Abstract
In the present study, the properties of endothelin-1 (ET-1) and platelet-activating factor (PAF) in inducing contraction and increased intracellular-free calcium level in rat mesenteric arteries and veins were studied. Furthermore, measurements of cytosolic ([Ca]c) and nuclear ([Ca]n) Ca2+ were performed by confocal microscopy.
PAF, at a concentration of 1 μM, and the selective ETB agonists, IRL-1620 and sarafotoxin S6C (100 nM), induced a marked constriction and increase in [Ca]i in the mesenteric vein but not in the artery. On the other hand, endothelin-1 (1–100 nM) induced a significant concentration-dependent nifedipine-insensitive increase in tension and [Ca]i in both arteries and veins.
Those responses to endothelin-1 were significantly reduced by the ETA receptor antagonist, BQ-123 (10−6 M), on both types of vessels, whereas the selective ETB receptor antagonist, BQ-788, inhibited only the venous responses. The mixed ETA/ETB receptor antagonist, SB 209670, reduced the ET-1-induced venous responses to the same level of that found in presence of BQ-123 or BQ-788. However, concomitant applications of BQ-123 and BQ-788 reduced the vasoconstriction below to that induced by ETA or ETB blockade without further affecting [Ca]i.
PAF and the selective ETB agonists IRL-1620, induced a sustained increase of [Ca]c and [Ca]n solely in venous cells and ET-1 in both arterial and venous smooth muscle cells.
Thus, PAF increases total intracellular calcium concentration and tension on the smooth muscle cells from venous origin only. Furthermore, ET-1-induced vasoactive as well as [Ca]i and [Ca]n increasing effects are mediated by distinct receptors on venous and arterial smooth muscles.
Keywords: Endothelin, platelet-activating factor, tension, calcium, mesenteric vessels, R-type Ca2+ channel
Introduction
Impairment of hydrostatic pressure by vasoactive agents leading to capillary plasma extravasation is believed to be an initiating factor in pro-inflammatory conditions of the vasculature. Qualitatively different responses of pre and post-capillary vascularization to various autacoids and hormones may affect hydrostatic pressure in the capillary and modulate albumin extravasation (Filep et al., 1991; 1993; D'Orléans-Juste et al., 1996; Plante et al., 1996). Among the different mediators, the potent vasoconstrictor, endothelin-1, and the inflammatory mediator, PAF, were both shown, via their respective receptors, to cause an increase in vascular permeability (Eibl et al., 2000; Hirayama et al., 1998; Sirois et al., 1992). In contrast, ET-1 has been shown to directly decrease permeability in intact isolated microvessels (Victorino et al., 1999). Since both arterial and venous vasculatures contribute in modulating intra-capillary hydrostatic pressure, and due to the difficulty in studying these phenomena in vivo and to the conflicting reports in the literature, it is of importance to characterize the vasoactive properties of ET-1 and PAF in pre and post-capillary vasculatures. We have shown that endothelin-1 and PAF induced an endothelium-dependent arterial vasodilation and endothelium-dependent venoconstriction, respectively, in the mesenteric vasculature of the rat (Claing et al., 1994; D'Orléans-Juste et al., 1993). Interestingly, arterial vasodilation and venoconstriction to PAF were sensitive to the dual voltage-dependent R and L-type calcium channel blocker, isradipine (Bkaily et al., 1993), but not to the L-type calcium channel blocker, nifedipine (Claing et al., 1994).
It is now believed that cytosolic and nuclear-free Ca2+ levels play an essential role in excitation, contraction and secretion coupling of many cell types, including vascular smooth muscle and endothelial cells (Bkaily et al., 1997a; 2000b; Jacques et al., 2000). Earlier studies from our group have illustrated that PAF increases total intracellular-free calcium ([Ca]i) in human and canine endothelial cells through the activation of the steady-state voltage-dependent sarcolemma R-type calcium channels (Bkaily et al., 1993). This increase of [Ca]i by PAF as well as by ET-1 was mainly nuclear (Bkaily et al., 1997a, 1997b). A role for voltage-dependent calcium entry in endothelial cell-dependent secretion-coupling mechanisms is a controversial issue, as dihydropiridine-sensitive channels are generally absent in that particular cell type (Bkaily et al., 1993).
Given the importance of ET-1 and PAF in the pathogenesis characterized by capillary leakage, we have verified the qualitatively distinct vasoactive effects of both agonists and their capacity to increase [Ca]i, [Ca]c and [Ca]n levels in tissues and cells of arterial and venous origins, the present study was performed in two steps. Firstly, we simultaneously monitored changes in tension and [Ca]i (Benchekroun et al., 1995; Claing et al., 1994) and, secondly, we visualized these changes using single cell confocal microscopy techniques, where Fluo-3 was used as an indicator of [Ca]c and [Ca]n levels (Bkaily et al., 1997a).
Our results in blood vessels and single cells allow us to conclude that ET-1 induces an increase in tension, [Ca]i, [Ca]c and [Ca]n via ETA receptors in the arterial vasculature, whereas ETB receptors are solely responsible for the same responses in the mesenteric vein. In addition, PAF, albeit an activator of venous structures, is inactive on the smooth muscle of the arterial mesenteric circuit.
