Abstract
We recently reported that nociceptin/orphanin FQ (N/OFQ) inhibited forskolin-stimulated adenylyl cyclase activity and increased basal enzyme activity in membranes of the external plexiform layer (EPL) and granule cell layer (GRL), respectively, of the rat main olfactory bulb. In the present study we have characterized the pharmacological profile of the inhibitory and stimulatory responses by examining the effects of various N/OFQ receptor agonists and antagonists.
N/OFQ(1 – 13)NH2 fully mimicked the inhibitory and stimulatory effects of N/OFQ with EC50 values of 0.9 and 6.5 nM, respectively. N/OFQ(1 – 7) was inactive at concentrations up to 1 μM, whereas Ac-RYYRIK-NH2 and [Phe1Ψ(CH2NH)Gly2]N/OFQ(1 – 13)-NH2 behaved as partial agonists in eliciting both responses.
The nonpeptidyl N/OFQ receptor antagonist J-113397 competitively counteracted the inhibitory and stimulatory effects of N/OFQ with pA2 values of 8.63 and 8.70, respectively. Similarly, the peptidyl antagonist [Nphe1]N/OFQ(1 – 13)NH2 potently antagonized the two effects with pA2 values of 8.03 and 8.45, respectively. None of the antagonists per se affected adenylyl cyclase activity.
These data show that in distinct layers of rat olfactory bulb both the inhibitory and stimulatory effects of N/OFQ on cyclic AMP formation display pharmacological properties consistent with the involvement of N/OFQ receptors.
Keywords: Nociceptin/orphanin FQ, nociceptin/orphanin FQ receptor agonists and antagonists, cyclic AMP, olfactory bulb
Introduction
Nociceptin, also named orphanin FQ, (N/OFQ) is a naturally occurring heptadecapeptide (Meunier et al., 1995; Reinscheid et al., 1995), which acts as an agonist of a G protein-coupled receptor termed ORL1 (human), MOR-C (mouse), LC132, ROR-C, XOR1, C3, and Hyp 8-1 (rat) by different investigators (for review, see Meunier, 2000; Calo' et al., 2000a). This receptor displays sequence homology to opioid receptors but does not bind classical opioid receptor ligands with high affinity. Like opioid receptors, the N/OFQ receptor has been shown to couple to G proteins of the Gi/Go family and to activate similar signal transduction pathways, including inhibition of forskolin (FSK)-stimulated adenylyl cyclase activity and voltage-activated calcium channels and opening of potassium channels (Meunier, 2000). The N/OFQ receptor shows a wide distribution in peripheral tissues and in different brain areas (Anton et al., 1996; Mollereau & Mouledous, 2000), suggesting the involvement in multiple physiological processes. Indeed, in vivo and in vitro studies have demonstrated that N/OFQ has a pleiotropic activity, regulating pain sensitivity, locomotion, food intake, learning and memory and emotional behavior (Meunier, 2000; Calo' et al., 2000a; Darland et al., 1998). In rodents, it has been found that N/OFQ exerts a bidirectional modulation of morphine-induced analgesia, causing antagonism at a supraspinal level and potentiation at the spinal cord (Tian et al., 1997). Moreover, N/OFQ elicits opposite effects on nociception and locomotion depending on the dose (Inoue et al., 1999; Nakano et al., 2000; Florin et al., 1996; Reinscheid et al., 1995; Jenk et al., 1997). This multiplicity of effects suggests a complex mechanism of action, possibly including the occurrence of receptor heterogeneity and the generation of multiple intracellular signals.
Although N/OFQ receptors have been initially characterized by their ability to inhibit cyclic AMP formation, we have recently observed that in distinct layers of the rat main olfactory bulb, N/OFQ elicited opposite effects on adenylyl cyclase activity (Onali et al., 2001). Thus, in membranes isolated from the glomerular layer N/OFQ inhibited basal adenylyl cyclase activity and the enzyme stimulations by corticotropin-releasing hormone, Ca2+/calmodulin and FSK. In membranes of the external plexiform layer (EPL) N/OFQ failed to affect basal adenylyl cyclase activity but markedly inhibited FSK-stimulated cyclic AMP formation, whereas in membranes of the granule cell layer (GRL) the peptide stimulated basal adenylyl cyclase activity and inhibited Ca2+/calmodulin- and FSK-stimulated enzyme activities. Evidence has been provided that the dual regulation likely results from the modulation of the activities of βγ-sensitive and insensitive adenylyl cyclase isoforms, which are differentially expressed in the olfactory bulb layers (Onali et al., 2001).
