Abstract
β-adrenoceptors mediate relaxation of bladder detrusor smooth muscle. This study investigates the contribution of β3-adrenoceptors to relaxation of the pig urinary bladder.
Cell membranes were prepared from detrusor muscle of the pig bladder dome and competition experiments with [3H]-dihydroalprenolol (DHA), a non-selective β-adrenoceptor antagonist was used as a specific radioligand to determine the presence of β-adrenoceptor subtypes. In functional experiments, isolated detrusor muscle strips were used to determine the potency of agonists and the affinity of antagonists.
In competition binding experiments, CGP20712A (β1-adrenoceptor selective) displaced [3H]-DHA from a single binding site with a low affinity. In contrast, displacement data for ICI 118551 (β2-adrenoceptor antagonist) and SR59230A (β3-adrenoceptor antagonist) best fitted a two-site model suggesting a predominant (70%) population of β3-adrenoceptors.
In functional studies, isoprenaline and salbutamol (β2-adrenoceptor agonist) relaxed KCl precontracted muscle strips with high potency (pEC50 7.7 and 7.2, respectively), whilst CGP12177 and BRL37344 (β3-adrenoceptor agonists) had low potency and were partial agonists. CGP20712A and atenolol (β1-adrenoceptor antagonists) antagonised responses with a low affinity. ICI118551 antagonized responses to isoprenaline and salbutamol with a high affinity (pKB=7.8 and 8.7, respectively), but the Schild slopes were low suggesting that responses were mediated by more than one β-adrenoceptor. The Schild plot for SR59230A was biphasic, apparent pKB values for 3 – 10 nM SR59230A being 8.6 and those for 30 nM – 1 μM being 7.7.
These data suggest that β3-adrenoceptors are the predominant β-adrenoceptor subtype present in the pig bladder and that β-adrenoceptor mediated responses of this tissue are mediated via both the β2- and β3-adrenoceptor subtypes.
Keywords: β-adrenoceptors, urinary bladder, [3H]-DHA, SR59230A, CGP20712A, detrusor relaxation
Introduction
For the pharmacological treatment of frequency, urgency and/or urge incontinence due to bladder overactivity, anticholinergics have been the treatment of choice. However, these drugs can exert severe side effects (accommodation paralysis, constipation, tachycardia, dryness of mouth and blurred vision) and some patients are refractory to their actions. Thus there is an urgent need for new drug therapies with novel mechanisms of action.
β-Adrenoceptors are found in the body of the bladder, where they mediate relaxation of detrusor muscle (Andersson, 1993; Morita et al., 1990; Li et al., 1992). The β-adrenoceptor subtype mediating relaxation appears to be species dependent, the predominant receptor being the β1-adrenoceptor in the cat and guinea-pig (Nergårdh et al., 1977; Li et al., 1992) and the β2-adrenoceptor in the rat, rabbit and human (Elmér, 1974; Levin et al., 1988; Morita et al., 1993; Oshita et al., 1997). However, Nergårdh et al. (1977) and Larsen (1979) have reported that relaxation of the human bladder is blocked by propranolol but not by practolol (β1-adrenoceptor antagonist) nor butoxamine (β2-adrenoceptor antagonist), suggesting the presence and functional characteristics of non β1- non β2-adrenoceptors. Recently, relaxation of the rat bladder smooth muscle has been reported to be mediated via β1, β2 and β3-adrenoceptors (Longhurst & Levendusky, 1999; Oshita et al., 1997). Yamazaki et al. (1998) have also reported that relaxation of the bladder smooth muscle is mediated mainly via β1-adrenoceptors in rabbits, both β1- and β3-adrenoceptors in rats, and β3-adrenoceptors in dogs. Thus, which β-adrenoceptor subtypes are present in the bladder and act to mediate relaxation remains controversial.
Pigs have been reported to be similar to humans with regard to urogenital adrenoceptor and muscarinic receptor expression (Goepel et al., 1997; Sellers et al., 2000; Yamanishi et al., 2000) and may be a useful large animal model to study the physiology and pathophysiology of the bladder (Mills et al., 2000). This study uses isolated muscle strips to identify the β-adrenoceptor subtype mediating relaxation of the pig urinary bladder.
Methods
Radioligand binding studies
Female pig urinary bladder was collected from the abattoir and immediately placed into 50 mM ice-cold Tris buffer (pH 7.4, 4°C). Strips of tissue were cut from the bladder dome, the mucosa and serosa removed and the tissues minced into small pieces using a razor blade. A crude membrane fraction was then homogenized with an Ultra-Turrax homogenizer, filtered through muslin, and then further homogenized with a Teflon homogenizer. Homogenates were centrifuged twice at 45,000×g for 10 min at 4°C and the subsequent pellet re-suspended in 5 ml of Tris buffer. Membranes were used immediately in radioligand assays performed in duplicate.