Methods
Tension and cytosolic-free calcium monitoring
Male Wistar rats (275–325 g) (Charles River) were sacrificed by stunning and exsanguination. The mesenteric artery and vein were carefully freed of connective tissues. The mesenteric vein was cut longitudinally, removed from the animal and placed in a physiological saline solution (PSS) gassed with a mixture of 95% O2 and 5% CO2 at room temperature. The mesenteric artery of the rat was cannulated with a polyethylene tube (PE-10), placed in the PSS and cut helicoidally. The endothelium of the vessels was gently removed with a humid cotton swab. The PSS solution contained (in mM): NaCl 123, KCl 4.67, MgCl2, 1.2, CaCl2, 2.5, KH2PO4 1.2, NaHCO3 15.5, glucose 11.5 and HEPES 10. The absence of vasodilatory responses to acetylcholine (10−7 M) was routinely controlled to ensure the complete removal of the endothelium. One artery and one vein per animal were tested. The loading of the vascular smooth muscle with Fura-2 AM was done using a standard technique (Benchekroun et al., 1995). In brief, the vessels were tied at one end with a metallic support and placed in a quartz cuvette containing the loading solution at room temperature and oxygenated (12 μM Fura-2/AM in DMEM containing 5% FBS in the presence of 0.1% cremophor EL and 1 mM probenecid). After the loading period, the tissues were rinsed and allowed to equilibrate with normal PSS for 45 min. The initial tensions of the mesenteric artery and vein were 2 g and 1 g, respectively. Experiments were then performed with a double-wavelength excitation fluorometer (Photon Technology International Inc., PTI), where the Fura-2 loaded strips were fixed horizontally in a quartz cuvette and attached to an isometric transducer (Benchekroun et al., 1995; Claing et al., 1994). Simultaneously, 510 nm fluorescence emitted by 340 nm and 380 nm excitation (F340 and F380, respectively) was measured by successive alternating illumination and the ratio of F340 to F380 was automatically calculated. The responses were expressed as increase in vessel tension (nM) and the variation of ratio (F340/F380) reflected the total cytoplasmic-free calcium level (Benchekroun et al., 1995; Himpens & Somlyo, 1998; Sato et al., 1988), which is the sum of cytosolic and nuclear calcium (Bkaily et al., 1997a; 2000a). The tension of the vessels was measured by an isometric transducer and recorded on a grass physiograph. For the effect of BQ-123, BQ-788 and SB 209670 on the ET-1 response, the strips were preincubated for 20 min with the receptor blockers before the application of the agonist. The total intracellular-free Ca2+ ([Ca]i) increase responses to PAF, ET-1, IRL-1620 and sarafotoxin S6C were analysed once both [Ca]i increase and contractions had reached a steady state. Furthermore, in the experiments where nifedipine was used, the calcium channel blocker was applied 30 min before the administration of ET-1. Finally, sodium nitroprusside was administered on the ET-1 precontracted vessels.
VSM culture
Cells were harvested from enzymatically dissociated rat mesenteric arteries and veins, according to the methods reported by Gunther et al. (1982) which were slightly modified in the present study. All procedures were carried out under aseptic conditions. Male Wistar rats were sacrificed by stunning and exsanguination. The superior mesenteric artery and vein were excised and placed in a petri dish containing ice-cold MEM with 0.2 mM added Ca2+. The adherent fat and connective tissue was removed by blunt dissection and the cleaned vessels were transferred into a flask. The vessels were then rinsed and incubated at 37°C for 1 h in a MEM enzyme dissociation mixture containing 0.2 mM added Ca2+, 15 mM HEPES (pH 7.2–7.4), 0.125 mg/ml elastase, 0.375 mg/ml soybean trypsin inhibitor, 2 mg ml−1 collagenase and 2 mg ml−1 BSA. After incubation at 37°C for 60 min, the suspension was aspirated into a 10 ml plastic syringe and triturated 10 times. The suspension was centrifuged in a siliconized conical glass tube (200×g, 5 min) and the pellet resuspended in 6 ml of DMEM containing 10% FBS, 2 mM L-glutamine, 25 mM HEPES buffer, 100 IU ml−1 penicillin and 100 μg ml−1 streptomycin. The dispersed cell suspension was aliquoted on 25 mm spheric lamelles used for confocal microscopy and then incubated at 37°C in a humidified 5% CO2, 95% air solution. After 5 h, the cultures were washed once with sterile PBS to remove non-adherent cells and debris and fed with fresh medium. The medium was routinely changed at 48 h intervals thereafter. Cells were used after 3–4 days of culture. Isolated VSM cells of mesenteric arteries and veins were marked with an antibody raised against α-actin to confirm their muscular origin.
Cytosolic and nuclear Ca2+ imaging with confocal microscopy
For free cytosolic and nuclear Ca2+ imaging, VSMCs were loaded with Fluo-3 (13.6 μM) reconstituted in DMSO and diluted to a final concentration of 13.6 μM in Tyrode-BSA solution using a standard technique (Bkaily et al., 1997a; 1999). The cells were incubated for 30 min at room temperature, washed and further incubated for 15 min at room temperature to complete hydrolysis for acetoxymethyl ester groups.
