Abstract
The effects of LASSBio 294, a new 3,4-methylenedioxybenzoyl-2-thienylhydrazone, on vascular tonus were investigated in isolated rat aortic rings.
LASSBio 294 induced a concentration-dependent relaxation of intact rat aortic rings with an inhibitory concentration (IC50) of 74 μM (95% confidence limits: 59 – 92). The mechanical removal of the endothelium abolished this effect.
In aortic rings with intact endothelium the effect of 100 μM LASSBio 294 was not altered by the pharmacological inhibition of NOS and cyclo-oxygenase pathways with 500 μM L-NAME and 10 μM indomethacin, respectively.
LASSBio 294 (100 μM) was able to relax aortic rings pre-contracted with high extracellular K+ (KCl 100 mM).
The relaxant effect of LASSBio 294 was fully reversed (and prevented) by the addition of 1 μM ODQ (1H-(1,2,4)oxadiazolo[4,3-a]quinoxaline-1-one), a selective inhibitor of soluble guanylate cyclase.
LASSBio 294 (100 μM) had no direct effect on PDE3 and PDE4 activities, however, it increased by 150% cyclic GMP content in aortic rings pre-treated with 100 μM L-NAME and 10 μM indomethacin, as did 1 μM zaprinast, a selective PDE5 inhibitor.
In conclusion, LASSBio 294 induced relaxation of isolated rat aorta probably by directly increasing cyclic GMP content, possibly as a consequence of PDE5 inhibition.
Keywords: Cyclic GMP, cyclic AMP, vascular smooth muscle, phosphodiesterase, LASSBio 294
Introduction
In recent studies identifying new anti-inflammatory lead candidates belonging to the class of N-acylhydrazone (NAH) derivatives (Figueiredo et al., 2000), has emerged LASSBio 294, a new 3,4-methylenedioxybenzoyl-2-thienylhydrazone synthesized from natural safrole (Figure 1). Since LASSBio 294 was designed as a phosphodiesterase (PDE) inhibitor, we decided to screen it for vasorelaxant properties with particular interest in evaluating a putative involvement of cyclic nucleotides.
Figure 1.
Structure of LASSBio 294.
Cyclic nucleotide signalling plays a regulatory role in the cardiovascular system (Delpy et al., 1996; Verde et al., 1999), with the intracellular levels of adenosine 3′5′ cyclic monophosphate (cyclic AMP) and guanosine 3′5′ cyclic monophosphate (cyclic GMP) being strictly regulated by both rates of synthesis and hydrolysis via cyclases and PDEs, respectively (Beavo, 1995; Soderling & Beavo, 2000).
Both particulate and soluble isoforms of guanylate cyclase are present in vascular smooth muscle cells. The soluble isoform is physiologically activated by nitric oxide (NO) released from adjacent endothelial cells in response to Ca2+ mobilizing agonists or shear stress, and pharmacologically by the NO donor sodium nitroprusside (Hobbs, 1997). An increase in the content of cyclic GMP, either in the endothelial cell or in the myocyte, causes activation of cyclic GMP-dependent protein kinase designated as PKG (Lohmann et al., 1997; Kwan et al., 2000). Two genes encoding mammalian PKG have so far been identified, namely type I and type II. Type I PKG exists as two isoforms (α and β) and PKG αI isoform is found in the aorta, heart, cerebellum and lung (Soff et al., 1997). PKG appears to cause vascular relaxation by lowering both [Ca2+]i and the Ca2+ sensitivity of the contractile elements (Salomone et al., 1996; Bolz et al., 1999). The reduction of [Ca2+]i in this tissue is attributed to a number of mechanisms including inhibition of the inositol 1,4,5-triphosphate receptor, the voltage-dependent L-type calcium channels (Pfeifer et al., 1999) and also activation of sarcoplasmic reticulum Ca2+ ATPase (SERCA) (Yoshida et al., 1999). Conversely, cyclic GMP, as well as cyclic AMP, is degraded by PDEs. In the last few years several isoforms and biological functions have been revealed for these enzymes. This superfamily includes 19 different genes subgrouped into 11 PDE families, according to the nucleotide preferentially hydrolyzed and to the regulatory properties of the enzyme. PDE1 (Ca2+-calmodulin-dependent), PDE2 (cyclic GMP-stimulated), PDE3 (cyclic GMP-inhibited), PDE4 (cyclic AMP-specific) and PDE5 (cyclic GMP-specific) are the best characterized (Beavo, 1995). However, a novel isoform called PDE9A has been recently described which, along with PDE5, is specific for cyclic GMP (Soderling & Beavo, 2000). In vascular smooth (and cardiac) muscles the main isoforms present are PDE1, PDE3, PDE4 and PDE5, while very small amounts of PDE2 are also present (Polson & Strada, 1996). In this way a cross-talk between cyclic GMP and cyclic AMP is possible in vivo. Although in vascular smooth muscle cyclic nucleotides cause vasodilatation by similar mechanisms, in cardiac muscle, cyclic AMP and low levels of cyclic GMP produce a positive inotropic effect (Kojda et al., 1997; Kojda & Kottenberg, 1999; Ohba & Kawata, 1999). Several previously synthesized PDE inhibitors, such as milrinone (a PDE3 selective inhibitor), have been indicated for the acute treatment of heart failure due to their positive inotropic and peripheral vasodilatory properties. In the present study we report that LASSBio 294, a newly synthesized agent with positive inotropic effects (Albuquerque et al., 1999), exerts vasodilatory properties in rat aorta, probably via an increase of cyclic GMP. Preliminary results have been previously presented (XVI Latin American Congress of Pharmacology, Brazil, September 2000).
