Abstract
This study investigates whether the cholinergic neurones, innervating the human proximal stomach, can be modulated by nitric oxide (NO) or vasoactive intestinal polypeptide (VIP), or via presynaptic muscarinic, α2- or 5-hydroxytryptamine4 (5-HT4-) receptors.
Circular muscle strips, without mucosa, were incubated with [3H]-choline to incorporate [3H]-acetylcholine into the cholinergic transmitter stores. The basal and electrically-induced release of tritium and [3H]-acetylcholine were analysed in a medium containing guanethidine (4×10−6 M), hemicholinium-3 (10−5 M), physostigmine (10−5 M) and atropine (10−6 M). Tissues were stimulated twice for 2 min (S1 and S2: 40 V, 1 ms, 4 Hz) and drugs were added before S2.
The NO synthase inhibitor L-NG-nitroarginine methyl ester (3×10−4 M) and the NO donor sodium nitroprusside (10−5 M), as well as VIP (10−7 M) did not influence the basal release nor the electrically-evoked release.
The α2-adrenoceptor agonist UK-14,304 (10−5 M) significantly inhibited the electrically-evoked release of [3H]-acetylcholine, and this was prevented by the α2-adrenoceptor antagonist rauwolscine (2×10−6 M).
The 5-HT4-receptor agonist prucalopride (3×10−7 M) significantly enhanced the electrically-evoked release of [3H]-acetylcholine, and the 5-HT4-receptor antagonist SB204070 (10−9 M) prevented this.
When atropine (10−6 M) was omitted from the medium and added before the second stimulation, it significantly increased the release of [3H]-acetylcholine.
These results suggest that the release of acetylcholine from the cholinergic neurones, innervating the circular muscle in the human proximal stomach, can be inhibited via presynaptic muscarinic auto-receptors and α2-adrenoceptors, and stimulated via presynaptic 5-HT4-receptors. No evidence for modulation by NO or VIP was obtained.
Keywords: Human proximal stomach, acetylcholine release, presynaptic modulation, nitric oxide, VIP, α2-adrenoceptors, 5-HT4-receptors, muscarinic receptors
Introduction
With regard to motility, the stomach can be divided into a proximal and a distal part. The proximal stomach consists of the fundus and the orad third of the corpus (Kelly, 1980). It acts as a reservoir for solid and liquid food, and plays a major role in the gastric emptying of liquids (see review Kelly, 1980). During a meal, the proximal stomach relaxes with minimal increases in intragastric pressure, as inhibitory non-adrenergic non-cholinergic (NANC) neurones become activated (Abrahamsson, 1986). Then, a tonic contraction of the proximal stomach generates a gastroduodenal pressure gradient that has been shown to play an important role in liquid emptying from the stomach (Kelly, 1980; Valenzuela & Liu, 1982). In man, in vivo experiments demonstrated that atropine reduces proximal gastric emptying, while the muscarinic agonist bethanechol tended to stimulate proximal stomach contractility (Parkman et al., 1999), suggesting that proximal gastric tone in man appears to be maintained, at least in part, by cholinergic input. In contrast, the proximal gastric relaxation during a meal, as mentioned above, depends on NANC neurones, of which the nitrergic neurones, releasing nitric oxide (NO), are the most important (Lefebvre, 1993; Tonini et al., 2000). Interaction between the nitrergic and cholinergic system might occur at the level of the stomach, as indirect evidence from functional experiments suggests that NO might inhibit the release of acetylcholine from intrinsic cholinergic nerve endings in rat, canine and rabbit gastric fundus (Lefebvre et al., 1992; Baccari et al., 1993; Paterson et al., 2000). In experimental animals, but not in humans, it has been shown that the release of acetylcholine at the level of the stomach can be modulated by different types of presynaptic receptors. Stimulation of presynaptic α2-adrenoceptors (Jansson & Lisander, 1969; Lefebvre et al., 1984; MacDonald et al., 1990; Leclere & Lefebvre, 2001) and vasoactive intestinal polypeptide (VIP)-receptors (Milenov et al., 1991; Baccari et al., 1994) inhibits the release of acetylcholine, while activation of 5-hydroxytryptamine4 (5-HT4)-receptors increases its release (Amemiya et al., 1996; Matsuyama et al., 1996; Takada et al., 1999). The possibility of auto-inhibition of acetylcholine release by stimulation of presynaptic muscarinic receptors has been demonstrated in the pig and guinea-pig stomach (Ogishima et al., 2000; Leclere & Lefebvre, 2001).
The aim of this study in the human proximal stomach was to measure acetylcholine release directly and to investigate possible presynaptic modulation of acetylcholine release by NO, and via presynaptic α2-adrenoceptors and muscarinic, VIP- and 5-HT4-receptors.
Methods
Tissue preparation
With the approval of the local ethics committee, macroscopically normal segments of gastric fundus (n=18) or corpus (n=2) were obtained from 20 patients (16 men, mean age 63 years (range 30 – 95)) undergoing surgery for oesophageal or gastric carcinoma. Experiments were carried out on isolated circular smooth muscle strips of the human gastric fundus or corpus. As no differences between fundus and corpus were observed, the results are pooled. As soon as possible, the stomach was incubated in physiological salt solution (PSS) and transported to the laboratory. After the mucosa was removed, full thickness strips of 1 to 1.5 cm in length and 0.3 cm in width (weight: 65±3 mg; n=99) were cut in the direction of the circular muscle. All strips were used within 24 h, except in one case when strips were used up to 36 h after surgery. Strips were mounted vertically between two platinum wire electrodes (40×0.5 mm) under a load of 2 g in 2 ml organ baths containing PSS (composition in mM: NaCl 112, KCl 4.7, MgCl2 1.2, KH2PO4 1.2, CaCl2 2.5, glucose 11.5, NaHCO3 25, choline 0.0015 and ascorbic acid 0.057), maintained at 37°C and gassed with carbogen (95% O2/5% CO2). Guanethidine (4×10−6 M) was present in the medium throughout all experiments. Electrical field stimulation (EFS) was applied by means of a Grass stimulator (S88, U.S.A.)