Saturation experiments were conducted using seven concentrations (0.25 to 16 nM) of [3H]-dihydroalprenolol (DHA), a non-selective β-adrenoceptor antagonist used as a specific radioligand. Samples (100 μl) of the membrane preparation were incubated at 37°C for 30 min with a range of radioligand concentrations to obtain a saturation curve. Non-specific binding represented [3H]-DHA bound in the presence of 10 μM of unlabelled propranolol. Incubations are terminated by addition of ice-cold Tris buffer followed by rapid filtration using a Brandel Cell Harvester. After washing of the filters to remove unbound radioligand, the radioactivity left was measured in a liquid scintillation analyser.
Competition binding experiments with [3H]-DHA (1.3 nM) used different concentrations of unlabelled 0.1 nM – 2 mM CGP20712A (β1-adrenoceptor selective antagonist), 0.1 nM – 1 μM ICI 118551 (β2-adrenoceptor selective antagonist) and 0.1 nM – 1 μM SR59230A (β3-adrenoceptor selective antagonist) to determine the ratio of β-adrenoceptor subtypes present in this tissue.
In vitro functional studies
Fresh pig urinary bladders from female pigs were immediately placed in cold (4°C) Krebs-bicarbonate solution (composition in mM: NaCl 118.4, KCl 4.7, CaCl2 1.9, NaHCO3 24.9, MgSO4 1.15, KH2PO4 1.15, glucose 11.7). Strips of tissue (10×3 mm) were cut from the bladder dome and the mucosa and serosa removed. The tissues were mounted in 30 ml organ baths containing Krebs solution which was maintained at 37°C and continuously gassed with 95% O2 and 5% CO2. The tissues were subjected to a resting tension of 1 g and allowed to equilibrate for 60 min, during which time they were washed every 10 min and the resting tension was adjusted. Isometric tension generated by the tissue was recorded via UF1 force transducers to a PC via a Cambridge Electronic Design (CED) interface using CHART software (CED).
After equibration the detrusor strips were precontracted with 50 mM potassium chloride. When the contraction had stabilized, increasing concentrations of β-adrenoceptor agonists were added cumulatively in 0.5 log unit increments and concentration-relaxation curves (CRCs) obtained to noradrenaline, isoprenaline (non-selective β-adrenoceptor agonists), salbutamol (selective β2-adrenoceptor agonist) and the β3-adrenoceptor selective agonists (BRL37344 and CGP12177). Experiments were performed in the presence of cocaine (10 μM), corticosterone (10 μM) and phentolamine (10 μM) to inhibit amine uptake and antagonize α-adrenoceptors respectively.
Following the first CRCs to isoprenaline, tissues were washed for about 30 min until a steady resting level of tension was attained. Tissues were then equilibrated for 30 min with Krebs solution containing the appropriate concentration of β-antagonist (propranolol, atenolol, ICI 118551 or SR59230A) or vehicle (time control), before construction of a second CRC to isoprenaline. In the control experiments, the two CRCs to isoprenaline and salbutamol were reproducible, but the third CRC varied probably because of tachyphylaxis. Thus two CRCs on each tissue were used to calculate affinity (pKB) values for each β-adrenoceptor antagonist.
Data analysis
In functional studies, the relaxation by β-adrenoceptor agonists was expressed as a percentage of maximum relaxation induced by 30 μM isoprenaline. Agonist potencies were expressed as mean pEC50±s.e.mean (−logarithm of the molar concentration of agonist resulting in 50% of the maximum response).
Antagonist dissociation constants (KB) were determined from the following equation: KB=antagonist concentration (molar)/(concentration ratio−1). pA2 values for the β-adrenoceptor antagonists were determined by Schild regression and taken as the x-intercept on the Schild plot (Arunlakshana & Schild, 1959). In radioligand binding studies the density of receptors (BMAX) and dissociation constants (KD) were calculated by Scatchard analysis of saturation curves and linear regression analysis using PRISM software (Graphpad). In displacement binding experiments the same software was used to determine Ki (inhibitory constant) values and analyse the displacement data for one- or two-site binding. Protein content of the membrane fractions was determined by the method of Lowry et al. (1951). Student's t-test was used to determine the statistical analysis difference between the two mean values. P values<0.05 were considered to be statistically significant (‘n' value refer to the number of animals).
