Abstract
The vasodilatory effects of nucleotides in the guinea-pig thoracic aorta were examined to determine the relationship between molecular expression and function of P2Y receptors.
In aortic rings precontracted with norepinephrine, vasodilatory responses to purine nucleotides exhibited a rank-order of potency of 2-methylthio-ATP>ADP>ATP. Responses to UTP, but not UDP suggested a functional role for P2Y4 but not P2Y6 receptors.
Aortic endothelial cells express at least four P2Y receptors; P2Y1, P2Y2, P2Y4 and P2Y6. In primary culture, these cells exhibit desensitizing transient calcium responses characteristic of P2Y1, P2Y2 and P2Y4, but not P2Y6 receptors. UDP had no effect on endothelial cell calcium.
The pyrimidinergic receptor agonist UTP is capable of eliciting robust vasodilation in aortic rings and causing calcium responses in cultured guineapig aortic endothelial cells. These responses are equivalent to the maximum responses observed to ATP and ADP.
Measurement of intracellular calcium release in response to ATP and 2-methylthio-ATP were similar, however only the 2-methylthio-ATP response was sensitive to the P2Y1 antagonist N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate (MRS2179). In aortic rings, vasodilatory responses to 2-methylthio-ATP, ATP and ADP were all blocked by pre-incubation of tissues with MRS2179.
MRS2179 pretreatment had no effect of the ability of UTP to cause relaxation of norepinephrine responses in aortic rings or the ability of UTP to cause calcium release in aortic endothelial cells.
We demonstrate robust effects of purine and pyrimidine nucleotides in guineapig aorta and provide functional and biochemical evidence that MRS2179 is a selective P2Y1 antagonist.
Keywords: Aorta; nucleotides; ATP; 2-methylthio-ATP; UTP; vasodilation; endothelial cells; calcium; 2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate (MRS 2179); guineapig
Introduction
Nucleotides have been established as extracellular vasoactive factors capable of regulating local blood vessel tone. Nucleotides orchestrate these actions by binding to so-called ionotropic P2X receptors and metabotropic P2Y receptors (Ralevic & Burnstock,1998). There are currently seven cloned P2X receptors (P2X1-7) and six cloned (von Kugelgen & Wetter, 2000) P2Y receptors (P2Y1,2,4,6,11) and the newly described platelet receptor P2Y12 (Savi et al., 2001). These receptors are categorized by nucleotide ligand binding preferences that vary across species (Ralevic & Burnstock,1998). Unlike the P2X ion channel receptors, P2Y receptors are GTP-binding protein coupled receptors and both subclasses of receptor, while partitioned among vascular cell types, are likely to mediate nucleotide responses in the vasculature. Although there are some distinctions in the coupling of P2Y receptors to response (Yang et al., 1996; Communi et al., 1996; von Kugelgen & Wetter, 2000), a precise understanding that explains the presence of multiple P2Y receptor subtypes located on a single cell type such as endothelium (Motte et al., 1993) or vascular smooth muscle (Erlinge et al., 1998) is still wanting. Indeed, the presence of both P2Y1 and P2Y2 receptors on endothelial cells appears to be redundant since both receptors bind ATP and ADP, and couple to the same GTP-binding protein to mobilize intracellular calcium and mediate relaxation. While this apparent redundancy has yet to be satisfactorily explained, it could be physiologically important based on the source of ATP versus UTP and the presence of disease. It is known that endothelial cells release ATP in response to vasoactive agonists or shear stress (Yang, et al., 1994; Oxhorn et al., 2000), and that this ATP is capable of acting in both an autocrine and paracrine fashion to signal vasodilation multiple times as ATP, or its active metabolites ADP and adenosine (ADO) (Buxton et al., 2001). Indeed, the intravascular actions of nucleotides in blood vessels involve a series of discrete actions that constitute a hormonal axis. The Nucleotide Axis Hypothesis (Oxhorn et al., 2000; Buxton et al., 2001) offers a framework within which to consider the actions of nucleotides and adenosine as responsible for the regulation of blood flow on a moment-to-moment basis directly and as permissive hormones in numerous vascular beds.
UTP release has been demonstrated from some cells upon mechanical stimulation (Lazarowski et al., 1997), is found in and released from platelet vesicles (Lazarowski & Harden, 1999), and is known to regulate blood vessel tone (Hardebo et al., 1987; Kong et al., 1996). Whether UTP is endothelial or platelet in origin, its actions in the undamaged blood vessels are most likely to be subserved by P2Y4 receptors expressed on endothelial cells (Yang et al., 1996). The P2Y4 receptor is a UTP-selective receptor coupled to intracellular calcium release (Charlton et al., 1996a; Yang et al., 1996). Homologues of the P2Y4 receptor have been cloned and characterized from human (Communi et al., 1996), rat (Bogdanov et al., 1998), and mouse (Suarez-Huerta et al., 2001) where UTP is a full agonist, but ATP is either a full agonist (rat and mouse), or partial agonist and even an antagonist (human). An extracellular origin for UTP together with the presence, on the endothelium, of an UTP-selective receptor establishes a role for UTP in the bloodstream as a regulatory hormone. Studies of nucleotide receptors have been complicated by the lack of selective agonists and antagonists, especially in systems where more than one receptor is present. Commonly, compounds such as pyridoxalphosphate 6-azophenyl 2′,4′-disulphonic acid (PPADS), suramin, and reactive blue-2 have been employed to antagonize both P2X and P2Y-mediated nucleotide responses in recombinant cell systems (Boyer et al., 1994; Charlton et al., 1996b), whole arteries (Brown et al., 1995), and vas deferens (McLaren et al., 1994). In studies of P2Y receptor-mediated IP3 accumulation in a model system, the turkey erythrocyte, N6-methyl-2′-deoxyadenosine-3′,5′-bisphosphate (MRS2179) was shown to antagonize the recombinant human P2Y1, with no observed effects on human P2Y2 or P2Y4, or on rat P2Y6 receptors (Boyer et al., 1998).
Here, in guinea-pig aorta, we show results of P2Y receptor expression, coupling of receptors to changes in endothelial cell calcium, the effects of receptor stimulation on blood vessel tone and the effect of receptor antagonism by MRS2179.
Methods
Aorta preparation and contraction experiments
The descending thoracic aorta, removed from female guineapigs following cervical dislocation under a protocol approved by the institutional animal care and use committee, were dissected free of connective and adipose tissue, cut as rings in 4 mm widths and suspended on tungsten wire triangles in 20 ml tissue baths (Radnoti, Monrovia, CA, U.S.A.). The preparation was suspended by silk thread between a fixed point and an isometric force transducer (Kent Scientific, Litchfield, CT, U.S.A.) for the measurement of tension. Transducer voltages were amplified and converted to digital signals by an analogue-to-digital board mounted within a computer running the Workbench® data acquisition system (Strawberry Tree, Sunnyvale, CA, U.S.A.). Rings were maintained at 37°C, aerated with 95% O2, 5% CO2, in a Krebs bicarbonate buffer containing (in mM): NaCl 118, KCl 4.75, KH2PO4 1.2, α-D-glucose 20, CaCl2 2.5, NaHCO3 25, and MgSO4 1.2 and loaded with initial tensions of 2 g mm−2. Initial tensions were based on established force-length relationships. Tissues were equilibrated in Krebs bicarbonate buffer (pH=7.4) for 1 h and precontracted twice to 60 mM potassium to standardize responses. Drugs were diluted 1 : 1000 upon addition into tissue baths. Only tissues that contracted to addition of 300 nM norepinephrine (NE) and subsequently relaxed to 2-methylthio-ATP were used to ensure that sample preparation had not damaged either smooth muscle or endothelial cells. Some tissues were mechanically denuded of endothelium (confirmed by contraction to acetylcholine) as a control for tissue-specific compartmentation of receptors. In these endothelium-denuded tissues, the contraction to NE was unaltered while the relaxation to purine and pyrimidine nucleotides was absent.
