selective with a single channel conductance (in the presence of calcium) of less than 1 pS. The whole-cell current showed marked inward rectification at negative membrane potentials, again serving to distinguish ICRAC from the store-operated currents recorded in either aortic or anococcygeal smooth muscle.
It is perhaps puzzling why store-operated currents have not been more frequently recorded in smooth muscle cells. The most obvious explanation is that the currents are so small in comparison with their receptor-operated cousins, with even the whole-cell store-operated current measuring little more than 10 pA. Indeed, it is possible that store-operated currents have been recorded in the past following application of receptor agonists, but have been swamped by receptor-operated currents activated simultaneously and so have remained unidentified. However, in some cases, SOCCs have been reported as absent despite determined attempts to detect them. For example, in A7r5 cells, two separate studies have failed to record any store-operated current, whilst being able to detect receptor-operated currents in response to endothelin-1 (Iwasawa et al., 1997; Iwamuro et al., 1999). This was despite the fact that thapsigargin produced an increase in [Ca2+]i comparable to, if not larger than that produced by the agonist. One possible explanation for this is offered by the results of experiments on ICRAC which suggest that, unlike receptor-operated currents, store-operated currents are likely to be inhibited by rises in [Ca2+]i. The regulation of ICRAC by intracellular calcium is complex (reviewed in Parekh & Penner, 1997) but perhaps the most prevalent mechanism is a rapid inactivation, occurring on a millisecond timescale, that appears to occur as a result of a localized rise in calcium near the cytoplasmic mouth of the channel, possibly by calcium entering through the channel itself (Hoth & Penner, 1993; Zweifach & Lewis, 1995; Fierro & Parekh, 1999). Should a similar mechanism affect store-operated channels in smooth muscle then it is possible that at any point in time a significant proportion of the available store-operated current is inactivated. This would be even more pronounced given the close association between the plasma membrane and that of the sarcoplasmic reticulum, which produces an enclosed microenvironment relatively inaccessible to intracellular calcium buffers such as EGTA used routinely to prevent calcium-dependent inhibitory processes. There is some evidence that in rat aorta, calcium entering via SOCCs does so into a non-contractile compartment of the cytoplasm envisaged to be associated with the superficial sarcoplasmic reticulum (Tosun et al., 1998) and one proposal put forward to explain the link between store depletion and the activation of SOCCs is that it results from a localized reduction in calcium concentrations in the narrow space between the SR and the internal mouth of the SOCC (Barritt, 1999; Putney et al., 2001). It is also possible that a significant component of the rise in calcium produced by SERCA pump inhibitors is due to disruption of the calcium buffering capacity of the SR (see above) such that the amount of calcium influx, and thus inward current, required to produce a given rise in [Ca2+]i is significantly less than that for a receptor agonist.
Pharmacology of ROCCs and SOCCs
Research into the relative importance of receptor- vs store-operated mechanisms in smooth muscle excitation-contraction coupling has been hampered by the paucity of selective blocking drugs for the respective channels. SKF96365, (1-[β-[3-(4-methoxyphenyl) propoxy]-4-methoxy-phenethyl]-1H-imidazole hydrochloride) the most commonly used inhibitor of store-operated calcium entry, is one of a series of N1-substituted imidazole compounds which also includes econazole and miconazole (Merritt et al., 1990; Clementi & Meldolesi, 1996). Certainly SKF96365 blocks the store-operated current in mouse anococcygeus cells (Wayman et al., 1996), but at a similar concentrations (IC50 approximately 10 μM) it also blocks the muscarinic receptor operated cation current in ileal smooth muscle (Zholos et al., 2000) and voltage-operated calcium channels in other tissues (Merritt et al., 1990; Clementi & Meldolesi, 1996). At higher concentrations, SKF96365 may also exert a range of other effects unrelated to its channel blocking activity (Mason et al., 1993; Jan et al., 1999). Recently, we have found that another N1-substituted imidazole, trifluoromethylphenylimidazole (TRIM), better known as an inhibitor of neuronal nitric oxide synthase (Moore & Handy, 1997), shows selectivity for store-operated calcium entry over voltage-operated calcium entry in mouse anococcygeus smooth muscle (Gibson et al., 2001), though whether this selectivity extends to receptor-operated currents, and to other smooth muscle tissues, remains to be established.
Several cations, most notably cadmium, nickel, gadolinium and lanthanum have also been shown to block both receptor and store-operated currents. The response to lanthanum appears to show a degree of tissue selectivity. The cation has been reported to inhibit receptor-operated calcium influx in some smooth muscles (Krautwurst et al., 1994) but to potentiate the ACh-induced cation current in ileal smooth muscle (Inoue et al., 1998). Similarly, lanthanum has been reported to inhibit store-operated responses in pulmonary smooth muscle (De La Fuente et al., 1995), but to have no effect on those in mouse anococcygeus (Wayman et al., 1996) or renal artery (Utz et al., 1999). Although it is generally accepted that inhibitors of voltage-operated calcium channels are highly selective, a recent report has shown that nifedipine, at low micromolar concentrations, is able to block store-operated calcium influx in arteriolar smooth muscle (Curtis & Scholfield, 2001). This may not be particularly surprising in light of what appears to be a high degree of structural homology between the pore-forming α1 subunits of the voltage-operated channels and the proteins thought to form their receptor-and store-operated counterparts. Overall, there is a pressing need for the identification of drugs which act selectively on either ROCCs or SOCCs to modify channel function.
Molecular structure of ROCCs and SOCCs – a unifying concept?
Whilst it is convenient to subdivide non-voltage-operated calcium channels into SOCCs and VOCCs on the basis of their activation mechanism, it is highly likely that this subdivision over-emphasizes what is a relatively minor difference between the underlying channels. As mentioned earlier, there is clear overlap between the functional roles played by store-operated and receptor-operated calcium entry; there is a subtle distinction between one response, initiated by receptor activation but potentiated by the release of calcium from intracellular stores and another initiated by store depletion as a consequence of receptor activation. In light of this, it is perhaps not surprising that emerging evidence suggests that, from a molecular viewpoint, both store-operated and receptor-operated channels may be formed from proteins belonging to the same family, being the mammalian homologues of so-called Transient Receptor Potential (TRP) channels, first cloned in Drosophila.
In Drosophila photoreceptors, activation of rhodopsin by light leads to the stimulation of phospholipase C, IP3 production and, ultimately, a sustained membrane depolarization (Montell, 1998). The depolarisation results from activation of two separate conductances, one calcium selective, the other permeable to both sodium and calcium. The calcium entering the photoreceptor via these pathways, in particular the calcium-selective pathway, is important for calcium-dependent light adaptation; mutant flies lacking a functional calcium-selective entry pathway are blinded by intense light. In these same mutants, exposure to light produces only a transient membrane depolarisation and as a result the mutation has been called transient receptor potential (trp). The functional similarity between the calcium entry pathway activated by light in Drosophila (and deficient in trp) and that activated following store depletion in mammalian cells led to the suggestion that the protein encoded by the trp gene might be analogous to that which supports store-operated calcium entry (Hardie & Minke, 1995). trp encodes an approximately 145 kDa protein (TRP) with several potential membrane spanning regions and significant structural similarity to the membrane spanning domains of the α1-subunit of VOCCs. Notably however TRP lacks the ‘fingerprint' S4 region containing numerous positively charged residues that are thought to form the voltage-sensor in voltage-operated channels. The non-selective cation channels activated along with TRP during the Drosophila light response have subsequently been named Transient Receptor Potential-Like (TRPL), encoded by the trpl gene (Phillips et al., 1992). TRPL is an approximately 127 kDa protein and shows significant sequence similarity to TRP, particularly in the N-terminal and membrane spanning domains. The proteins differ markedly however in their C-terminal regions and it has been shown that the C-terminal domain of TRP is essential for activation of the channels following intracellular calcium store-depletion (Sinkins et al., 1996).