Methods
The investigation was in accord with the local ethics committee for animal care (Federal University of Rio de Janeiro). Male Wistar rats (250 – 300 g) were anaesthetized with ether and killed by decapitation. The thoracic aorta was quickly removed, placed in physiological solution (composition (mM): NaCl 122, KCl 5, NaHCO3 15, glucose 11.5, MgCl2 1.25, CaCl2 1.25 and KH2PO4 1.25) bubbled with 95% O2/5% CO2 and maintained at 37°C. Tissues were cleaned of connective tissue and cut into rings. In some experiments endothelium was removed by mechanical rubbing of the intimal surface.
Measurement of contractile responses
Aortic rings (3 mm wide) were fixed in an organ bath chamber filled with physiological solution under a resting tension of 20 mN, and left to equilibrate for 60 min during which time the physiological solution was changed once. The developed active tension was measured isometrically using a Grass Transducer (FT-03). Data were acquired and analysed using Chart 3.4/s software (MacLab, U.S.A). In K+-rich depolarising solutions an isosmotic replacement of NaCl was performed. Tissues were precontracted by 1 μM noradrenaline (intact vessels) and the relaxant drugs (or solvent; time-matched control) were added at the plateau of the tonic contraction. For the rings which had been pretreated with L-NAME or indomethacin, final concentrations of noradrenaline of 0.3 and 3 μM, respectively, were used to keep the same level of active force as the non-treated tissues. The first contraction evoked by 1 μM noradrenaline was used to check the endothelial integrity; vessels that relaxed about 50% in response to 1 μM acetylcholine were considered as possessing an intact endothelium (Salomone et al., 1996). After wash-out the vessels were left to equilibrate for a further 60 min.
Cyclic GMP content determination
Intact aortic rings (5 mm wide) were placed in aerated physiological solution at 37°C and left to equilibrate for 30 min in the presence of 100 μM L-NAME and 10 μM indomethacin. The rings were then randomly divided into 3 groups and incubated for 10 min with 100 μM LASSBio 294 or 1 μM zaprinast. Following this, the rings were immediately frozen in liquid nitrogen to prevent cyclic GMP degradation. For the extraction of cyclic GMP, rings were homogenized in cold trichloroacetic acid (6%) and the homogenate centrifuged (2000×g for 15 min at 4°C). The pellet was used to determine protein content according to a method adapted for insoluble proteins (Lowry et al., 1951). The supernatant was washed four times with five volumes of water-saturated diethyl ether. The upper layer was discharged after each wash. The aqueous phase was then evaporated to dryness (Delpy et al., 1996). The dried extract was dissolved in a suitable volume of assay buffer and cyclic GMP content determined colorimetrically using an enzyme immunoassay kit (Amersham Pharmacia Biotech, U.S.A). Data are expressed as fmol of cyclic GMP μg−1 of protein.
PDE 3 and 4 activity
An assay for phosphodiesterase inhibitors was designed, using rat or rabbit heart membrane preparations enriched in PDE 3 or PDE 4 isoforms, respectively (Shahid et al., 1990), as further validated by the difference in the maximal inhibition exerted by rolipram, a selective PDE 4 inhibitor (see Figure 7). Cyclic AMP-PDE activity was measured using [3H]-cyclic AMP as a substrate and a two-step procedure for isolation and quantification of [3H]-adenosine (Cheffoy de courcelles et al., 1992).