Because of the peculiarity of N/OFQ effects on cyclic AMP formation in the olfactory bulb layers and the presence in these areas of opioid receptors similarly coupled to cyclic AMP (Olianas & Onali, 1994), it was important to assess that the effects were mediated by specific N/OFQ receptors. In the present study, we used various N/OFQ receptor agonists and antagonists to investigate the pharmacological properties of N/OFQ-induced inhibition of FSK-stimulated adenylyl cyclase activity in EPL membranes and of N/OFQ-induced stimulation of basal enzyme activity in GRL membranes. Part of this study has previously been presented in an abstract form (Olianas et al., 2000).
Methods
Microdissection of olfactory bulb and membrane preparation
Male Sprague-Dawley rats (200 – 300 g) were used. Animals were maintained in a 12 h light/dark cycle with food and water ad libitum. Experiments were performed according to the principles of laboratory animal care (Law on animal experiments in Italy, D.L. 116/92). Rats were killed by decapitation and the olfactory bulbs were rapidly removed and immersed into an ice-cold phosphate-buffered saline solution (pH 7.4). With the use of a tissue slicer the bulbs were cut into 300 μm thick coronal sections, which were kept in the same saline solution at ice-bath temperature. Each slice was then placed on a cooled glass slide and with the aid of a stereoscopic microscope equipped with a diascopic illuminator base, the EPL and the GRL were free-hand dissected. The tissue layers from individual slices were pooled and homogenized in an ice-cold buffer containing (in mM): HEPES-NaOH 10, EGTA 1, MgCl2 1 and dithiothreitol (DTT) 1 (pH 7.40) using a teflon-glass tissue grinder. For two olfactory bulbs, the dissection procedure lasted up to a maximum of 40 – 50 min. The homogenate was centrifuged at 27,000×g for 20 min at 4°C. The pellet was resuspended in the same buffer at a protein concentration of 0.8 – 1.0 mg ml−1 and used immediately for adenylyl cyclase assays. For each experiment, a fresh tissue preparation was used.
Adenylyl cyclase assay
The adenylyl cyclase activity was measured by monitoring the conversion of [α-32P]ATP to [32P]cyclic AMP. The reaction mixture (final volume 100 μl) contained 50 mM HEPES/NaOH (pH 7.4), 2.3 mM MgCl2, 0.3 mM DTT, 0.3 mM EGTA, 0.2 mM [α-32P]ATP (50 c.p.m. pmol−1), 0.5 mM [3H]cyclic AMP (80 c.p.m. nmol−1), 1 mM 3-isobutyl-1-methylxanthine, 5 mM phosphocreatine, 50 u/ml creatine phosphokinase, 100 μM GTP, 50 μg of bovine serum albumin (BSA), 10 μg of bacitracin, 10 μM bestatin and 10 kallikrein inhibitor units of aprotinin. When FSK was used, it was dissolved in dimethylsulphoxide and included in the reaction mixture at the final concentration of 10 μM. Dimethylsulphoxide, at the final concentration of 0.5%, failed to affect adenylyl cyclase activity. The reaction was started by adding the tissue preparation (30 – 40 μg of protein) and was carried out at 30°C for 10 min. The reaction was stopped by adding 200 μl of a solution containing 2% of sodium dodecyl sulphate, 45 mM ATP and 1.3 mM cyclic AMP (pH 7.5). Cyclic AMP was isolated by sequential chromatography on Dowex 50W-X4 and on neutral alumina as described by Salomon et al. (1974). The recovery of [32P] cyclic AMP from each sample was calculated on the basis of the recovery of [3H]cyclic AMP. The enzyme activity appeared linear with tissue protein concentration and with time (at least up to 10 min). Assays were carried out in duplicate. Protein content was determined by the method of Bradford (1976).
Statistical analysis
Results are reported as mean±s.e.mean. Data from agonist concentration-response curves were analysed by a least-squares curve fitting computer programme (Graph Pad Prism, San Diego, CA, U.S.A.) Antagonist pA2 values were calculated from Arunlakshana-Schild regressions (Arunlakshana & Schild, 1959), in which the log of dose ratios – 1 (DR-1) is plotted as a function of the antagonist concentration. In each experiment, agonist concentration-response curves were constructed in the presence of either vehicle (control) or at least three different antagonist concentrations ranging 30 – 100 fold. For each antagonist the pA2 values were calculated by using the PHARM/PCS programme of Tallarida & Murray (1987). Statistical significance of the difference between mean values was determined by Student's t-test.