Fluo-3 loaded cells were examined with a Molecular Dynamics (Sunnyvale, CA, U.S.A.) Multi Probe 2001 Confocal Argon Laser Scanning System (CSLM) equipped with a Nikon Diaphot epifluorescence inverted microscope and a 60× (1.4 NA) Nikon and Plan achromat objective. The Argon laser line (9.0 mV) was directed to the sample via a 510 nm primary dichroic filter and attenuated with a 1–3% neutral density filter to reduce photobleaching. The pinhole size was set at 100 μM. The image size was 512×512 pixels with a pixel size of 0.11 μM. Laser line intensity, photometric gain, PMT setting and filter attenuation were kept constant throughout the experimental procedures. Serial optical scans were performed 2 min after addition of each agent. A total of 12–15 scans (512×512) were performed for each series with a step size of 0.8–1.0 μM. The number of sections and step size were rigorously maintained during the course of each experiment, in order to localize calcium variations within the boundaries of the nucleus (Bkaily et al., 1997a; 1999). Once agent additions completed, 30 mM KCl and 10 mM EGTA were added to the bath medium to ensure that cells were still responsive to external stimuli. Quantification of the intracellular ion concentration (cytosolic and nuclear) using the non-ratiometric dye, Fluo-3, was done using a procedure described previously (Bkaily et al., 2000a). In brief, sarcolemna and nuclear envelope membranes were perforated with 0.1% Triton×100 solution. The perforated cells were then exposed to different concentrations of Ca2+ buffer containing 13.5 μM Fluo acid. The Ca2+-Fluo-3 fluorescence intensity curve was then constructed (Bkaily et al., 2000a).
Nuclear staining
At the end of each experiment, the nucleus was stained with 100 nM of live cell nucleic acid stain Syto 11. Serial optical scans were taken immediately after development of the stain (approximately 8–10 min), while maintaining positioning, number of sections and step size identical to those used for calcium uptake. 3-D reconstructions of the nucleus were performed, as described by Bkaily et al. (1997a), and used as templates to delineate nuclear from cytosolic-free calcium.
Materials
The fluorescent probe, Fura-2/AM, was purchased from Calbiochem and Fluo-3 from Molecular Probes. Endothelin-1, sarafotoxin 6C, BQ-123 and BQ-788 were purchased from American Peptide Co. IRL-1620 and PAF were obtained from Bachem. All other materials, if not stated otherwise, were purchased from Sigma Chemical Co. Care of animals conform to the guiding principles for animal experimentation, as enunciated by the Canadian Council on Animal Care and approved by the Ethical Committee on Animal Research of the Medical School of the Université de Sherbrooke.
Statistics
All results are expressed as mean values±s.e.mean. When applicable, statistical significance was determined using the variance analysis ANOVA for matched or unmatched values, followed by the proper multiple comparison test suggested by the program ‘GraphPad Instat'™ (V2.04a) to assess statistical significance of the results. For Figure 5, a student t-test for matched values was performed. P values of less than 0.05 were considered significant.
Figure 5.
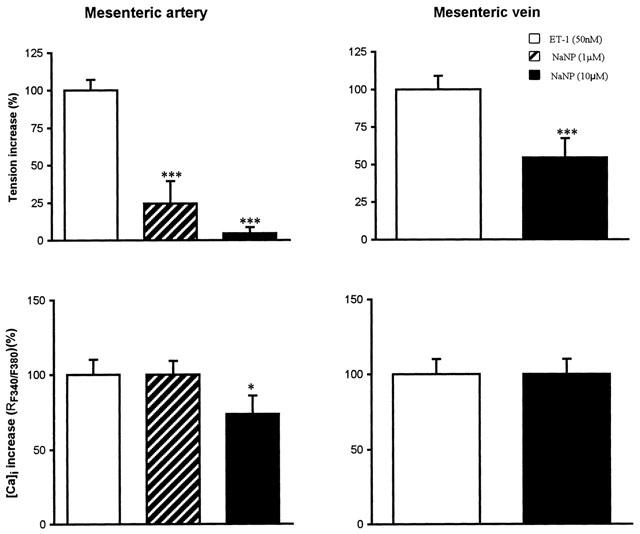
Effect of a curative treatment with sodium nitroprussiate (1 μM and 10 μM) against the response induced by ET-1 (50 nM). Data are the mean±s.e.mean from 5–8 different tissues. ***P<0.001 and *P<0.05 when compared to control values with ET-1 alone.
Results
Effect of ET-1, selective ETB agonists or PAF on the change of tension and total free calcium ([Ca]i)
In the first series of experiments, the simultaneous measurement of tension and [Ca]i was performed in order to determine the effective concentrations of ET-1 and PAF on the mesenteric artery and vein of the rat.
Figure 1 illustrates the concentration-dependent increase in tension and [Ca]i level induced by ET-1 (1–100 nM) on the mesenteric artery and vein. Increasing the concentrations of ET-1 from 100 nM up to 1 μM did not further increase the tension and [Ca]i in both preparations (results not shown). Significant increase in both tension and [Ca]i in the mesenteric artery was seen at 10 nM of ET-1. However, in the mesenteric vein, significant increases in [Ca]i were detected at a concentration of 5 nM of ET-1. Thus, only concentrations of ET-1 that showed significant increase in both tension and [Ca]i were used in the series of experiments with ET receptor antagonists as well as those in single cell confocal microscopy. Figure 2 illustrates the contractile and [Ca]i-increasing properties of two selective ETB agonists, IRL-1620 and sarofotoxin S6C (0.1 μM each), in the mesenteric vein but not in the arterial counterpart. A higher concentration of IRL-1620 or sarafotoxin S6C (1 μM) did not further increase tension or [Ca]i levels in the mesenteric vein (results not shown).
