Abstract
Neuropeptides acting on tachykinin NK receptors play an important role in the amplification of inflammatory responses. We have assessed the effects of tachykinin NK receptor blockade on the injuries following intestinal ischaemia and reperfusion (I/R) in rats.
The tachykinin NK1 receptor antagonist SR140333 dose-dependently (0.05 to 0.5 mg kg−1) suppressed the local (intestine) and remote (lung) increases in vascular permeability and neutrophil recruitment following mild I/R injury. A structurally-distinct NK1 receptor antagonist, CP99,994, but not tachykinin NK2 or NK3 receptor antagonists also suppressed mild I/R injury.
Neonatal pretreatment with capsaicin effectively depleted sensory neurons and abrogated the injuries following mild I/R.
Treatment with SR140333 (0.5 mg kg−1) significantly reversed severe reperfusion-induced local and remote increases in vascular permeability, neutrophil recruitment, intestinal haemorrhage and blood neutropaenia, but did not prevent the lethality associated with severe I/R.
Post-ischaemic treatment with SR140333 significantly inhibited the elevations of TNF-α in the intestine and lung, but not serum, following severe I/R. The increase in the concentrations of IL-10 in the lung and serum were also suppressed.
Post-ischaemic blockade of tachykinin NK1 receptors markedly inhibited the local and remote injuries, but not lethality, following reperfusion of the SMA in rats. Neuropeptides, possibly substance P, released from sensory nerves appear to account for the activation of these tachykinin NK1 receptors. Antagonists of the tachykinin NK1 receptor may be useful adjuncts in the treatment of the injuries which occur following reperfusion of an ischaemic vascular territory.
Keywords: Capsaicin, IL-10, ischaemia and reperfusion injury, NK receptors, substance P, tachykinins, TNF-α
Introduction
The reduction in blood flow, ischaemia, to an organ or a vascular bed can lead to significant tissue injury and cell death if it is prolonged. Thus, the restoration of blood flow, i.e. the ‘reperfusion' of the ischaemic vascular bed, is necessary to save ischaemic tissue following an acute vascular event (Lefer & Lefer, 1996; Willerson, 1997). However, reperfusion may lead to recruitment and activation of leukocytes, release of mediators of the inflammatory process and injury to the affected vascular bed, limiting the beneficial effects of blood flow restoration (Lefer & Lefer, 1996; Willerson, 1997). Neutrophils and TNF-α are thought to be key players in the pathophysiology of ischaemia and reperfusion (I/R) injury (e.g. Kubes et al., 1995; Gilmont et al., 1996; Cornejo et al., 1997; Souza et al., 2000b). Thus, strategies which limit neutrophil accumulation and pro-inflammatory cytokine production induced by the reperfusion process may be useful adjuncts in the treatment of ischaemic disorders in various organs.
Several studies have now demonstrated that the release and action of neuropeptides, especially substance P, may play an important role in the amplification of acute and chronic inflammatory responses (e.g. Bhatia et al., 1998; Bozic et al., 1996; reviewed by Quartara & Maggi, 1998). The main source of these neuropeptides in the periphery appears to be sensory nerve endings, but there is much evidence demonstrating the ability of inflammatory cells themselves to release neuropeptides (Maggi, 1997). The neuropeptides released appear to function mainly via the activation of tachykinin NK1 receptors, but there is also evidence suggesting a role for both tachykinin NK2 and NK3 receptor activation (Cutrufo et al., 2000; Nenan et al., 2001). The role of tachykinin NK receptor activation during I/R injury is largely unknown, but acute ishemic events are accompanied by sensory nerve activation, significant sensation of pain and substance P release (Maggi, 1997).
We have recently described the local and remote reperfusion injuries which occur following ischaemia of the superior mesenteric artery (SMA) in the rat (Souza et al., 2000a, 2000b). Following I/R of the SMA, we observed significant local (intestine and mesentery) and remote (lung) oedema and neutrophil accumulation, and marked systemic alterations, which included hypotension, neutropaenia and death, specially after more prolonged I/R periods (Souza et al., 2000a, 2000b). Moreover, we detected significant pro-inflammatory cytokine production in tissues and serum after reperfusion of the ischaemic SMA (Souza et al., 2000b). Here, we have investigated the potential role of sensory nerve-derived tachykinins on the local and remote reperfusion injuries following ischaemia of the SMA. Initial experiments were designed to examine the dose-dependent effects of antagonists at the tachykinin NK receptors (NK1, NK2 and NK3) on the injuries following mild I/R of the SMA. After demonstration that mild I/R was sensitive to capsaicin depletion and tachykinin NK1 receptor blockade, we investigated the effects of treatment with the NK1 receptor antagonist SR140333 on the local and systemic injuries following severe I/R injury.
Methods
Animals
Male wistar rats (200–200 g) obtained from the Bioscience unit of our Institution were housed in standard conditions and had free access to commercial chow and water. All the procedures described had prior approval from the local animal ethics committee.
Ischaemia and reperfusion injury
Rats were anaesthetized with urethane (140 mg kg−1, i.p.) and laparotomy was performed. The superior mesenteric artery (SMA) was isolated and ischaemia was induced by totally occluding the SMA for 30 or 120 min. After ischaemia, reperfusion was initiated by removal of the occlusion. Animals made ischaemic for 30 or 120 min were allowed to reperfuse for 30 (mild I/R) or 120 (severe I/R) min, respectively. The durations of I/R were based upon previous experiments (Souza et al., 2000a, 2000b) and were optimal for mild and severe reperfusion injuries. Sham-operated animals or animals only made ischaemic were used as controls for the reperfusion-induced injury.
Initial experiments were carried out in the mild reperfusion injury model to examine the dose-dependent effect of tachykinin NK1 (SR140333, 0.05 to 0.5 mg kg−1 and CP99,994, 4.0 mg kg−1), NK2 (SR48968, 0.6 to 6.0 mg kg−1) and NK3 (SR142801, 1.0 to 9.0 mg kg−1) receptor antagonists. In these experiments, drugs were administered i.v. 5 min prior to the reperfusion of the SMA. We then tested the effects of the administration of SR140333 (0.5 mg kg−1, i.v., 5 min prior to reperfusion) in the more severe I/R model. None of the drugs used in the present study had any significant effects on basal parameters (data not shown) and to simplify the graphs presented, basal data obtained in vehicle or drug treated animals have been pooled for presentation.