Figure 7.
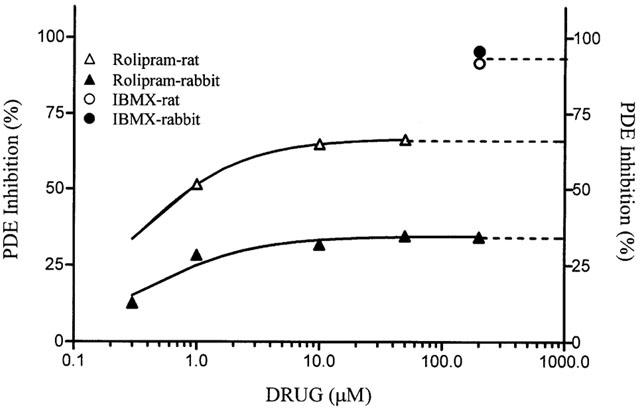
Cyclic AMP-dependent phosphodiesterase inhibition by rolipram. Cyclic AMP-dependent phosphodiesterase activity present either in rat or rabbit heart membrane preparations was measured in the presence of increasing concentrations of rolipram, a PDE4 selective inhibitor, or in the presence of a saturating concentration of IBMX, a non-selective PDE inhibitor.
Drugs
LASSBio 294, synthesized at the LASSBio laboratory of the Federal University of Rio de Janeiro (Albuquerque et al., 1999), was dissolved in 100% dimethylsulphoxide (DMSO) to obtain a 100 mM stock solution. The final concentration of DMSO in the organ bath never exceeded 0.1% (v v−1). Noradrenaline, NG-nitroarginine methyl ester (L-NAME), IBMX, methylene blue, acetylcholine and indomethacin were purchased from SIGMA Chemical Co. (U.S.A). ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one) and zaprinast were purchased from Tocris (U.S.A). All drugs were dissolved in water on the day of the experiment, except indomethacin which was dissolved in 5% sodium carbonate.
Statistical analysis
Results of the experiments are expressed as arithmetic means±s.e.mean (% of effect) or geometric means with 95% confidence intervals (IC50). Drug concentrations producing 50% inhibition of the responses (IC50) were calculated independently for each experiment by non-linear regression analysis (GraphPad Prism, GraphPad software Inc. San Diego, CA, U.S.A). A Student's t-test was performed using Primer software (McGraw-Hill Co., New York, NY, U.S.A). Vasodilator responses are expressed as percentages of the maximal contraction induced by noradrenaline. Differences were considered significant at P<0.05.
Results
Aortic relaxation induced by LASSBio 294
In noradrenaline-precontracted aortic rings with a functional endothelium LASSBio 294 (30 – 300 μM) induced a concentration-dependent relaxation (Figure 2) with an inhibitory concentration (IC50) of 74 μM (95% confidence limit 59 – 92, n=8). The time required to reach the maximal effect at each concentration was about 10 min. In respect of this we selected the concentration of 100 μM, close to the IC50 value, for future experiments. Since this relaxation might have been due to the release of endothelial NO we investigated the role of the functional endothelium. In endothelium-denuded aorta, as validated by the absence of relaxant responses to 1 μM acetylcholine, LASSBio 294-induced relaxation was almost completely abolished indicating an endothelial contribution to its effect (Figure 3). IBMX (100 μM), a non-selective PDE inhibitor, almost fully relaxed these vessels indicating that PDE activity and related relaxation pathways were functional in these rubbed aorta (Figure 3). When aortic rings with functional endothelium were pre-contracted with 100 mM KCl, LASSBio294 produced a similar relaxation (35.5±5.0%, n=8, P<0.05 versus maximal contraction and DMSO) to that observed when 1 μM noradrenaline was used as the contracting agent (49.5±5.9%, n=9). This result indicates that hyperpolarization of myocytes due to the release of endothelium-derived hyperpolarizing factor (EDHF) is not a possible mechanism to explain the relaxation induced by LASSBio 294. In order to establish the possible involvement of the L-arginine/nitric oxide (NO) pathway we pre-treated aortic rings with the nitric oxide synthase (NOS) inhibitor L-NAME (500 μM) for 30 min before inducing the contraction of intact aorta with 0.3 μM noradrenaline. Since this treatment did not alter the relaxation induced by 100 μM LASSBio 294 (Figure 4), we conclude that there is no direct role for basal NO in this effect. Since PGI2 released by endothelial cells could be another mediator involved in the relaxation response of LASSBio 294 we also tested the influence of cyclo-oxygenase pathway inhibition by pre-incubating the aorta with 10 μM indomethacin. As previously observed for L-NAME, the pre-treatment with indomethacin did not alter the relaxant effect of LASSBio 294 (Figure 4).