Figure 1.

Concentration-dependent effect on tension and [Ca]i of ET-1 (1–100 nM) on the rat mesenteric artery and vein. Data are the mean±s.e.mean from 6–15 different tissues.
Figure 2.

Two selective ETB agonists, IRL-1620 and sarafotoxin S6C (S6C) (100 nM) induce a vasoconstriction and [Ca]i of the mesenteric vein but not artery. Data is mean±s.e.mean of six different tissues.
Similarly, PAF at a concentration of 1 μM was able to induce a significant increase in tension (1.25±0.29 mN) and [Ca]i (RF340/F380: 0.07±0.013) in the mesenteric vein (n=6 different tissue preparations). In contrast, PAF (1 μM) was unable to change either tension or [Ca]i in the arterial vessels (n=10 different tissue preparations; results not shown). Thus, PAF at a concentration of 1 μM was used in the single cell confocal microscopy experiments.
Effect of selective agonists, antagonists and non-selective ETA/ETB antagonist on ET-1-induced increase of tension and [Ca]i
In a third series of experiments, the contribution of ETA and ETB receptors in the endothelin-1-induced events was identified through the use of selective or non-selective antagonists.
The selective ETA antagonist, BQ-123 (1 μM), markedly reduced the contraction induced by 10 nM of ET-1 by 93.0±5.3% (n=6, ***P<0.001) and the increase of [Ca]i by 90.4±11.2% (n=6, ***P<0.001) on the mesenteric artery. The blockade of the ETA receptors also reduced the increase in tension induced by 10 nM of ET-1 on the mesenteric vein by 74.0±6.1% (n=8, ***P<0.001) (Figure 3).
Figure 3.
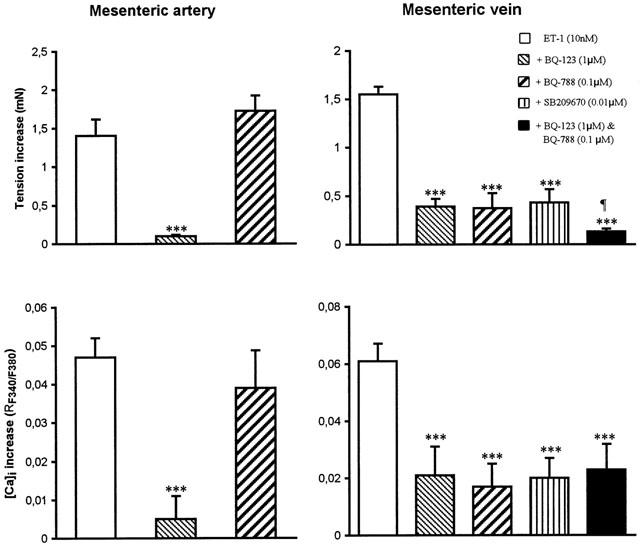
Effect of BQ-123 (1 μM), BQ-788 (0.1 μM), SB209670 (0.1 μM) and BQ-123 (1 μM)+BQ-788 (0.1 μM) on the response to ET-1 (10 nM) in the mesenteric artery and vein. ***P<0.001 responses to ET-1 in presence of BQ-123, BQ-788 or SB209670 are significantly different from control values (ET-1 without antagonists). The mixture of BQ-123 and BQ-788 induced an antagonism of ET-1 contraction, which is significantly greater than treatment with BQ-123, BQ-788 or SB209670 administered alone (¶P<0.05). Data are the mean±s.e.mean from 6–12 different tissues.
The selective ETB receptor antagonist, BQ-788 (0.1 μM), did not alter the response induced by ET-1 (10 nM) on the mesenteric artery. In contrast, BQ-788 reduced by 73.4±10.0% the increase of tension and by 72.2±7.6% the total intracellular calcium level induced by ET-1 (Figure 3) in the mesenteric vein. The non-selective ETA/ETB antagonist, SB 209670 (0.01 μM), did not further reduce the contractile response nor the increase of [Ca]i induced by ET-1 (10 nM) on the vein (Figure 3), when compared to BQ-123 or BQ-788. However, the simultaneous treatment with BQ-123 (1 μM) and BQ-788 (0.1 μM) of the same preparation further reduced the contractile (n=5, ***P<0.001) but not the [Ca]i response triggered by ET-1 (Figure 3).
Effect of an L-type calcium channel blocker on ET-1-induced increase of tension and [Ca]i
A 30-min pre-treatment with the specific L-type calcium channel blocker, nifedipine (0.1–1 μM), was unable to affect the contractile and [Ca]i response induced by ET-1 in both arteries and veins (n=5) (Figure 4), although it abolished (at 0.1 μM) the contractile response and the increase of [Ca]i induced by the selective L-type calcium channel opener, Bay K 8644, (5nM) in the artery (control tension: 0.72±0.21 mN; [Ca]i : 0.06±0.01 R; + nifedipine : tension : 0; [Ca]i : 0, n=6, P<0.001) as well as in the vein (control tension: 1.37±0.09 mN; [Ca]i : 0.1±0.02 R; + nifedipine : tension :0; [Ca]i : 0, n=12, P<0.001).
Figure 4.

Effect of pre-treatment with nifedipine (0.1 μM and 1 μM) on the response to ET-1 (10 nM) on the mesenteric artery and vein. Data are the mean±s.e.mean from 6–10 different tissues.