Tissues were contracted with 300 nM NE and then treated with 1 μM MRS2179 where indicated. In order to study the effects of UTP over a range of concentrations up to 1 mM without altering the standard addition volume of 20 μl (1 : 1000 dilution), changes of the bath solution were performed at the peak of contraction with standard Krebs-bicarbonate buffer supplemented to mimic pre-bath change conditions or provide a new stimulus in addition to the pre-bath change condition. We term this procedure wash-in. As a control for the wash-in procedure, some tissues experienced wash-in at the peak of contraction with Krebs-bicarbonate buffer supplemented only with 300 nM NE. Parallel controls were performed with 1 mM UTP +/− 1 μM MRS2179, in the presence and absence of endothelium.
Endothelial cell preparation and culture
Guinea-pig aortic endothelial cells (GPAEC) were prepared by longitudinal dissection of aorta in standard phosphate buffered saline (PBS) followed by physical removal of the endothelium with a cell scraper (Fischer Scientific, PA, U.S.A.). Cells were centrifuged at 2000×g for 10 min, the PBS removed and the pellet resuspended in Dulbecco's Modified Eagle Medium supplemented with 10% foetal bovine serum as we have previously described (Yang et al., 1994). Cell isolates were panned on fibronectin-coated surface for 120 min and potential smooth muscle cell contamination removed by washing and confirmed by microscopic examination. Endothelial cells were cultured in modified 2 ml culture dishes (of our own design) with glass bottoms to allow UV excitation and grown at 37°C in 95%O2/5%CO2. Cells were allowed to grow for 1 week following isolation prior to experimentation. The maintenance of endothelial phenotype in culture was independently confirmed by flow cytometric analysis of PECAM 1 (CD31) expression and binding of the plant lectin UEA (Buxton et al., 2001). Only cell preparations that tested positive for both determinants of endothelial phenotype were employed in calcium experiments.
Fura-2 loading and calcium imaging
GPAEC were grown to 25% confluence, washed with a MOPS buffer containing (in mM): NaCl 120, KCl 5, MgCl2 1, CaCl2 1, glucose 6, NaHCO3 5, Na-MOPS (pH=7.4) 10 and loaded with the permeant acetoxymethyl-ester of the calcium indicator dye Fura-2 (Fura-2AM, 2 μM; Molecular Probes, OR, U.S.A.) in the same buffer supplemented with 0.1 mg/ml bovine serum albumen, 0.02% Cremophor EL and 10 nM neostigmine bromide. Cells were loaded for 1 h at 21°C, rinsed with MOPS buffer and allowed to recover at 37°C for 20 min. Fields of 6 to 10 Fura-2 loaded cells were illuminated first with 340 nm followed by 380 nm light using a Photon Technology Inc. Delta Ram coupled to a Nikon inverted microscope. Fluorescence emission at 510 nm was collected by a 40× fluorescence objective and imaged by an intensified CCD at video rates (Photon Technology Inc., NJ, U.S.A.). A ratio of these images was calculated to obtain information on the relative change in cytoplasmic calcium with a temporal resolution of 66 msec. Agonists were introduced to the cultures by carefully removing 1 ml of buffer and replacing it with 1 ml buffer containing the desired agonists at specific 2× concentrations. This procedure was controlled by removal and replacement of buffer alone and found to be preferable to addition of concentrated agonist. Addition of the well-known endothelial agonist bradykinin (1 nM) at the termination of each experiment served to provide a reference calcium transient. In some experiments, addition of ionomycin followed by addition of Mn2+ to quench fluorescence served to reference the size of the calcium transients seen with nucleotides and bradykinin. Precise calibration of intracellular calcium concentrations was not desirable or performed.
RNA isolation and RT–PCR
Total RNA was prepared using the TRIzol method (Gibco BRL) from 50 mg (wet wt.) of guinea-pig thoracic aorta prepared as described above. Aorta was either used intact or mechanically denuded of endothelium prior to RNA isolation. Resulting RNA was resuspended in 30 μl DEPC treated H2O. Any DNA contamination remaining was removed by treatment at 37°C with 10 units RNAse-free DNAse I (Promega, U.S.A). The enzyme was then inactivated by adding 25 mM EDTA with heating at 55°C for 10 min. cDNA was synthesized from 1 μg of total RNA using 500 ng oligo-dT15 (Promega), 2 mM dNTPs, 10 mM DTT and 200 units Superscript II reverse transcriptase (Gibco BRL).
Primers for PCR of P2Y receptors were designed from regions of high homology between human, mouse, and/or rat homologues for use on guineapig templates. The sequences employed were: mouse P2Y1 (Genbank accession number AJ245636, C. Leon); human P2Y1 (AJ006945, C. Leon); human P2Y2 (NM002564, C.E. Parr et al.); rat P2Y2 (9U56839, C.I. Seye et al.); human P2Y4 (X91852, D. Communi et al.); rat P2Y4 (Y14705, Y.D. Bogdanov et al.); human P2Y6 (NM004154, R. Maier et al.); and rat P2Y6 (AA778919, L. Hillier et al.). The expected products for P2Y1,2,4,6 from these primer pairs are 191, 203, 260, and 100 bp, respectively. Thermal cycling was performed for 40 cycles at 94°C for 45 s, 53°C for 45 s, 72°C for 45s; followed by 7 min at 72°C as a final extension. For analysis, 5 μl of each resulting product was separated by electrophoresis for 1 h–100 V on a 1.25% agarose gel. Gels were bathed in 2 mg/ml−1 ethidium bromide for 10 min and imaged with UV light in an gel-documentation apparatus from Alpha Innotech (U.S.A). Differences in resulting RT–PCR patterns obtained from intact tissue and denuded aorta were then ascribed to the presence of endothelium in the intact tissue.
Data analysis
Data from contractile experiments, logged as digital data files from the QuickLog program (Strawberry Tree Software, Sunnyvale, CA, U.S.A.), were normalized by considering baseline tension 0% and peak tension after contractile stimulus as 100%. Relaxations to specific agonists were then compared as the total reduction from the 100% level achieved after their addition. For concentration response curves, triplicate experiments of four tissues each were performed and the relaxed tensions were averaged for each vasodilator at different non-cumulative concentrations. Averages±standard error of the mean (s.e.mean) were plotted and curves were fitted as sigmoidal concentration response curves using the data analysis and presentation software Prism®, from GraphPad Software (Carlsbad, CA, U.S.A.). Data from calcium experiments were imported as ratios of the measured 510 nm emissions at 340/380 nm excitations for each region of interest (each ROI is a single cell) into Prism software. Total calcium changes were observed and plotted as a continuous trace of the gathered data points for representation of typical calcium responses. All observations were performed in triplicate.