Figure 2.
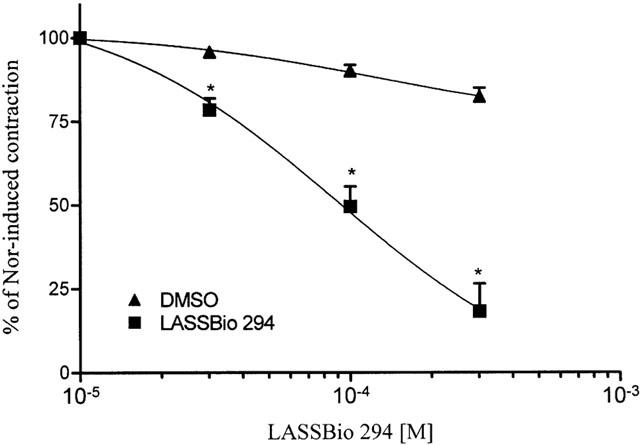
Concentration-response curve for LASSBio 294 in endothelium-intact rat aorta precontracted with noradrenaline. LASSBio 294 or its solvent DMSO (0.01 – 0.3%) were added at the plateau of the contraction induced by 1 μM noradrenaline (Nor) and left for 10 min to achieve a maximal effect. Curves were fitted by non-linear regression analysis using the one-site competition equation. Top and bottom were fixed at 100 and 0, respectively. P<0.05 (n=8).
Figure 3.
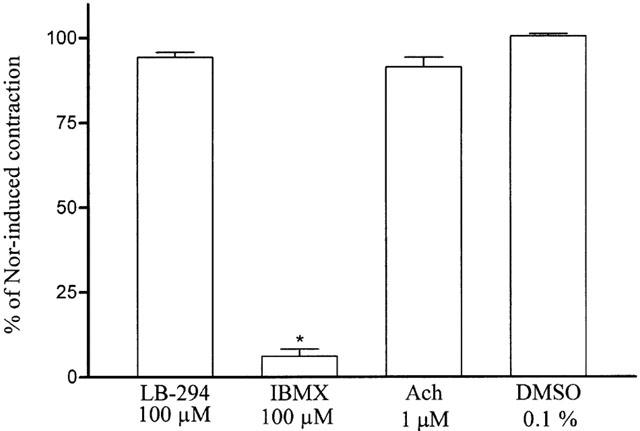
Effect of 100 μM LASSBio 294 in endothelium-denuded rat aorta contracted with noradrenaline. LASSBio 294 (LB-294), IBMX, acetylcholine (Ach) or DMSO were added at the plateau of the contraction induced by 1 μM noradrenaline (Nor) and left for 10 min. The endothelium was removed by mechanical rubbing. *P<0.001 in relation to contraction induced by noradrenaline and DMSO (n=9).
Figure 4.
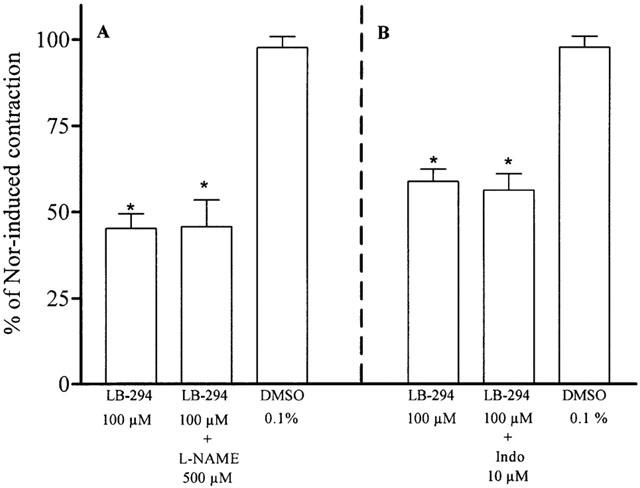
Effect of L-NAME and indomethacin on LASSBio 294-induced vasorelaxant effect. The effect of LASSBio 294 was observed before and after the incubation of intact aortic rings with L-NAME (A) or indomethacin (Indo) (B) for 30 min. The contraction was induced by 0.3 or 3 μM noradrenaline (Nor), respectively. LASSBio 294 (LB-294) or DMSO were then added at the plateau and left for 10 min. *P<0.001 in relation to DMSO (n=7 – 9).