Following these initial findings in Drosophila, a number of groups have cloned homologues of genes encoding TRP and TRPL in both Caenorhabditis elegans and mammals. Based on sequence similarity, the products of these genes in C. elegans have been divided into three TRP channel subfamilies termed short (S), long (L) and Osm (O) (Harteneck et al., 2000). The mammalian equivalents have tentatively been called C, V and M respectively (Clapham et al., 2001). ‘V' and ‘M' in this nomenclature refer to vanilloid receptor-like and melastatin-like sub-families, whilst C is a historical reference to the first mammalian TRP gene product reported to form a cation channel. It should be noted however that members of the V and M families also form such channels and it is likely that a more logical nomenclature will emerge soon.
Although it is generally accepted that members of the TRP protein family form the channels supporting store-operated calcium entry, exactly which members are involves remains open to debate. Most attention has focused on the short, TRPC subfamily although a recent report has suggested that CaT1, a member of the TRPV subfamily, may also play a role (Yue et al., 2001). Within the TRPC subfamily there are at least seven members, denoted TRPC1-7. By analogy to voltage-operated channels, it is likely that functional channels are formed by a tetrameric assembly of TRPC proteins which includes the likelihood of hetero-tetrameric assembly. Results, obtained by a number of groups using a variety of expression systems, have shown that all seven TRPC proteins, whether expressed alone or in combination, can form calcium permeable cation channels. However, only rarely has it been possible to reproduce the phenotype of the calcium entry pathway in a native cell by transfecting one or more of the TRPC genes into a cell expression system. In particular, it has proved difficult to form a consensus view as regards the ‘normal' activation mechanisms for these channels (Reviews; Putney & McKay, 1999; Clapham et al., 2001; Putney et al., 2001) with conflicting reports for almost every subfamily member on whether activation involves store-dependent or store-independent mechanisms.
TRPC1 (Zitt et al., 1996), TRPC2 (Vannier et al., 1999), TRPC3 (Zhu et al., 1996), TRPC4 (Warnat et al., 1999) and TRPC5 (Philipp et al., 1998) have all at some stage been reported to form channels activated by store depletion. Recently, an antibody raised against an epitope near the proposed pore-forming region of the TRPC1 peptide has been shown to reduce store-operated calcium entry in arteriolar smooth muscle cells (Xu & Beech, 2001). Antibody binding was shown to be localized to the plasma membrane and appeared to be extracellular as it did not require prior permeabilization of the smooth muscle cells.
Although, for the historical reasons detailed earlier, initial interest revolved around a potential role for these TRP proteins in forming store-operated channels, many have subsequently been shown to have properties consistent with their forming receptor-operated channels. Thus, for example, co-expression of TRPC5 with either TRPC1 (Strübing et al., 2001) or TRPC4 (Schaefer et al., 2000) forms non-selective cation channels activated by G-protein coupled receptors, but not store depletion, when expressed in HEK-293 cells, as does expression of TRPC6 alone (Boulay et al., 1997; Inoue et al., 2001). In all three cases, the current-voltage relationships for the agonist-induced responses had the distinctive ‘S-shape' typical of receptor-operated currents recorded in smooth muscle (see earlier), and had reversal potentials indicative of non-selective cation currents. Indeed, the remarkable similarity between the properties of the current seen in HEK293 cells expressing TRPC6 and the phenylephrine-induced current in rabbit portal vein led Inoue et al. (2001) to suggest that this homologue was an ‘essential component' of the native current. TRP6 mRNA, and the resultant protein, were both detected in portal vein myocytes and the phenylephrine-induced current was inhibited in cells treated with TRPC6 antisense oligonucleotides. Interestingly, the current activated by carbachol in cells co-expressing TRPC1 and TRPC5 was potentiated by lanthanum (Strübing et al., 2001) as is the acetylcholine-induced receptor-operated current in ileal smooth muscle (Inoue et al., 1998). TRPC6 has been reported to form channels activated directly by diacylglycerol (DAG; Hofmann et al., 1999). Similar results have been obtained with TRPC3 (Hofmann et al., 1999) and TRPC7 (Okada et al., 1999) and these findings raise the possibility that this signalling pathway might be involved in mediating receptor-operated currents in smooth muscle, although at this stage there is little direct evidence to support this.
Although a number of TRPC genes have been reported to be expressed in smooth muscle tissues (Zhu et al., 1995; 1996; McKay et al., 2000) such reports often have to be interpreted with caution since experiments have been performed on whole tissues containing cell types other than smooth muscle cells. With the exception of the two studies mentioned above (Inoue et al., 2001; Xu & Beech, 2001) there is little information available on the expression of TRPC genes, or the translated proteins, specifically in smooth muscle cells. One recent study has reported a differential distribution of TRPC mRNA in a range of murine and canine gastrointestinal and vascular smooth muscles (Walker et al., 2001). These workers performed RT – PCR on RNA isolated from identified smooth muscle cells, dissociated enzymatically from the whole muscle, and ‘harvested' using a modified patch pipette. TRPC4 was most abundantly expressed in the smooth muscles studied whilst TRP6 and TRPC7 were expressed at lower levels. TRPC3 was detected in small amounts in canine renal artery whilst mRNA for TRPC1, TRPC2 and TRPC5 was not detected. In a separate study, McDaniel et al. (2001) reported the expression of mRNA for TRPC1, TRPC2, TRPC4, TRPC5 and TRPC6 in rat pulmonary artery smooth muscle cells.
Overall, therefore, whilst there is much evidence to suggest that TRPC proteins can form both receptor-operated and store-operated channels in cell expression systems, whether these channels underlie the native currents in smooth muscle is less clear. Nonetheless, they do provide a unifying concept linking ROCCs and SOCCs within the same ion channel family, and their diversity provides a possible explanation for the wide variety of receptor-operated and store-operated responses recorded in smooth muscle. For example, as depicted in Figure 1, one putative model is that TRPC proteins might fall into at least two broad classes – one responsive to receptor activation but not store depletion (TRPCr), the other responsive to store depletion (TRPCs). The subunit composition of the complete channel (assuming a tetrameric structure) would then determine its relative sensitivity to activation by receptor-operated or store-operated mechanisms (or both). This model also highlights a developing, and as yet unresolved, problem of classification and nomenclature within this whole area – the term ROCC covers an increasingly diverse range of channel activation mechanisms, ranging from true ligand-gated receptor-channel macromolecules (P2X), through receptor-G protein-channel processes, to SOCCs themselves (since the normal physiological agent causing depletion of the sarcoplasmic reticulum in smooth muscle would be IP3 generated by receptor activation). However, at present, the most pressing need is for more detailed information on the differential expression of the TRPC proteins in smooth muscle cells and the identification of which combination(s) come together to form the functional channels.