Materials
Growth media and serum were obtained from Atlanta Biologicals (Norcross, GA, U.S.A.). TRIzol was obtained from Invitrogen (Carlsbad, CA, U.S.A.). The antibody to endothelial PECAM1 was obtained from Biogenesis (Brentwood, NH, U.S.A.). Fura-2 AM was obtained from Molecular Probes (Eugene, OR, U.S.A.). Unless otherwise noted, all other buffers, drugs and reagents were obtained from Sigma Chemical Co. (St Louis, MO, U.S.A.) including nucleotides, analogues, and MRS2179.
Results
Guinea-pig aortae contracted in the presence of 300 nM NE are relaxed in a dose-dependent fashion following addition of nucleotides or nucleotide analogues. The P2Y1 agonist 2-MeS-ATP relaxed guinea-pig aorta by 13% of peak contraction at 3 nM (Figure 1) and had a maximum vasodilatory effect of 90% at 300 nM (Figure 2). Similar experiments were performed for ATP, ADP and UTP to characterize the ability of nucleotides to relax guinea-pig aorta and concentration response curves were constructed (Figure 2). Apparent EC50 values were determined by computer analysis for 2-MeS-ATP, ADP, ATP and UTP of 10 nM, 320 nM, 3 μM, and 550 μM respectively. This rank order of potency suggests a dominant role for the P2Y1 purinergic receptor in guinea-pig aorta.
Figure 1.
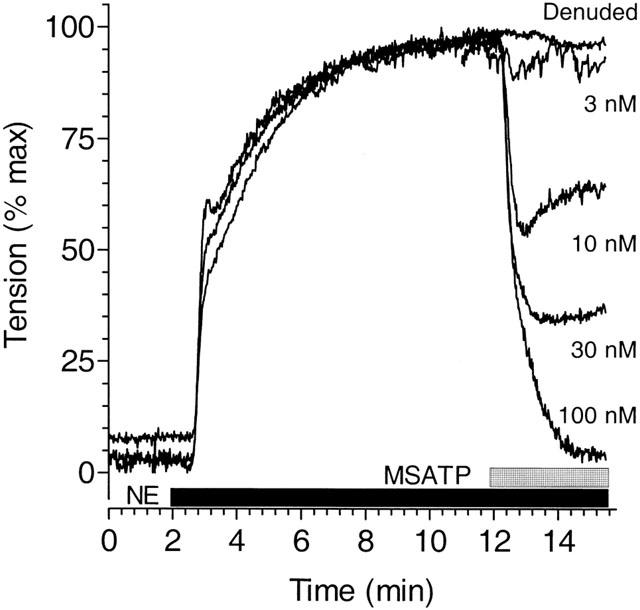
Relaxation of guinea-pig aorta by the P2Y1 agonist 2-MeS-ATP. Thoracic aortic rings from male guinea-pigs were contracted with NE (300 nM) as shown by the solid bar and peak tension normalized to 100% in order to compare results from disparate tissues. Addition of the P2Y1-selective agonist, 2-MeS-ATP (stippled bar) at various concentrations in the continued presence of NE, led to the immediate relaxation of tension except when endothelium was removed (Denuded) and the vessel stimulated with 100 μM 2-MeS-ATP. The possible role of β-adrenergic receptors in the response to nucleotides was controlled by conducting some experiments with phenylephrine rather than NE as the contractile agonist. The ability of nucleotides to relax agonist-mediated contraction in guinea-pig aorta was not altered by the choice of the contractile agonist. The tracings presented are typical and representative of observations in three experiments performed in quadruplicate.
Figure 2.
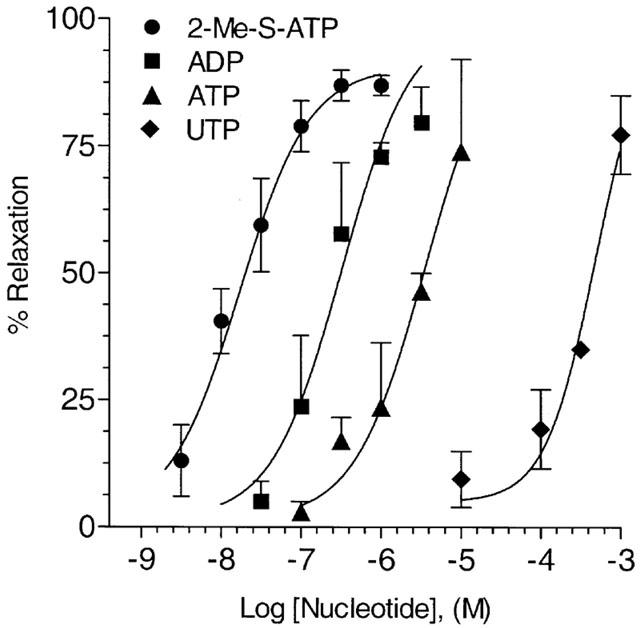
Relaxation of NE-induced contraction in guineapig aorta by nucleotides. Concentration response curves for 2-MeS-ATP •, ADP ▪amp;, ATP ▴, and UTP ♦ mediated relaxation of NE-induced (300 nM) contraction in guinea-pig aorta were constructed from data obtained in experiments, conducted as presented in Figure 1. The mean relaxation achieved in vessels treated at various concentrations of nucleotides in a non-cumulative manner is plotted in an up-going fashion as percentage of relaxation achieved, as is our convention. Data points are mean tension±s.e.mean as derived from computer fits of the data employing an equation describing a sigmoid curve using the data analysis and presentation program Prism®. All data points are the mean of at least n=3–9 observations for each nucleotide at each concentration.
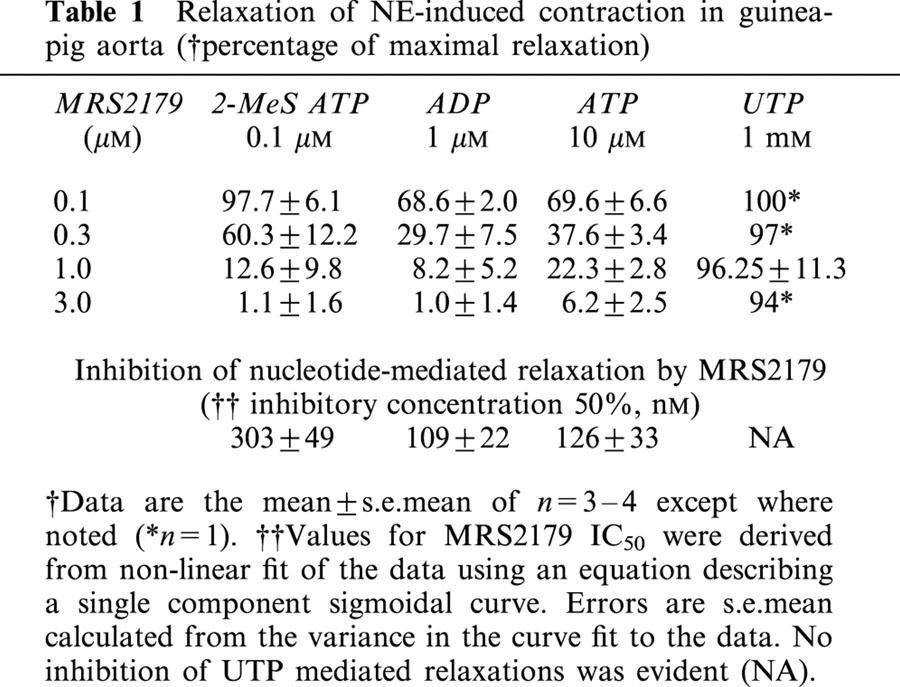
In order to investigate the role of P2Y1 receptors in the vasodilatory responses presented here, the ability of nucleotides to relax NE-induced contraction was observed in the presence of the antagonist MRS2179. MRS2179 had little effect on 2-MeS-ATP induced relaxation when employed at equimolar concentrations (100 nM) where 2-MeS-ATP is maximally effective, but significantly reduced vasodilation to 100 nM 2-MeS ATP in a dose-dependent fashion between 0.3 μM and 3 μM (Figure 3a; Table 1). MRS2179 was also able to block the responses to ADP and ATP in a similar fashion with near complete inhibition at 3 μM (Figure 3b,c; Table 1). The apparent IC50s for MRS2179 on purinergic agonists at their maximally effective concentrations varied between 109–303 nM (Table 1).