Materials
[α-32P]ATP (30 – 40 Ci mmol−1) and [2,8-3H]cyclic AMP (25 Ci mmol−1) were from New England Nuclear (Boston, MA, U.S.A.) N/OFQ, N/OFQ(1 – 13)NH2 and [F/G]N/OFQ were obtained from Neosystem (Strasbourg, France). J-113397 (1-[(3R, 4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzamidazol-2-one) was kindly provided by Dr S. Ozaki, Banyu Tsukuba Research Institute, Tsukuba, Japan. [Nphe1]N/OFQ(1 – 13)NH2 (Nphe) was either generously provided by Drs G. Calo' and R. Guerrini, University of Ferrara, Italy, or purchased from Neosystem. Ac-RYYRIK-NH2 and N/OFQ(1 – 7) were from Tocris (Bristol, U.K.) FSK was from Calbiochem, (La Jolla, CA, U.S.A.) Agonist and antagonists solutions were freshly prepared in 0.1% BSA from concentrated stock solutions just before the beginning of the experiment. Aprotinin, bestatin, bacitracin and the other reagents were from Sigma Chemical Company (St. Louis, MO, U.S.A.)
Results
Effects of N/OFQ receptor agonists
In membranes of EPL, N/OFQ inhibited FSK-stimulated adenylyl cyclase activity in a concentration-dependent manner (Figure 1). N/OFQ(1 – 13)NH2 was as potent and effective as N/OFQ, whereas N/OFQ(1 – 7) was completely inactive at concentrations up to 1 μM. [F/G]N/OFQ and Ac-RYYRIK-NH2 elicited a concentration-dependent inhibition of adenylyl cyclase activity, but their maximal effects were lower than that produced by N/OFQ (Table 1).
Figure 1.
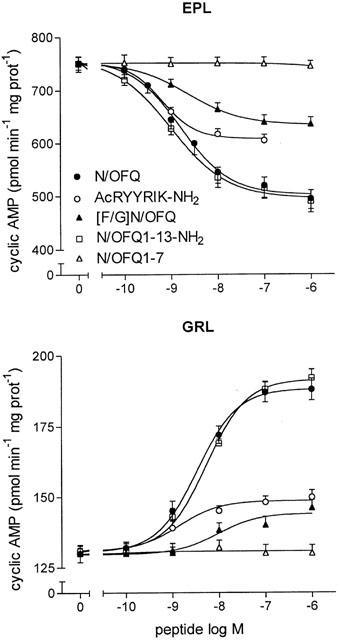
Concentration-dependent effects of N/OFQ receptor agonists on FSK-stimulated and basal adenylyl cyclase activities in EPL and GRL membranes, respectively. Values are the mean±s.e.mean of three to eight experiments.
Table 1.
Properties of N/OFQ receptor agonists and antagonists in regulating adenylyl cyclase (a.c.) activity in distinct layers of rat olfactory bulb
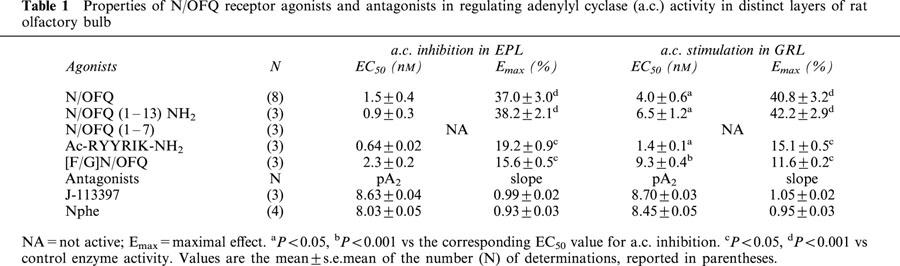
In membranes of GRL, N/OFQ produced a concentration-dependent and saturable stimulation of basal adenylyl cyclase activity (Figure 1). N/OFQ(1 – 13)NH2 fully mimicked the stimulatory effect of N/OFQ, whereas N/OFQ(1 – 7) was inactive. Both [F/G]N/OFQ and Ac-RYYRIK-NH2 produced an increase of adenylyl cyclase activity with maximal effects lower than that of N/OFQ (Table 1). A comparison of the agonists EC50 values indicated that each compound was significantly more potent in inhibiting FSK-stimulated adenylyl cyclase activity in EPL than in stimulating the basal enzyme activity in GRL (Table 1).