Neonatal capsaicin treatment
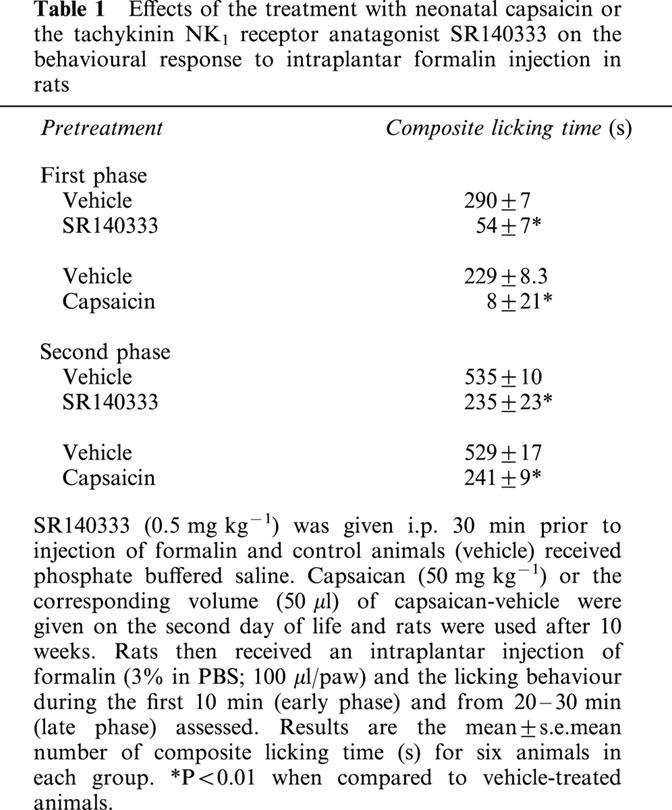
The protocol for capsaicin depletion was similar to that of Jancsó et al. (1977). Briefly, rats were pretreated on the second day of life with capsaicin, at which time they weighed approximately 200 g. As a positive control for the depletion of capsaicin-sensitive sensory nerves, rats received an intraplantar injection of formalin (3% in PBS; 100 μl/paw) and the composite licking behaviour during the first 10 min (early phase) and from 20 to 30 min (late phase) assessed (Shibata et al., 1989). Formalin-induced licking behaviour has been previously demonstrated to be dependent on the release of NK1-acting neuropeptides from sensory nerves (Sakurada et al., 1993; Rupniak et al., 1996; Damas & Liegeois, 1999). To confirm the latter point, animals were treated with the tachykinin NK1 receptor antagonist (0.5 mg kg−1, i.p.) 30 min prior to the injection of formalin.
Evaluation of changes in vascular permeability
The extravasation of Evans blue dye into the tissue was used as an index of increased vascular permeability (Saria & Lundberg, 1983; de matos et al., 1999). Evans blue (20 mg kg−1) was administered i.v. (1 ml kg−1) via a femoral vein 2 min prior to reperfusion of the ischaemic artery. Thirty min (in the mild model) or 120 min (in the severe model) after reperfusion, segments of the duodenum (10 cm) were cut open and allowed to dry in a petri dish for 24 h at 37°C. The dry weight of the tissue was calculated and Evans blue extracted using 3 ml of formamide (24 h at room temperature). The amount of Evans blue in the tissue was obtained by comparing the extracted absorbance with that of a standard Evans blue curve read at 620 nm in an ELISA plate reader. Results are presented as the amount of Evans blue per μg per 100 mg of tissue. The mesentery was also extracted en bloc, halved and a similar extraction procedure was performed. The right ventricle was flushed with 20 ml of phosphate buffered saline to wash the intravascular Evans blue in the lungs. The left lung was then excised and used for Evans blue extraction. The right lung was used for the determination of myeloperoxidase as described below.
Myeloperoxidase levels
The extent of neutrophil accumulation in the intestine, mesentery and right lung tissue was measured by assaying myeloperoxidase activity as previously described (de matos et al., 1999). Briefly, a portion of duodenum, half the mesentery and the flushed right lung of animals that had undergone I/R injury were removed and snap frozen in liquid nitrogen. Upon thawing, the tissue (1 g of tissue per 19 ml of buffer) was homogenized in pH 4.7 buffer (NaCl 0.1 M NaPO4 0.02 M NaEDTA 0.015 M), centrifuged at 260×g for 10 min and the pellet subjected to hypotonic lysis (15 ml of 0.2% NaCl solution followed 30 s later by addition of an equal volume of a solution containing 1.6% NaCl and 5% glucose). After a further centrifugation, the pellet was resuspended in 0.05 M NaPO4 buffer (pH 5.4) containing 0.5% hexadecyltrimethylammonium bromide (HTAB) and re-homogenized. One ml aliquots of the suspension were transferred into 1.5 ml-Eppendorf tubes followed by three freeze–thaw cycles using liquid nitrogen. The aliquots were then centrifuged for 15 min at 10,000×g, the pellet was resuspended to 1 ml and samples of intestine, mesentery and lung were diluted prior to assay. Myeloperoxidase activity in the resuspended pellet was assayed by measuring the change in optical density (OD) at 450 nm using tetramethylbenzidine (1.6 mM) and H2O2 (0.5 mM). Results were expressed as total number of neutrophils by comparing the OD of tissue supernatant with the OD of rat peritoneal neutrophils processed in the same way. To this end, neutrophils were induced in the peritoneum of rats by injecting 3 ml of casein 5%. A standard curve of neutrophil (>95% purity) numbers versus OD was obtained by processing purified neutrophils as above and assaying for MPO activity.
Determination of the concentration of circulating leukocytes
The total number of circulating leukocytes and neutrophils were evaluated in blood samples obtained via a cannula in the femoral artery. Samples were collected prior to ischaemia (time 0), 120 min after ischaemia and 30 and 120 min after reperfusion. The number of total circulating leukocytes was determined by counting leukocytes in a modified Neubauer chamber after staining with Turk's solution, and differential counts by evaluating the percentage of each leukocyte on blood films stained with May–Grunwald–Giemsa.
Measurement of haemoglobin concentrations
The determination of the concentrations of haemoglobin in tissue was used as an index of tissue haemorrhage. After washing and perfusing the intestines to remove excess blood in the intravascular space, a sample of approximately 100 mg of duodenum was removed and homogenized in Drabkin's colour reagent according to instructions of the manufacturer (Analisa, Belo Horizonte, Brazil). This reagent measures the total haemoglobin content (in intact red blood cells or not) of a tissue. The suspension was centrifuged for 15 min at 3000×g and filtered using 0.2 μm filters. The resulting solution was read using an ELISA plate reader at 520 nm and compared against a standard curve of haemoglobin.