Experimental protocol
Basically, the same method was used as described for the labelling of acetylcholine pools in pig gastric fundus (Leclere & Lefebvre, 2001). Briefly, during 60 min, the tissues were superfused at a rate of 2 ml min−1, using a peristaltic pump (Gilson Minipuls, France). During the last 20 min the strips were subjected to continuous EFS (40 V, 1 ms, 0.5 Hz). After this equilibration period, superfusion was stopped and the preparations were incubated for 30 min with [3H]-choline (5 μCi ml−1) during which the tissues were stimulated electrically (40 V, 1 ms, 2 Hz) in order to label their cholinergic transmitter stores.
After the labelling procedure, the strips were superfused (2 ml min−1) for 90 min with PSS to remove loosely bound radioactivity. From now on the PSS contained in addition 10−5 M hemicholinium-3 to prevent the re-uptake of choline, 10−5 M physostigmine to prevent the hydrolysis of acetylcholine and, except in one series of experiments, 10−6 M atropine to prevent the auto-inhibition of acetylcholine release.
After the washout period, the strips were no longer superfused but the content of the organ bath (2 ml) was collected and replaced each 3 min. A total of 35 samples was collected. One millilitre of the samples was mixed with 4 ml of the scintillator containing solution Ultima Gold (Canberra Packard, U.S.A.) The strips were stimulated twice for 2 min (S1 and S2; 40 V, 1 ms, 4 Hz), at 13 min (S1, 5th sample), and 73 min (S2, 25th sample) after the end of the washout period. Tetrodotoxin, calcium-free medium, L-NG-nitroarginine methyl ester (L-NAME), sodium nitroprusside (SNP), VIP and atropine were added 30 min (15th sample) before S2. The α2-adrenoceptor antagonist rauwolscine was added 30 min (15th sample) before S2 and the α2-adrenoceptor agonist, UK-14,304 was added 3 min (24th sample) before S2. The 5-HT4-receptor antagonist, SB204070 was added 36 min (13th sample) before S2 and the 5-HT4-receptor agonist, prucalopride was added 15 min (20th sample) before S2. The α2- and 5-HT4-receptor antagonists and agonists were either given to the same tissue, or to separate tissues. Once added, drugs remained present until the end of the experiment. At the end of the experiment, tissues were blotted and weighed.
Measurement of radioactivity and separation by HPLC of radioactive compounds
Radioactivity of all samples was measured by liquid scintillation counting (Packard Tri-Carb 2100 TR, Canberra Packard, U.S.A.) External standardization was used to correct for counting efficiency. Electrical stimulation induced an increase in tritium overflow, not only in samples 5 (S1) and 25 (S2) but also in the five samples after that with stimulation. The stimulation-induced increase in tritium overflow was calculated by subtracting basal tritium overflow. Basal tritium overflow during the period of enhanced tritium overflow was calculated by fitting a regression line through the values of the four samples just before stimulation and the values of the four samples starting from the sixth sample after stimulation.
The amount of [3H]-acetylcholine, [3H]-choline and [3H]-phosphorylcholine in the samples was analysed by reverse phase HPLC (Bischoff Chromatography, Germany; Hyperchrome-HPLC-column, 250×4.6 mm, prepacked with HYPERSIL-ODS 5.0 μm). A 0.1 M phosphate buffer (pH 4.7) was used, containing methanol (8 vol %) and tetramethylammonium (0.2 mM). The flow was 0.5 ml min−1 and the effluent was collected in 1 min fractions. This is a suitable method to separate the different components as we have demonstrated previously (Leclere & Lefebvre, 2001).
HPLC was performed on one sample before S1 and S2 (sample 3 before S1 and sample 23 before S2), and on the sample during stimulation (sample 5 and 25). One hundred microlitres of the sample was injected into the HPLC; 27 fractions were collected, and each fraction was mixed with 2 ml of Ultima Gold. Fractions 7 to 12 contained the peaks of [3H]-phosphorylcholine and [3H]-choline and were taken together to calculate the amount of [3H]-phosphorylcholine and [3H]-choline, as both peaks could not be separated completely with the phosphate buffer we used. Fractions 14 to 25 were summed to calculate the amount of [3H]-acetylcholine. The real amount of [3H]-phosphorylcholine plus [3H]-choline and of [3H]-acetylcholine was calculated by subtracting the background counting. Background counting was calculated by fitting a regression line through the values of the first five fractions and fractions 26 and 27. Finally, the percentage of [3H]-acetylcholine in each sample was calculated.
Drugs and radiochemicals
L-ascorbic acid, atropine sulphate, choline chloride, guanethidine sulphate, L-NG-nitroarginine methyl ester, sodium nitroprusside and vasoactive intestinal polypeptide were obtained from Sigma (St. Louis, U.S.A.), hemicholinium-3-bromide from RBI (Natick, U.S.A.), methanol from Lab-Scan (Dublin, Ireland), [methyl-3H]-choline chloride (2775 GBq/mmol) from NEN (Boston, U.S.A.), physostigmine salicylate from Federa (Brussels, Belgium), prucalopride (gift from Janssen Research Foundation, Beerse, Belgium), rauwolscine hydrochloride from Carl Roth KG (Karlsruhe, Germany), SB204070 ((1-butyl-4-piperidinyl)-methyl-8-amino-7-chloro1,4-benzodioxane-5-carboxylate HCl) (gift from SmithKline Beecham, Worthing, U.K.), tetramethylammonium chloride from Merck-Schuchardt (Hohenbrunn, Germany), tetrodotoxin from Alomone Labs (Jerusalem, Israel) and UK-14,304 tartrate (5-bromo-6-[-2-imidazolin-2-ylamino]-quinoxaline) (gift from Pfizer, Sandwich, U.K.)
Drugs were dissolved and diluted with distilled water. Stock solutions of 10−3 M tetrodotoxin, 10−2 M prucalopride and 10−3 M SB204070 were kept frozen at −20°C. Dilutions were made the day of the experiment.