Effects of N/OFQ receptor antagonists
As shown in Figure 2, in EPL membranes the addition of increasing concentrations of the nonpeptidyl N/OFQ receptor antagonist J-113397 (Kawamoto et al., 1999; Ozaki et al., 2000) caused a progressive shift to the right of the N/OFQ concentration-response curve in inhibiting adenylyl cyclase activity. Schild analysis of J-113397 antagonism yielded a pA2 value of 8.63. In GRL membranes, J-113397 counteracted the N/OFQ stimulation of adenylyl cyclase activity with a similar potency (Table 1). The pseudopeptide antagonist Nphe (Guerrini et al., 2000; Calo' et al., 2000b) was quite potent in antagonizing the dual regulation of cyclic AMP by N/OFQ (Figure 3). In EPL and GRL membranes, Nphe blocked the N/OFQ inhibitory and stimulatory effects, respectively, with potencies in the low nanomolar range (Table 1). At the concentrations used, both J-113397 and Nphe failed per se to affect cyclic AMP formation.
Figure 2.
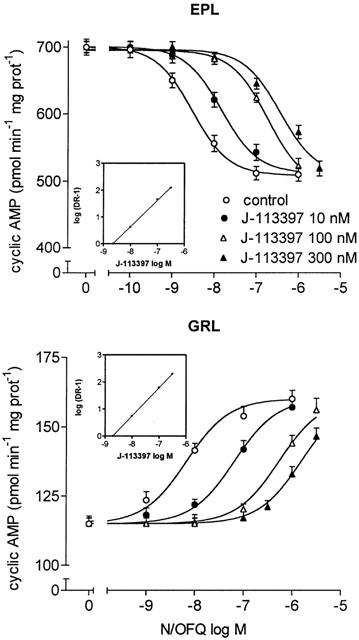
Antagonism by J-113397 of N/OFQ inhibition and stimulation of cyclic AMP formation in distinct layers of rat olfactory bulb. FSK-stimulated and basal adenylyl cyclase activities were assayed in EPL and GRL membranes, respectively, at the indicated concentrations of N/OFQ in the absence (control) and in the presence of different concentrations of J-113397. Values are the mean±s.e.mean of three experiments. Insets: Schild plots of J-113397 antagonism.
Figure 3.
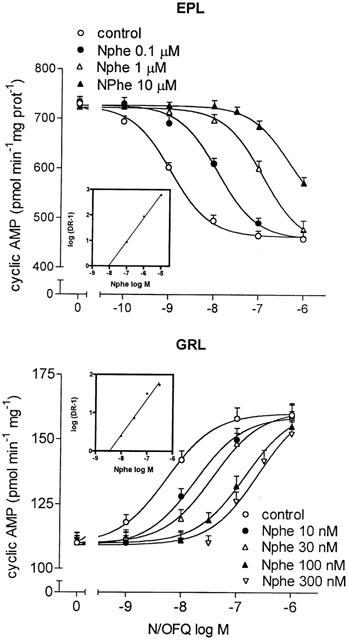
Antagonism by Nphe of N/OFQ inhibition and stimulation of cyclic AMP formation in distinct layers of rat olfactory bulb. FSK-stimulated and basal adenylyl cyclase activities were assayed in EPL and GRL membranes, respectively, at the indicated concentrations of N/OFQ in the absence (control) and in the presence of different concentrations of Nphe. Values are the mean±s.e.mean of four experiments. Insets: Schild plots of Nphe antagonism.
Discussion
The aim of the present study was to investigate the possible involvement of N/OFQ receptors in the N/OFQ-induced inhibition and stimulation of cyclic AMP formation in distinct layers of rat olfactory bulb. Pharmacologically, this issue is relevant for several reasons. In mouse brain membranes, Mathis et al. (1997) previously found that N/OFQ inhibited FSK-stimulated adenylyl cyclase activity by acting on naloxone-sensitive sites, suggesting the involvement of heterogeneous N/OFQ receptors. In membranes prepared from rat cerebral cortex, cerebellum and brain stem, Okawa et al. (1998) reported that N/OFQ failed to affect either basal or FSK-stimulated adenylyl cyclase activity, possibly because of receptor-effector uncoupling during membrane preparation. Moreover, the olfactory bulb expresses μ and δ opioid receptors coupled to both inhibition and stimulation of cyclic AMP (Olianas & Onali, 1994), a condition that makes crucial the demonstration that specific N/OFQ receptors mediate the dual effects of N/OFQ on cyclic AMP.