Figure 3.
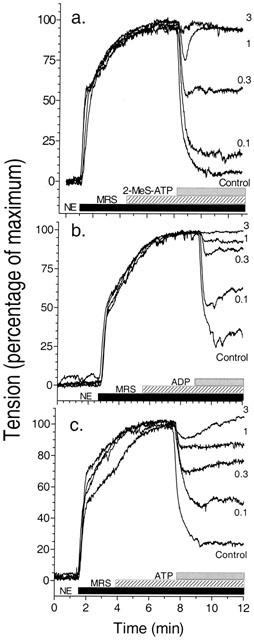
Effects of MRS2179 on nucleotide-mediated relaxation of NE-induced contraction in guineapig aorta. Experiments, conducted as described in Figure 1, were carried out in the presence of the nucleotide antagonist MRS2179 (hatched bar) added as a pretreatment in increasing concentrations prior to addition of P2Y receptor agonists (stippled bar) as shown. (a) MRS2179 inhibition at 100 nM to 3 μM of addition of a maximal dose of 2-MeS-ATP (100 nM). (b) MRS2179 inhibition at 100 nM to 3 μM of a maximal dose of ADP (1 μM). (c) MRS2179 inhibition of a maximal dose of ATP (10 μM) from 100 nM to 3 μM. Graphs are representative of three experiments conducted for each nucleotide.
Table 1.
Relaxation of NE-induced contraction in guinea-pig aorta (†percentage of maximal relaxation)
For endothelium, the ability of vasoactive agonists to relax blood vessels is correlated with their ability to raise intracellular calcium leading to the formation and release of nitric oxide (Hutcheson & Griffith, 1997). In guinea-pig aorta, pretreatment of tissues with L-NAME (0–30 μM) had no effect on the NE-induced contraction, but blunted the ATP-induced relaxation in a concentration-dependent fashion (10 μM, 16%; 30 μM, 60%: n=1). Thus, we imaged single-cell Fura-2 fluorescence in primary cultures of aortic endothelial cells. Control experiments confirmed that the nucleotide-dependent transient elevations in intracellular calcium we measure are dependent on release from intracellular stores as they can be measured in the absence of extracellular calcium in the incubation buffer. Consistent with our earlier observations in cardiac endothelial cells (Yang et al., 1996), addition of 1 μM 2-MeS-ATP causes the rapid release of calcium from intracellular stores that desensitizes in the continuing presence of agonist, to further addition of agonist (Figure 4). A second desensitizing response (P2Y2) is observed upon addition of 1 μM ATP, and this receptor becomes desensitized after a second treatment with 10 μM ATP. Further additions of ADP at 1 and 10 μM are then incapable of eliciting additional responses, suggesting the functional presence of only P2Y1 and P2Y2 receptors for purine nucleotides.
Figure 4.
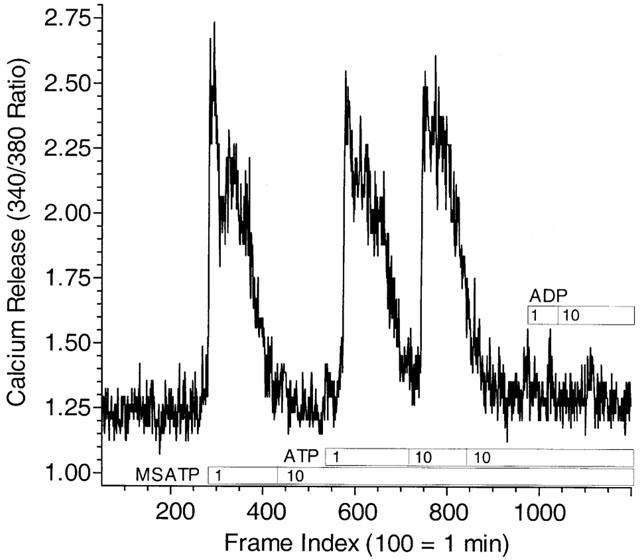
Single-cell recording of nucleotide-induced Fura-2 fluorescence in GPAEC under nucleotide receptor desensitizing conditions. Primary cultures of endothelial cells grown on glass-bottom plates of our own design were loaded with the calcium indicator dye Fura-2 as described in Methods and imaged in order to determine the ability of nucleotides to release calcium from intracellular stores. Single-cell recording of Fura-2 fluorescence is presented as a continuous representative tracing. Cells were bathed in MOPS buffer containing 1 mM calcium and agonists introduced in 2× concentration as described in Methods. Calcium release stimulated by addition of 2-MeS-ATP (1 μM) desensitizes in the continued presence of the nucleotide and to further addition of a large concentration of the agonist as we have described previously (Yang et al., 1996). Addition of ATP, however, in the continued presence of 2-MeS-ATP in the bathing buffer is still able to stimulate calcium release in this cell consistent with action at a P2Y2 receptor. Desensitization of the ATP response by repeated addition of 10 μM ATP followed by addition of ADP does not result in a response to ADP at 1 or 10 μM. Controls using addition of bradykinin demonstrated that the calcium stores were not depleted by this desensitization protocol (data not shown). The single-cell fluorescence tracing shown, indexed to image frames, is representative of 5–8 cells imaged in three independent experiments.
Under conditions where 2-MeS-ATP stimulates calcium release, subsequent responses to the agonist after washout (non-desensitizing conditions) are blocked by MRS2179. The failure of cells to respond to P2Y1 receptor stimulation was not the result of desensitization under these conditions since 1 μM 2-MeS-ATP could stimulate calcium release following washout of MRS2179 (Figure 5). Unlike the case for the effects of 2-MeS-ATP, however, MRS2179 failed to block the calcium transient seen following addition of ATP consistent with a selective effect of MRS2179 on the P2Y1, but not the P2Y2 receptor. MRS2179 also failed to prevent ADP stimulation of calcium transients under these conditions (data not shown).
Figure 5.