Drugs and chemicals
[3H]-dihydroalprenolol (DHA) (specific activity 111.8 Ci mmol−1) was purchased from NEN Life Science Products, Inc. (Boston, MA, U.S.A.), and was stored at −20°C. Noradrenaline, (±)-Isoprenaline HCl, (±)-propranolol HCl and atenolol were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.) Salbutamol sulphate, BRL37344 and CGP12177 were purchased from Tocris Cookson Ltd. (Bristol, U.K.) SR59230A, 3-(2-ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2S)-2- propanol oxalate was synthesized in our laboratory (Dokkyo University).
Results
Radioligand binding studies
In saturation binding experiments, binding of [3H]-DHA was saturable and displaceable by propranolol. Analysis of [3H]-DHA saturation curves (n=8) demonstrated a single population of binding sites with a mean dissociation constant (KD) of 1.40±0.18 nM and a density of 154.4±46.2 fmol mg−1 protein.
Competition experiments with CGP20712A (n=4) best fitted displacement from a single binding site with a low affinity (mean pKi value of 5.02±0.23), suggesting that β1-adrenoceptors were not present. In contrast, competition curves for ICI 118551 were shallow (Hill slope of 0.56±0.09) and in six out of eight experiments the data fitted a two-site model of binding better than a single-site model. Similarly for SR59230A, competition curves were shallow (Hill slope of 0.39±0.14) and data best fitted a two-site model of binding in five of the eight experiments. The percentage of sites having a high affinity for ICI118551 was 21% and the value for SR58230A was 69% (Table 1).
Table 1.
Competition data for [3H]-DHA binding to pig detrusor membranes
Functional studies in vitro
Agonist potencies
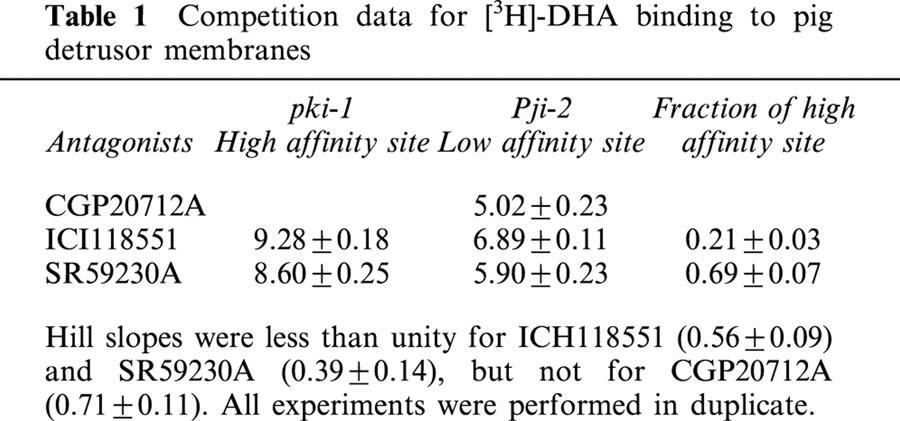
Isoprenaline (non-selective β-adrenoceptor agonist) induced relaxation with high potency with a mean pEC50 values of 7.7±0.02 (n=24). Noradrenaline (non-selective α- and β-adrenoceptor agonist) in the presence of phentolamine, an α-adrenoceptor antagonist, had a lower potency with a mean pEC50 value of 5.8±0.05, but with a mean maximum relaxation of 98% of that obtained to 30 μM isoprenaline (n=22). Salbutamol (β2-adrenoceptor agonist) also induced relaxation with a high potency (pEC50 value of 7.2±0.03) and with a mean maximum relaxation of 86±1.9% of that obtained to 30 μM isoprenaline (n=13). However the β3-adrenoceptor agonists CGP12177 (n=4) and BRL37344 (n=7) had a lower potency (pEC50=4.0±0.12 and 6.9±0.24, respectively), and both were partial agonists with maximum relaxation responses of 40±3.3 and 59±2.3% of that to 30 μM isoprenaline, respectively (Figure 1).
Figure 1.
Mean cumulative concentration-relaxation curves to β-adrenoceptor agonists (isoprenaline, n=24; salbutamol, n=13; noradrenaline, n=22; BRL 37344, n=7; CGP 12177, n=4) and time control (n=4). Responses are plotted as a percentage of the maximum response to 30 μM isoprenaline. The mean relaxant responses to 30 μM isoprenaline were 75% (range 60 – 100%) of responses to 50 mM KCl.