The analysis of the effects of different N/OFQ analogues showed that the N-terminal tridecapeptide N/OFQ(1 – 13)NH2 was as potent and effective as N/OFQ in eliciting either the inhibition or the stimulation of adenylyl cyclase activity in EPL and GRL, respectively. On the other hand, the shorter N/OFQ fragment N/OFQ(1 – 7) was completely inactive in both responses. These data agree with the reported pharmacological activity of the two peptides at the cloned ORL1 receptor, N/OFQ(1 – 13)NH2 being a full agonist with a potency (pEC50=9.8) similar to that of N/OFQ (Calo' et al., 2000a) and N/OFQ(1 – 7) displaying negligible affinity and functional activity (Butour et al., 1997).
The acetylated hexapeptide Ac-RYYRIK-NH2 has been identified by Dooley et al. (1997) as a high-affinity (Ki=1.5 nM) N/OFQ receptor ligand from a synthetic combinational library. In functional assays, Ac-RYYRIK acted as a partial agonist (Dooley et al., 1997; Bigoni et al., 1999). In agreement with these data, the present study shows that Ac-RYYRIK-NH2 behaved as a partial agonist both in inhibiting FSK-stimulated cyclic AMP formation in EPL and in stimulating basal cyclic AMP formation in GRL membranes, with potencies (EC50 values of 0.64 and 1.4 nM, respectively) consistent with its affinity for the cloned N/OFQ receptor.
The pseudopeptide [F/G]N/OFQ also displayed a partial agonist activity. Guerrini et al. (1998) originally reported that this synthetic N/OFQ analogue acted as a selective antagonist of N/OFQ receptors in guinea-pig ileum and rat vas deferens, but soon after Butour et al. (1998) found that in CHO cells transfected with the cloned human ORL1 receptor cDNA [F/G]N/OFQ acted as a pure receptor agonist. Subsequently, a number of behavioural and biochemical studies (reviewed by Calo' et al., 2000a) have reported that [F/G]N/OFQ can act either as an antagonist, agonist or partial agonist. We have previously shown that in mouse N1E-115 neuroblastoma cells, which express N/OFQ receptor mRNA and high-affinity N/OFQ binding sites, [F/G]N/OFQ behaved as a partial agonist in stimulating G protein activation and inhibiting cyclic AMP accumulation (Olianas et al., 1999). The present study shows that [F/G]N/OFQ displayed a similar pharmacological activity in eliciting the dual regulation of cyclic AMP in olfactory bulb layers with potencies (EC50 values=2.3 – 9.3 nM) close to that found for the inhibition of cyclic AMP formation in CHO cells expressing the human ORL1 receptor (IC50=7.5 nM) (Butour et al., 1998).
Both full and partial agonists were significantly more potent in inhibiting the FSK-stimulated adenylyl cyclase activity in EPL than in stimulating the basal enzyme activity in GRL. Several factors may account for this difference. Radioligand binding assays have previously demonstrated the presence of a higher density of [3H]N/OFQ binding sites in EPL than in GRL (Onali et al., 2001). Moreover, the two responses appear to be mediated by different molecular mechanisms, which may involve differences in receptor-effector coupling efficiency. The inhibitory effect in EPL is possibly due to attenuation of the activity of adenylyl cyclases type I and V/VI (Onali et al., 2001), which are potently inhibited by G protein βγ subunits and α subunits of Gi/Go, respectively, and highly sensitive to stimulation by FSK (Sunahara et al., 1996). On the other hand, evidence has been provided that in GRL the N/OFQ stimulation is due to activation of adenylyl cyclases type II/IV by βγ subunits acting synergistically with Gs (Onali et al., 2001). It is possible that, to produce sufficient βγ subunits to enhance type II/IV cyclases, N/OFQ receptors need to activate a larger pool of Gi/Go than that required for the inhibitory effect. Thus, differences in receptor density and receptor-effector coupling efficiency may explain the difference in agonist EC50 values for the stimulatory and inhibitory responses.
Two different N/OFQ receptor antagonists, J-113397 and Nphe, were examined for their ability to block N/OFQ-induced inhibition and stimulation of adenylyl cyclase activity in EPL and GRL membranes. Kawamoto et al. (1999) and Ozaki et al. (2000) reported that J-113397 bound with high affinity (IC50=2.3 nM) to the cloned ORL1 receptor and with much lower affinity to either μ, δ or κ opioid receptors (IC50 values > 1 μM). Similar results were obtained by Bigoni et al. (2000), showing that J-113397 behaved as a selective, competitive and potent antagonist of N/OFQ effects in different peripheral organs and cloned ORL1 receptor. Accordingly, in the present study J-113397 counteracted the N/OFQ-induced inhibition and stimulation of cyclic AMP formation with pA2 values of 8.63 and 8.70, which are close to the pA2 values (7.52 – 8.07) reported by Bigoni et al. (2000). Moreover, the present pA2 values agree with the high potency of J-113397 observed by Kawamoto et al. (1999) and Ozaki et al. (2000) in blocking N/OFQ activation of the ORL1 receptor (IC50 values=5.6 – 26 nM). The slope values of the Schild regressions were close to unity, indicating that J-113397 acted as a competitive antagonist.