Measurement of cytokine concentrations in serum, intestine and lungs
TNF-α, IL-1β, IL-6 and IL-10 concentrations were measured in serum and intestine of animals using ELISA techniques previously described (Rees et al., 1999a, 1999b; Hagan et al., 1993; Francischi et al., 2000; Ball et al., 2001). Serum was obtained from coagulated blood (15 min at 37°C, then 30 min at 4°C) and stored at −20°C until further analysis. Serum samples were analysed at a 1 : 1 dilution in PBS. One hundred mg of duodenum or lung of sham-operated and reperfused animals were homogenized in 1 ml of PBS (NaCl 0.4 m and NaPO4 10 mM) containing anti-proteases (mM: PMSF 0.1; benzethonium chloride 0.1, EDTA 10 and aprotinin A 20 KI) and 0.05% Tween 20. The samples were then centrifuged for 10 min at 3000×g and the supernatant immediately used for ELISA assays at a 1 : 5 dilution in PBS. ELISA plates (Nunc MaxiSorb) were coated with sheep anti-rat TNF-α/IL-1β/IL-6 or IL-10 polyclonal antibodies (1–2 μg ml−1) overnight. The plates were washed thrice and then blocked with 1% bovine serum albumin. After a further wash, plates were incubated with samples or recombinant rat cytokine and incubated overnight. The biotinylated polyclonal antibodies were used at a 1 : 1000 to 1 : 2000 dilution and the assays had a sensitivity of 16 pg ml−1.
Drugs and reagents
The following drugs were obtained from Sigma (U.S.A.): urethane, Evans blue, capsaicin, hexadecyltrimethylammonium bromide. SR140333, SR48968 and SR142801 were a kind gift of Sanofi Rechearche, Lilly, France. CP99,994 was a gift of Pfizer Ltd., Sandwich, Kent.
Statistical analysis
Results are shown as the mean±s.e.mean. Per cent inhibition was calculated by subtracting the background levels of Evans blue extravasation or myeloperoxidase (obtained in sham-operated animals) from control and treated animals. Differences were compared by using analysis of variance (ANOVA) followed by Student–Newman–Keuls post-hoc analysis. Results with a P<0.05 were considered significant.
Results
Dose-dependent effects of tachykinin NK receptor antagonists in a mild model of I/R injury
Initial experiments evaluating the dose-dependent effects of tachykinin NK receptor antagonists were conducted in a mild model of I/R injury. Post-ischaemic treatment of animals with the tachykinin NK1 receptor antagonist SR140333 dose-dependently inhibited both the increase in vascular permeability and the recruitment of neutrophils in the intestine and lung following reperfusion of the ischaemic SMA (Figure 1). Maximal inhibition occurred at the dose of 0.5 m kg−1. Post-ischaemic treatment with the tachykinin NK2 receptor antagonist SR48968 had no effect on the recruitment of neutrophils into the lung or intestine following reperfusion injury (Figure 1). Moreover, SR48968 only marginally inhibited the increase in vascular permeability in the intestine, but not lung, of reperfused animals (Figure 1). In a similar manner, the tachykinin NK3 receptor antagonist SR142801 had no effect on the increase in vascular permeability or neutrophil accumulation in the lung and intestine following mild I/R injury (Figure 1).
Figure 1.
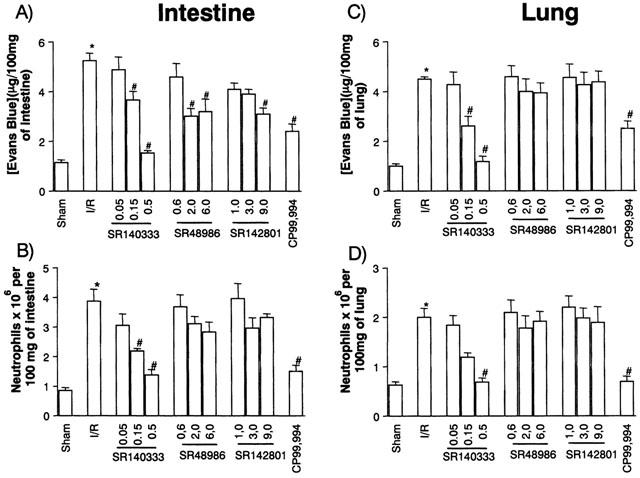
Dose-dependent effects of the treatment with tachykinin NK receptor antagonists on the increase in vascular permeability and recruitment of neutrophils in the intestine (A,B) and lung (C,D) following mild ischaemia (30 min) and reperfusion (30 min) injury of the superior mesenteric artery. Changes in vascular permeability (A,C) were assessed by evaluating the extravasation of Evans blue dye and neutrophil recruitment (B,D) was assessed by evaluating the tissue levels of myeloperoxidase. Tachykinin NK1 (SR140333, 0.05–0.5 mg kg−1 and CP99,994, 4.0 mg kg−1), NK2 (SR48986, 0.6–6.0 mg kg−1) or NK3 (SR142801, 1.0–9.0 mg kg−1) receptor antagonists were given i.v. 5 min prior to reperfusion. Control animals (I/R) received phosphate buffered saline. Results are shown as μg Evans blue or as number of neutrophils per 100 mg of tissue and are the mean±s.e.mean of 5–6 animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.05 when compared to mild I/R animals.
In order to confirm an important role for the tachykinin NK1 receptor, animals were treated with CP99,994, a non-peptide tachykinin NK1 receptor antagonist structurally distinct from SR140333. As seen with SR140333, post-ischaemic treatment with CP99,994 effectively inhibited both the increase in vascular permeability and the recruitment of neutrophils following mild I/R injury (Figure 1).
Effect of sensory nerves depletion by neonatal capsaicin treatment in a mild model of I/R injury
Sensory nerves are a major source of neuropeptides during inflammatory conditions (Holzer, 1991). In order to investigate the potential contribution of sensory nerves for the release of tachykinin NK1-acting neuropeptides and consequent development of mild I/R injury, animals were treated neonatally with capsaicin (Holzer, 1991). As a positive control for the effectiveness of neonatal capsaicin at depleting sensory nerves, we evaluated a behavioural effect, the licking response, after the intraplantar injection of formalin. Formalin induced a biphasic behavioural response that was sensitive to inhibition by SR140333. At the dose used (0.5 mg kg−1), the inhibitory effects of SR140333 were of greater magnitude during the early phase (first 10 min) after formalin injection (82% inhibition) but persisted during the late phase (20–30 min) (56% inhibition, Table 1). Similarly to the effects of the tachykinin NK1 receptor antagonist, neonatal treatment with capsaicin inhibited by 96 and 54%, the early and late phase, respectively (Table 1).