Figure 1.
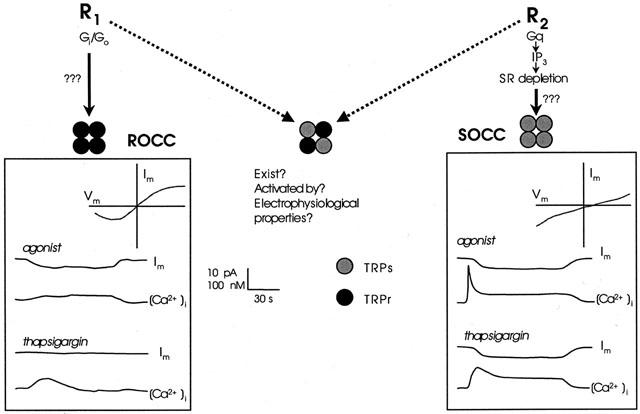
Comparison of the responses mediated by ROCCs and SOCCs in smooth muscle. The left hand panel shows hypothetical changes in membrane current (Im) and intracellular calcium [Ca2+]i produced by a receptor agonist and the SERCA pump inhibitor thapsigargin in a cell possessing only ROCCs. The top right corner of the panel shows the current-voltage relationship for the receptor operated current. The functional channel is proposed to be formed from four ‘receptor coupled' (TRPCr) subunits (black circles). The right hand panel shows the equivalent responses but in this case from a cell possessing only SOCCs, formed from four ‘store-coupled' (TRPCs) subunits (grey circles). Note the difference in the current-voltage relationship and in the response to thapsigargin. A channel formed from a mixture of TRPs and TRPr subunits is depicted in the middle of the figure though firm evidence for the existence of such channels is lacking. See text for further details.
Conclusion and future directions
This review has attempted to highlight the important role of non-voltage-gated mechanisms, specifically ROCCs and SOCCs, in smooth muscle function and to present the recent evidence that ROCCs and SOCCs may in fact be members of the same ion channel family, differing only in their composition of TRPC protein subunits. However, many questions remain unanswered and amongst the most important awaiting full resolution are the following:
Activation mechanisms
There is as yet no clear picture of the molecular mechanisms that link receptor activation to the opening of non-ligand-gated ROCCs (G proteins, diacylglycerol, phospholipids?) or store-depletion to the opening of SOCCs (diffusible messenger, conformational coupling, secretory mechanisms, sub-plasmalemmal calcium concentration?). In both cases care must be taken in extrapolating results obtained from experiments on TRP proteins expressed in cell lines to native smooth muscle cells. A recent report has shown that the so-called capacitative influx factor (CIF) – the soluble intracellular messenger purported to be released on calcium store depletion and responsible for activating capacitative calcium influx in the diffusible messenger model – can activate store-operated cation channels in mouse aortic smooth muscle (Trepakova et al., 2000). That said, the close association between the plasma membrane and that of the sarcoplasmic reticulum (van Breemen et al., 1995), often in association with cavaeoli, would be able to support other models by which the signal is passed from the depleted store to the (presumably) TRP channel protein (reviewed in Putney et al., 2001).
Pharmacology
Can we develop selective activators/inhibitors of ROCCs or SOCCs that would provide important tools for the further investigation of these channels and compounds with potential therapeutic application? L-type calcium channel blockers have proved very useful therapeutic agents by allowing modification of smooth muscle function in cardiovascular disease. However, they have little effect on smooth muscles (or related diseases) controlled predominantly by non-voltage-gated mechanisms (for example, airways smooth muscle in asthma); selective blockers of ROCCs or SOCCs might provide this capability. The importance of such agents might not be limited simply to the control of excitation-contraction coupling; there is for example, growing evidence that SOCCs (and TRP proteins) may be up-regulated during smooth muscle proliferation (Golovina, 1999; Golovina et al., 2001; Wang et al., 2001; Dreja et al., 2001).
Structure
As mentioned earlier, elucidation of the complete molecular structure of native ROCCs and SOCCs will be a crucial step towards a full understanding of channel function.
The answers to the above questions will determine whether ROCCs and SOCCs are indeed members of the same ion channel family, linked by activation mechanisms, protein subunit composition and pharmacology. In addition to identifying potential drug targets for treatment of conditions such as prostatic hyperplasia and pulmonary hypertension this will also lead to the development of much clearer classification/nomenclature guidelines than exist at present.
Acknowledgments
Work in our laboratories is supported by the Wellcome Trust and BBSRC.
Abbreviations
- ACh
acetylcholine
- ATP
adenosine triphosphate
- [Ca2+]i
concentration of intracellular calcium
- CPA
cyclopiazonic acid
- EGTA
ethylene glycol-bis (b-amino ethyl ether) tetraacetic acid acid
- ICRAC
calcium release-activated current
- IP3
inositol (1,4,5) trisphosphate
- mRNA
messenger ribonucleic acid
- ROCC
receptor-operated calcium channel
- SERCA
sarco-endoplasmic reticulum calcium ATPase
- SKF96365
1-[β-[3-(4-methoxyphenyl) propoxy]-4-methoxy-phenethyl]-1H-imidazole hydrochloride
- SOCC
store-operated calcium channel
- TRIM
trifluoromethylphenylimidazole
- TRP
transient receptor potential
- TRPC
transient receptor potential channel
- TRPL
transient receptor potential like
- VOCC
voltage-operated calcium channel
References
- AHN S.C., KIM S.J., SO I., KIM K.W. Inhibitory effect of phorbol 12, 13 dibutyrate on carbachol-activated nonselective cationic current in guinea-pig gastric myocytes. Pflügers Arch. 1997;434:505–507. doi: 10.1007/s004240050429. [DOI] [PubMed] [Google Scholar]
- ALBERT A.P., AROMOLARAN A.S., LARGE W.A. Agents that increase tyrosine phosphorylation activate a non-selective cation current in single rabbit portal vein smooth muscle cells. J. Physiol. 2000;530:207–217. doi: 10.1111/j.1469-7793.2001.0207l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMÉDÉE T., BENHAM C.D., BOLTON T.B., BYRNE N.G., LARGE W.A. Potassium, chloride and non-selective cation conductances opened by noradrenaline in rabbit ear artery cells. J. Physiol. 1990;423:551–568. doi: 10.1113/jphysiol.1990.sp018039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMÉDÉE T., LARGE W.A. Microelectrode study on the ionic mechanisms which contribute to the noradrenaline-induced depolarization in isolated cells of the rabbit portal vein. Br. J. Pharmacol. 1989;97:1331–1337. doi: 10.1111/j.1476-5381.1989.tb12596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AROMOLARAN A.S., ALBERT A.P., LARGE W.A. Evidence for myosin light chain kinase mediating noradrenaline-evoked cation current in rabbit portal vein myocytes. J. Physiol. 2000;524:853–863. doi: 10.1111/j.1469-7793.2000.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AROMOLARAN A.S., LARGE W.A. Comparison of the effects of divalent cations on the noradrenaline-evoked cation current in rabbit portal vein smooth muscle cells. J. Physiol. 1999;520:771–782. doi: 10.1111/j.1469-7793.1999.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRITT G.J. Receptor-activated Ca2+ inflow in animal cells: a variety of pathways tailored to meet different intracellular Ca2+ signalling requirements. Biochem. J. 1999;337:153–169. [PMC free article] [PubMed] [Google Scholar]