The pseudopeptide Nphe is also a highly selective antagonist of the N/OFQ receptor, displaying nanomolar affinity for the recombinant receptor (pKi=8.39) and very low affinity for the opioid receptors (Calo' et al., 2000b). In EPL membranes, Nphe antagonized the N/OFQ-induced inhibition of cyclic AMP formation with a pA2 of 8.03, whereas in GRL it counteracted the N/OFQ-induced stimulation of cyclic AMP with a pA2 of 8.45. These values agree quite well with the reported affinity of Nphe for the recombinant N/OFQ receptor. However, they are about 100 fold higher than those previously obtained for the antagonism of N/OFQ inhibition of electrically-induced twitches in peripheral organs (pA2 values=6.0 – 6.4) and FSK-stimulated cyclic AMP accumulation in CHO cells expressing the recombinant N/OFQ receptor (pA2=5.96) (Calo' et al., 2000b). At the present, the reason of this difference is not understood. It is possible that in the olfactory bulb layers the N/OFQ effects on cyclic AMP are mediated by receptors displaying high sensitivity to Nphe. Alternatively, unknown tissue factors or differences in the experimental conditions may be responsible for the discrepancy in the potency of Nphe between the present and the previous studies. Whatever the reason, the Nphe pA2 values obtained in the present study correlate well with the reported affinity constant of Nphe for the recombinant N/OFQ receptor determined in radioligand binding assays (pKi=8.39) (Calo' et al., 2000b), as expected for a receptor antagonist (Kenakin et al., 1992).
In conclusion, the present study shows that in distinct layers of rat main olfactory bulb both the inhibitory and the stimulatory effects of N/OFQ on adenylyl cyclase activity display a pharmacological profile consistent with the involvement of specific N/OFQ receptors. These observations provide the first demonstration that pharmacologically defined N/OFQ receptors generate opposite signals on cyclic AMP formation in the brain and may constitute an important framework for the understanding of the role of N/OFQ in the modulation of olfactory function.
Acknowledgments
We thank Drs G. Calo' and R. Guerrini, University of Ferrara, Italy, for providing Nphe and Dr S. Ozaki, Banyu Tsukuba Research Institute, Tsukuba, Japan, for providing J-113397.
Abbreviations
- BSA
bovine serum albumin
- DTT
dithiothreitol
- EPL
external plexiform layer of the rat main olfactory bulb
- FSK
forskolin
- GRL
granule cell layer of the rat main olfactory bulb
- N/OFQ
nociceptin/orphanin FQ
- Nphe
[Nphe1]N/OFQ(1 – 13)NH2
- ORL1
the cloned human N/OFQ receptor
- [F/G]N/OFQ
[Phe1Ψ(CH2-NH)Gly2]N/OFQ(1 – 13)-NH2
References
- ANTON B., FEIN J., TO T., LI X., SILBERSTEIN L., EVANS C.J. Immunohistochemical localization of ORL-1 in the central nervous system of the rat. J. Comp. Neurol. 1996;368:229–251. doi: 10.1002/(SICI)1096-9861(19960429)368:2<229::AID-CNE5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIGONI R., CALO' G., RIZZI A., DE RISI R., DE RISI C., HASHIMOTO Y., HASHIBA E., LAMBERT D.G., REGOLI D. In vitro characterization of J113397, a non-peptide nociceptin/orphanin FQ receptor antagonist. NS. Arch. Pharmacol. 2000;361:565–568. doi: 10.1007/s002100000220. [DOI] [PubMed] [Google Scholar]