Table 1.
Effects of the treatment with neonatal capsaicin or the tachykinin NK1 receptor anatagonist SR140333 on the behavioural response to intraplantar formalin injection in rats
In capsaicin-treated animals, there was little increase in vascular permeability following mild I/R injury (Figure 2). Likewise, the recruitment of neutrophils following mild I/R was markedly inhibited in comparison to animals that were treated neonatally with vehicle (Figure 2).
Figure 2.
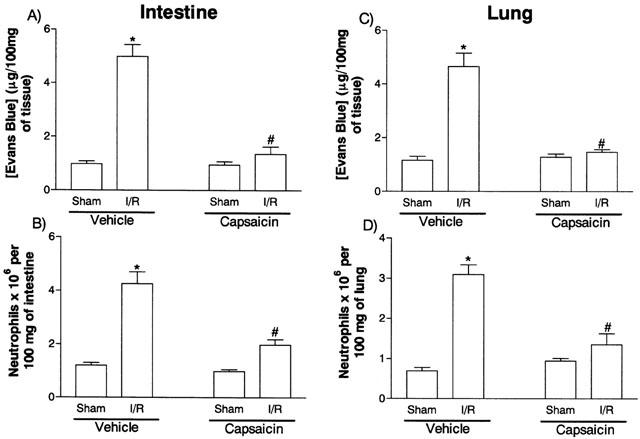
Effects of the neonatal treatment with capsaicin on the increase in vascular permeability and recruitment of neutrophils in the intestine (A,B) and lung (C,D) following mild ischemia (30 min) and reper (30 min) injury of the superior mesenteric artery. Changes in vascular permeability (A,C) were assessed by evaluating the extravasation of Evans blue dye and neutrophil recruitment (B,D) was assessed by evaluating the tissue levels of myeloperoxidase. Capsaicin (50 mg kg−1) or the corresponding volume (50 μl) of capsaicin-vehicle were given on the second day of life and rats were used after 10 weeks. Results are shown as μg Evans blue or as number of neutrophils per 100 mg of tissue and are the mean±s.e.mean of six animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.05 when compared to vehicle-treated animals.
Effects of a tachykinin NK1 receptor antagonist on the local, remote and systemic injuries in a model of severe I/R injury
The next series of experiments was carried out in a model of severe I/R injury where in addition to the changes in vascular permeability and neutrophil accumulation we could observe tissue haemorrhage, leukopenia, changes in cytokine levels in tissue and blood and significant lethality (Souza et al., 2000b). For these experiments we chose to use the tachykinin NK1 receptor antagonist SR140333 at a dose shown to be the maximally inhibitory in the mild I/R model (0.5 mg kg−1). Post-ischaemic treatment with SR140333 virtually abrogated the increase in vascular permeability and neutrophil recruitment in the intestine and in the lung following severe I/R injury (Figure 3). Treatment with SR140333 also abrogated the intestinal increase of haemoglobin, a marker of tissue haemorrhage (Figure 4).
Figure 3.
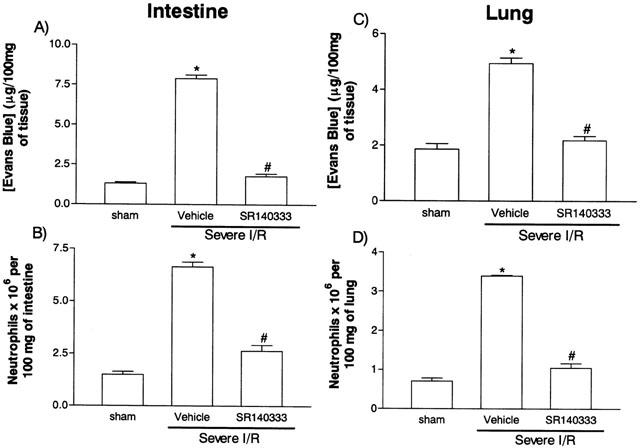
Effects of the treatment with the tachykinin NK1 receptor antagonist SR140333 on the increase in vascular permeability and recruitment of neutrophils in the intestine (A,B) and lung (C,D) following severe ischaemia (120 min) and reperfusion (120 min) injury of the superior mesenteric artery. Changes in vascular permeability (A,C) were assessed by evaluating the extravasation of Evans blue dye and neutrophil recruitment (B,D) was assessed by evaluating the tissue levels of myeloperoxidase. SR140333 (0.5 mg kg−1) was given i.v. 5 min prior to reperfusion. Control animals (vehicle) received phosphate buffered saline. Results are shown as μg Evans blue or as number of neutrophils per 100 mg of tissue and are the mean±s.e.mean of 5–6 animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.05 when compared to mild I/R animals.
Figure 4.
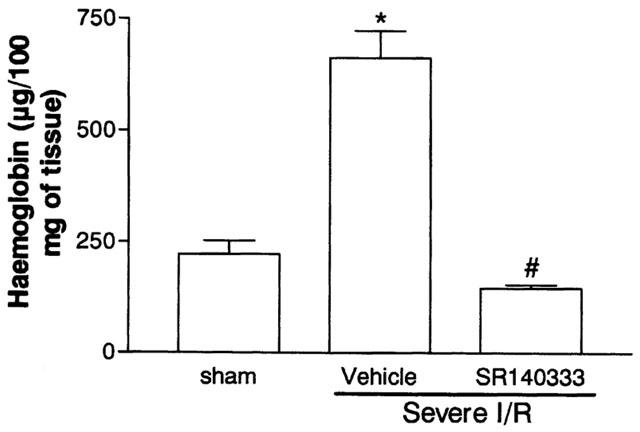
Effects of the treatment with the tachykinin NK1 receptor antagonist SR140333 on the haemorrhage observed in the intestine following severe ischemia (120 min) and reperfusion (120 min) of the superior mesenteric artery. Tissue haemorrhage was assessed by evaluating the tissue levels of haemoglobin. SR140333 (0.5 mg kg−1) was given i.v. 5 min prior to reperfusion. Control animals (vehicle) received phosphate buffered saline. Results are shown as μg haemoglobin per 100 mg of tissue and are the mean±s.e.mean of 5–6 animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.01 when compared to severe I/R animals.