- BENHAM C.D., BOLTON T.B., BYRNE N.G., LARGE W.A. Action of externally applied adenosine triphosphate on single smooth muscle cells dispersed from rabbit ear artery. J. Physiol. 1987;387:473–488. doi: 10.1113/jphysiol.1987.sp016585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENHAM C.D., BOLTON T.B., LANG R.J. Acetylcholine activates an inward current in single smooth mammalian smooth muscle cells. Nature. 1985;316:345–347. doi: 10.1038/316345a0. [DOI] [PubMed] [Google Scholar]
- BENHAM C.D., TSIEN R.W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- BERRIDGE M.J. Capacitative calcium entry. Biochem. J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOLTON T.B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979;59:606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- BOLTON T.B., LARGE W.A. Are junction potentials essential? Dual mechanism of smooth muscle cell activation by transmitter released from autonomic nerves. Quarterly J. Exp. Physiol. 1986;71:1–28. doi: 10.1113/expphysiol.1986.sp002960. [DOI] [PubMed] [Google Scholar]
- BOULAY G., ZHU X., PEYTON M., JIANG M., HURST R., STEFANI E., BIRNBAUMER L. Cloning and expression of a novel mammalian homolog of Drosophila Transient Receptor Potential (Trp) involved in calcium entry secondary to activation of receptors coupled by the Gq class of G protein. J. Biol. Chem. 1997;272:29672–29680. doi: 10.1074/jbc.272.47.29672. [DOI] [PubMed] [Google Scholar]
- BURT R.P., CHAPPLE C.R., MARSHALL I. The role of capacitative Ca2+ influx in the α1B-adrenoceptor-mediated contraction to phenylephrine of the rat spleen. Br. J. Pharmacol. 1995;116:2327–2333. doi: 10.1111/j.1476-5381.1995.tb15073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYRNE N.G., LARGE W.A. Membrane ionic mechanisms activated by noradrenaline in cells isolated from the rabbit portal vein. J. Physiol. 1988;404:557–573. doi: 10.1113/jphysiol.1988.sp017306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYRON K.L., TAYLOR C. Vasopressin stimulation of Ca2+ mobilization, two bivalent cation entry pathways and Ca2+ efflux in A7r5 rat smooth muscle cells. J. Physiol. 1995;485:455–468. doi: 10.1113/jphysiol.1995.sp020742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTEELS R., DROOGMANS G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells of rabbit ear artery. J. Physiol. 1981;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN C., WAGONER P.K. Endothelin induces a nonselective cation current in vascular smooth muscle cells. Circ Res. 1991;69:447–454. doi: 10.1161/01.res.69.2.447. [DOI] [PubMed] [Google Scholar]
- CHEN Q., VAN BREEMEN C. The superficial buffer barrier in venous smooth muscle sarcoplasmic reticulum refilling and unloading. Br. J. Pharmacol. 1993;109:336–343. doi: 10.1111/j.1476-5381.1993.tb13575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAPHAM D.E., RUNNELS L.W., STRÜBING C. The TRP ion channel family. Nature Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- CLEMENTI E., MELDOLESI J. Pharmacological and functional properties of voltage-independent Ca2+ channels. Cell Calcium. 1996;19:269–279. doi: 10.1016/s0143-4160(96)90068-8. [DOI] [PubMed] [Google Scholar]
- COHEN R.A., WEISBROD R.M., GERICKE M., YAGHOUBI M., BIERL C., BOLOTINA V.M. Mechanism of nitric oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ. Res. 1999;84:210–219. doi: 10.1161/01.res.84.2.210. [DOI] [PubMed] [Google Scholar]
- CURTIS T.M., SCHOLFIELD C.N. Nifedipine blocks Ca2+ store refilling through a pathway not involving L-type Ca2+ channels in rabbit arteriolar smooth muscle. J. Physiol. 2001;532:609–623. doi: 10.1111/j.1469-7793.2001.0609e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LA FUENTE P.G., SAVINEAU J.-P., MARTHAN R. Control of pulmonary vascular smooth muscle tone by sarcoplasmic reticulum Ca2+ pump blockers: thapsigargin and cyclopiazonic acid. Pflügers Arch. 1995;429:617–624. doi: 10.1007/BF00373982. [DOI] [PubMed] [Google Scholar]
- DREJA K., BERGDAHL A., HELLSTRAND P. Increased store-operated Ca2+ entry into contractile vascular smooth muscle following organ culture. J. Vasc. Res. 2001;38:324–331. doi: 10.1159/000051063. [DOI] [PubMed] [Google Scholar]
- ENOKI T., MIWA S., SAKAMOTO A., MINOWA T., KOMURO T., KOBAYASHI S., NINOMIYA H., MASAKI T. Long-lasting activation of cation current by low concentration of endothelin-1 in mouse fibroblasts and smooth muscle cells of rabbit aorta. Br. J. Pharmacol. 1995;115:479–485. doi: 10.1111/j.1476-5381.1995.tb16358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIERRO L., PAREKH A.B. Fast calcium-dependent inactivation of calcium release-activated calcium current (CRAC) in RBL-1 cells. J. Memb. Biol. 1999;168:9–17. doi: 10.1007/s002329900493. [DOI] [PubMed] [Google Scholar]
- FRIEL D.D. An ATP-sensitive conductance in single smooth muscle cells from the rat vas deferens. J. Physiol. 1988;401:361–380. doi: 10.1113/jphysiol.1988.sp017167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., FERNANDES F., WALLACE P., MCFADZEAN I. Selective inhibition of thapsigargin-induced contraction and capacitative calcium entry in mouse anococcygeus by trifluoromethylphenylimidazole (TRIM) Br. J. Pharmacol. 2001;134:233–236. doi: 10.1038/sj.bjp.0704286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., MCFADZEAN I., TUCKER J.F., WAYMAN C. Variable potency of nitrergic-nitrovasodilator relaxations of the mouse anococcygeus against different forms of induced tone. Br. J. Pharmacol. 1994;113:1494–1500. doi: 10.1111/j.1476-5381.1994.tb17165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON A., MCFADZEAN I., WALLACE P., WAYMAN C.P. Capacitative Ca2+ entry and the regulation of smooth muscle tone. Trends Pharmacol. Sci. 1998;19:266–269. doi: 10.1016/s0165-6147(98)01222-x. [DOI] [PubMed] [Google Scholar]