Effect of sodium nitroprusside on the vasoconstriction and increase of [Ca]i induced by ET-1 in the mesenteric artery and vein
In ET-1 precontracted arteries, sodium nitroprusside (1 and 10 μM) induced a concentration-dependent relaxation of the vessel (n=5–8, ***P<0.001) and a reduction of [Ca]i at the highest concentration only. On the mesenteric vein, sodium nitroprusside reduced the contraction induced by ET-1 without affecting the increase of [Ca]i (Figure 5).
Effect of ET-1, IRL-1620 and PAF on [Ca]c and [Ca]n
The following series of experiments were performed on single vascular smooth muscle cells in which changes in [Ca]c and [Ca]n induced by PAF, ET-1 or IRL-1620 were monitored through confocal microscopy. Figure 6 shows the cytosolic and nuclear-free calcium increase by PAF (1 μM) on VSM cells isolated from mesenteric arteries and veins. Similarly, ET-1 (0.1 μM) induced an increase in cytosolic and nuclear levels in both VSM cells from arteries and veins (Figure 7). The selective ETB receptor agonist, IRL-1620 (0.1 μM), increased [Ca]c and [Ca]n in the VSM cells from the vein without any effect on the arterial cells (Figure 8). Furthermore, in all these cells, sustained depolarization of the cell membrane with continued superfusion with 30 mM [K]o induced sustained increase of [Ca]c and [Ca]n; extracellular application of the Ca2+ chelator, EGTA (10 mM), decreased both levels to near the control values. Finally, staining with Syto 11 illustrates that a large portion of [Ca]i increases occur within the nucleus of venous smooth muscle cells, as previously demonstrated in arterial counterparts (Bkaily et al., 1999).
Figure 6.

Cross-sectional view of a 3-D reconstructed isolated vascular smooth muscle cell from arterial (A–E) and venous (F–L) origin. This figure illustrates the intracellular calcium distribution of VSM cells isolated from arteries in response to PAF (1 μM; B,C,G,H,I and J) (A: control; B: 2 min; C: 10 min; D: KCl, 30 s and E: EGTA, 5 min) and VSM cells isolated from veins (F: control; G: 2 min; H: 5 min; I: 10 min; J: 15 min; K: KCl, 30 s and L: EGTA, 5 min). The white scale bar represents length in μm. The colour scale represents pseudocolor intensity levels of the complex Fluo-3 calcium fluorescence from 0–255 and [Ca] concentrations from 0.01 to 40 μM. This figure is representative of three other experiments.
Figure 7.

Cross-sectional view of a 3-D reconstructed isolated vascular smooth muscle cell from arterial (A to H) and venous (I to P) origin. This figure illustrates the intracellular calcium distribution of VSM cells isolated from arteries in response to ET-1 (0.1 μM) (B,C,D,E,F,J,K,L and M) (A: control; B: 2 min; C: 5 min; D: 10 min; E: 15 min; F: 18 min; G: KCl, 30 s and H: EGTA, 5 min) and VSM cells isolated from veins (I: control; J: 5 min; K: 15 min; L: 20 min; M: 25 min; N: KCl, 30 s; O: EGTA, 2 min and P: Syto-11). The white scale bar represents length in μm. The colour scale represents pseudocolor intensity levels of Fluo-3 dye from 0–255 and [Ca] concentrations from 0.01 to 40 μM. This picture is representative of three other experiments.
Figure 8.

Cross-sectional view of a 3-D reconstructed isolated vascular smooth muscle cell from arterial (A to D) and venous origin (E to J). This figure illustrates the intracellular calcium distribution of VSM cells isolated from arteries in response to IRL-1620 (1 μM; B,C,F and G) (A: control; B: 5 min; C: 10 min) or ET-1 (0.1 μM) (D: 10 min) and VSM cells isolated from veins (E: control; F: 5 min; G: 10 min; H: KCl, 30 s, I: EGTA, 5 min; J: Syto: 11). The white scalebar represents length in μm. The colour scale represents pseudocolor, intensity levels of Fluo-3 from 0–255 and Ca concentrations from 0.01 to 40 μM. This figure is representative of three other experiments.
Discussion
The data presented here shows that endothelin-1 induces an increase in cytosolic calcium and a concomitant increase in tension. These results are in accordance with data reported in porcine coronary arteries (Yasutsune et al., 1999). Since the Ca2+ is the same for both cytosolic and nuclear-free Ca2+, the increase of [Ca]i induced by 5 nM of ET-1 (in absence of changes in tension) in the mesenteric artery may substantially reflect the increase of nuclear Ca2+ due to the cytosolic Ca2+ buffering capacity of the nuclei (Bkaily et al., 1997a). On the other hand, our results suggest that the ET-1-induced constrictive effects are mediated through ETA receptors on the arterial smooth muscle and through both ETA and ETB receptors on the venous smooth muscle. This observation supports previous studies from our laboratory (D'Orléans-Juste et al., 1993), illustrating the role of both receptor types in the constrictive properties of endothelins in both arterial and venous mesenteric beds of the rat. The ETA-mediated vasoconstriction by ET-1 in the rat mesenteric arteries was also shown by Rizzoni et al. (1997). We have previously reported the predominant endothelium-dependant pre-capillary vasodilation and post-capillary constriction in the mesenteric circuit in response to endothelin-1 (D'Orléans-Juste et al., 1993). Nonetheless, other reports (Mickley et al., 1997; Touyz et al., 1995) suggested the presence of contractile ETB receptors on rat mesenteric arteries. Recently, it was shown that in freshly isolated rat mesenterial segments, only ETA receptors were characterized, while ETB receptors only appeared following organ culture (Adner et al., 1998). These results would suggest that ETB receptors, when present, only play a minor role, if any (Rizzoni et al., 1997). In the present study, we were unable to detect ETB-dependent contractions in the rat mesenteric arterial strips or any IRL-1620-induced [Ca]i increase in muscle strips or cultured VSM cells of the same tissular origin. The conflicting observations reported among the different groups, including ours, could be attributed to differences in the size of the mesenteric arteries studied (1st versus 2nd or 3rd generation) and to the method of tissue preparation for contraction studies (rings versus strips).