Data analysis
The ratios S2/S1 for total radioactivity (TR) and for tritiated acetylcholine were calculated. Experimental data are expressed as means±s.e.mean and n refers to the number of tissues. Results were compared by the unpaired t-test or by ANOVA followed by a t-test corrected for multiple comparisons (Bonferroni procedure) when more than two responses had to be compared. P values of less than 0.05 were considered statistically significant.
Results
Control experiments
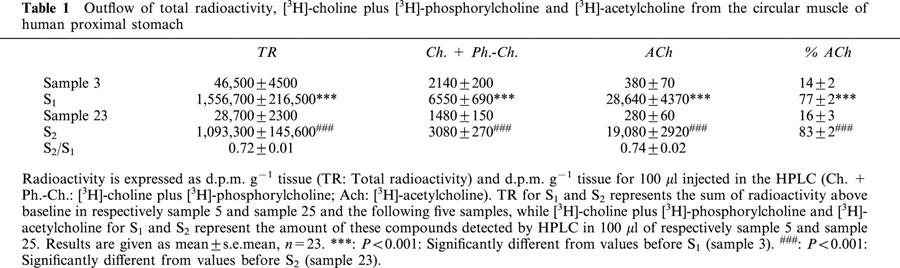
Tissues were electrically stimulated in PSS containing guanethidine (4×10−6 M), hemicholinium-3 (10−5 M), physostigmine (10−5 M) and atropine (10−6 M) at 40 V, 1 ms, 4 Hz for 2 min after incubation with [3H]-choline for 30 min. Field stimulation caused a clearcut increase in total radioactivity (TR) and 15 min were required after stimulation to re-establish the basal release of tritium. [3H]-Acetylcholine could be detected when HPLC was used to separate the different components present in the samples. The mean amounts of TR, [3H]-choline plus [3H]-phosphorylcholine and [3H]-acetylcholine before and during stimulation, as well as the percentage of [3H]-acetylcholine released are given in Table 1. The S2/S1 ratios for TR and [3H]-acetylcholine were respectively 0.72±0.01 and 0.74±0.02 (n=23).
Table 1.
Outflow of total radioactivity, [3H]-choline plus [3H]-phosphorylcholine and [3H]-acetylcholine from the circular muscle of human proximal stomach
Tetrodotoxin (3×10−6 M; n=3) or removal of extracellular calcium (n=3) did not influence basal release of TR, but they nearly abolished the electrically-evoked tritium release as compared with control (P<0.001). In control tissues (n=3), the S2/S1 ratios for release of TR and [3H]-acetylcholine were 0.71±0.06 and 0.69±0.03, respectively. After superfusion with tetrodotoxin or calcium-free medium, the S2/S1 ratios for TR were respectively 0.08±0.04 and 0.05±0.02, while those for [3H]-acetylcholine were respectively 0.01±0.02 and 0.00±0.02.
The effects of L-NG-nitroarginine methyl ester, sodium nitroprusside and VIP
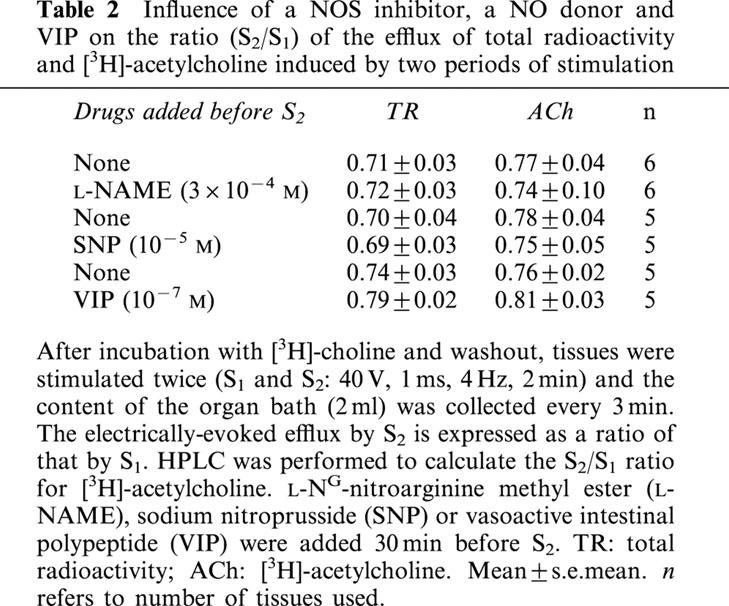
The NO synthase inhibitor L-NAME (3×10−4 M; n=6) or the NO donor SNP (10−5 M; n=5), when added 30 min before the second stimulation, had no influence on basal release of TR. L-NAME or SNP also had no effect on the electrically-evoked increase of TR or [3H]-acetylcholine released (Table 2). Similarly, VIP (10−7 M; n=5), added 30 min before S2, was without effect on the basal release of TR and on the electrically-evoked increase in release of TR or [3H]-acetylcholine (Table 2).
Table 2.
Influence of a NOS inhibitor, a NO donor and VIP on the ratio (S2/S1) of the efflux of total radioactivity and [3H]-acetylcholine induced by two periods of stimulation
The effects of UK-14,304 and rauwolscine
The α2-adrenoceptor antagonist rauwolscine (2×10−6 M; n=9) did not alter the basal efflux of TR, nor had it any effect per se on the S2/S1 ratio for TR (S2/S1 ratios for control and rauwolscine: 0.66±0.04 and 0.66±0.05, respectively, P>0.05; n=4).
The selective α2-adrenoceptor agonist UK-14,304 (10−5 M), added 3 min before the second stimulation period, did not alter the basal efflux of TR (n=6). However, UK-14,304 significantly reduced the amount of TR and [3H]-acetylcholine released upon electrical stimulation. In control tissues, the S2/S1 ratios for release of TR and [3H]-acetylcholine were 0.72±0.02 and 0.70±0.03 respectively (Figure 1A), while in the presence of UK-14,304 the ratios were 0.33±0.03 and 0.31±0.04 (P<0.001; n=6; Figure 1B). Rauwolscine prevented the inhibition of the electrically-evoked release of TR and [3H]-acetylcholine by UK-14,304 (S2/S1 ratios for TR and [3H]-acetylcholine in the presence of rauwolscine: 0.67±0.06 and 0.58±0.04 respectively, P>0.05 compared with control, n=5; Figure 1C).