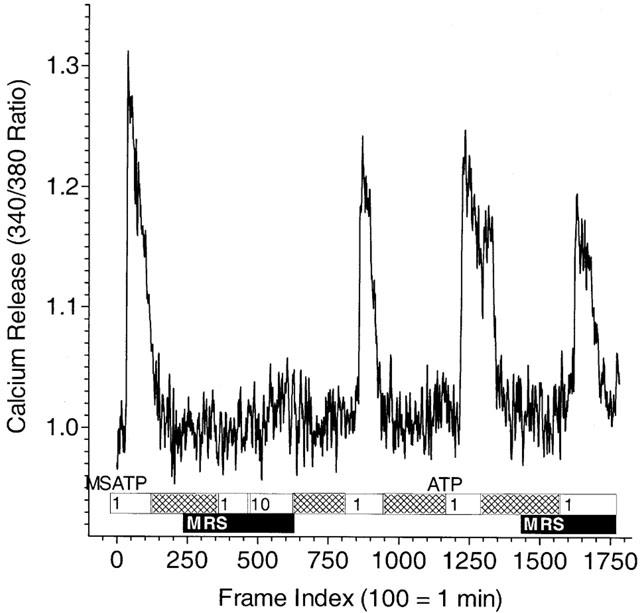
The effect of MRS2179 on nucleotide-stimulated single-endothelial cell calcium release under non-desensitizing conditions. Single-cell recording of Fura-2 fluorescence is presented as a continuous representative tracing. Cells were bathed in MOPS buffer containing 1 mM calcium and agonists introduced in 2× concentration as described in Methods. Addition of 2-MeS-ATP (1 μM) results in the release of calcium that is blocked by subsequent addition of the agonist following washout (hatched bar) and in the presence of 3 μM MRS2179 (MRS; solid bar). Re-addition of 2-MeS-ATP (1 μM) following washout of the MRS2179 once again results in calcium release. The effect of ATP to stimulate calcium release is not blocked by MRS2179 pretreatment (solid bar). Data, indexed to image frames, are from a single cell and are representative of five to eight such recordings in a single experiment repeated three times.
Since MRS2179 antagonized nucleotide receptor activated calcium transients selectively in aortic endothelial cells but was capable of antagonizing the effects of each purine nucleotide tested in tissue studies, we sought to discover what receptors for nucleotides are present in the guinea-pig thoracic aorta. RT–PCR demonstrates the presence of the predicted P2Y1 and P2Y2 receptors on endothelium (Figure 6). Receptor expression was determined to be endothelial because transcript detection is lost upon physical denuding of the endothelium from the blood vessel and subsequent testing of the vascular smooth muscle. Furthermore, both P2Y4 and P2Y6 receptor cDNA were also detected. P2Y6 receptor transcripts are endothelial in origin, as are those for P2Y1 and P2Y2, while P2Y4 receptors may be expressed in both the endothelium and the smooth muscle. These data suggest that in addition to the calcium-mediated vasodilatory responses to 2-MeS-ATP, ADP, and ATP, there may be a role for actions of UTP in guinea-pig aorta as well.
Figure 6.
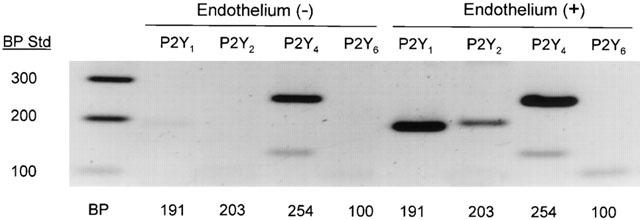
Molecular expression of P2Y receptors in guinea-pig aorta. Gel electrophoresis of RT–PCR products from intact and endothelium-denuded guinea-pig descending thoracic aorta was performed on pooled aorta samples with (3) and without (3) endothelium. RNA was purified as described in Methods and RT–PCR performed using specific primers designed from known sequence using Vector NTI Suite (InforMax, San Francisco, CA, U.S.A.). Intact vessels demonstrate RT–PCR products for P2Y1,2,4,6 (expected band sizes are illustrated below lanes). Denuded vessels only demonstrate a positive RT–PCR product for P2Y4. Therefore P2Y1,2,6 expression is concluded to be primarily endothelial. Gel image is representative of three separate RNA isolations and subsequent RT–PCR experiments.
Indeed, under conditions where P2Y1 receptor mediated calcium release was blocked by MRS2179, both ATP and UTP were able to elicit calcium transients (Figure 7). In the simultaneous presence of MRS2179, 2-MeS-ATP and ATP, the subsequent stimulation with UTP (100 μM) is capable of causing an additional calcium response of similar magnitude to that seen to addition of ATP providing functional evidence of a receptor disparate from P2Y1 or P2Y2 with pharmacology consistent with P2Y4. No calcium mobilization could be detected in response to the addition of UDP up to 100 μM (data not shown).
Figure 7.
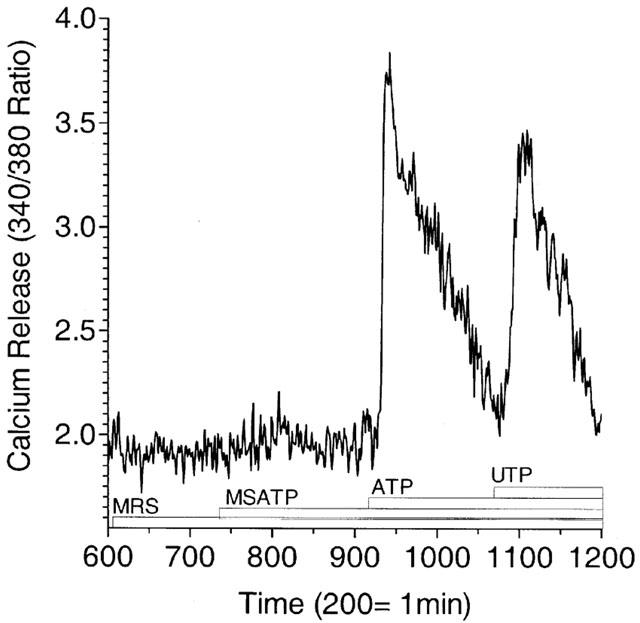
Single-cell recording of UTP-stimulated increase in Fura-2 fluorescence in GPAEC under desensitizing conditions. Single-cell recording of Fura-2 fluorescence is presented as a continuous representative tracing. Cells were bathed in MOPS buffer containing 1 mM calcium and agonists introduced in 2× concentration as described in Methods and illustrated in the figure with a labelled bar beneath the tracing. MRS2179 added at 10 μM at the start of the experiment antagonizes 1 μM 2-MeS-ATP but has no effect on 1 μM ATP-stimulated calcium release. Subsequent addition of 100 μM UTP causes a calcium response in the presence of MRS2179, 2-MeS-ATP, and ATP that is similar in intensity to the calcium response observed following earlier addition of 1 μM ATP. In companion experiments (not shown) re-addition of ATP at 1 μM failed to elicit a response suggesting effective desensitization of the ATP response. Data, indexed to image frames, are from a single cell and are representative of five to eight such recordings in a single experiment repeated three times.