Antagonist affinities
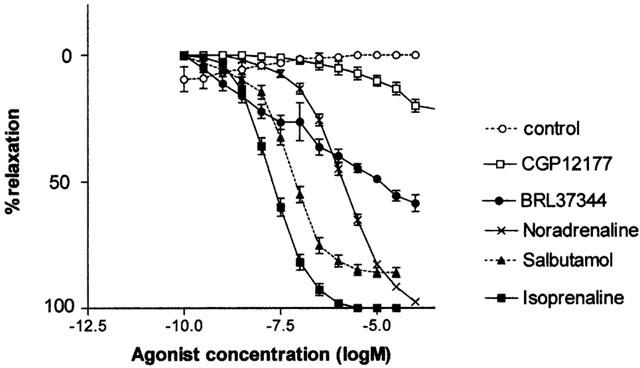
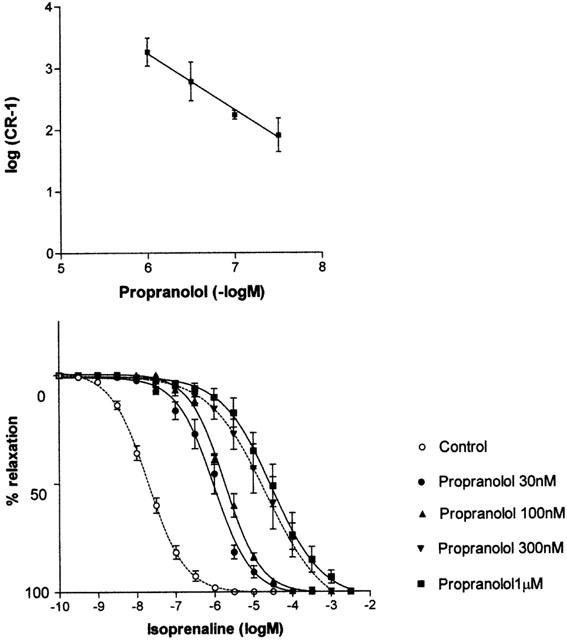
Propranolol (30 nM – 1 μM) antagonized CRCs to isoprenaline in a competitive manner (pKB=9.3±0.12) without affecting maximum responses and the Schild plot having a slope close to unity (0.92±0.06) (Figure 2, Table 2). The β1-adrenoceptor selective antagonist atenolol (0.3 – 3 μM) antagonized responses to isoprenaline with a low affinity (pKB=6.1±0.08) and without affecting maximum responses. The resulting Schild plot had a slope not significantly different from unity (0.83±0.12). Another β1-adrenoceptor selective antagonist CGP20712A failed to antagonise these responses below 30 μM (Figure 3).
Figure 2.
(Lower panel) Effect of propranolol (30 nM to 1 μM) on concentration-response curves to isoprenaline in pig detrusor muscle. (Upper panel) Schild plot for the antagonism of responses to isoprenaline by propranolol. The slope of the plot is 0.92±0.06, and the X-intercept (PA2) is 9.5.
Table 2.
In vitro antagonist affinity estimates using isoperenaline, salbutamol or noradrenaline as the agonist
Figure 3.
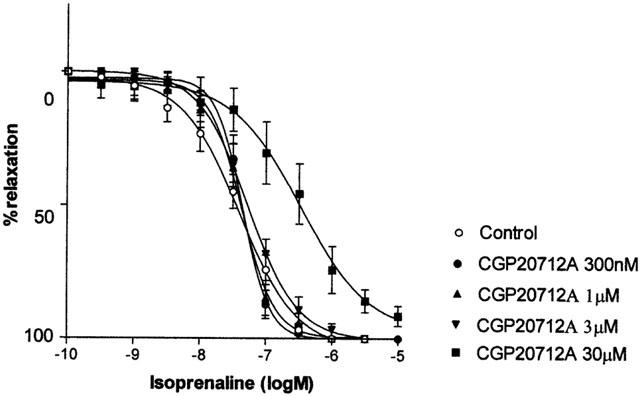
Effects of CGP 20712A (300 nM – 30 μM) on concentration-response curves to isoprenaline in pig bladder.