Figure 1.
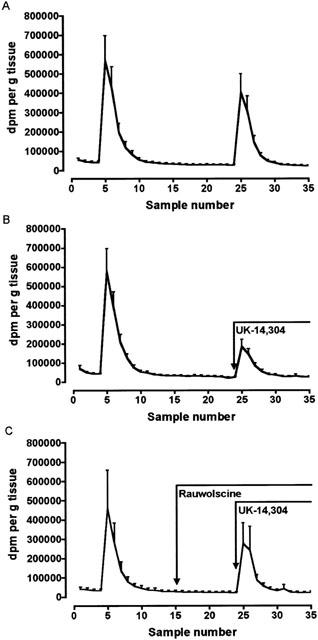
Effects of UK-14,304 and UK-14,304 in the presence of rauwolscine on the electrically-evoked release of total radioactivity (TR) from preparations of human proximal stomach pre-incubated with [3H]-choline. Tissues were stimulated twice (S1 and S2: 40 V, 1 ms, 4 Hz, 2 min), and the content of the organ bath (2 ml) was collected in 3 min samples for TR. (A) TR outflow of control experiments (n=6). (B) Release of TR when UK-14,304 (10−5 M) was added 3 min before S2 (n=6). (C) Release of TR when rauwolscine (2×10−6 M) was added 30 min and UK-14,304 (10−5 M) 3 min before S2 (n=5). The results are given as mean±s.e.mean.
The effects of prucalopride and SB204070
The 5-HT4-receptor antagonist SB204070 (10−9 M; n=8) did not alter the basal release of TR, nor did SB204070 influence the S2/S1 ratio for TR versus control (0.64±0.06 and 0.66±0.04, respectively, P>0.05; n=4).
The selective 5-HT4-receptor agonist prucalopride (3×10−7 M) did not alter the basal efflux of TR. However, prucalopride significantly enhanced the amount of TR (P<0.001) and [3H]-acetylcholine (P<0.01) released upon electrical stimulation (n=6; Figure 2). When SB204070 was added before prucalopride, it completely prevented the increase of the electrically-evoked release of TR and [3H]-acetylcholine by prucalopride (P>0.05; n=4; Figure 2).
Figure 2.
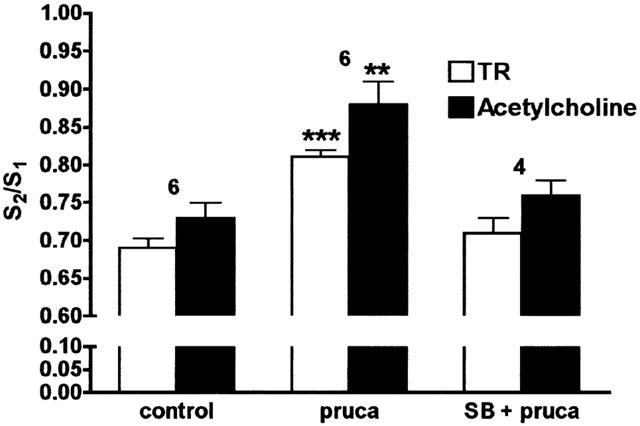
Effects of prucalopride (pruca) and prucalopride in the presence of SB204070 (SB) on the electrically-evoked release of total radioactivity (TR) and [3H]-acetylcholine from preparations of human proximal stomach pre-incubated with [3H]-choline. Tissues were stimulated twice (S1 and S2: 40 V, 1 ms, 4 Hz, 2 min); SB204070 (10−9 M) was added 36 min and prucalopride (3×10−7 M) 15 min before S2. The electrically-evoked efflux by S2 is expressed as a ratio of that by S1. HPLC was performed to calculate the S2/S1 ratio for [3H]-acetylcholine. Each column represents the mean±s.e.mean. **P<0.01; ***P<0.001: Significantly different from control. Numbers above the columns refer to number of tissues used.
The effect of atropine
Until now, all experiments were performed in the presence of the muscarinic antagonist atropine. To study whether the released acetylcholine is able to influence its own release, a series of experiments was done in the absence of atropine. In the absence of atropine, the mean basal release of TR before S1 (sample 3) was 54,200±14,600 d.p.m. g−1 tissue (n=6), which is not significantly different from the basal release in the presence of atropine. However, the mean increase in TR released during the first stimulation was 563,600±153,400 d.p.m. g−1 tissue, a significant decrease in comparison to tissues stimulated in the presence of atropine (1,556,700±216,500 d.p.m. g−1 tissue, see Table 1, P<0.05), suggesting that acetylcholine inhibits its own release. This was confirmed by the observation that atropine (10−6 M), added 30 min before S2, significantly increased the S2/S1 ratios for both TR and [3H]-acetylcholine released upon electrical stimulation, without having an effect on basal release. In control tissues, the S2/S1 ratios for release of TR and [3H]-acetylcholine were 0.82±0.05 (n=6) and 0.92±0.04 (n=5) respectively, while the ratios were 2.03±0.22 (P<0.001; n=6) and 2.70±0.52 (P<0.01; n=5) when atropine was added between S1 and S2.
Discussion
This study investigated whether the release of acetylcholine in the human proximal stomach can be influenced by NO or VIP, or via presynaptic muscarinic, α2- or 5-HT4-receptors, as assessed by direct measurement of [3H]-acetylcholine release. Experiments were conducted on preparations which had been incubated with [3H]-choline to incorporate [3H]-acetylcholine into the cholinergic transmitter stores.
Control experiments
Incubation of the human proximal stomach with [3H]-choline resulted in the synthesis of [3H]-acetylcholine, that was released during field stimulation. The field-stimulated release of TR and [3H]-acetylcholine was prevented by tetrodotoxin or by the removal of extracellular calcium, indicating a neuronal release dependent upon respectively, the opening of sodium channels, and the presence of calcium in the external medium. Basal release of TR was not influenced in the presence of tetrodotoxin or the absence of extracellular calcium, indicating a low degree of spontaneous activity of the cholinergic neurones during rest. This implies a low exchange of newly synthesized [3H]-acetylcholine against unlabelled acetylcholine, suggesting that electrical stimulation during labelling is necessary, as it is in the rat myenteric plexus and the pig gastric fundus (Wessler & Werhand, 1990; Leclere & Lefebvre, 2001).