Inhibition of soluble guanylate cyclase reverses LASSBio 294-induced relaxant effects
After discarding NO, EDHF and PGI2 as possible candidates involved in the vascular relaxant action of LASSBio 294, we investigated the possible involvement of cyclic nucleotides. The addition of 1 μM ODQ, a selective inhibitor of soluble guanylate cyclase, reversed (Figure 5) and also prevented (data not shown) the relaxant effect of 100 μM LASSBio 294, indicating that this action might be due to an increase in cyclic GMP.
Figure 5.
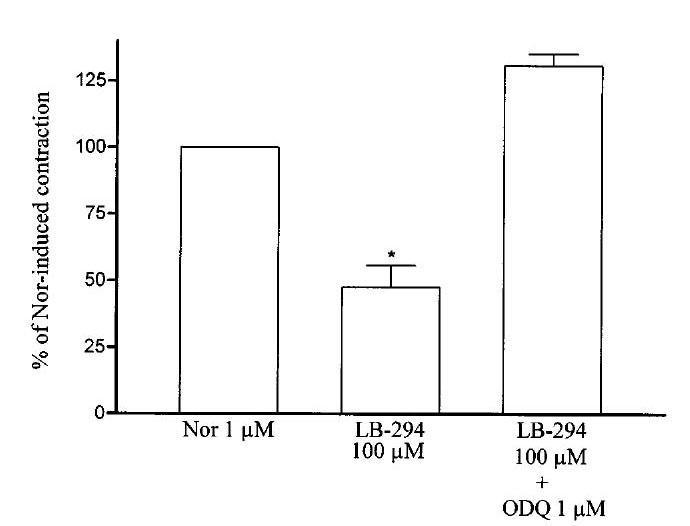
Effect of ODQ on rat aortic relaxation induced by LASSBio 294. LASSBio 294 (LB-294) was added at the plateau of the contraction induced by 1 μM noradrenaline (Nor) and left for 10 min. ODQ was then added and the aortic ring left again for 10 min before new measurement of the tension. *P<0.001 in relation to noradrenaline (n=4).
LASSBio 294 increases cyclic GMP content
Using a direct assay for dosing cyclic GMP content in rat aortic rings incubated essentially under the same conditions used for the contractile measurements, we observed that 100 μM LASSBio 294 increased the aortic cyclic GMP content by 150%, as did 1 μM zaprinast, a selective PDE5 inhibitor used as a positive control (Figure 6).
Figure 6.
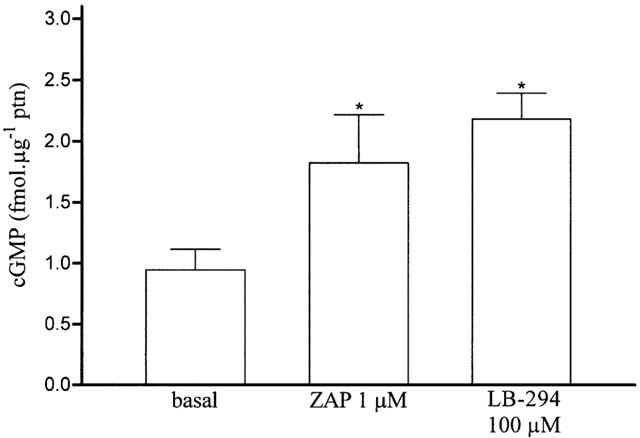
Effect of LASSBio 294 on rat aortic cyclic GMP content. Intact aortic rings (5 mm) were incubated as for tension measurement for 30 min in the presence of 100 μM L-NAME and 10 μM indomethacin. Physiological solution (basal), LASSBio 294 (LB-294) or zaprinast (ZP) were then added for 10 min before tissue freezing and processing for cyclic GMP content measurement (see Methods). P⩽0.05 in relation to basal (n=5).