We have previously shown an increase in the concentration of blood neutrophils during the ischaemic period, and a rapid drop in neutrophil levels once reperfusion occurs (Souza et al., 2000b). As SR140333 was administered just prior to reperfusion, the concentration of circulating neutrophils at 120 min of ischaemia were similar and markedly greater than sham-operated animals (Figure 5). In vehicle-treated animals, reperfusion of the ischaemic SMA induced a rapid fall of circulating neutrophils to levels observed in sham-operated animals (Figure 5). Pretreatment with SR140333 partially reversed the rapid neutropenia with approximately 30% inhibition at 120 min (Figure 5).
Figure 5.
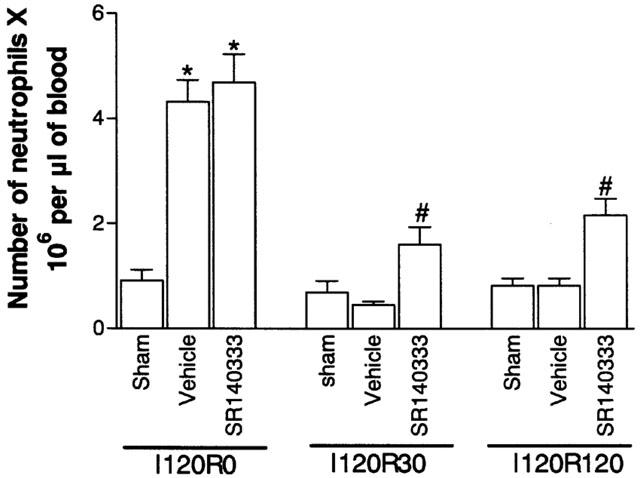
Effects of the treatment with the tachykinin NK1 receptor antagonist SR140333 on the concentration of circulating neutrophils following severe ischaemia (120 min) and reperfusion (120 min) of the superior mesenteric artery. Neutrophils were counted in May–Grunwald–Giemsa-stained blood smears just prior to (I120R0) and at 30 (I120R30) and 120 (I120R120) min after reperfusion. SR140333 (0.5 mg kg−1) was given i.v. 5 min prior to reperfusion. Control animals (vehicle) received phosphate buffered saline. Results are shown as the number of neutrophils×106 per μl of blood and are the mean±s.e.mean of 5–6 animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.01 when compared to severe I/R animals.
The levels of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) and the anti-inflammatory cytokine IL-10 are markedly elevated in serum and tissues after severe I/R injury in rats (Figure 6; Souza et al., 2000b). Post-ischaemic treatment with SR140333 significantly inhibited the elevations of TNF-α in the intestine and lung of severe I/R animals but failed to alter the elevation of the concentration of this cytokine in serum (Figure 6). The elevations of the concentration of IL-6 or IL-1β in tissue and serum were not affected by SR140333 treatment, with the exception of a 40% inhibition of the increase of IL-6 levels in the intestine of animals submitted to severe I/R injury (data not shown). Interestingly, SR140333 abrogated the increase in the concentrations of IL-10 in the lung and serum after severe I/R injury (Figure 6).
Figure 6.
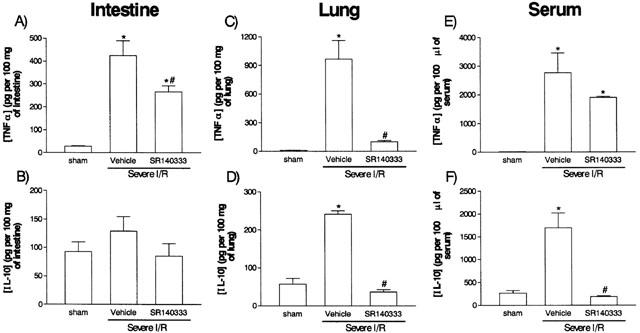
Effects of the treatment with the tachykinin NK1 receptor antagonist SR140333 on the concentrations of TNF-α and IL-10 in the intestine, lung and serum following severe ischaemia (120 min) and reperfusion (120 min) of the superior mesenteric artery. TNF-α (A,C,E) and IL-10 (B,D,F) were assessed in the intestine (A,B), lung (C,D) and serum (E,F) by using specific ELISA. SR140333 (0.5 mg kg−1) was given i.v. 5 min prior to reperfusion. Control animals (vehicle) received phosphate buffered saline. Results are shown as pg TNF-α or IL-10 per ml of plasma or as pg TNF-α or IL-10 per 100 mg of tissue and are the mean±s.e.mean of 5–6 animals in each group. *P<0.01 when compared to sham-operated animals and #P<0.05 when compared to severe I/R animals.
Reperfusion after 120 min of ischaemia of the SMA artery leads to the death of approximately 60% of the animals (Souza et al., 2000b). In the present series of experiments, treatment with SR140333 had no significant effect on the lethality in the severe I/R model (Vehicle-treated, 58% lethality after 120 min, n=12; SR140333, 39% lethality, n=8; P>0.05).
Discussion
The restoration of blood flow of an ischaemic vascular territory, i.e. reperfusion, is a major therapeutic objective following ischaemia of an organ or tissue. However, the reperfusion of an ischaemic territory may activate the cascade of inflammation locally and systemically, limiting the potential benefits of blood flow restoration. Thus, strategies which limit the injury caused by the reperfusion process may be useful adjuncts in the treatment of acute ischaemic disorders in various organs. Here, we have evaluated the involvement of neuropeptides and the potential use of tachykinin NK receptor antagonists during intestinal I/R injury. Intestinal ischaemia is a common clinical syndrome of medical importance with a high mortality rate (Endean et al., 2001). Surgery is the treatment of choice and reperfusion injury may follow these surgical approaches.