- GOLOVINA V.A. Cell proliferation is associated with enhanced capacitative Ca2+ entry in human arterial myocytes. Am. J. Physiol. 1999;277:C343–C349. doi: 10.1152/ajpcell.1999.277.2.C343. [DOI] [PubMed] [Google Scholar]
- GOLOVINA V.A., PLATOSHYN O., BAILEY C.L., WANG J., LIMSUWAN A., SWEENEY M., RUBIN L.J., YUAN J.X. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. Am. J. Physiol. Heart. Circ. Physiol. 2001;280:H746–H755. doi: 10.1152/ajpheart.2001.280.2.H746. [DOI] [PubMed] [Google Scholar]
- HARDIE R.C., MINKE B. Phosphoinositide-mediated phototransduction in Drosophila photoreceptors: the role of Ca2+ and trp. Cell Calcium. 1995;18:256–274. doi: 10.1016/0143-4160(95)90023-3. [DOI] [PubMed] [Google Scholar]
- HARTENECK C., PLANT T.D., SCHULTZ G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- HELLIWELL R.M., LARGE W.A. Dual effect of external Ca2+ on noradrenaline-activated cation current in rabbit portal vein smooth muscle cells. J. Physiol. 1996;492:75–88. doi: 10.1113/jphysiol.1996.sp021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLIWELL R.M., LARGE W.A. Facilitatory effect of Ca2+ on the noradrenaline-evoked cation current in rabbit portal vein smooth muscle cells. J. Physiol. 1998;512:731–741. doi: 10.1111/j.1469-7793.1998.731bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFMANN T., OBUKHOV A.G., SCHAEFER M., HARTENECK C., GUNDERMANN T., SCHULTZ G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- HOTH M., PENNER R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature. 1992;355:353–356. doi: 10.1038/355353a0. [DOI] [PubMed] [Google Scholar]
- HOTH M., PENNER R. Calcium release-activated calcium current in rat mast cells. J. Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE R., ISENBERG G. Effect of membrane potential on acetylcholine-induced inward current in guinea-pig ileum. J. Physiol. 1990a;424:57–71. doi: 10.1113/jphysiol.1990.sp018055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE R., ISENBERG G. Intracellular calcium ions modulate acetylcholine-induced inward current in guinea-pig ileum. J. Physiol. 1990b;424:73–92. doi: 10.1113/jphysiol.1990.sp018056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE R., KITAMURA K., KURIYAMA H. Acetylcholine activates single sodium channels in smooth muscle cells. Pflügers Arch. 1987;410:69–74. doi: 10.1007/BF00581898. [DOI] [PubMed] [Google Scholar]
- INOUE R., KURIYAMA H. Dual regulation of cation-selective channels by muscarinic and alpha 1-adrenergic receptors in the rabbit portal vein. J. Physiol. 1993;465:427–448. doi: 10.1113/jphysiol.1993.sp019685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE R., MORITA H., YANAGIDA H., ITO Y. Potentiating actions of lanthanum on ACh-induced cation current in guinea-pig ileal smooth muscle cells. J. Smooth Musc. Res. 1998;34:69–81. doi: 10.1540/jsmr.34.69. [DOI] [PubMed] [Google Scholar]
- INOUE R., OKADA T., ONOUE H., HARA Y., SHIMIZU S., NAITOH S., ITO Y., MORI Y. The transient receptor potential protein homologue TRP6 is the essential component of vascular α1-adrenoceptor-activated Ca2+-permeable cation channel. Circ. Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- IWAMURO Y., MIWA S., ZHANG X.-F., MINOWA T., ENOKI T., OKAMOTO Y., HASEGAWA H., FURUTANI H., OKAZAWA M., ISHIKAWA M., HASHIMOTO N., MASAKI T. Activation of three types of voltage-independent Ca2+ channel in A7r5 cells by endothelin-1 as revealed by a novel Ca2+ channel blocker LOE 908. Br. J. Pharmacol. 1999;126:1107–1114. doi: 10.1038/sj.bjp.0702416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IWASAWA K., NAKAJIMA T., HAZAMA H., GOTO A., SHIN W.S., TOYO-OKA T., OMATA M. Effects of extracellular pH on receptor-mediated Ca2+ influx in A7r5 rat smooth muscle cells: involvement of two different types of channel. J. Physiol. 1997;503:237–251. doi: 10.1111/j.1469-7793.1997.237bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAN C.R., HO C.M., WU N., TSENG C.J. Multiple effects of 1-[β-[3-(4-methoxyphenyl) propoxy]-4-methoxy-phenethyl]-1H-imidazole hydrochloride (SKF 96365) on Ca2+ signaling in MDCK cells: depletion of thapsigargin-sensitive Ca2+ store followed by capacitative Ca2+ entry, activation of direct Ca2+ entry, and inhibition of thapsigargin-induced capacitative Ca2+ entry. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;359:92–101. doi: 10.1007/pl00005336. [DOI] [PubMed] [Google Scholar]
- KHAKH B.S., BURNSTOCK G., KENNEDY C., KING B.F., NORTH R.A., SÉGUÉLA P., VOIGHT M., HUMPHREY P.A. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- KIM S.J., AHN S.C., SO I., KIM K.W. Role of calmodulin in the activation of carbachol-activated cationic current in guinea-pig gastric antral myocytes. Pflügers Arch. 1995;430:757–762. doi: 10.1007/BF00386173. [DOI] [PubMed] [Google Scholar]
- KIM Y.C., KIM S.J., KANG T.M., SUH S.H., SO I., KIM K.W. Effects of myosin light chain kinase inhibitors on carbachol-activated nonselective cationic current in guinea-pig gastric myocytes. Pflügers Arch. 1997;434:346–353. doi: 10.1007/s004240050407. [DOI] [PubMed] [Google Scholar]
- KIM Y.C., KIM S.J., KIM J.H., CHO C.H., JUHNN Y.-S., SUH S.H., SO I., KIM K.W. Suppression of the carbachol-activated nonselective cationic current by antibody against alpha subunit of Go protein in guinea-pig gastric myocytes. Pflügers Arch. 1998a;436:494–496. doi: 10.1007/s004240050663. [DOI] [PubMed] [Google Scholar]
- KIM Y.C., KIM S.J., SIM J.H., JUN J.Y., KANG T.M., SUH S.H., SO I., KIM K.W. Protein kinase C mediates the desensitization of CCh-activated nonselective cationic current in guinea-pig gastric myocytes. Pflügers Arch. 1998c;436:1–8. doi: 10.1007/s004240050597. [DOI] [PubMed] [Google Scholar]