The venous mesenteric vasculature of the rat predominantly responds to endothelin-1 via the activation of ETA receptors. However, a small population of ETB receptors was also illustrated, as demonstrated by the venoconstrictive effects of a selective ETB agonist, IRL-1620 (D'Orléans-Juste et al., 1993). The same ETB-dependent phenomenon seen in the intact mesenteric vasculature occurs at the level of the main venous, but not arterial, mesenteric vessels, as illustrated in the present study with IRL-1620 or sarafotoxin S6C. Both receptor types (ETA on the arterial, ETA and ETB receptors on the venous side) contribute to the increased [Ca]i level and tension. The fact that the ETA/ETB receptor antagonist SB209670 decreased ET-1-induced increase of tension and [Ca]i to the same extent as BQ-123 or BQ-788, could be due to the fact that the former antagonist possesses a higher affinity for ETA (pA2 for 9.22) than ETB receptors (pA2 for 8.09) (Maurice et al., 1997). It is possible that the further decrease of tension and absence of effect on [Ca]i simultaneous blockade of ETA and ETB receptors by BQ-123 and BQ-788 could be due to the higher affinity of the mixture for ETB receptors (pA2 of BQ-788 9.01) than ETA receptors (pA2 of BQ-123 6.41) (Maurice et al., 1997). Finally, the superior effect of the mixture of BQ-123 and BQ-788 may not be due to a lack of selectivity of BQ-788, since that particular antagonist did not affect at all the contraction of ET-1 on the mesenteric artery in the present study.
In another series of experiments, we have shown that the mesenteric vein but not the artery responds to PAF by an increase in [Ca]i and a contraction. These results are in accordance with those reported by Claing et al. (1994), who have shown in the double-perfused arterial and venous vasculature of the rat that PAF induces an endothelium-dependent vasodilation solely in the pre-capillary and a constriction of the post-capillary circuits.
In order to avoid the contaminating contribution of elastin, collagen, endothelium and perivascular nerves, we have analysed single smooth muscle cells of arterial and venous origin challenged with endothelin-1 and the selective ETB receptor agonist, IRL-1620. In absence of any drug, our results showed that in rat VSM cells, nuclear-free Ca2+ was higher than that of the cytosol. This finding is similar to those reported in the rabbit and human heart, VSM and endothelial cells (Bkaily et al., 1997a). In our experiments, endothelin-1 was found to markedly increase intracellular calcium, which was largely nuclear in both the arterial and venous single cells; IRL-1620 was found active only in smooth muscle cells of venous origin. These results are in accordance with those recently reported for ET-1 and PAF in human and rabbit aortic VSM cells (Bkaily et al., 1997a; 2000a).
Based on the present observations, we suggest that the potent vasoactive agents, PAF and endothelin-1, trigger tissue-specific cytosolic and nuclear calcium increase and myocontractions. The effect of these factors on single cells mimicks the response found in arterial and venous strips, as well as the intact mesenteric circuits (Claing et al., 1994; D'Orléans-Juste et al., 1993). It is also worthy of notice that both mesenteric arteries and veins possess functional L-type calcium channels, as illustrated by the nifedipine-sensitive contraction induced by Bay K-8644 in these tissues. However, this type of channel does not seem to be involved in the ET-1 and PAF-mediated actions.
Despite the observations that cytosolic calcium increase seems to be essential for the initiation of contraction, we report a reduction in vascular tone with sodium nitroprusside that was not correlated with a reduction in cytosolic calcium. These observations may be explained by the fact that although cytosolic calcium is important for the coupling of troponin and myosin elements via the activity of the myosin-light chain kinase (MLCK), vasodilation may occur at a step beyond the coupling of a cytosolic calcium-calmodulin entity to the MLCK, as recently shown in rabbit aortas (Bkaily et al., 2000b).
Finally, the contribution of vascular endothelium in the vasoactive effects of both PAF and endothelin was not studied. We have previously reported that the endothelium acts as a humoral barrier in the rat perfused mesenteric arterial and venous circuits (Claing et al., 1994) and can modulate vascular responses to vasoactive agents.