Electrical stimulation not only caused an increase in the release of [3H]-acetylcholine, but also a moderate increase in the outflow of [3H]-phosphorylcholine and [3H]-choline. Our results are in contrast with results in the guinea-pig and rat myenteric plexus, where electrical field stimulation only caused an increase in [3H]-acetylcholine release (Wessler & Werhand, 1990; Hebeiß & Kilbinger, 1996), but are in agreement with results in the canine ileum, pig gastric fundus and rat and guinea-pig trachea (Wessler et al., 1990; 1991; Hryhorenko et al., 1994; Leclere & Lefebvre, 2001). As the S2/S1 ratio for [3H]-acetylcholine was systematically similar to that for TR, it will no longer be necessary to separate all radioactive components in future experiments with this tissue as the results of TR reflect those of [3H]-acetylcholine.
Presynaptic modulation of acetylcholine release
Acetylcholine
In this type of experiment, the presence of an acetylcholinesterase inhibitor (physostigmine) is required to be able to determine the amount of [3H]-acetylcholine released. Our previous experiments in the pig gastric fundus illustrated that the process of auto-inhibition of acetylcholine release via stimulation of presynaptic muscarinic auto-receptors on the cholinergic neurones is increased in the presence of physostigmine. This auto-inhibition was prevented by adding atropine together with physostigmine (Leclere & Lefebvre, 2001), and this condition was also used in the actual human proximal stomach experiments. The presence of inhibitory presynaptic muscarinic auto-receptors in the human proximal stomach was confirmed in the experiments in the absence of atropine in the basal medium since: (1) when only physostigmine was present, about three times less tritium was liberated during electrical stimulation; (2) the ratio S2/S1 for TR and [3H]-acetylcholine was significantly increased when atropine was added before S2 compared to control strips in the presence of physostigmine alone. The presence of presynaptic muscarinic auto-receptors on the cholinergic neurones of human proximal stomach is in agreement with the general concept that presynaptic muscarinic receptors inhibit the release of acetylcholine from peripheral ends of parasympathetic nerve fibres in tissues of different species (see review Starke et al., 1989).
NO and VIP
In several species and tissues it has been demonstrated that NO can act presynaptically on cholinergic neurones to enhance the basal release of acetylcholine and to inhibit the electrically-evoked release of acetylcholine (Kilbinger, 1996). Functional experiments in the human gastric fundus led to the proposal that endogenous NO might tonically inhibit the release of acetylcholine (Tonini et al., 2000). From functional studies, however, it is not possible to determine with certainty the site(s) of action of NO (presynaptic inhibition of acetylcholine release versus postsynaptic functional antagonism). For this reason, the effects of a NO synthase inhibitor and a NO donor on [3H]-acetylcholine release in the human proximal stomach were determined. Blockade of NO synthase, or addition of SNP did not significantly affect the basal release of TR or the electrically-evoked overflow of TR and [3H]-acetylcholine, suggesting that NO does not modulate acetylcholine release in the human proximal stomach. This is in agreement with the findings in other species and tissues where NO donors and NO synthase inhibitors do not modify [3H]-acetylcholine release in either tracheal or intestinal preparations (Brave et al., 1991; Ward et al., 1993; 1996; Milenov & Kalfin, 1996; Rae et al., 1998; Leclere & Lefebvre, 2001). Still, as the strips are cut in the direction of the circular muscle layer, it cannot be excluded that longitudinally directed interneurones and/or sensory neurones are not fully assessed; modulation of acetylcholine released, by NO might still be present at this level.
As VIP is co-localized with NO in the majority of the myenteric neurones in the human gastric fundus, and VIP is released during electrical field stimulation (Tonini et al., 2000), the effect of VIP on the electrically-evoked release of acetylcholine was investigated. The concept that VIP can inhibit cholinergic neurotransmission via a presynaptic mechanism has indeed been proposed for gastrointestinal and respiratory tissue (Hakoda & Ito, 1990; Milenov et al., 1991; Baccari et al., 1994). VIP did not significantly affect the basal release of TR or electrically-evoked overflow of TR and [3H]-acetylcholine, suggesting that VIP does not modulate [3H]-acetylcholine release in human proximal stomach. This is in agreement with observations in various gastrointestinal and respiratory tissues (Lefebvre et al., 1992; Ward et al., 1993; Sekizawa et al., 1993), although modulation of acetylcholine released, by VIP might still be present at the level of the longitudinally directed interneurones and/or sensory neurones. Indeed, it has been demonstrated that PACAP and VIP can stimulate the spontaneous and inhibit the electrically-evoked release of [3H]-acetylcholine of guinea-pig longitudinal muscle myenteric plexus preparations (Katsoulis et al., 1993).
Presynaptic α2-adrenoceptors
It has already been shown that presynaptic inhibitory α2-adrenoceptors are present on cholinergic neurones in various gastrointestinal and other tissues (see Introduction; for review see de ponti et al., 1996). In the actual study, the α2-adrenoceptor agonist UK-14,304 significantly reduced the stimulation-induced efflux of TR and [3H]-acetylcholine in the human proximal stomach, as it did in guinea-pig ileum and pig gastric fundus (Funk et al., 1995; Leclere & Lefebvre, 2001). The incubation period of 3 min for UK-14,304 is sufficient as increasing this period to 30 min did not increase the inhibitory effect of UK-14,304 (results not shown). The inhibition of the stimulated overflow produced by UK-14,304 was antagonized by rauwolscine, a selective α2-adrenoceptor antagonist (Weitzell et al., 1979), indicating that cholinergic nerves of the human proximal stomach are endowed with α2-adrenoceptors, causing inhibition of acetylcholine release. This corresponds with the general idea that endogenous noradrenaline is able to inhibit non-sphincteric muscle in the gastrointestinal tract by inhibition of acetylcholine release from the cholinergic motor neurones via presynaptic α2-adrenoceptors (McIntyre & Thompson, 1992). Whether endogenous noradrenaline is able to influence acetylcholine release in the human proximal stomach within the experimental conditions was not assessed, as guanethidine was continuously present in the PSS, preventing the release of endogenous noradrenaline.