The effect of LASSBio 294 on PDE activity
Since both the synthetic strategy and present experimental data indicated that PDEs are potential molecular targets of LASSBio 294, we initiated experiments to address this question. At the moment we are able to test a direct inhibition of LASSBio 294 on PDE activity using rat and rabbit heart membrane preparations that were reported to be enriched in PDE3 or PDE4 isoforms, respectively (Shahid et al., 1990). As shown in Figure 7, rolipram maximally inhibited 1/3 and 2/3 of cyclic AMP specific PDE activity in rabbit and rat preparations, respectively, indicating a low contribution of PDE4 (rolipram-sensitive, cyclic AMP or cyclic GMP hydrolyzing PDE) isoform in rabbit (in which PDE3 is supposed to be the predominant cyclic AMP hydrolyzing PDE). On the other hand, PDE4 should be the principal isoform contributing for cyclic AMP hydrolysis in the rat heart particular preparation. Note that a high concentration of the non-selective inhibitor IBMX fully inhibited the cyclic AMP-dependent PDE activity in both preparations (Figure 7). When tested at 100 μM in these two preparations, LASSBio 294 had no significant effect (n=3), indicating that it lacks a direct effect on two of the most important PDE isoforms found in vascular smooth muscle.
Discussion
In the present study we investigated the effect of LASSBio 294, an original synthetic drug designed to inhibit phosphodiesterase, in vascular smooth muscle. Using a classical model for screening drug effects on isolated vessels, we report here that LASSBio 294 relaxes intact aortic rings in a concentration- and endothelium-dependent manner. However, despite the endothelial dependence, LASSBio 294 (100 μM) was able to relax vessels pre-treated with either the nitric oxide inhibitor (NOS) L-NAME or the cyclo-oxygenase inhibitor indomethacin, a fact that leads us to exclude any dependence of LASSBio 294 effect on the basal release of either endothelial NO or prostanoids, classical mediators of vasodilating agents (Cocks, 1996). Endothelial cells might also release a not yet identified endothelium-derived hyperpolarizing factor (EDHF), even if this appears not to be the main endothelial relaxant mediator in rat thoracic aorta (Cocks, 1996). Hyperpolarization could also occur via activation of the large conductance K+ channel (BKCa) (Bolotina et al., 1994), which along with the ATP-sensitive K+ channel (KATP) is the main channel/current present in vascular smooth muscle cells. Therefore, we investigated the effect of LASSBio 294 in intact vessels precontracted with 100 mM extracellular K+. In the presence of raised extracellular potassium the opening of these channels and subsequent hyperpolarization of the vascular myocyte is impaired (Magnon et al., 1998), and such conditions are also known to inhibit the L-NAME-resistant endothelium-dependent relaxation generally attributed to EDHF (Cowan et al., 1993). LASSBio 294 was able to relax KCl-precontracted rings in a similar concentration range to that observed for tissues precontracted with noradrenaline. Based on these data we excluded any direct hyperpolarizing effect as the mechanism of LASSBio 294-induced vasodilatation.
We subsequently decided to investigate any possible involvement of cyclic nucleotides in respect of their extensive regulatory role in the cardiovascular system (Delpy et al., 1996; Verde et al., 1999). The relaxation induced by LASSBio 294 was fully reversed by the addition of ODQ, a selective inhibitor of soluble guanylate cyclase, and also prevented by pre-treatment with this inhibitor, indicating that LASSBio 294 may exert its relaxant effect via an increase in aortic cyclic GMP. Interestingly, Delpy & le Monnier de Gouville (1996) showed that DMPPO, a selective inhibitor of cyclic GMP-specific PDE 5, lost its relaxant effect following removal of endothelium, in a similar manner to that observed here with LASSBio 294. Concerning phosphodiesterase activities, although LASSBio 294 had no direct effect on PDE3 and PDE4 isoforms and hence probably did not alter cyclic AMP directly (an inhibition of the PDE1 isoform cannot be discarded at the moment), it was able to increase cyclic GMP levels in intact aortic rings, as did the PDE5 inhibitor zaprinast. Delpy et al. (1996) showed that in vascular smooth muscle the addition of methylene blue, a non-selective inhibitor of soluble guanylate cyclase, impaired isoprenaline-induced relaxation. Since isoprenaline-induced relaxation is mediated by the activation of adenylate cyclase the authors concluded that when a tissue expresses more than one phosphodiesterase isoform there might be a cross talk between cyclic AMP and cyclic GMP. Moreover, they showed that the inhibition of PDE5 caused an increase in the aortic levels of cyclic AMP. According to these authors, this increase was due to an indirect inhibition of PDE3, an isoform with a high Vmax for cyclic AMP hydrolysis, which is inhibited by cyclic GMP. This cross talk has been reported in other tissues such as cardiac muscle in which PDE5 is not the main isoform. In this issue a low concentration of glyceryl trinitrate increases cyclic GMP, which preferentially inhibits the PDE3 isoform, improving myocardial contraction through an increase in cyclic AMP and activation of a cyclic AMP-dependent protein kinase (PKA) (Kojda & Kottenberg, 1999). In this way, the present data could be interpreted on the basis of a LASSBio 294-induced inhibition of PDE5. The increase in intracellular levels of cyclic GMP may also indirectly increase cyclic AMP levels in tissues such as rat aorta where PDE3 and PDE5 are co-expressed. Our present hypothesis does not discard other molecular target(s) such as PDE9A, a novel high-affinity cyclic GMP-specific phosphodiesterase isoform recently identified (Soderling & Beavo, 2000). However, its tissue distribution and biological function have yet to be established.