In summary, our study demonstrates that PAF, although inactive in the endothelium-denuded arterial blood vessels, is able to trigger an increase in tension and both cytosolic and nuclear calcium entry in the venous mesenteric vasculature. Our data suggests that this calcium entry is mediated by a nifedipine-insensitive calcium channel. The use of endothelin-1 and the selective ETB receptor agonists, IRL-1620 and sarafotoxin S6C, demonstrates the functional presence of only ETA receptors in the arterial vessels and of both ETA and ETB receptors in the venous smooth muscles which are responsible for increase in both tension and cytosolic calcium entry through a nifedipine-insensitive Ca2+ channel. Finally, it is of interest that PAF (venous VSM cells only) and ET-1 trigger a particularly marked increase in both [Ca]c and [Ca]n. In fact, there is an increasing body of evidence supporting the contribution of nuclear and intracellular calcium in maintaining cellular calcium homeostasis. Furthermore, receptors such as AngII and ET-1 have been shown to be present and functional within the nuclear envelope membranes (Bkaily et al., 1997a,1997b; 2000a; Jacques et al., 2000). Thus, it is possible that both PAF and ET-1 effects observed in our experiments at the tissular and cellular levels could also be due in part to ligand stimulation of nuclear membrane receptors (via receptor-ligand complex internalization) (Bkaily et al., 2000a). Further experiments are required in order to pursue that particular hypothesis.
Acknowledgments
The authors acknowledge Helen Morin and Pierre Pothier for secretarial and technical assistance, respectively. This project was financed by a Canadian Institutes for Health Research Group Grant for the Study of Immunocardiovascular Interactions (GR-13915).
Abbreviations
- ET
endothelin
- PAF
platelet-activating factor
- VSMC
vascular smooth muscle cell
References
- ADNER M., GEARY G.G., EDVINSSON L. Appearance of contractile endothelin-B receptors in rat mesenteric arterial segments following organ culture. Acta. Physiol. Scand. 1998;163:121–129. doi: 10.1046/j.1365-201X.1998.00369.x. [DOI] [PubMed] [Google Scholar]
- BENCHEKROUN M.T., GROS-LOUIS N., BKAILY G., D'ORLEANS-JUSTE P. R-type calcium channel involved in endothelin-1-induced contraction of rabbit aorta. J. Cardiovasc. Pharmacol. 1995;26:S300–S302. [PubMed] [Google Scholar]
- BKAILY G., D'ORLÉANS-JUSTE P., NAIK R., PERODIN J., STANKOVA J., ABDULNOUR E., ROLA-PLESZCZYNSKI M. PAF activation of a voltage-gated R-type Ca2+ channel in human and canine aortic endothelial cells. Br. J. Pharmacol. 1993;110:519–520. doi: 10.1111/j.1476-5381.1993.tb13841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BKAILY G., POTHIER P., D'ORLÉANS-JUSTE P., SIMAAN M., JACQUES D., JAALOUK D., BELZILE F., HASSAN G., BOUTIN C., HADDAD G., NEUGEBAUER W. The use of confocal microscopy in the investigation of cell structure and function in the heart, vascular endothelium and smooth muscle cells. Mol. Cell. Biochem. 1997a;172:171–194. [PubMed] [Google Scholar]
- BKAILY G., D'ORLÉANS-JUSTE P., POTHEIR P., CALIXTO J.B., YUNES R. Nuclear membrane receptors and channel: potential therapeutical targets for drug action. Drug Devel. Res. 1997b;42:211–222. [Google Scholar]
- BKAILY G., JACQUES D., POTHIER P. Use of confocal microscopy to investigate cell structure and function. Methods in Enzymology. 1999;307:119–135. doi: 10.1016/s0076-6879(99)07010-x. [DOI] [PubMed] [Google Scholar]
- BKAILY G., JACQUES D., D'ORLÉANS-JUSTE P., HASSAN G., CHOUFANI S.Using confocal microscopy imaging to measure changes in intracellular ions Receptors: A practical approach 2000aLondon: Oxford University Press; 209–232.Ed. Stanford, S.C. and Horton, R.W. pp [Google Scholar]
- BKAILY G., SHBAKLO H., TAOUDI, BENCHEKROUN M., SADLER S., DUVAL M., JACQUES D., D'ORLÉANS-JUSTE P. Nitric oxide relaxes the vascular smooth muscle independently of ET-1- and U46619-induced intracellular increase of calcium. Journal of Cardiovascular Pharmacology. 2000b;36:S110–S116. doi: 10.1097/00005344-200036051-00036. [DOI] [PubMed] [Google Scholar]
- CLAING A., BKAILY G., BERTHIAUME N., SIROIS P., ROLA-PLESZCZYNSKI M., D'ORLEANS-JUSTE P. Role of R-type calcium channels in the response of the perfused arterial and venous mesenteric vasculature of the rat to platelet-activating factor. Br. J. Pharmacol. 1994;112:1202–1208. doi: 10.1111/j.1476-5381.1994.tb13211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ORLEANS-JUSTE P., CLAING A., WARNER T.D., YANO M., TELEMAQUE S. Characterization of receptors for endothelins in the perfused arterial and venous mesenteric vasculatures of the rat. Br. J. Pharmacol. 1993;110:687–692. doi: 10.1111/j.1476-5381.1993.tb13866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'ORLEANS-JUSTE P., CLAING A., REGOLI D., SIROIS P., PLANTE G.E. Endothelial and smooth muscle pharmacology of pre- and post-capillary microcirculation: correlation with plasma extravasation. Prostaglandins Leukot. Essent. Fatty Acids. 1996;54:31–57. doi: 10.1016/s0952-3278(96)90078-2. [DOI] [PubMed] [Google Scholar]