The β2-antagonist ICI118551 (30 nM – 1 μM) antagonized responses to isoprenaline with a relatively high affinity (apparent pKB=7.8±0.09) and without affecting maximum responses, but the Schild slope was significantly (P<0.05) less than unity (0.54±0.12) suggesting that responses were mediated by more than one receptor (Figure 4). ICI 118551 had the same effects on concentration-relaxation curves to isoprenaline (apparent pKB=8.4±0.21 and Schild slope=0.62±0.03) in the presence of the β1-adrenoceptor antagonist CGP20712A (1 μM). ICI118551 (3 – 30 nM) also antagonized responses to salbutamol with high affinity (apparent pKB=8.7±0.08), but the Schild slopes were again low (0.77±0.20).
Figure 4.
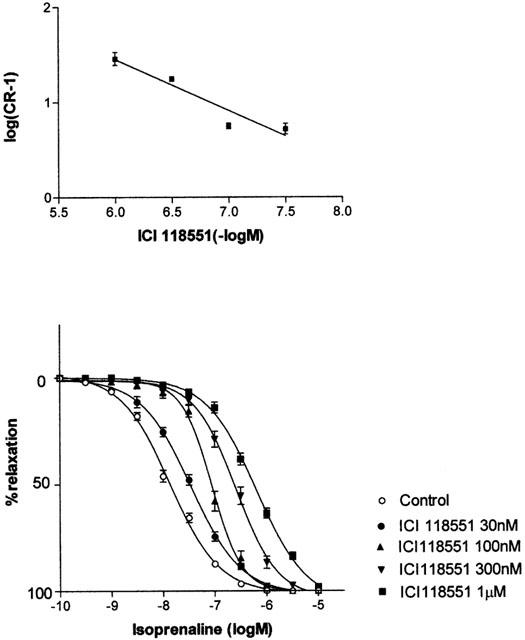
(Lower panel) Effects of ICI 118551(30 nM – 1 μM) on concentration-response curves to isoprenaline in pig bladder. (Upper panel) Schild plot for the antagonism of responses to isoprenaline by ICI 118551. The slope of the plot is 0.54±0.12.
The selective β3-antagonist SR59230A at low concentrations (3 – 10 nM) antagonized responses in a competitive manner (Schild slope 0.93±0.03) and with a high affinity (pKB=8.6±0.06). At higher concentrations (30 nM – 1 μM) SR59230A antagonized responses with an apparently lower affinity (apparent pKB=7.7±0.10) and the Schild plot had a low slope (0.36±0.04) (Figure 5). SR59230A had similar effects on CRCs to noradrenaline: SR59230A at low concentrations (3 – 30 nM) antagonized responses with a high affinity (pKB=8.61±0.11) and with a Schild slope of 1.24±0.04, but at higher concentrations (30 – 300 nM) it antagonized responses with an apparently lower affinity (apparent pKB=8.09±0.19) and the Schild plot had a low slope (0.28±0.03).
Figure 5.
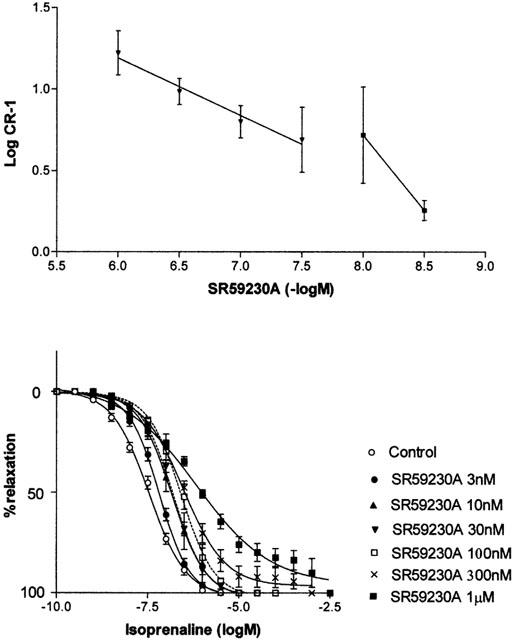
(Lower panel) Effects of SR59230A (3 nM – 1 μM) on concentration-response curves to isoprenaline in pig bladder. (Upper panel) Schild plot for the antagonism of responses to isoprenaline by SR59230A.
Discussion
Levin et al. (1988) have demonstrated a predominance of β2-adrenoceptors using radioligand binding studies in human detrusor smooth muscle, and Goepel et al. (1997) have reported that β2-adrenoceptors predominate in the pig and human detrusor. In our present radioligand binding studies, displacement of [3H]-DHA with CGP20712A had a very low affinity (pKi=5.0), indicating that β1-adrenoceptors were not present in pig bladder. Displacement with ICI 118551 and SR59230A demonstrated that the fraction of high affinity site (β2-, and β3-adrenoceptors, respectively) was 0.21 and 0.69, respectively. Thus our results suggested that about 70% of the β-adrenoceptor subtypes appeared to be β3-adrenoceptors.