- KIM S.J., KOH E.-M., KANG T.M., KIM Y.C., SO I., ISENBERG G., KIM K.W. Ca2+ influx through carbachol-activated non-selective cation channels in guinea-pig gastric myocytes. J. Physiol. 1998b;513:749–760. doi: 10.1111/j.1469-7793.1998.749ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHDA M., KOMORI S., UNNO T., OHASHI H. Carbachol-induced [Ca2+]i oscillations in single smooth muscle cells of guinea-pig ileum. J. Physiol. 1996;492:315–328. doi: 10.1113/jphysiol.1996.sp021311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., BOLTON T.B. Role of G-proteins in muscarinic receptor inward and outward currents in rabbit jejunal smooth muscle. J. Physiol. 1990;427:395–419. doi: 10.1113/jphysiol.1990.sp018178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOMORI S., KAWAI M., PACAUD P., OHASHI H., BOLTON T.B. Oscillations of receptor-operated cationic current and internal calcium in single guinea-pig ileal smooth muscle cells. Pflügers Arch. 1993;424:431–438. doi: 10.1007/BF00374905. [DOI] [PubMed] [Google Scholar]
- KOMORI S., KAWAI M., TAKEWAKI T., OHASHI H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J. Physiol. 1992;450:105–126. doi: 10.1113/jphysiol.1992.sp019118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOTLIKOFF M.I., DHULIPALA P., WANG Y.X. M2 signaling in smooth muscle cells. Life Sci. 1999;64:437–442. doi: 10.1016/s0024-3205(98)00583-9. [DOI] [PubMed] [Google Scholar]
- KRAUTWURST D., DEGTIAR V.E., SCHULTZ G., HESCHELER J. The isoquinoline derivative LOE 908 selectively blocks vasopressin-activated nonselective cation currents in A7r5 aortic smooth muscle cells. Naunyn Schmiedebergs Arch. Pharmacol. 1994;349:301–7. doi: 10.1007/BF00169297. [DOI] [PubMed] [Google Scholar]
- KWAN C.Y., CHAUDHARY R., ZHENG X.F., NI J., LEE R.M. Effects of sarcoplasmic reticulum calcium pump inhibitors on vascular smooth muscle. Hypertension. 1994;23:I156–I160. doi: 10.1161/01.hyp.23.1_suppl.i156. [DOI] [PubMed] [Google Scholar]
- LEE H.K., BAYGUINOV O., SANDERS K.M. Role of nonselective cation current in muscarinic responses to canine colonic muscle. Am. J. Physiol. 1993;265:C1463–C1471. doi: 10.1152/ajpcell.1993.265.6.C1463. [DOI] [PubMed] [Google Scholar]
- LEE H.K., SHUTTLEWORTH C.W., SANDERS K.M. Tachykinins activate nonselective cation currents in canine colonic myocytes. Am. J. Physiol. 1995;269:C1394–C1401. doi: 10.1152/ajpcell.1995.269.6.C1394. [DOI] [PubMed] [Google Scholar]
- LOIRAND G., PACAUD P. Mechanism of the ATP-induced rise in cytosolic Ca2+ in freshly isolated smooth muscle cells from human saphenous vein. Pflügers Arch. 1995;430:429–436. doi: 10.1007/BF00373919. [DOI] [PubMed] [Google Scholar]
- LOW A.M., KOTECHA N., NEILD T.O., KWAN C.Y., DANIEL E.E. Relative contributions of extracellular Ca2+ and Ca2+ stores to smooth muscle contraction in arteries and arterioles of rat, guinea-pig, dog and rabbit. Clin. Exp. Pharmacol. Physiol. 1996;23:310–316. doi: 10.1111/j.1440-1681.1996.tb02829.x. [DOI] [PubMed] [Google Scholar]
- MASON M.J., MAYER B., HYMEL L.J. Inhibition of Ca2+ transport pathways in thymic lymphocytes by econazole, miconazole, and SKF 96365. Am. J. Physiol. 1993;264:C654–C662. doi: 10.1152/ajpcell.1993.264.3.C654. [DOI] [PubMed] [Google Scholar]
- MCDANIEL S.S., PLATOSHYN O., WANG J., YU Y., SWEENEY M., KRICK S., RUBIN L.J., YUAN J.X.-J. Capacitative Ca2+ entry in agonist-induced pulmonary vasoconstriction. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L870–L880. doi: 10.1152/ajplung.2001.280.5.L870. [DOI] [PubMed] [Google Scholar]
- MCKAY R.R., SZYMECZEK-SEAY C.L., LIEVREMONT J.-P., BIRD G.ST.J., ZITT C., JÜNGLING E., LÜCKHOFF A., PUTNEY J.W. Cloning and expression of the human transient receptor potential 4 (TRP4) gene: localization and functional expression of human TRP4 and TRP3. Biochem. J. 2000;351:735–746. [PMC free article] [PubMed] [Google Scholar]
- MERRITT J.E., ARMSTRONG W.P., BENHAM C.D., HALLAM T.J., JACOB R., JAXA-CHAMIEC A., LEIGH B.K., MCCARTHY S.E., MOORES R., RINK T.J. SKF96365, a novel inhibitor of receptor-mediated calcium entry. Biochem. J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTELL C. TRP trapped in fly signalling web. Curr. Opin. Neurobiol. 1998;8:389–397. doi: 10.1016/s0959-4388(98)80066-4. [DOI] [PubMed] [Google Scholar]
- MOORE P.K., HANDY R.L.C. Selective inhibitors of neuronal nitric oxide synthase–is no NOS really good NOS for the nervous system. Trends Pharmacol. Sci. 1997;18:204–211. doi: 10.1016/s0165-6147(97)01064-x. [DOI] [PubMed] [Google Scholar]
- MULRYAN K., GITTERMAN D.P., LEWIS C.J., VIAL C., LECKIE B.J., COBB A.L., BROWN J.E., CONLEY E.C., BUELL G., PRITCHARD C.A., EVANS R.J. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- MUNRO D.D., WENDT I.R. Effects of cyclopiazonic acid on [Ca2+]i and contraction in rat urinary bladder smooth muscle. Cell Calcium. 1994;15:369–380. doi: 10.1016/0143-4160(94)90012-4. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA T., HAZAMA H., HAMADA E., WU S.N., IGARASHI K., YAMASHITA T., SEYAMA Y., OMATA M., KURACHI Y. Endothelin-1 and vasopressin activate Ca2+-permeable non-selective cation channels in aortic smooth muscle cells: mechanism of receptor-mediated Ca2+ influx. J. Mol. Cell Cardiol. 1996;28:707–722. doi: 10.1006/jmcc.1996.0066. [DOI] [PubMed] [Google Scholar]
- NAKAJIMA T., IWASAWA K., HAZAMA H., ASANO M., OKUDA Y., OMATA M. Extracellular Mg2+ inhibits receptor-mediated Ca2+-permeable non-selective cation currents in aortic smooth muscle cells. Eur. J. Pharmacol. 1997;320:81–86. doi: 10.1016/s0014-2999(96)00873-4. [DOI] [PubMed] [Google Scholar]
- NAKAZAWA K., MATSUKI N. Adenosine triphosphate-activated inward current in isolated smooth muscle cells from rat vas deferens. Pflügers Arch. 1987;409:644–646. doi: 10.1007/BF00584668. [DOI] [PubMed] [Google Scholar]
- NOGUERA M.A., IVORRA M.D., CHULIA S., D'OCON P. Capacitative Ca2+ entry associated with α1-adrenoceptors in rat aorta. Naunyn-Schmiedeberg's Arch. Pharmacol. 1997;356:83–89. doi: 10.1007/pl00005033. [DOI] [PubMed] [Google Scholar]
- NOGUERA M.A., MADRERO Y., IVORRA M.D., D'OCON P. Characterisation of two different Ca2+ entry pathways dependent on depletion of internal Ca2+ pools in rat aorta. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;357:92–99. doi: 10.1007/pl00005156. [DOI] [PubMed] [Google Scholar]