Presynaptic 5-HT4-receptors
Stimulation of gastrointestinal 5-HT4-receptors induces contraction or relaxation depending on the tissue and species studied. Contractile responses are generally ascribed to 5-HT4-receptors localized on cholinergic neurones, facilitating acetylcholine release (see e.g. Elswood et al., 1991; Kilbinger & Wolf, 1992; Briejer & Schuurkes, 1996) while the relaxant responses are due to 5-HT4-receptors localized on smooth muscle (see e.g. Tam et al., 1995; Prins et al., 2000). In the stomach, facilitatory 5-HT4-receptors on cholinergic neurones are present in the guinea-pig (Buchheit & Buhl, 1994; Matsuyama et al., 1996) and rat (Amemiya et al., 1996). Whereas nervous 5-HT4-receptors seem present in the rat gastric fundus (Amemiya et al., 1996), a recent study in the guinea-pig stomach suggested a regional distribution of the nervous 5-HT4-receptors, being present in the corpus and antrum but absent in the fundus (Takada et al., 1999). In this study, we assessed the possible presence of facilitatory 5-HT4-receptors on the cholinergic nerves by use of the selective 5-HT4-receptor agonist prucalopride (Briejer et al., 1998). Prucalopride significantly increased the stimulation-induced efflux of TR and [3H]-acetylcholine in the human proximal stomach. This increase was completely antagonized by SB204070, a selective 5-HT4-receptor antagonist (Wardle et al., 1994). This indicates that cholinergic nerves of the human proximal stomach are endowed with 5-HT4-receptors stimulating acetylcholine release. A stimulatory effect of gastroprokinetic agents such as cisapride can thus also be expected at the level of the human proximal stomach. As SB204070 did not influence the S2/S1 ratio for TR, it is unlikely that electrical stimulation causes the release of endogenous 5-HT which might act on 5-HT4-receptors to enhance [3H]-acetylcholine release.
In conclusion, the data provided by this study indicate that measurement of tritium release after incubation with [3H]-choline can be used to reflect endogenous acetylcholine release in response to cholinergic neuron stimulation in the human proximal stomach. The results indicated the presence of presynaptic inhibitory α2-adrenoceptors and muscarinic auto-receptors, and excitatory 5-HT4-receptors. No evidence for the modulation of acetylcholine release by NO or VIP was obtained.
Acknowledgments
The study was financially supported by grant No. 3G0031.96 from the Fund for Scientific Research Flanders, grant O11A1696 from the Special Investigation Fund of the Ghent University and by the Interuniversity Pole of Attraction Programme P4/16 (Services to the Prime Minister-Federal Services for Scientific, Technical and Cultural Affairs). We thank Prof Dr B. de Hemptinne, Prof Dr U. Hesse, Prof Dr P. Pattyn and Dr I. Kerremans from the Department of Surgery of the Ghent University for providing the human tissue samples.
Abbreviations
- 5-HT
5-hydroxytryptamine
- EFS
electrical field stimulation
- L-NAME
L-NG-nitroarginine methyl ester
- NANC
non-adrenergic non-cholinergic
- NO
nitric oxide
- PPS
physiological salt solution
- SNP
sodium nitroprusside
- TR
total radioactivity
- VIP
vasoactive intestinal polypeptide
References
- ABRAHAMSSON H. Non-adrenergic non-cholinergic nervous control of gastrointestinal motility patterns. Arch. Int. Pharmacodyn. 1986;280 Suppl:50–61. [PubMed] [Google Scholar]
- AMEMIYA N., HATTA S., TAKEMURA H., OHSHIKA H. Characterization of the contractile response induced by 5-methoxytryptamine in rat stomach fundus strips. Eur. J. Pharmacol. 1996;318:403–409. doi: 10.1016/s0014-2999(96)00777-7. [DOI] [PubMed] [Google Scholar]
- BACCARI M.C., BERTINI M., CALAMAI F. Effects of L-NG-nitro arginine on cholinergic transmission in the gastric muscle of the rabbit. Neuroreport. 1993;4:1102–1104. [PubMed] [Google Scholar]
- BACCARI M.C., CALAMAI F., STADERINI G. Modulation of cholinergic transmission by nitric oxide, VIP and ATP in the gastric muscle. Neuroreport. 1994;5:905–908. doi: 10.1097/00001756-199404000-00013. [DOI] [PubMed] [Google Scholar]
- BRAVE S.R., HOBBS A.J., GIBSON A., TUCKER J.F. The influence of L-NG-nitro-arginine on field stimulation induced contractions and acetylcholine release in guinea pig isolated tracheal smooth muscle. Biochem. Biophys. Res. Commun. 1991;179:1017–1022. doi: 10.1016/0006-291x(91)91920-8. [DOI] [PubMed] [Google Scholar]
- BRIEJER M.R., MEULEMANS A.L., BOSMANS J.-P., VAN DAELE P., SCHUURKES J.A.J. In vitro pharmacology of the novel enterokinetic R093877. Gastroenterology. 1998;112:A704. [Google Scholar]
- BRIEJER M.R., SCHUURKES J.A.J. 5-HT3 and 5-HT4 receptors and cholinergic and tachykininergic neurotransmission in the guinea-pig proximal colon. Eur. J. Pharmacol. 1996;308:173–180. doi: 10.1016/0014-2999(96)00297-x. [DOI] [PubMed] [Google Scholar]
- BUCHHEIT K., BUHL T. Stimulant effects of 5-hydroxytryptamine on guinea-pig stomach preparations in vitro. Eur. J. Pharmacol. 1994;262:91–97. doi: 10.1016/0014-2999(94)90031-0. [DOI] [PubMed] [Google Scholar]