Initial dose-response experiments were carried out in a mild model of I/R injury (Souza et al., 2000a). Post-ischaemic treatment of animals with SR140333, a tachykinin NK1 receptor antagonist, inhibited both the increase in vascular permeability and neutrophil recruitment following mild I/R injury. Similarly, pretreatment with a structurally-unrelated tachykinin NK1 receptor antagonist, CP-99,994, markedly inhibited mild I/R injury. Of note, not only were local (intestine) injury inhibited, but there was also a striking inhibition of remote (lung) reperfusion injury. In contrast, pretreatment with antagonists of tachykinin NK2 or NK3 receptors had little or no effect on the increase in vascular permeability or neutrophil influx following mild I/R injury. In fact, only a marginal inhibition of vascular permeability in the intestine was observed following treatment with SR48986, a tachykinin NK2 receptor antagonist. In addition to inhibiting mild I/R injury, post-ischaemic treatment with SR140333 effectively suppressed the reperfusion-induced increase in vascular permeability, neutrophil influx, tissue haemorrhage and blood neutropaenia. Moreover, SR140333 inhibited local (intestine) and remote (lung) increase in the concentrations of the pro-inflammatory cytokine TNF-α observed after severe I/R injury. The concentration of TNF-α in the serum of reperfused animals was not significantly affected. Similarly to our findings with phosphodiesterase type 4 inhibitors (Souza et al., 2001), the lack of effect of the tachykinin NK1 receptor antagonist on serum concentrations of TNF-α correlated with the lack of effect of the drug on reperfusion-induced lethality. Overall, the above results are first to demonstrate an important role of the activation of tachykinin NK1 receptors in the development of the local, remote and systemic injuries following intestinal I/R. Of note, the antagonists were given prior to reperfuse and at the end of the ischaemic period. This time frame of drug administration keeps in mind the real clinical situation when patients are seen with the ischaemic syndrome and will undergo a procedure to reperfusion the ischaemic vascular bed. In support of our present findings, tachykinin NK1 receptor antagonism has been shown to reduce oxidative injury and improve functional recovery following in vitro myocardial I/R injury in magnesium-deficient animals (Kramer et al., 1997). Similarly, ‘myocardial stunning' was prevented by tachykinin NK1 receptor antagonism in an in vitro guinea-pig model (Chiao & Caldwell, 1996).
There are at least two possibilities that may explain the beneficial effects of tachykinin NK1 receptor antagonists in our I/R model – inhibition of neutrophil influx and/or inhibition of pro-inflammatory cytokine release. As neutrophil recruitment underlies both local and remote injury (Souza et al., 2000a, 2000b), the prevention of the recruitment of this cell type may explain the anti-inflammatory effects of tachykinin NK1 receptor antagonists. This possibility is in line with other studies demonstrating an important role of neuropeptides and the tachykinin NK1 receptor for neutrophil activation and/or recruitment in different models of inflammation (e.g. Ahluwalia et al., 1998; Cao et al., 2000; Medeiros et al., 2001). However, this still leaves an open question as to whether tachykinin NK1 receptors on the neutrophil or on an intermediate cell type (e.g. mast cell) (Maggi, 1997) mediate the action of locally-released neuropeptides. In addition to blocking neutrophil influx, tachykinin NK1 receptor antagonists also prevented the release of TNF-α. This is in agreement with other studies demonstrating similar effects of these antagonists on LPS-induced systemic cytokine release (Dickerson et al., 1998). As inhibition of the pro-inflammatory cytokine TNF-α is accompanied by abrogation of I/R injuries in our model (Souza et al., 2001), this inhibitory effect could underline the marked suppressive effects of tachykinin NK1 receptor antagonists in our I/R injury model. However, local, but not systemic, TNF-α release is dependent on the influx of neutrophils (Souza et al., 2000b) suggesting that the suppression of the migration of that cell type may be a more dominant action in our model.
Substance P acting on tachykinin NK1 receptors of macrophages markedly agumented LPS-induced production of the anti-inflammatory cytokine IL-10 (Ho et al., 1996). Since IL-10 may be induced in reperfused tissue and may modulate reaction to I/R injury (Frangogiannis et al., 2000), we measured the concentrations of this cytokine following severe I/R injury in control and SR140333-treated animals. There was a significant elevation of IL-10 concentrations in the serum and lung, but not intestine, following reperfusion of the ischaemic SMA. However, treatment with SR140333 markedly inhibited the elevations in IL-10 concentrations. These results demonstrate that an increase in IL-10 does not explain the inhibitory effects of tachykinin NK1 receptor antagonists and are in agreement with the dependency of IL-10 production on local TNF-α release (Souza et al., 2001).
Sensory nerves are a major source of tachykinin NK1 receptor-acting neuropeptides, especially substance P (Holzer, 1991; Quartara & Maggi, 1998). In addition, substance P may be released by leukocytes, such as macrophages, and endothelial cells (reviewed by Maggi, 1997). To investigate the role of sensory nerves as a source of neuropeptides in our system, animals were pretreated neonatally with capsaicin (Holzer, 1991). At the dose used, capsaicin markedly suppressed the neuropeptide-dependent behavioural response to formalin injection and also effectively suppressed the injuries following mild intestinal I/R. These data suggest that sensory nerves are responsible for the majority of neuropeptides released during I/R injury. Indeed, there is evidence demonstrating the activation of cardiac vagal nerve chemosensitive endings in rats undergoing I/R (Ustinova & Schultz, 1994). Our data do not allow any conclusions in respect to the molecular mechanisms responsible for the activation of sensory nerves and subsequent neuropeptide release and action on tachykinin NK1 receptors. However, there are several possibilities which could account for the activation of sensory nerves during I/R. Previous studies have demonstrated that sensory nerves release tachykinins in response to ischaemia or low pH (reviewed in Maggi, 1995). In addition, locally released prostaglandins may participate in sensory nerve activation during ischaemia, but not during reperfusion (Ustinova & Schultz, 1994). Finally, bradykinin and leukotriene release during I/R injury may account for sensory nerve activation in this setting (Longhurst & Dittman, 1987; Souza et al., 2000a; 2001; Montuschi et al., 2000).
In addition to our present findings, previous studies from our group have shown that antagonism of PAF or LTB4 receptors inhibited the development of I/R injury in rats (Souza et al., 2000a, 2000b). Moreover, we have recently shown that anti-TNF-α antibodies prevented both injury and lethality following severe I/R injury (Souza et al., 2001). This leaves a question as to how these different mediators interact in our system to mediate I/R injury. Our working hypothesis is that with the onset of reperfusion, there is a marked local release of oxygen-derived metabolites which activate different tissue cells (including endothelial cells, macrophages, mast cells and nerve endings) to release inflammatory mediators (e.g. TNF-α, PAF) which will start the recruitment of neutrophils. This early neutrophil recruitment appears to be PAF- (Souza et al., 2000a) and TNF-α-dependent (Souza et al., 2001) whereas LTB4 plays a greater role in the activation of neutrophils (Souza et al., 2000b). One possibility to explain the importance of tachykinin NK1 receptor activation in the system is that the activation of these receptors by neuronal-derived neuropeptides may underlie the initial release of lipid mediators. Alternatively, NK1 receptor activation may occur later and be necessary for the amplification of the inflammatory response after the onset of reperfusion, i.e. after a first wave of incoming leukocytes (specially neutrophils) and released mediators, neuropeptide release and NK1 receptor activation may facilitate further TNF-α release, leukocyte influx and mediator release. The released mediators and recruited leukocytes may then, in cooperation, achieve sufficient intensity to cause tissue injury and lethality.