- OHTA T., KAWAI K., ITO S., NAKAZATO Y. Ca2+ entry activated by emptying of intracellular Ca2+ stores in ileal smooth muscle of the rat. Br. J. Pharmacol. 1995;114:1165–1170. doi: 10.1111/j.1476-5381.1995.tb13329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OIKE M., KITANURA K., KURIYAMA H. Protein kinase C activates the non-selective cation channel in rabbit portal vein. Pflügers Arch. 1993;424:159–164. doi: 10.1007/BF00374607. [DOI] [PubMed] [Google Scholar]
- OKADA T., INOUE R., YAMAZAKI K., MAEDA A., KUROSAKI T., YAMAKUNI T., TANAKA I., SHIMIZU S., IKENAKA K., IMOTO K., MORI Y. Molecular and functional characterization of a novel mouse transient receptor potential protein homologue TRP7. J. Biol. Chem. 1999;274:27359–27370. doi: 10.1074/jbc.274.39.27359. [DOI] [PubMed] [Google Scholar]
- OONUMA H., NAKAJIMA T., NAGATA T., IWASAWA K., WANG Y., HAZAMA H., MORITA Y., YAMAMOTO K., NAGAI R., OMATA M. Endothelin-1 is a potent activator of nonselective cation currents in human bronchial smooth muscle cells. Am. J. Respir. Cell. Mol. Biol. 2000;23:213–221. doi: 10.1165/ajrcmb.23.2.3868. [DOI] [PubMed] [Google Scholar]
- PACAUD P., BOLTON T.B. Relation between muscarinic receptor cationic current and internal calcium in guinea-pig jejunal smooth muscle cells. J. Physiol. 1991;441:477–499. doi: 10.1113/jphysiol.1991.sp018763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAREKH A.B., PENNER R. Store depletion and calcium influx. Physiol. Rev. 1997;77:901–930. doi: 10.1152/physrev.1997.77.4.901. [DOI] [PubMed] [Google Scholar]
- PETKOV G.V., BOEV K.K. The role of sarcoplasmic reticulum and sarcoplasmic reticulum Ca2+-ATPase in the smooth muscle tone of the cat gastric fundus. Pflügers Arch. 1996a;431:928–935. doi: 10.1007/s004240050087. [DOI] [PubMed] [Google Scholar]
- PETKOV G.V., BOEV K.K. Cyclopiazonic acid-induced changes in contractile activity of smooth muscle strips isolated from cat and guinea-pig stomach. Eur. J. Pharmacol. 1996b;318:109–115. doi: 10.1016/s0014-2999(96)00764-9. [DOI] [PubMed] [Google Scholar]
- PHILIPP S., HAMBRECHT J., BRASLAVSKI L., SCHROTH G., FREICHEL M., MARAKAMI M., CAVALIÉ A., FLOCKERZI V. A novel capacitative calcium entry channel expressed in excitable cells. EMBO J. 1998;17:4274–4282. doi: 10.1093/emboj/17.15.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILLIPS A.M., BULL A., KELLY L.E. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W. Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W., BROAD L.M., BRAUN F.-J., LIEVREMONT J.-P., BIRD G.S. Mechanisms of capacitative calcium entry. J. Cell Sci. 2001;114:2223–2229. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W., MCKAY R.R. Capacitative calcium entry channels. BioEssays. 1999;21:38–46. doi: 10.1002/(SICI)1521-1878(199901)21:1<38::AID-BIES5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- SCHAEFER M., PLANT T.D., OBUKHOV A.G., HOFMANN T., GUNDERMANN T., SCHULTZ G. Receptor-mediated regulation of the nonselective cation channels TRPC4 and TRPC5. J. Biol. Chem. 2000;275:17517–17526. doi: 10.1074/jbc.275.23.17517. [DOI] [PubMed] [Google Scholar]
- SEKIGUCHI F., SHIMAMURA K., AKASHI M., SUNANO S. Effects of cyclopiazonic acid and thapsigargin on electromechanical activities and intracellular Ca2+ in smooth muscle of carotid artery of hypertensive rats. Br. J. Pharmacol. 1996;118:857–864. doi: 10.1111/j.1476-5381.1996.tb15478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIMS S.M. Cholinergic activation of a non-selective cation current in canine gastric smooth muscle is associated with contraction. J. Physiol. 1992;449:377–398. doi: 10.1113/jphysiol.1992.sp019091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SINKINS W.G., VACA L., HU Y., KUNZE D.I., SCHILLINGS W.P. The COOH-terminal domain of Drosophila TRP channels confers thapsigargin sensitivity. J. Biol. Chem. 1996;271:2955–2960. doi: 10.1074/jbc.271.6.2955. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Electromechanical and pharmacomechanical coupling in vascular smooth muscle. J. Pharmacol. Exp. Ther. 1968;159:129–145. [PubMed] [Google Scholar]
- STRÜBING C., KRAPIVINSKY G., KRAPIVINSKY L., CLAPHAM D.E. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- SUZUKI H. Electrical responses to smooth muscle cells of the rabbit ear artery to adenosine triphosphate. J. Physiol. 1985;359:401–415. doi: 10.1113/jphysiol.1985.sp015592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEMOTO M., TAKAGI K., OGINO K., TOMITA T. Comparison of contractions produced by carbachol, thapsigargin and cyclopiazonic acid in the guinea-pig tracheal muscle. Br. J. Pharmacol. 1998;124:1449–1454. doi: 10.1038/sj.bjp.0701993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEPEL M., RUESS C., MEHRING N., NEUSSER M., ZIDEK W. Effect of inhibition of sarcoplasmic Ca2+-ATPase on vasoconstriction and cytosolic Ca2+ in aortic smooth muscle from spontaneously hypertensive and normotensive rats. Clin. Exp. Hypertens. 1994;16:493–506. doi: 10.3109/10641969409067958. [DOI] [PubMed] [Google Scholar]
- TOSUN M., PAUL R.J., RAPOPORT R.M. Coupling of store-operated Ca2+ entry to contraction in rat aorta. J. Pharmacol. Exp. Therap. 1998;285:759–766. [PubMed] [Google Scholar]
- TRAPANI A., MATSUKI N., ABEL P.W., HERMSMEYER K. Norepinephrine produces tension through electromechanical coupling in rabbit ear artery. Eur. J. Pharmacol. 1981;72:87–91. doi: 10.1016/0014-2999(81)90301-0. [DOI] [PubMed] [Google Scholar]
- TREPAKOVA E.S., CSUTORA P., HUNTON D.L., MARCHASE R.B., COHEN R.A., BOLOTINA V.M. Calcium influx factor directly activates store-operated cation channels in vascular smooth muscle cells. J. Biol. Chem. 2000;275:26158–26163. doi: 10.1074/jbc.M004666200. [DOI] [PubMed] [Google Scholar]
- TREPAKOVA E.S., GERICKE M., HIRAKAWA Y., WEISBROD R.M., COHEN R.A., BOLOTINA W.M. Properties of native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells. J. Biol. Chem. 2001;276:7782–7790. doi: 10.1074/jbc.M010104200. [DOI] [PubMed] [Google Scholar]
- UTZ J., ECKERT R., TRAUTWEIN W. Changes of intracellular calcium concentrations by phenylephrine in renal arterial smooth muscle cells. Pflügers Arch. 1999;438:725–731. doi: 10.1007/s004249900091. [DOI] [PubMed] [Google Scholar]