In preliminary functional experiments in vitro, both potassium chloride and carbachol were examined as precontracting agents. In these experiments, the tension developed to 50 mM potassium chloride was more stable and was maintained for longer than that following contraction with carbachol. Thus subsequent experiments were performed using 50 mM potassium chloride to precontract tissues. In our functional studies, isoprenaline and the β2-adrenoceptor selective agonist salbutamol demonstrated relaxation with high potency (pEC50 7.7 and 7.2, respectively). However CGP12177, which is a partial β3-adrenoceptor agonist and an antagonist at β1- and β2-adrenoceptors, and also BRL37344, a selective β3-adrenoceptor agonist (Kaumann & Molenaar, 1996) exhibited low potency on the bladder. The maximum relaxation effects of these agonists were 40 – 60% of those to isoprenaline, being comparable to those for the human bladder reported by Takeda et al. (1999). In our present study, β1-adrenoceptors did not appear to be involved in mediating responses because the β1-adrenoceptor antagonists, atenolol and CGP20712A antagonized isoprenaline-relaxation responses with a low affinity. The present study in pig detrusor smooth muscle therefore suggests an involvement of β2-adrenoceptors and an additional non-β1, non-β2 adrenoceptor, because ICI118551, a selective β2-adrenoceptor antagonist (Kaumann & Molenaar, 1996) antagonized responses to isoprenaline with a high affinity (pKB=7.8), but the Schild slope was significantly less than unity. ICI118551 had the same effects on concentration-relaxation curves to isoprenaline in the presence of the β1-receptor antagonist CGP20712A (1 μM). The non-β1, non-β2 adrenoceptor would appear to be the β3-adrenoceptor subtype, since SR59230A antagonized responses at low concentrations yielding a high affinity estimate (pKB=8.6) comparable with values obtained for this antagonist at rat colonic β3-adrenoceptors (8.7, Manara et al., 1996). Furthermore, the Schild plot for SR59230A over these low concentrations had a slope of unity. At higher concentrations (>30 nM) SR59230A antagonized responses less than that expected for an action of isoprenaline at one receptor and the Schild slope for antagonism with SR59230A at concentrations above 30 nM was significantly less than unity, again suggesting the involvement of more than one receptor. It is interesting to note that SR59230A did not appear to have a uniform effect on the concentration-response curves to isoprenaline, but produced a greater antagonism of responses to higher isoprenaline concentrations than responses to low concentrations of this agonist (see Figure 5). This again would suggest the involvement of both β2- and β3-adrenoceptors in mediating responses.
Although mRNA encoding for the β3-adrenoceptors as well as β1- and β2-adrenoceptors has been found in the bladders of several species including human (Takeda et al., 1999; Fujimura et al., 1999; Igawa et al., 1999), the functional role of β3-adrenoceptors in the bladder still remains unclear. Igawa et al. (1999; 2001) have reported that neither dobutamine (β1- and β2-adrenoceptor agonist) nor procaterol (β2-adrenoceptor agonist) at concentrations up to 10 μM produce any significant relaxation of human bladder and that BRL 37344 and CGP 12177A (β3-adrenoceptor agonists) only induced significant relaxation at concentrations greater than 1 μM, the mean pD2 (pEC50) values for these agonists being 6.4 and 5.7, respectively. The pEC50 value of BRL 37344 we obtained in pig bladder (6.9) is similar to that of Igawa et al. (1999), but the value for CGP 12177A (4.0) was substantially lower. These authors have also reported that, in the presence of CGP 20712A (100 nM) and ICI 118551 (100 nM), SR58894A (β3-antagonist) antagonised isoprenaline concentration-relaxation curves in a concentration-dependent manner, but with a low affinity (pA2 value of 6.2) and produced a Schild plot with a low slope (0.68) (Igawa et al., 1999). The pA2 value of 6.2 obtained by these authors for SR58894A is close to that obtained on the guinea-pig trachea (β2-receptors) and far less than that obtained at the β3-receptors of the rat proximal colon (pA2 value of 8.1) (Manara et al., 1996). Takeda et al. (1999) have reported that BRL37344 and CGP12177A exhibited relaxant effects in human detrusor smooth muscle with pD2 values of 6.3 and 5.9, respectively, and intrinsic activity values relative to isoprenaline of 0.48 and 0.50, respectively. However they have reported that ZD-7144, another putative β3-agonist elicited only a slight effect (intrinsic activity value relative to isoprenaline being 0.09). Therefore it is still unclear whether β3-adrenoceptors have a major role in β-adrenoceptor mediated relaxation of the human bladder.