In aortic rings with or without endothelium intact and contracted with 300 nM NE, dramatic effects of UTP to relax the tissue in a MRS2179-insensitive manner could be reproducibly measured using a wash-in protocol where treatments were applied by removing the contents of the bath and replacing them with pre-diluted agonists (Figure 8). Four wash-in treatment groups were employed: 300 nM NE (control); 300 nM NE plus 1 mM UTP; 300 nM NE plus 1 mM UTP and 1 μM MRS2179; and 300 nM NE plus 1 mM UTP on endothelium denuded aorta. The effect of UTP to relax the NE-induced contraction was endothelium-dependent and unaffected by MRS2179 (Figure 8). Addition of MRS2179 at concentrations up to 3 μM had no effect on 1 mM UTP induced vasodilation. This same concentration of MRS2179 caused an 87.4% reduction in the response to 100 nM 2-MeS ATP, a 91.8% reduction in the response to 1 μM ADP, and a 77.7% reduction in the response to 10 μM ATP (Table 1). UDP (10 μM to 1 mM) was incapable of inducing vasodilation (data not shown) despite expression of a P2Y6 receptor in guinea-pig aorta.
Figure 8.
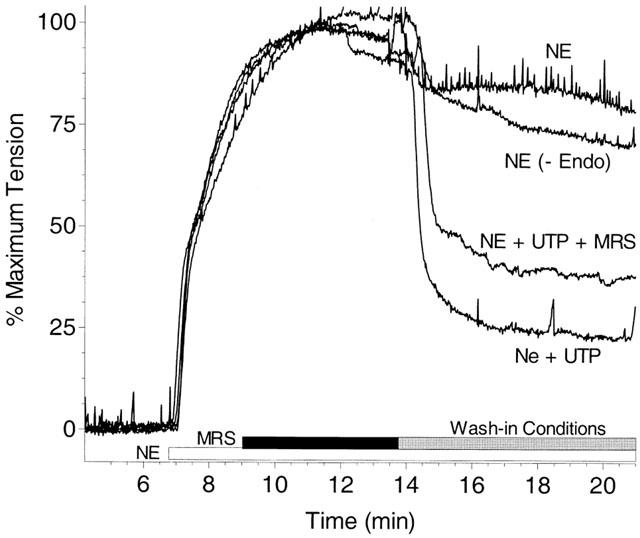
Pyrimidinergic receptor-mediated relaxation of guinea-pig aorta. Guinea-pig aortic rings are pre-contracted with 300 nM NE as in Figure 1, and allowed to develop significant tension prior to addition of the nucleotide receptor antagonist MRS2179 (1 μM) to one tissue bath. All samples are allowed to reach steady-state tension prior to initiation of the wash-in procedure (detailed in Methods) to all tissue baths. The control tissue (NE) is washed-in with Krebs-bicarbonate buffer containing 300 nM NE, as was added initially. A denuded tissue (-Endo) demonstrates similar tension to the control despite the presence of 1 mM UTP in the wash-in buffer indicating the endothelium dependence of the UTP response. Similar traces are obtained for intact tissues washed-in with 300 nM NE plus 1 mM UTP, regardless of the presence of 1 μM MRS2179 (difference not significant n=3). Graph is representative of three independent experiments performed in quadruplicate.
Discussion
Many UTP receptors have been cloned to date including homologues from human, rat, and turkey (Bogdanov et al., 1998; Boyer et al., 1997; Communi et al., 1996). Interestingly, the pharmacology varies markedly for each underscoring the importance of a complete pharmacological characterization of these receptors in multiple systems. Receptor coupling to inositol phosphate generation indicates that the human receptor has a ligand potency order of UTP>UDP= deoxy-UTP>5-bromo-UTP>ITP>ATP (Communi et al., 1996). The expressed rat and turkey P2Y4 receptors appear to be much less selective for UTP, responding to both UTP and ATP, as well as other nucleotide analogues with equal potency (Boyer et al., 1997; Communi et al., 1996). Based on the pharmacology observed in GPAEC, the UTP response demonstrated here appears to be more like that seen with the human receptor, rather than the rat and turkey receptors. ATP pretreatment and functional desensitization of ATP-responsive P2Y receptors does not reduce the effect of UTP on intracellular calcium. Endothelial cell Ca2+ transients are equal in response to exogenous UTP treatment or to ATP treatment, and the UTP response is not diminished by prior exposures to ATP while that of ATP is completely desensitized. This suggests unique, if equally important roles, for each nucleotide in the bloodstream as regulators of blood vessel tone.
Since the original observations that separate pyrimidinergic and purinergic receptors exist (Seifert & Schultz, 1989), UTP has been examined for vasoactive properties in various preparations. It has been demonstrated that some cells may respond to mechanical stimulation by releasing UTP extracellularly (Lazarowski et al., 1997). This, together with the knowledge that UTP may be present in locally high concentrations during the platelet release reaction (Lazarowski & Harden, 1999) provides a basis for the expectation that UTP may act on endothelial cells and serve to maintain blood flow during pathological states (Buxton et al., 2001). Nucleotides in the vasculature have been demonstrated to have two effects on vessel tone. In norepinephrine-contracted rat aorta for example, UTP is capable of causing dose-dependent, endothelium-dependent vasodilation of up to 65% (Bultmann et al., 1998), while in resting tissues the same concentrations (10–300 μM) are capable of inducing significant contractions that rival norepinephrine-induced tone (Abbracchio & Burnstock, 1994; Garcia-Velasco et al., 1995; Urquilla, 1978). Interestingly, in rat aorta, the vasodilation to similar concentrations of ATP is not wholly endothelium-dependent (Garcia-Velasco et al., 1995). In our study of guinea-pig aorta, nucleotide-induced vasodilation is entirely dependent on intact endothelium, and no nucleotide-mediated contraction is seen in naive or contracted vessels with or without endothelium. While we think it unlikely that the P2Y4 receptor cDNA detected in denuded vessel RT–PCR experiments (Figure 6) is from RNA contributed by endothelium contamination (based on the absence of PCR products for other receptor subtypes in the smooth muscle sample), it is possible that P2Y4 receptors exist in the smooth muscle cell, but are not coupled to regulation of contraction. Whatever the case, it is clear that the endothelial P2Y4 receptor subserves the relaxation to UTP in guineapig aorta in the presence of endothelium.
The concentration response curves generated from NE contracted guineapig aorta for each nucleotide species results in a rank order of potency of 2-MeS-ATP>ADP>ATP>>UTP. UTP is the least potent nucleotide with near-maximal effects seen at 1 mM (Figure 8). This is consistent with published potency profiles from rat aorta where 2-MeS-ATP>ADP>ATP=UTP; however, the EC50 values are quite different. In rat aorta 2-MeS-ATP has an EC50 of 91 nM (Dol-Gleizes et al., 1999). In our studies in the guinea-pig, 2-MeS-ATP is far more potent (0.11 of the effect in rat) with an EC50 value of 10 nM. In both this study of guineapig aorta and the recent Dol-Gleizes study in rat aorta, the EC50 for ADP (320 v 600 nM) and ATP (3.0 v 1.1 μM) are readily comparable. Unlike rat aorta, relaxation to UTP in guinea-pig aorta is not equipotent with ATP. While this distinction cannot be explained here, it is clear in both species that UTP, while efficacious, is not a potent agonist. If UTP were present in vivo only upon its release from platelets, it may stand to reason that endothelium would see locally high concentrations that may reach millimolar levels (Lazarowski et al., 1997; Buxton et al., 2001). In general, aorta may be predominated functionally by P2Y1 receptor-mediated vasodilation in response to purine nucleotides.