- VAN BREEMEN C., CHEN Q., LAHER I. Superficial buffer barrier function of smooth muscle sarcoplasmic reticulum. Trends Pharmacol. Sci. 1995;16:98–105. doi: 10.1016/s0165-6147(00)88990-7. [DOI] [PubMed] [Google Scholar]
- VAN BREEMEN C., AARONSON P., LOUTZENHIZER R. Sodium-calcium interactions in mammalian smooth muscle. Pharmacol Rev. 1979;30:167–208. [PubMed] [Google Scholar]
- VANNIER B., PEYTON M., BOULAY G., BROWN D., QIN N., JIANG M., ZHU X., BIRNBAUMER L. Mouse trp2, the homologue of the human trpc2 pseudogene encodes mTrp2, a store-depletion-activated capacitative Ca2+ entry channel. Proc. Natl. Acad. Sci. 1999;96:2060–2064. doi: 10.1073/pnas.96.5.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGALIS F., SANDERS K.M. Cholinergic stimulation activates a non-selective cation current in canine pyloric circular muscle cells. J. Phyiol. 1990;429:223–236. doi: 10.1113/jphysiol.1990.sp018253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER R.L., HUME J.R., HOROWITZ B. Differential expression and alternative splicing of TRP channel genes in smooth muscles. Am. J. Physiol. Cell Physiol. 2001;280:C1184–C1192. doi: 10.1152/ajpcell.2001.280.5.C1184. [DOI] [PubMed] [Google Scholar]
- WALLACE P., AYMAN S., MCFADZEAN I., GIBSON A. Thapsigargin-induced tone and capacitative calcium influx in mouse anococcygeus smooth muscle cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:368–375. doi: 10.1007/s002109900100. [DOI] [PubMed] [Google Scholar]
- WANG Q., LARGE W.A. Noradrenaline-evoked cation conductance recorded with the nystatin whole-cell method in rabbit portal vein cells. J. Physiol. 1991;435:21–39. doi: 10.1113/jphysiol.1991.sp018496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y., CHEN J., WANG Y., TAYLOR C.W., HIRATA Y., HAGIWARA H., MIKOSHIBA K., TOYO-OKA T., OMATA M., SAKAKI Y. Crucial role of type 1, but not type 3, inositol 1,4,5-trisphosphate (IP3) receptors in IP3-induced Ca2+ release, capacitative Ca2+ entry, and proliferation of A7r5 vascular smooth muscle cells. Circ. Res. 2001;88:202–209. doi: 10.1161/01.res.88.2.202. [DOI] [PubMed] [Google Scholar]
- WANG Y.-X., DHULIPALA P.D.K., LI L., BENOVIC J.L., KOTLIKOFF M.I. Coupling of M2 muscarinic receptors to membrane ion channels via a phosphoinositide 3-kinase γ and atypical protein kinase C. J. Biol. Chem. 1999;274:13859–13864. doi: 10.1074/jbc.274.20.13859. [DOI] [PubMed] [Google Scholar]
- WANG Y.-X., KOTLIKOFF M.I. Signalling pathway for histamine activation of non-selective cation channels in equine tracheal myocytes. J. Physiol. 2000;523:131–138. doi: 10.1111/j.1469-7793.2000.t01-3-00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAYMAN C., MCFADZEAN I., GIBSON A., TUCKER J.F. Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in smooth muscle cells of the mouse anococcygeus. Br. J. Pharmacol. 1996;117:566–572. doi: 10.1111/j.1476-5381.1996.tb15228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAYMAN C.P., WALLACE P., GIBSON A., MCFADZEAN I. Correlation between store-operated cation current and capacitative calcium influx in smooth muscle cells from mouse anococcygeus. Eur. J. Pharmacol. 1999;376:325–329. doi: 10.1016/s0014-2999(99)00400-8. [DOI] [PubMed] [Google Scholar]
- WARNAT J., PHILIPP S., ZIMMER S., FLOCKERZI V., CAVALIÉ A. Phenotype of a recombinant store-operated channel: highly selective permeation of Ca2+ J. Physiol. 1999;518:631–638. doi: 10.1111/j.1469-7793.1999.0631p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIONG Z., KITAMURA K., KURIYAMA H. ATP activates cationic currents and modulates the calcium current through GTP-binding proteins in rabbit portal vein. J. Physiol. 1991;440:143–165. doi: 10.1113/jphysiol.1991.sp018701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU S.-Z., BEECH D.J. TrpC1 is a membrane-spanning subunit of store-operated Ca2+ channels in native vascular smooth muscle cells. Circ. Res. 2001;88:84–87. doi: 10.1161/01.res.88.1.84. [DOI] [PubMed] [Google Scholar]
- XUAN Y.-T., WANG O.-L., WHORTON R. Thapsigargin stimulates Ca2+ entry in vascular smooth muscle cells: nicardipine-sensitive and -insensitive pathways. Am. J. Physiol. Cell Physiol. 1992;262:C1258–C1265. doi: 10.1152/ajpcell.1992.262.5.C1258. [DOI] [PubMed] [Google Scholar]
- YUE L., PENG J.-B., HEDIGER M.A., CLAPHAM D.E. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature. 2001;410:705–709. doi: 10.1038/35070596. [DOI] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. G-protein control of voltage dependence as well as gating of muscarinic metabotropic channels in guinea-pig ileum. J. Physiol. 1994;478:195–202. doi: 10.1113/jphysiol.1994.sp020242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Effects of divalent cations on muscarinic receptor cationic current in smooth muscle from guinea-pig small intestine. J. Physiol. 1995;486:67–82. doi: 10.1113/jphysiol.1995.sp020791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. Br. J. Pharmacol. 1997;122:885–893. doi: 10.1038/sj.bjp.0701438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., TSYTSYURA Y.D., PHILYPPOV I.B., SHUBA M.F., BOLTON T.B. Voltage-dependent inhibition of the muscarinic cationic current in guinea-pig ileal cells by SK&F 96365. Br. J. Pharmacol. 2000;129:695–702. doi: 10.1038/sj.bjp.0703115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHU X., CHU P.B., PEYTON M., BIRNBAUMER L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193–198. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]
- ZHU X., JIANG M., PEYTON M., BOULAY G., HURST R., STEFANI E., BIRNBAUMER L. trp, a novel mammalian gene family essential for agonist-activated capacitative Ca2+ entry. Cell. 1996;85:661–671. doi: 10.1016/s0092-8674(00)81233-7. [DOI] [PubMed] [Google Scholar]
- ZITT C., ZOBEL A., OBUKHOV A.G., HARTENECK C., KALKBRENNER F., LÜCKHOFF A., SCHULTZ G. Cloning and functional expression of a human Ca2+-permeable cation channel activated by calcium store depletion. Neuron. 1996;16:1189–1196. doi: 10.1016/s0896-6273(00)80145-2. [DOI] [PubMed] [Google Scholar]
- ZWEIFACH A., LEWIS R.S. Rapid inactivation of depletion-activated calcium current (ICRAC) due to local calcium feedback. J. Gen. Physiol. 1995;105:209–226. doi: 10.1085/jgp.105.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]