- DE PONTI F., GIARONI C., COSENTINO M., LECCHINI S., FRIGO G. Adrenergic mechanisms in the control of gastrointestinal motility: from basic science to clinical applications. Pharmacol. Ther. 1996;69:59–78. doi: 10.1016/0163-7258(95)02031-4. [DOI] [PubMed] [Google Scholar]
- ELSWOOD C.J., BUNCE K.T., HUMPHREY P.P.A. Identification of putative 5-HT4 receptors in guinea-pig ascending colon. Eur. J. Pharmacol. 1991;196:149–155. doi: 10.1016/0014-2999(91)90421-l. [DOI] [PubMed] [Google Scholar]
- FUNK L., TRENDELENBURG A.-U., LIMBERGER N., STARKE K. Subclassification of presynaptic α2-adrenoceptors: α2D-autoreceptors and α2D-adrenoceptors modulating release of acetylcholine in guinea-pig ileum. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:58–66. doi: 10.1007/BF00169190. [DOI] [PubMed] [Google Scholar]
- HAKODA H., ITO Y. Modulation of cholinergic neurotransmission by the peptide VIP, VIP antiserum and VIP antagonists in dog and cat trachea. J. Physiol. 1990;428:133–154. doi: 10.1113/jphysiol.1990.sp018204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEBEIß K., KILBINGER H. Differential effects of nitric oxide donors on basal and electrically evoked release of acetylcholine from guinea-pig myenteric neurones. Br. J. Pharmacol. 1996;118:2073–2078. doi: 10.1111/j.1476-5381.1996.tb15646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRYHORENKO L.M., WOSKOWSKA Z., FOX-THRELKELD J.-A.E.T. Nitric oxide (NO) inhibits release of acetylcholine from nerves of isolated circular muscle of the canine ileum: relationship to motility and release of nitric oxide. J. Pharmacol. Exp. Ther. 1994;271:918–926. [PubMed] [Google Scholar]
- JANSSON G., LISANDER B. On adrenergic influences on gastric motility in chronically vagotomized cats. Acta Physiol. Scand. 1969;76:463–471. doi: 10.1111/j.1748-1716.1969.tb04493.x. [DOI] [PubMed] [Google Scholar]
- KATSOULIS S., CLEMENS A., SCHWÖRER H., CREUTZFELDT W., SCHMIDT W.E. PACAP is a stimulator of neurogenic contraction in guinea pig ileum. Am. J. Physiol. 1993;265:G295–G302. doi: 10.1152/ajpgi.1993.265.2.G295. [DOI] [PubMed] [Google Scholar]
- KELLY K.A. Gastric emptying of liquids and solids: roles of proximal and distal stomach. Am. J. Physiol. 1980;239:G71–G76. doi: 10.1152/ajpgi.1980.239.2.G71. [DOI] [PubMed] [Google Scholar]
- KILBINGER H. Modulation of acetylcholine release by nitric oxide. Progress Brain Res. 1996;109:219–224. doi: 10.1016/s0079-6123(08)62105-6. [DOI] [PubMed] [Google Scholar]
- KILBINGER H., WOLF D. Effects of 5-HT4 receptor stimulation on basal and electrically evoked release of acetylcholine from guinea-pig myenteric plexus. Naunyn-Schmiedeberg's Arch. Pharmacol. 1992;345:270–275. doi: 10.1007/BF00168686. [DOI] [PubMed] [Google Scholar]
- LECLERE P.G., LEFEBVRE R.A. Influence of nitric oxide donors and of the α2-agonist UK-14,304 on acetylcholine release in the pig gastric fundus. Neuropharmacology. 2001;40:270–278. doi: 10.1016/s0028-3908(00)00123-4. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A. Non-adrenergic non-cholinergic neurotransmission in the proximal stomach. Gen. Pharmacol. 1993;24:257–266. doi: 10.1016/0306-3623(93)90301-d. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A., DE VRIESE A., SMITS G.J.M. Influence of vasoactive intestinal polypeptide and NG-nitro-L-arginine methyl ester on cholinergic neurotransmission in the rat gastric fundus. Eur. J. Pharmacol. 1992;221:235–242. doi: 10.1016/0014-2999(92)90707-b. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A., WILLEMS J.L., BOGAERT M.G. Inhibitory effect of dopamine on canine gastric fundus. Naunyn-Schmiedebergs Arch. Pharmacol. 1984;326:22–28. doi: 10.1007/BF00518774. [DOI] [PubMed] [Google Scholar]
- MACDONALD A., KELLY J., DETTMAR P.W. Pre- and post-junctional α-adrenoceptor-mediated responses in the rat gastric fundus in vitro. J. Pharm. Pharmacol. 1990;42:752–757. doi: 10.1111/j.2042-7158.1990.tb07015.x. [DOI] [PubMed] [Google Scholar]
- MATSUYAMA S., SAKIYAMA H., NEI K., TANAKA C. Identification of putative 5-hydroxytryptamine4 (5-HT4) receptors in guinea pig stomach: the effect of TKS159, a novel agonist, on gastric motility and acetylcholine release. J. Pharmacol. Exp. Ther. 1996;276:989–995. [PubMed] [Google Scholar]
- MCINTYRE A.S., THOMPSON D.G. Review article: adrenergic control of motor and secretory function in the gastrointestinal tract. Aliment. Pharmacol. Ther. 1992;6:125–142. doi: 10.1111/j.1365-2036.1992.tb00257.x. [DOI] [PubMed] [Google Scholar]
- MILENOV K., KALFIN R. Cholinergic-nitrergic interactions in the guinea-pig gastric fundus. Neuropeptides. 1996;30:365–371. doi: 10.1016/s0143-4179(96)90026-8. [DOI] [PubMed] [Google Scholar]
- MILENOV K., KALFIN R., MANDREK K. Effect of vasoactive intestinal peptide (VIP) on the mechanical activity and [3H] acetylcholine release in guinea-pig gastric muscle. Acta Physiol. Pharmacol. Bulg. 1991;17:13–18. [PubMed] [Google Scholar]