In this study, we present transcriptional expression of several P2Y receptors by the endothelium in guinea-pig aorta. The functional expression of a UTP specific receptor not desensitized by ATP or antagonized by MRS2179 is consistent with the expression of the P2Y4 receptor. Despite clear RT–PCR evidence of P2Y2 expression in whole tissue we do not see an MRS2179 independent response to ATP in the aortic ring experiments. We do see MRS2179-independent, ATP-induced calcium release in cultured aortic endothelial cells consistent with the presence of a P2Y2 receptor. Our studies indicating equal UTP responsiveness with or without ATP desensitization suggest that the guineapig P2Y2 receptor does not recognize UTP. While this may be unexpected based on the pharmacology of the P2Y2 (originally named P2U) receptor described elsewhere (Ralevic & Burnstock, 1998), it is clear that the pharmacology of P2 receptor homologues is disparate among tissues from various species.
MRS2179 has been employed effectively here and elsewhere (Jimenez et al., 2000) as an alternative to P2Y1 desensitization, facilitating the study of individual receptors in multi-receptor systems where nucleotide responses appear to be promiscuous. MRS2179, and therefore P2Y1 antagonism, inhibits the ability of ADP to evoke platelet shape change, aggregation, and intracellular calcium mobilization (Baurand et al., 2001). Interestingly, platelet signalling via ADP may require the presence of more then one P2Y receptor. MRS2179 pretreatment failed to prevent adenylyl cyclase inhibition by ADP in the same study (Baurand et al., 2001). In addition, in platelets, the P2Y1 specificity of MRS2179 has been successfully employed in the characterization of the elusive P2Y12 (P2YAT) receptor (Savi et al., 2001) now suggested to be the therapeutic target of clopidigrel.
The results of this study suggest that UTP is an effective vasodilator in guinea-pig aorta capable of causing vasodilation comparable to that seen with more traditional P2Y nucleotide agonists like 2-MeS-ATP, ADP and ATP. Unlike these purine nucleotides, however, UTP acts via expression of a receptor other than P2Y1, which we suggest is the P2Y4 receptor. In calcium imaging experiments we demonstrate the utility of MRS2179 as a P2Y1-specific antagonist and demonstrate the functional expression of at least three different P2Y receptor subtypes (P2Y1, 2 and 4). Using this information about the receptor expression of cultured endothelial cells we applied MRS2179 to aortic rings to determine the effect of P2Y1 antagonism in whole tissues. We found a contrast between the ability of nucleotides to elicit calcium responses in endothelial cells, and their ability to cause vasodilation in tissue. The responses to 2-MeS-ATP, ADP, and ATP are MRS2179 sensitive in tissue where only 2-MeS-ATP is sensitive to MRS2179 in cultured cells. Only the response to UTP was insensitive to MRS2179 in both cell and tissue systems. This study not only questions the link between what can be deduced in cell culture about the reality of receptor signalling in tissue, but verifies the role of UTP in either paradigm, even when ATP and ADP act at disparate sites.
Finally, in detailing knowledge of the action of UTP in guinea-pig aorta, we extend the evidence that the existence of nucleotides in the blood stream to act on extracellularly-directed receptors to subserve changes in blood flow through vasodilation is consistent with a role for nucleotides as permissive hormones. Nucleotides could act well beyond their site of origin due to extracellular reactions that favor their formation. The presence on the endothelial cell of a cascade of extracellular nucleotide metabolism that can employ nucleotide triphosphates as phosphoryl donor for the transphosphorylation of nucleotide diphosphates (Buxton et al., 2001; Yegutkin et al., 2001) provides for the maintenance of nucleotide levels that may serve to maintain the actions of ATP and adenosine as arterial vasodilators. While the particular role of UTP in this axis in humans cannot be judged from the data provided here, it is of interest that, on a biochemical basis, it can be proposed that the release of UTP in conduit vessels under pathophysiological conditions may subserve increases in the local concentrations of ATP by supporting the reformation of ATP from ADP by the ecto enzyme, Nm23-H1. Since ATP can act as ATP per se as well as ADP once metabolized, and since some fraction of ADP may be converted to ATP by the transphosphorylation reaction supported by UTP, it is possible to propose that the actions of UTP, both at P2Y4 receptors and at the Nm23 ecto-enzyme would result in upstream vasodilation and promote the actions of ATP, ADP and adenosine that carry the vasodilation forward in the blood vessel.
Acknowledgments
The authors are grateful to Brian Oxhorn for assistance in the measurement of intracellular calcium and Paul Carlson for assistance with contractile studies. This work was supported in part by grants from the Robert Z. Hawkins Foundation, and the NIH (HL35416) to I.L.O. Buxton; and a pre-doctoral fellowship from the American Heart Association Western States Affiliate to R.A. Kaiser.
Abbreviations
- cDNA
complimentary deoxyribonucleic acid
- DEPC
diethyl pyrocarbonate
- EDTA
ethylenediaminetetraacetic acid
- Fura-2AM
5-oxazolecarboxylic acid, 2-(6-(bis(2-((acetyloxy)methoxy)-2-oxoethyl)amino)-5- (2-(bis(2-((acetyloxy)methoxy)-2-oxoethyl)amino)-5-methylphenoxy) ethoxy)-2-benzofuranyl)-, (acetyloxy)methyl ester
- MRS2179
2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate
- PECAM1
Platelet-endothelial cell adhesion molecule 1
- RT–PCR
reverse transcriptase-polymerase chain reaction
- TRIzol®
octrizole=2-(2H-Benzotriazol-2-yl)-4-(1,1,3,3-tetramethylbutyl)phenol
References
- ABBRACCHIO M.P., BURNSTOCK G. Purinoceptors: are there families of P2X and P2Y purinoreceptors. Pharmacol. Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- BAURAND A., RABOISSON P., FREUND M., LEON C., CAZENAVE J., BOURGUIGNON J., GACHET C. Inhibition of platelet function by administration of MRS2179, a P2Y(1) receptor antagonist. Eur. J. Pharmacol. 2001;412:213–221. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- BOGDANOV Y., WILDMAN S.S., CLEMENTS M.P., KING B.F., BURNSTOCK G. Molecular cloning and characterization of Rat P2y4 nucleotide receptor. Br. J. Pharmacol. 1998;124:428–430. doi: 10.1038/sj.bjp.0701880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER J.L., MOHANRAM A., CAMAIONI E., JACOBSON K.A., HARDEN T.K. Competitive and selective antagonism of P2Y1 receptors by N6-Methyl 2′-deoxyadenosine 3′,5′-bisphosphate. Br. J Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER J.L., WALDO G.L., HARDEN T.K. Molecular cloning and functional expresssion of an Avian G protein coupled P2Y receptor. Molecular Pharmacology. 1997;52:928–934. doi: 10.1124/mol.52.6.928. [DOI] [PubMed] [Google Scholar]