- BIGONI R., GIULIANI S., CALO' G., RIZZI A., GUERRINI R., SALVADORI S., REGOLI D., MAGGI C.A. Characterization of nociceptin receptors in the periphery: in vitro and in vivo studies. NS. Arch. Pharmacol. 1999;359:160–167. doi: 10.1007/pl00005338. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- BUTOUR J.-L., MOISAND C., MAZARGUIL H., MOLLERAU C., MEUNIER J.-C. Recognition and activation of the opioid receptor-like ORL1 receptor by nociceptin, nociceptin analogs and opioids. Eur. J. Pharmacol. 1997;321:97–103. doi: 10.1016/s0014-2999(96)00919-3. [DOI] [PubMed] [Google Scholar]
- BUTOUR J.-L., MOISAND C., MOLLERAU C., MEUNIER J.-C. [Phe1ψ (CH2-NH)Gly2]nociceptin-(1–13)-NH2 is an agonist of the nociceptin (ORL1) receptor. Eur. J. Pharmacol. 1998;349:R5–R6. doi: 10.1016/s0014-2999(98)00273-8. [DOI] [PubMed] [Google Scholar]
- CALO' G., GUERRINI R., BIGONI R., RIZZI A., MARZOLA G., OKAWA H., BIANCHI C., LAMBERT D.G., SALVADORI S., REGOLI D. Characterization of [Nphe1]nociceptin(1–13)NH2, a new selective nociceptin receptor antagonist. Br. J. Pharmacol. 2000b;129:1183–1193. doi: 10.1038/sj.bjp.0703169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALO' G., GUERRINI R., RIZZI A., SALVATORI S., REGOLI D. Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br. J. Pharmacol. 2000a;129:1261–1283. doi: 10.1038/sj.bjp.0703219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARLAND T., HEINRICHER M.M., GRANDY D.K. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- DOOLEY C.T., SPAETH C.G., BERZETEI-GURSKE I.P., CRAYMER K., ADAPA I.D., BRANDT S.R., HOUGHTEN R.A., TOLL L. Binding and in vitro activities of peptides with high affinity for the nociceptin/orphanin FQ receptor, ORL1. J. Pharmacol. Exp. Ther. 1997;283:735–741. [PubMed] [Google Scholar]
- FLORIN S., SUAUDEAU C., MEUNIER J.-C., COSTENTIN J. Nociceptin stimulates locomotion and exploratory behaviour in mice. Eur. J. Pharmacol. 1996;317:9–13. doi: 10.1016/s0014-2999(96)00707-8. [DOI] [PubMed] [Google Scholar]
- GUERRINI R., CALO' G., BIGONI R., RIZZI A., VARANI K., TOTH G., GESSI S., HASHIBA E., HASHIMOTO Y., LAMBERT D.G., BOREA P.A., TOMATIS R., SALVADORI S., REGOLI D. Further studies on nociceptin-related peptides: discovery of new chemical template with antagonistic activity on the nociceptin receptor. J. Med. Chem. 2000;43:2805–2813. doi: 10.1021/jm990075h. [DOI] [PubMed] [Google Scholar]
- GUERRINI R., CALO' G., RIZZI A., BIGONI R., BIANCHI C., SALVADORI S., REGOLI D. A new selective antagonist of the nociceptin receptor. Br. J. Pharmacol. 1998;123:163–165. doi: 10.1038/sj.bjp.0701640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INOUE M., SHIMOHIRA I., YOSHIDA A., ZIMMER A., TAKESHIMA H., SAKURADA T., UEDA H. Dose-related opposite modulation by nociceptin/orphanin FQ of Substance P nociception in the nociceptors and spinal cord. J. Pharmacol. Exp. Ther. 1999;291:308–313. [PubMed] [Google Scholar]
- JENK F., MOREAU J.-L., MARTIN J.R., KILPATRICK G.J., REINSCHEID R.K., MONSMA F.J., NOTHACKER H.-P., CIVELLI O. Orphanin FQ acts as an anxiolitic to attenuate behavioural responses to stress. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14854–14858. doi: 10.1073/pnas.94.26.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAMOTO H., OZAKI S., ITOH Y., MIYAJI M., ARAI S., NAKASHIMA H., KATO T., OHTA H., IWASAWA Y. Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzamidazol-2-one (J-113397) J. Med. Chem. 1999;42:5061–5063. doi: 10.1021/jm990517p. [DOI] [PubMed] [Google Scholar]
- KENAKIN T.P., BOND R.A., BONNER T.I. Definition of pharmacological receptors. Pharmacol. Rev. 1992;44:351–362. [PubMed] [Google Scholar]
- MATHIS J.P., RYAN-MORO J., CHANG A., HOM J.S.H., SCHEINBERG D.A., PASTERNAK G.W. Biochemical evidence for orphanin FQ/nociceptin receptor heterogeneity in mouse brain. Biochem. Biophys. Res. Commun. 1997;230:462–465. doi: 10.1006/bbrc.1996.5867. [DOI] [PubMed] [Google Scholar]