In conclusion, our data demonstrate that the post-ischaemic blockade of tachykinin NK1 receptors markedly inhibited the local and remote injuries, but not lethality, following reperfusion of the SMA in rats. Neuropeptides, possibly substance P, released from sensory nerves appear to account for the activation of these tachykinin NK1 receptors. Altogether our results suggest that antagonists of the tachykinin NK1 receptor may be useful adjuncts in the treatment of the injuries which occur following reperfusion of an ishcemic vascular territory.
Acknowledgments
This work received financial support from FAPEMIG and CNPq (Brazil).
Abbreviations
- I/R
ischaemia and reperfusion
- MPO
myeloperoxidase
- OD
optical density
- SMA
superior mesenteric artery
References
- AHLUWALIA A., DE FELIPE C., O'BRIEN J., HUNT S.P., PERRETTI M. Impaired IL-1beta-induced neutrophil accumulation in tachykinin NK1 receptor knockout mice. Br. J. Pharmacol. 1998;124:1013–1015. doi: 10.1038/sj.bjp.0701978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALL V., VIGUES S., GEE C.K., POOLE S., BRISTOW A.F. Rat interleukin-10: production and characterisation of biologically active protein in a recombinant bacterial expression system. Eur. Cytokine Netw. 2001;12:187–193. [PubMed] [Google Scholar]
- BHATIA M., SALUJA A.K., HOFBAUER B., FROSSARD J.L., LEE H.S., CASTAGLIUOLO I., WANG C.C., GERARD N., POTHOULAKIS C., STEER M.L. Role of substance P and the neukinin 1 receptor in acute pancreatitis and pancreatitis-associated lung injury. Proc. Natl. Acad. Sci. 1998;95:4760–4765. doi: 10.1073/pnas.95.8.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOZIC C.R., LU B., HOPKEN U.E., GERARD C., GERARD N.P. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- CAO T., PINTER E., AL-RASHEED S., GERARD N., HOULT J.R., BRAIN S.D. Neurokinin-1 receptor agonists are involved in mediating neutrophil accumulation in the inflamed, but not normal, cutaneous microvasculature: an in vivo study using neurokinin-1 receptor knockout mice. J. Immunol. 2000;164:5424–5429. doi: 10.4049/jimmunol.164.10.5424. [DOI] [PubMed] [Google Scholar]
- CHIAO H., CALDWELL R.W. The role of substance P in myocardial dysfunction during ischemia and reperfusion. Naunyn Schmiedebergs Arch. Pharmacol. 1996;353:400–407. doi: 10.1007/BF00261436. [DOI] [PubMed] [Google Scholar]
- CORNEJO C.J., WINN R.K., HARLAN J.M. Anti-adhesion therapy. Adv. Pharmacol. 1997;39:99–141. doi: 10.1016/s1054-3589(08)60070-8. [DOI] [PubMed] [Google Scholar]
- CUTRUFO C., EVANGELISTA S., CIRILLO R., CIUCCI A., CONTE B., LOPEZ G., MANZINI S., MAGGI C.A. Protective effect of the tachykinin NK2 receptor antagonist nepadutant in acute rectocolitis induced by diluted acetic acid in guinea-pigs. Neuropeptides. 2000;34:355–359. doi: 10.1054/npep.2000.0819. [DOI] [PubMed] [Google Scholar]
- DAMAS J., LIEGEOIS J.F. The inflammatory reaction by formalin in the rat paw. Naunyn Schmiedebergs Arch. Pharmacol. 1999;359:220–227. doi: 10.1007/pl00005345. [DOI] [PubMed] [Google Scholar]
- DE MATOS I.M., SOUZA D.G., SEABRA D.G., FREIRE-MAIA L., TEIXEIRA M.M. Effects of tachykinin NK1- or PAF-receptor blockade on the lung injury induced by scorpion venom. Eur. J. Pharmacol. 1999;376:293–300. doi: 10.1016/s0014-2999(99)00382-9. [DOI] [PubMed] [Google Scholar]
- DICKERSON C., UNDEM B., BULLOCK B., WINCHURCH R.A. Neuropeptide regulation of proinflammatory cytokine responses. J. Leukoc. Biol. 1998;63:602–605. doi: 10.1002/jlb.63.5.602. [DOI] [PubMed] [Google Scholar]
- ENDEAN E.D., BARNES S.L., KWOLEK C.J., MINION D.J., SCHWARCZ T.H., MENTZER R.M., Jr Surgical management of thrombotic acute intestinal ischemia. Ann Surg. 2001;233:801–808. doi: 10.1097/00000658-200106000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANCISCHI J.N., YOKORO C.M., CUNHA F.Q., TAFURI Wg.L, TEIXEIRA M.M. Effects of the PDE4 inhibitor rolipram in a rat model of arthritis. Eur. J. Pharmacol. 2000;399:243–249. doi: 10.1016/s0014-2999(00)00330-7. [DOI] [PubMed] [Google Scholar]
- FRANGOGIANNIS N.G., MENDOZA L.H., LINDSEY M.L., BALLANTYNE C.M., MICHAEL L.H., SMITH C.W., ENTMAN M.L. IL-10 induced in the reperfused myocardium and may modulate the reaction to injury. J. Immunol. 2000;165:2798–2808. doi: 10.4049/jimmunol.165.5.2798. [DOI] [PubMed] [Google Scholar]
- GILMONT R.R., DARDANO A., ENGLE J.S., ADAMSON B.S., WELSH M.J., LI T., REMICK D.G., SMITH D.J., REES R.S. TNF-α potentiates oxidant and reperfusion-induced endothelial cell injury. J. Surg. Res. 1996;61:175–182. doi: 10.1006/jsre.1996.0101. [DOI] [PubMed] [Google Scholar]
- HAGAN P., POOLE S., BRISTOW A.F. Endotoxin-stimulated production of rat hypothalamic interleukin-1 beta in vivo and in vitro, measured by specific immunoradiometric assay. J. Mol. Endocrinol. 1993;11:31–36. doi: 10.1677/jme.0.0110031. [DOI] [PubMed] [Google Scholar]
- HO W.Z., KAUFMAN D., UVAYDOVA M., DOUGLAS S.D. Substance P augments interleukin-10 and tumor necrosis factor-α release by human cord blood monocytes and macrophages. J. Neuroimmunol. 1996;71:73–80. doi: 10.1016/s0165-5728(96)00132-4. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: Cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- JANCSÓ G., KIRALY E., JANCSO-GABOR A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- KRAMER J.H., PHILLIPS T.M., WEGLICKI W.B. Magnesium-deficiency-enhanced post-ischemic myocardial injury is reduced by substance P receptor blockade. J. Mol. Cell. Cardiol. 1997;29:97–110. doi: 10.1006/jmcc.1996.0255. [DOI] [PubMed] [Google Scholar]