In conclusion, we report here that LASSBio 294 is able to elicit vascular relaxation by increasing intracellular cyclic GMP levels, possibly as a consequence of PDE5 inhibition. Such an effect is potentially useful for decreasing the after load in the treatment of cardiac congestive failure, in conjunction with its inotropic effect.
Acknowledgments
The authors thank the following institutions for financial support: PRONEX (FINEP 41960888-CNPq 661420/1996-5), FUJB (8992-3), CNPq, & FAPERJ. The authors also thank Eliana Freitas for technical assistance.
Abbreviations
- IBMX
3-Isobutyl-1-methylxanthine
- L-NAME
NG-nitroarginine methyl ester
- ODQ
1H-(1,2,4)oxadiazolo[4,3-a]quinoxaline-1-one
References
- ALBUQUERQUE E.X., BARREIRO E.J., SUDO R.T.LASSBio 294 A novel digitalis-like compound with potential anti-fatigue activity US Patent Office 199917024lProvisional Number
- BEAVO J.A. Cyclic nucleotide phosphodiesterases: Functional implications of multiple isoforms. Physiol. Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- BOLOTINA V.M., NAJIBI S., PALACINO J.J., PAGANO P.J., COHEN R.A. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- BOLZ S-S., WIT C., POHL U. Endothelium-derived hyperpolarizing factor but not NO reduces smooth muscle Ca2+ during acetylcholine-induced dilation of microvessels. Br. J. Pharmacol. 1999;128:124–134. doi: 10.1038/sj.bjp.0702775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEFFOY DE COURCELLES D., DE LOORE K., FREYNE E., JANSSEN P.A.J. Inhibition of human cardiac cyclic AMP-phosphodiesterases by R 80122, a new selective cyclic AMP-phosphodiesterase III inhibitor: a comparison with other cardiotonic compounds. J. Pharmacol. Exp. Ther. 1992;263:6–14. [PubMed] [Google Scholar]
- COCKS T.M.Endothelium-dependent vasodilator mechanisms Pharmacology of vascular smooth muscle 1996Oxford University Press, New York; 233–251.ed. Garland, C. J. & Angus, J. A. pp [Google Scholar]
- COWAN C.L., PALACINO J.J., NAJIBI S., COHEN R.A. Potassium channel-mediated relaxation to acetylcholine in rabbit arteries. J. Pharmacol. Exp. Ther. 1993;266:1482–1489. [PubMed] [Google Scholar]
- DELPY E., LE MONNIER DE GOUVILLE A.-C. Cardiovascular effects of a novel, potent and selective phosphodiesterase 5 inhibitor, DMPPO: in vitro and in vivo characterisation. Br. J. Pharmacol. 1996;118:1377–1384. doi: 10.1111/j.1476-5381.1996.tb15548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELPY E., COSTE H., LE MONNIER DE GOUVILLE A.-C. Effects of cyclic GMP elevation on isoprenaline-induced increase in cyclic AMP and relaxation in rat aortic smooth muscle: role of phosphodiesterase. Br. J. Pharmacol. 1996;119:471–478. doi: 10.1111/j.1476-5381.1996.tb15696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FIGUEIREDO J.M., CAMARA C.A., AMARANTE E.G., MIRANDA A.L.P., SANTOS F.M., RODRIGUES C.R., FRAGA C.A.M., BARREIRO E.J. Design and synthesis of novel potent antinociceptive agents: N-methyl-imidazolyl N-acylhydrazone derivatives. Bioorg. Med. Chem. Lett. 2000;8:2243–2248. doi: 10.1016/s0968-0896(00)00152-8. [DOI] [PubMed] [Google Scholar]
- HOBBS A.J. Soluble guanylate cyclase: The forgotten sibling. Trends Pharmacol. Sci. 1997;18:484–491. doi: 10.1016/s0165-6147(97)01137-1. [DOI] [PubMed] [Google Scholar]
- KOJDA G., KOTTENBERG K., NOACK E. Inhibition of nitric oxide and soluble guanylate cyclase induces cardiodepressive effects in normal rat hearts. Eur. J. Pharmacol. 1997;334:181–190. doi: 10.1016/s0014-2999(97)01168-0. [DOI] [PubMed] [Google Scholar]