In our studies, propranolol antagonised concentration-relaxation curves to isoprenaline in a competitive manner with high affinity (pKB=9.31). Propranolol has been reported to be highly selective for β1-, and β2-adrenoceptors over the β3-subtype (Seguchi et al., 1998), and it is surprising that the Schild plot had a slope close to unity. However, Nergårdh et al. (1977) and Larsen (1979) have suggested the presence of non β1-, non β2-adrenoceptors in the human detrusor, reporting that isoprenaline-induced relaxation was antagonized by propranolol but not by practolol (β1-antagonist) or butoxamine (β2-antagonist). These results may show similarity between pig and human bladder, with both species apparently possessing a non β1-, non-β2-adrenoceptor which has a high affinity for propranolol.
The possibility of heterogeneity of β3-adrenoceptors has also been suggested (Longhurst & Levendusky, 1999; Blin et al., 1994). Longhurst & Levendusky (1999) have reported that BRL 37344 relaxed rat urinary bladder with high potency, but SR 58611A, another β3-adrenoceptor agonist was less potent and they speculated the existence of atypical β-adrenoceptors in rat bladder, in addition to the β3-subtype.
In conclusion, our data suggest that both β2- and β3-adrenoceptors are present in the pig urinary bladder with the β3-adrenoceptor subtype predominating (70%). Our data in vitro suggest that β-adrenoceptor mediated responses of the pig bladder are mediated via both β2- and β3-adrenoceptors.
Abbreviations
- [3H]-DHA
[3H]-dihydroalprenolol
- CRC
concentration-relaxation curve
- SR59230A
3-(2-ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronaphth-1-ylamino]-(2S)-2-propanol oxalate
References
- ANDERSSON K.E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol. Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIN N., NAHMIAS C., DRUMARE M.F., STROSBERG A.D. Mediation of most atypical effects by species homologues of the β3-adrenoceptor. Br. J. Pharmacol. 1994;112:911–919. doi: 10.1111/j.1476-5381.1994.tb13167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELMÉR M. Inhibitory β-adrenoceptors in the urinary bladder of the rat. Life Sci. 1974;15:273–280. doi: 10.1016/0024-3205(74)90217-3. [DOI] [PubMed] [Google Scholar]
- FUJIMURA T., TAMURA K., TSUTSUMI T., YAMAMOTO T., NAKAMURA K., KOIBUCHI Y., KOBAYASHI M., YAMAGUCHI O. Expression and possible functional role of the β3-adrenoceptor in human and rat detrusor muscle. J. Urol. 1999;161:680–685. [PubMed] [Google Scholar]
- GOEPEL M., WITTMANN A., RÜBBEN H., MICHEL M.C. Comparison of adrenoceptor subtype expression in porcine and human bladder and prostate. Urol. Res. 1997;25:199–206. doi: 10.1007/BF00941983. [DOI] [PubMed] [Google Scholar]
- IGAWA Y., YAMAZAKI Y., TAKEDA H., HAYAKAWA K., AKAHANE M., AJISAWA Y., YONEYAMA T., NISHIZAWA O., ANDERSSON K.-E. Functional and molecular biological evidence for a possible β3-adrenoceptor in the human detrusor muscle. Br. J. Pharmacol. 1999;126:819–825. doi: 10.1038/sj.bjp.0702358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGAWA Y., YAMAZAKI Y., TAKEDA H., KAIDOH K., AKAHANE M., AJISAWA Y., YONEYAMA T., NISHIZAWA O., ANDERSSON K.-E. Relaxation effects of isoproterenol and selective β3-adrenoceptor agonists on normal, low compliant and hyperreflexic human bladders. J. Urol. 2001;165:240–244. doi: 10.1097/00005392-200101000-00071. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., MOLENAAR P. Differences between the third cardiac β-adrenoceptor and the colonic β3-adrenoceptor in the rat. Br. J. Pharmacol. 1996;118:2085–2098. doi: 10.1111/j.1476-5381.1996.tb15648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARSEN J.-J. α- and β-adrenoceptors in the detrusor muscle and bladder base of the pig and β-adrenoceptors in the detrusor muscle of man. Br. J. Pharmacol. 1979;65:215–222. doi: 10.1111/j.1476-5381.1979.tb07821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN R.M., RUGGIERU M.R., WEIN A.J. Identification of receptor subtypes in the rabbit and human urinary bladder by selective radio-ligand binding. J. Urol. 1988;139:844–848. doi: 10.1016/s0022-5347(17)42659-0. [DOI] [PubMed] [Google Scholar]