- MEUNIER J.C. The potential therapeutic value of nociceptin receptor agonists and antagonists. Exp. Opin. Ther. Patents. 2000;10:371–388. [Google Scholar]
- MEUNIER J.C., MOLLEREAU C., TOLL L., SUAUDEAU C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.C., FERRARA P., MONSERRAT B., MAZARGUIL H., VASSART G., PARMENTIER M., COSTENTIN J. Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MOLLEREAU C., MOULEDOUS L. Tissue distribution of the opioid receptor-like (ORL1) receptor. Peptides. 2000;21:907–917. doi: 10.1016/s0196-9781(00)00227-8. [DOI] [PubMed] [Google Scholar]
- NAKANO H., MINAMI T., ABE K., ARAI T., TOKUMURA M., IBII N., OKUDA-ASHITAKA E., MORI H., ITO S. Effect of intrathecal nocistatin on the formalin-induced pain in mice versus that of nociceptin/orphanin FQ. J. Pharmacol. Exp. Ther. 2000;292:331–336. [PubMed] [Google Scholar]
- OKAWA H., HIRST R.A., SMART D., MCKNIGHT A.T., LAMBERT D.G. Rat central ORL-1 receptor uncouples from adenylyl cyclase during membrane preparation. Neurosci. Lett. 1998;246:46–52. doi: 10.1016/s0304-3940(98)00228-6. [DOI] [PubMed] [Google Scholar]
- OLIANAS M.C., MAULLU C., CALO' G., GUERRINI G., ONALI P. Potent blockade of neuronal nociceptin receptors coupled to cyclic AMP by [Nphe1]nociceptin(1–13)NH2. Proc. Soc. Neurosci. 2000;26:1159. [Google Scholar]
- OLIANAS M.C., MAULLU C., INGIANNI A., ONALI P. [Phe1ψ(CH2-NH)Gly2]nociceptin-(1–13)NH2 acts as a partial agonist at ORL1 receptor endogenously expressed in mouse N1E-115 neuroblastoma cells. NeuroReport. 1999;10:1127–1131. doi: 10.1097/00001756-199904060-00041. [DOI] [PubMed] [Google Scholar]
- OLIANAS M.C., ONALI P. Activation of opioid and muscarinic receptors stimulates basal adenylyl cyclase but inhibits Ca2+/calmodulin- and forskolin-stimulated enzyme activities in rat olfactory bulb. J. Neurochem. 1994;63:161–168. doi: 10.1046/j.1471-4159.1994.63010161.x. [DOI] [PubMed] [Google Scholar]
- ONALI P., INGIANNI A., OLIANAS M.C. Dual coupling of opioid receptor-like (ORL1) receptors to adenylyl cyclase in the different layers of the rat main olfactory bulb. J. Neurochem. 2001;77:1520–1530. doi: 10.1046/j.1471-4159.2001.00371.x. [DOI] [PubMed] [Google Scholar]
- OZAKI S., KAWAMOTO H., ITOH Y., MIYAJI M., IWASAWA Y., OHTA H. A potent and highly selective nonpeptidyl nociceptin/orphanin FQ receptor (ORL1) antagonist: J-113397. Eur. J. Pharmacol. 2000;387:R17–R18. doi: 10.1016/s0014-2999(99)00822-5. [DOI] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J., CIVELLI O. Orphanin FQ: A neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- SALOMON Y., LONDOS D., RODBELL M. A highly sensitive adenylate cyclase assay. Anal. Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- SUNAHARA R.K., DESSAUER C.W., GILMAN A.G. Complexity and diversity of mammalian adenylyl cyclases. Annu. Rev. Pharmacol. Toxicol. 1996;36:461–480. doi: 10.1146/annurev.pa.36.040196.002333. [DOI] [PubMed] [Google Scholar]
- TALLARIDA R.J., MURRAY R.B. Manual of Pharmacologic Calculations with Computer Programs. Springer-Verlag, New York; 1987. [Google Scholar]
- TIAN J.-H., XU W., FANG Y., MOGIL J.S., GRISEL J.E., GRANDY D.K., HAN J.-S. Bidirectional modulatory effects of orphanin FQ on morphine-induced analgesia: antagonism in brain and potentiation in spinal cord of the rat. Br. J. Pharmacol. 1997;120:676–680. doi: 10.1038/sj.bjp.0700942. [DOI] [PMC free article] [PubMed] [Google Scholar]