- OGISHIMA M., KAIBARA M., UEKI S., KURIMOTO T., TANIYAMA K. Z-338 facilitates acetylcholine release from enteric neurons due to blockade of muscarinic autoreceptors in guinea pig stomach. J. Pharmacol. Exp. Ther. 2000;294:33–37. [PubMed] [Google Scholar]
- PARKMAN H.P., TRATE D.M., KNIGHT L.C., BROWN K.L., MAURER A.H., FISHER R.S. Cholinergic effects on human gastric motility. Gut. 1999;45:346–354. doi: 10.1136/gut.45.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATERSON C.A., ANVARI M., TOUGAS G., HUIZINGA J.D. Nitrergic and cholinergic vagal pathways involved in the regulation of canine proximal gastric tone: an in vivo study. Neurogastroenterol. Mot. 2000;12:301–306. doi: 10.1046/j.1365-2982.2000.00209.x. [DOI] [PubMed] [Google Scholar]
- PRINS N.H., SHANKLEY N.P., WELSH N.J., BRIEJER M.R., LEFEBVRE R.A., AKKERMANS L.M.A., SCHUURKES J.A.J. An improved in vitro bioassay for the study of 5-HT4 receptors in the human isolated large intestinal circular muscle. Br. J. Pharmacol. 2000;129:1601–1608. doi: 10.1038/sj.bjp.0703254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAE M.G., KHOYI M.A., KEEF K.D. Modulation of cholinergic neuromuscular transmission by nitric oxide in canine colonic circular smooth muscle. Am. J. Physiol. 1998;275:G1324–G1332. doi: 10.1152/ajpgi.1998.275.6.G1324. [DOI] [PubMed] [Google Scholar]
- SEKIZAWA K., FUKUSHIMA T., IKARASHI Y., MARUYAMA Y., SASAKI H. The role of nitric oxide in cholinergic neurotransmission in rat trachea. Br. J. Pharmacol. 1993;110:816–820. doi: 10.1111/j.1476-5381.1993.tb13885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STARKE K., GÖTHERT M., KILBINGER H. Modulation of transmitter release by presynaptic autoreceptors. Physiol. Rev. 1989;69:864–989. doi: 10.1152/physrev.1989.69.3.864. [DOI] [PubMed] [Google Scholar]
- TAKADA K., SAKURAI-YAMASHITA Y., YAMASHITA K., KAIBARA M., HAMADA Y., NAKANE Y., HIOKI K., TANIYAMA K. Regional difference in correlation of 5-HT4 receptor distribution with cholinergic transmission in the guinea pig stomach. Eur. J. Pharmacol. 1999;374:489–494. doi: 10.1016/s0014-2999(99)00321-0. [DOI] [PubMed] [Google Scholar]
- TAM F.S.F., HILLIER K., BUNCE K.T., GROSSMAN C. Differences in response to 5-HT4 receptor agonists and antagonists of the 5-HT4-like receptor in human colon circular smooth muscle. Br. J. Pharmacol. 1995;115:172–176. doi: 10.1111/j.1476-5381.1995.tb16335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONINI M., DE GIORGIO R., DE PONTI F., STERNINI C., SPELTA V., DIONIGI P., BARBARA G., STANGHELLINI V., CORINALDESI R. Role of nitric oxide- and vasoactive intestinal polypeptide-containing neurones in human gastric fundus strip relaxations. Br. J. Pharmacol. 2000;129:12–20. doi: 10.1038/sj.bjp.0702977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENZUELA J.E., LIU D.P. The effect of variations in intragastric pressure and gastric emptying of a saline meal in humans. Scand. J. Gastroenterol. 1982;17:293–296. doi: 10.3109/00365528209182056. [DOI] [PubMed] [Google Scholar]
- WARD J.K., BELVISI M.G., FOX A.J., MIURA M., TADJKARIMI S., YACOUB M.H., BARNES P.J. Modulation of cholinergic neural bronchoconstriction by endogenous nitric oxide and vasoactive intestinal peptide in human airways in vitro. J. Clin. Invest. 1993;92:736–743. doi: 10.1172/JCI116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD S.M., DALZIEL H.H., KHOYI M.A., WESTFALL A.S., SANDERS K.M., WESTFALL D.P. Hyperpolarization and inhibition of contraction mediated by nitric oxide released from enteric inhibitory neurones in guinea-pig taenia coli. Br. J. Pharmacol. 1996;118:49–56. doi: 10.1111/j.1476-5381.1996.tb15365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARDLE K.A., ELLIS E.S., BAXTER G.S., KENNETT G.A., GASTER L.M., SANGER G.J. The effects of SB204070, a highly potent and selective 5-HT4 receptor antagonist, on guinea-pig distal colon. Br. J. Pharmacol. 1994;112:789–794. doi: 10.1111/j.1476-5381.1994.tb13148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEITZELL R., TANAKA T., STARKE K. Pre- and postsynaptic effects of yohimbine stereoisomers on noradrenergic transmission in the pulmonary artery of the rabbit. Naunyn-Schmiedeberg's Arch. Pharmacol. 1979;308:127–136. doi: 10.1007/BF00499054. [DOI] [PubMed] [Google Scholar]
- WESSLER I., HELLWIG D., RACKÉ K. Epithelium-derived inhibition of [3H]acetylcholine release from the isolated guinea-pig trachea. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;342:387–393. doi: 10.1007/BF00169454. [DOI] [PubMed] [Google Scholar]
- WESSLER I., KLEIN A., POHAN D., MACLAGAN J., RACKÉ K. Release of [3H]acetylcholine from the isolated rat or guinea-pig trachea evoked by preganglionic nerve stimulation; a comparison with transmural stimulation. Naunyn-Schmiedeberg's Arch. Pharmacol. 1991;344:403–411. doi: 10.1007/BF00172579. [DOI] [PubMed] [Google Scholar]
- WESSLER I., WERHAND J. Evaluation by reverse phase HPLC of [3H]acetylcholine release evoked from the myenteric plexus of the rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;341:510–516. doi: 10.1007/BF00171730. [DOI] [PubMed] [Google Scholar]