- EIBL G., HOTZ H.G., FAULHABER J., KIRCHENGAST M., BUHR H.J., FOITZIK T. Effect of endothelin and endothelin receptor blockade on capillary permeability in experimental pancreatitis. Gut. 2000;46:390–394. doi: 10.1136/gut.46.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILEP J.G., SIROIS M.G., ROUSSEAU A., FOURNIER A., SIROIS P. Effects of endothelin-1 on vascular permability in the conscious rat: interactions with platelet-activating factor. Br. J. Pharmacol. 1991;104:797–804. doi: 10.1111/j.1476-5381.1991.tb12509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILEP J.G., SIROIS M.G., FOLDES-FILEP E., ROUSSEAU A., PLANTE G.E., FOURNIER A., YANO M., SIROIS P. Enhancement by endothelin-1 of microvascular permeability via the activation of ETA receptors. Br. J. Pharmacol. 1993;109:880–886. doi: 10.1111/j.1476-5381.1993.tb13657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNTHER S., ALEXANDER R.W., ATKINSON W.J., GIMBRONE M.A., Jr Functional angiotensin II receptors in cultured vascular smooth muscle cells. J. Cell. Biol. 1982;92:289–298. doi: 10.1083/jcb.92.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIMPENS B., SOMLYO A.P. Free-calcium and force transients during depolarization and pharmacomechanical coupling in guinea-pig smooth muscle. J. Physiol (Lond) 1988;395:507–530. doi: 10.1113/jphysiol.1988.sp016932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAYAMA T., OGAWA Y., TOBISE K., KIKUCHI K. Mechanism of endothelium-dependent vasorelaxation evoked by lysophosphatidylcholine. Hypertens Res. 1998;21:137–145. doi: 10.1291/hypres.21.137. [DOI] [PubMed] [Google Scholar]
- JACQUES D., SADER S., CHOUFANI S., D'ORLÉANS-JUSTE P., CHAREST P. Endothelin-1 regulates cytosolic and nuclear Ca2+ in human endocardial endothelium. J. Cardiovasc. Pharmacol. 2000;36:S397–S400. doi: 10.1097/00005344-200036051-00116. [DOI] [PubMed] [Google Scholar]
- MAURICE M.C., GRATTON J.P., D'ORLÉANS-JUSTE P. Pharmacology of two new mixed ETA/ETB receptor antagonists, BQ-928 and 238, in monoreceptorial vascular preparations and perfused kidney of the rabbit. Br. J. Pharmacol. 1997;120:319–325. doi: 10.1038/sj.bjp.0700895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICKLEY E.J., GRAY G.A., WEBB D.J. Activation of endothelin ETA receptors masks the constrictor role of endothelin ETB receptors in rat isolated small mesenteric arteries [published erratum appears in Br J Pharmacol 1997 Jul; 121(6): 1239] Br. J. Pharmacol. 1997;120:1376–1382. doi: 10.1038/sj.bjp.0701036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PLANTE G.E., LEHOUX S., SIROIS P. Drug-induced alteration of endothelial permeability in the rat aorta. Potential consequences on the vessel wall. Adv. Exp. Med. Biol. 1996;416:249–253. doi: 10.1007/978-1-4899-0179-8_41. [DOI] [PubMed] [Google Scholar]
- RIZZONI D., PORTERI E., PICCOLI A., CASTELLANO M., BETTONI G., PASINI G., AGABITI-ROSEI E. The vasoconstriction induced by endothelin-1 is mediated only by ET(A) receptors in mesenteric small resistance arteries of spontaneously hypertensive rats and Wistar Kyoto rats. J. Hypertens. 1997;15:1653–1657. doi: 10.1097/00004872-199715120-00068. [DOI] [PubMed] [Google Scholar]
- SATO K., OZAKI H., KARAKI H. Changes in cytosolic calcium level in vascular smooth muscle strip measured simultaneously with contraction using fluorescent calcium indicator fura 2. J. Pharmacol. Exp. Ther. 1988;246:294–300. [PubMed] [Google Scholar]
- SIROIS M.G., FILEP J.G., ROUSSEAU A., FOURNIER A., PLANTE G.E., SIROIS P. Endothelin-1 enhances vascular permeability in conscious rats: role of thromboxane A2. Eur. J. Pharmacol. 1992;214:119–125. doi: 10.1016/0014-2999(92)90108-g. [DOI] [PubMed] [Google Scholar]
- TOUYZ R.M., DENG L.Y., SCHIFFRIN E.L. Endothelin subtype B receptor-mediated calcium and contractile responses in small arteries of hypertensive rats. Hypertension. 1995;26:1041–1045. doi: 10.1161/01.hyp.26.6.1041. [DOI] [PubMed] [Google Scholar]
- VICTORINO G.P., WISNER D.H., TUCKER V.L. Direct actions of endothelin-1 on single vessel hydraulic permeability [published erratum appears in J Trauma 1999 Dec; 47(6):1164] J. Trauma. 1999;47:713–718. doi: 10.1097/00005373-199910000-00016. [DOI] [PubMed] [Google Scholar]
- YASUTSUNE T., KAWAKAMI N., HIRANO K., NISHIMURA J., YASUI H., KITAMURA K., KANAIDE H. Vasorelaxation and inhibition of the voltage-operated Ca2+ channels by FK506 in the porcine coronary artery. Br. J. Pharmacol. 1999;126:717–729. doi: 10.1038/sj.bjp.0702339. [DOI] [PMC free article] [PubMed] [Google Scholar]