- KUBES P., JUTILA M., PAYNE D. Therapeutic potential of inhibiting leukocyte rolling in ischemia/reperfusion. J. Clin. Invest. 1995;95:2510–2519. doi: 10.1172/JCI117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEFER A.M., LEFER D.J. The role of nitric oxide and cell adhesion molecules on the microcirculation in ischaemia-reperfusion. Cardiovasc. Res. 1996;327:43–51. [PubMed] [Google Scholar]
- LONGHURST J.C., DITTMAN L.E. Hypoxia, bradykinin, and prostaglandins stimulate ischemically sensitive visceral afferents. Am. J. Physiol. 1987;253:H556–H567. doi: 10.1152/ajpheart.1987.253.3.H556. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. Tachykininis and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Proc. Neurobiol. 1995;45:1–98. doi: 10.1016/0301-0082(94)e0017-b. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. The effects of tachykinin on inflammatory and immune cells. Regul. Pept. 1997;70:75–90. doi: 10.1016/s0167-0115(97)00029-3. [DOI] [PubMed] [Google Scholar]
- MEDEIROS M.V., MACEDO-SOARES M.F., DE LUCA I.M., HYSLOP S., DENUCCI G., ANTUNES E. Contribution of C-fibers to leucocyte recruitment in bronchoalveolar lavage fluid and pleural cavity in the rat. Eur. J. Pharmacol. 2001;421:133–140. doi: 10.1016/s0014-2999(01)01018-4. [DOI] [PubMed] [Google Scholar]
- MONTUSCHI P., PREZIOSI P., CIABATTONI G. Thachykinins-eicosanoid crosstalk in airway inflammatory. Trends Pharmacol. Sci. 2000;21:336–340. doi: 10.1016/s0165-6147(00)01520-0. [DOI] [PubMed] [Google Scholar]
- NENAN S., GERMAIN N., LAGENTE V., EMONDS-ALT X., ADVENIER C., BOICHOT E. Inhibition of inflammatory cell recruitment by the tachykinin NK3-receptor antagonist, SR142081, in a murine model of asthma. Eur. J. Pharmacol. 2001;421:201–205. doi: 10.1016/s0014-2999(01)01036-6. [DOI] [PubMed] [Google Scholar]
- QUARTARA L., MAGGI C.A. The tachykinin NK1 receptor. Part II: distribution and pathophysiological roles. Neuropeptides. 1998;32:1–49. doi: 10.1016/s0143-4179(98)90015-4. [DOI] [PubMed] [Google Scholar]
- REES G.S., BALL C., WARD H.L., GEE C.K., TARRANT G., MISTRY Y., POOLE S., BRISTOW A.F. Rat interleukin 6: expression in recombinant Escherichia coli, purification and development of a novel ELISA. Cytokine. 1999a;11:95–103. doi: 10.1006/cyto.1998.0408. [DOI] [PubMed] [Google Scholar]
- REES G.S., GEE C.K., WARD H.L., BALL C., TARRANT G., MISTRY Y., POOLE S., BRISTOW A.F. Rat tumour necrosis factor-alpha: expression in recombinant Pichia pastoris, purification, characterization and development of a novel ELISA. Eur. Cytokine Netw. 1999b;10:383–392. [PubMed] [Google Scholar]
- RUPNIAK N.M., CARLSON E., BOYCE S., WEBB J.K., HILL R.G. Enantioselective inhibition of the formalin paw late phase by the NK1 receptor antagonist L-733,060 in gerbils. Pain. 1996;67:189–195. doi: 10.1016/0304-3959(96)03109-0. [DOI] [PubMed] [Google Scholar]
- SAKURADA T., KATSUMATA K., YOGO H., TAN-NO K., SAKURADA S., KISARA K. Antinociception induced by CP 96,345, a non-peptide NK-1 receptor antagonist, in the mouse formalin and capsaicin tests. Neurosci. Lett. 1993;151:142–145. doi: 10.1016/0304-3940(93)90006-7. [DOI] [PubMed] [Google Scholar]
- SARIA A., LUNDBERG J.M. Evans blue fluorescence: quantitative and morphological evaluation of vascular permeability in animal tissue. J. Neurosci. Meth. 1983;8:41–49. doi: 10.1016/0165-0270(83)90050-x. [DOI] [PubMed] [Google Scholar]
- SHIBATA M., OHKUBO T., TAKAHASHI H., INOKI R. Modified formalin test: charecteristic biphasic pain response. Pain. 1989;38:347–352. doi: 10.1016/0304-3959(89)90222-4. [DOI] [PubMed] [Google Scholar]
- SOUZA D.G., CARA D.C., CASSALI G.D., COUTINHO S.F., SILVEIRA M.R., ANDRADE S.P., POOLE S.P., TEIXEIRA M.M. Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries following ischaemia mesenteric artery in the rat. Br. J. Pharmacol. 2000b;131:1800–1808. doi: 10.1038/sj.bjp.0703756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., CASSALI G.D., POOLE S., TEIXEIRA M.M. Effects of inhibition of PDE4 and TNF-a on local and remote injuries following ischemia and reperfusion injury. Br. J. Pharmacol. 2001;134:985–994. doi: 10.1038/sj.bjp.0704336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., COUNTINHO S.F., SILVEIRA M.R., CARA D.C., TEIXEIRA M.M. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischemia of the superior mesenteric artery in rats. Eur. J. Pharmacol. 2000a;403:121–128. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- USTINOVA E.E., SCHULTZ H.D. Activation of cardiac vagal afferents in ischemia and reperfusion. Prostaglandins versus oxygen-derived free radicals. Circ. Res. 1994;74:904–911. doi: 10.1161/01.res.74.5.904. [DOI] [PubMed] [Google Scholar]
- WILLERSON J.T. Pharmacological approaches to reperfusion injury. Adv. Pharmacol. 1997;39:291–312. doi: 10.1016/s1054-3589(08)60074-5. [DOI] [PubMed] [Google Scholar]