- KOJDA G., KOTTENBERG K. Regulation of basal myocardial function by NO. Cardiovasc. Res. 1999;41:514–523. doi: 10.1016/s0008-6363(98)00314-9. [DOI] [PubMed] [Google Scholar]
- KWAN H.Y., HUANG Y., YAO X. Store-operated calcium entry in vascular endothelial cells is inhibited by cGMP via a protein kinase G-dependent mechanism. J. Biol. Chem. 2000;275:6758–6753. doi: 10.1074/jbc.275.10.6758. [DOI] [PubMed] [Google Scholar]
- LOHMANN S. M., VAANDRAGER A. B., SMOLENSKI A., WALTER U., DEJONGE H.R. Distinct and specific functions of c-GMP-dependent protein kinases. Trends Biochem. Sci. 1997;22:307–312. doi: 10.1016/s0968-0004(97)01086-4. [DOI] [PubMed] [Google Scholar]
- LOWRY A.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MAGNON M., CALDERONE V., FLOCH A., CAVERO I. Influence of depolarisation on vasorelaxant potency and efficacy of Ca2+ entry blockers, K+ channel openers, nitrate derivatives, salbutamol and papaverine in rat aortic rings. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:452–463. doi: 10.1007/pl00005278. [DOI] [PubMed] [Google Scholar]
- OHBA M., KAWATA H. Biphasic nature of inotropic action of nitric oxide donor NOC7 in guinea-pig ventricular trabeculae. Jpn. J. Physiol. 1999;49:389–394. doi: 10.2170/jjphysiol.49.389. [DOI] [PubMed] [Google Scholar]
- PFEIFER A., RUTH P., DOSTMANN W., SAUSBIER M., KLATT P., HOFMANN F. Structure and function of cGMP-dependent protein kinases. Rev. Physiol. Biochem. Pharmacol. 1999;135:105–149. doi: 10.1007/BFb0033671. [DOI] [PubMed] [Google Scholar]
- POLSON J.B., STRADA S.J. Cyclic nucleotide phosphodiesterases and vascular smooth muscle. Ann. Rev. Pharmacol. Toxicol. 1996;36:403–427. doi: 10.1146/annurev.pa.36.040196.002155. [DOI] [PubMed] [Google Scholar]
- SALOMONE S., SILVA C.L.M., MOREL N., GODFRAIND T. Facilitation of the vasorelaxant action of calcium antagonists by basal nitric oxide in depolarised artery. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;354:505–512. doi: 10.1007/BF00168443. [DOI] [PubMed] [Google Scholar]
- SHAHID M., WILSON M., NICHOLSON C.D., MARSHALL R.J. Species-dependent differences in the properties of particulate cyclic nucleotide phosphodiesterase from rat and rabbit ventricular myocardium. J. Pharm. Pharmacol. 1990;42:283–284. doi: 10.1111/j.2042-7158.1990.tb05409.x. [DOI] [PubMed] [Google Scholar]
- SODERLING S.H., BEAVO J.A. Regulation of cAMP and cGMP signalling: new phosphodiesterases and new functions. Curr. Opin. Cell Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- SOFF G.A., CORNWELL T.L., CUNDIFF D.L., GATELY S., LINCOLN T.M. Smooth muscle cell expression of type I cyclic GMP-dependent protein kinase is supressed by continuous exposure to nitrovasodilators, theophyline, cyclic GMP and cyclic AMP. J. Clin. Invest. 1997;100:2580–2587. doi: 10.1172/JCI119801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERDE I., VANDECASTEELE G., LEZOUALC'H F., FISCHMEISTER R. Characterisation of the cyclic nucleotide phosphodiesterase subtypes involved in the regulation of the L-type Ca2+ current in rat ventricular myocytes. Br. J. Pharmacol. 1999;127:65–74. doi: 10.1038/sj.bjp.0702506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIDA Y., TOYOSATO A., ISLAM M.O., KOGA T., FUJITA S., IMAL S. Stimulation of plasma membrane Ca2+-pump ATPase of vascular smooth muscle by cGMP-dependent protein kinase: functional reconstitution with purified proteins. Mol. Cell Biochem. 1999;190:157–167. [PubMed] [Google Scholar]