- LI J.H., YASAY G.D., KAU S.T. β-adrenoceptor subtypes in the detrusor of guinea pig urinary bladder. Pharmacology. 1992;44:13–18. doi: 10.1159/000138868. [DOI] [PubMed] [Google Scholar]
- LONGHURST P.A., LEVENDUSKY M. Pharmacological characterization of β-adrenoceptors mediating relaxation of the rat urinary bladder in vitro. Br. J. Pharmacol. 1999;127:1744–1750. doi: 10.1038/sj.bjp.0702709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY K.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MANARA L., BADONE D., BARONI M., BOCCARDI G., CECCHI R., CROCI T., GIUDICE A., GUZZI U., LANDI M., LE FUR G. Functional identification of rat atypical β-adrenoceptors by the first β3-selective antagonists, aryloxypropanolaminotetralins. Br. J. Pharmacol. 1996;117:435–442. doi: 10.1111/j.1476-5381.1996.tb15209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLS I.W., NOBLE J.G., BRADING A.F. Radiotelemetered cystometry in pigs: validation and comparison of natural filling versus diuresis cystometry. J. Urol. 2000;164:1745–1750. [PubMed] [Google Scholar]
- MORITA T., ANDO M., KIHARA K., OSHIMA H. Species differences in cAMP production and contractile response induced by β-adrenoceptor subtypes in urinary bladder smooth muscle. Neurourol. Urodyn. 1993;12:185–190. doi: 10.1002/nau.1930120213. [DOI] [PubMed] [Google Scholar]
- MORITA T., DOHKITA S., KONDO S., NISHIMOTO T., HIRANO S., TSUCHIDA S. Cyclic adenosine monophosphate production and contractile response induced by beta-adrenoceptor subtypes in rabbit urinary bladder smooth muscle. Urol. Int. 1990;45:10–15. doi: 10.1159/000281650. [DOI] [PubMed] [Google Scholar]
- NERGÅRDH A., BORÉUS L.O., NAGLO A.-S. Characterization of the adrenergic beta-receptor in the urinary bladder of man and cat. Acta. Pharmacol. Toxicol. 1977;40:14–21. doi: 10.1111/j.1600-0773.1977.tb02049.x. [DOI] [PubMed] [Google Scholar]
- OSHITA M., HIRAOKA Y., WATANABE Y. Characterization of β-adrenoceptors in urinary bladder: comparison between rat and rabbit. Br. J. Pharmacol. 1997;122:1720–1724. doi: 10.1038/sj.bjp.0701562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEGUCHI H., NISHIMURA J., ZHOU Y., NIIRO N., KUMAZAWA J., KANAIDE H. Expression of β3-adrenoceptors in rat detrusor smooth muscle. J. Urol. 1998;159:2197–2201. doi: 10.1016/S0022-5347(01)63305-6. [DOI] [PubMed] [Google Scholar]
- SELLERS D.J., YAMANISHI T., CHAPPLE C.R., COULDWELL C., YASUDA K., CHESS-WILLIAMS R. M3 muscarinic receptors but not M2 mediate contraction of the porcine detrusor muscle in vitro. J. Auton. Pharmacol. 2000;20:171–176. doi: 10.1046/j.1365-2680.2000.00181.x. [DOI] [PubMed] [Google Scholar]
- TAKEDA M., OBARA K., MIZUSAWA T., TOMITA Y., ARAI K., TSUTSUI T., HATANO A., TAKAHASHI K., NOMURA S. Evidence for β3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: Analysis by molecular biological and pharmacological methods. J. Pharmacol. Exp. Ther. 1999;288:1367–1373. [PubMed] [Google Scholar]
- YAMANISHI T., YASUDA K., CHAPPLE C.R., CHESS-WILLIAMS The role of M2-muscarinic receptors in mediating contraction of the pig urinary bladder in vitro. Br. J. Pharmacol. 2000;131:1482–1488. doi: 10.1038/sj.bjp.0703719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAZAKI Y., TAKEDA H., AKAHANE M., IGAWA Y., NISHIZAWA O., AJISAWA Y. Species differences in the distribution of β-adrenoceptor subtypes in bladder smooth muscle. Br. J. Pharmacol. 1998;124:593–599. doi: 10.1038/sj.bjp.0701870. [DOI] [PMC free article] [PubMed] [Google Scholar]