- BOYER J.L., ZOHN I.E., JACOBSON K.A., HARDEN T.K. Differential effects of P2-purinoceptor antagonists on phospholipase c- and adenylyl cyclase-coupled P2Y-purinoceptors. Br. J. Pharmacol. 1994;113:614–620. doi: 10.1111/j.1476-5381.1994.tb17034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN C.A., TANNA B., BOARDER M.R. PPADS: an antagonist at endothelial P2Y-purinoceptors but not P2U- purinoceptors. Br. J. Pharmacol. 1995;116:2413–2416. doi: 10.1111/j.1476-5381.1995.tb15088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULTMANN R., TULUC F., STARKE K. On the suitability of adenosine 3′-phosphate 5′-phosphosulphate as a selective P2Y receptor antagonist in intact tissues. Eur. J. Pharmacol. 1998;359:95–101. doi: 10.1016/s0014-2999(98)00600-1. [DOI] [PubMed] [Google Scholar]
- BUXTON I.L.O., KAISER R.A., OXHORN B.C., CHEEK D.J. Evidence supporting the nucleotide axis hypothesis: ATP release and metabolism by coronary endothelium. Am. J. Physiol. 2001;281:H1657–H1666. doi: 10.1152/ajpheart.2001.281.4.H1657. [DOI] [PubMed] [Google Scholar]
- CHARLTON S.J., BROWN C.A., WEISMAN G.A., TURNER J.T., ERB L., BOARDER M.R. Cloned and transfected P2Y4 receptors: characterization of a suramin and PPADS-insensitive response to UTP. Br. J. Pharmacol. 1996a;119:1301–1303. doi: 10.1111/j.1476-5381.1996.tb16038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHARLTON S.J., BROWN C.A., WEISMAN G.A., TURNER J.T., ERB L., BOARDER M.R. PPADS and suramin as antagonists at cloned P2Y- and P2U-purinoceptors. Br. J. Pharmacol. 1996b;118:704–710. doi: 10.1111/j.1476-5381.1996.tb15457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMMUNI D., MOTTE S., BOEYNAEMS J.-M., PIROTTON S. Pharmacological characterization of the human P2Y4 receptor. Eur. J. Pharmacol. 1996;317:383–389. doi: 10.1016/s0014-2999(96)00740-6. [DOI] [PubMed] [Google Scholar]
- DOL-GLEIZES F., MARES A.M., SAVI P., HERBERT J.M. Relaxant effect of 2-methyl-thio-adenosine diphosphate on rat thoracic aorta: effect of clopidogrel. Eur J Pharmacol. 1999;367:247–253. doi: 10.1016/s0014-2999(98)00985-6. [DOI] [PubMed] [Google Scholar]
- ERLINGE D., HOU M., WEBB T.E., BARNARD E.A., MOLLER S. Phenotype changes of the vascular smooth muscle cell regulate P2 receptor expression as measured by quantitative RT–PCR. Biochem. Biophys. Res. Commun. 1998;248:864–870. doi: 10.1006/bbrc.1998.9083. [DOI] [PubMed] [Google Scholar]
- GARCIA-VELASCO G., SANCHEZ M., HIDALGO A., GARCIA D.B.M. Pharmacological dissociation of UTP- and ATP-elicited contractions and relaxations in isolated rat aorta. Eur. J. Pharmacol. 1995;294:521–529. doi: 10.1016/0014-2999(95)00576-5. [DOI] [PubMed] [Google Scholar]
- HARDEBO J.E., KAHRSTROM J., OWMAN C., SALFORD L.G. Endothelium-dependent relaxation by uridine tri- and diphosphate in isolated human pial vessels. Blood Vessels. 1987;24:150–155. doi: 10.1159/000158690. [DOI] [PubMed] [Google Scholar]
- HUTCHESON I.R., GRIFFITH T.M. Central role of intracellular calcium stores in acute flow- and agonist-evoked endothelial nitric oxide release. Br. J Pharmacol. 1997;122:117–125. doi: 10.1038/sj.bjp.0701340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIMENEZ A.I., CASTRO E., COMMUNI D., BOEYNAEMS J.M., DELICADO E.G., MIRAS-PORTUGAL M.T. Coexpression of several types of metabotropic nucleotide receptors in single cerebellar astrocytes. J Neurochem. 2000;75:2071–2079. doi: 10.1046/j.1471-4159.2000.0752071.x. [DOI] [PubMed] [Google Scholar]
- KONG I.D., CHUNG H.S., PARK K.S., HAN J.K., LEE J.W. Hemodynamic characteristics of extracellular UTP in the perfused rat liver. Yonsei Med. J. 1996;37:262–269. doi: 10.3349/ymj.1996.37.4.262. [DOI] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., HARDEN T.K. Quantitation of extracellular UTP using a sensitive enzymatic assay. Br. J Pharmacol. 1999;127:1272–1278. doi: 10.1038/sj.bjp.0702654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAZAROWSKI E.R., HOMOLYA L., BOUCHER R.C., HARDEN T.K. Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J. Biol. Chem. 1997;272:24348–24354. doi: 10.1074/jbc.272.39.24348. [DOI] [PubMed] [Google Scholar]
- MCLAREN G.J., LAMBRECHT G., MUTSCHLER E., BAUMERT H.G., SNEDDON P., KENNEDY C. Investigation of the actions of PPADS, a novel P2x-purinoceptor antagonist, in the guinea-pig isolated vas deferens. Br. J. Pharmacol. 1994;111:913–917. doi: 10.1111/j.1476-5381.1994.tb14825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOTTE S., PIROTTON S., BOEYNAEMS J.M. Heterogeneity of ATP receptors in aortic endothelial cells. Involvement of P2y and P2u receptors in inositol phosphate response. Circ. Res. 1993;72:504–510. doi: 10.1161/01.res.72.3.504. [DOI] [PubMed] [Google Scholar]
- OXHORN B.C., CHEEK D.J., BUXTON I.L.O. Role of nucleotides and nucleosides in the regulation of cardiac blood flow. AACN Clinical Issues. 2000;11:241–251. doi: 10.1097/00044067-200005000-00007. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- SAVI P., LABOURET C., DELESQUE N., GUETTE F., LUPKER J., HERBERT J.M. P2y(12), a new platelet ADP receptor, target of clopidogrel. Biochem. Biophys. Res. Commun. 2001;283:379–383. doi: 10.1006/bbrc.2001.4816. [DOI] [PubMed] [Google Scholar]
- SEIFERT R., SCHULTZ G. Involvement of pyrimidinoceptors in the regulation of cell functions by uridine and by uracil nucleotides. Trends. Pharmacol Sci. 1989;10:365–369. doi: 10.1016/0165-6147(89)90009-6. [DOI] [PubMed] [Google Scholar]
- SUAREZ-HUERTA N., POUILLON V., BOEYNAEMS J., ROBAYE B. Molecular cloning and characterization of the mouse P2Y(4) nucleotide receptor. Eur J Pharmacol. 2001;416:197–202. doi: 10.1016/s0014-2999(01)00875-5. [DOI] [PubMed] [Google Scholar]
- URQUILLA P.R. Prolonged contraction of isolated human and canine cerebral arteries induced by uridine 5′-triphosphate. Stroke. 1978;9:133–136. doi: 10.1161/01.str.9.2.133. [DOI] [PubMed] [Google Scholar]
- VON KUGELGEN I, WETTER A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- YANG S., BUXTON I.L.O., PROBERT C.B., TALBOT J.N., BRADLEY M.E. Evidence for a discrete UTP receptor in cardiac endothelial cells. Br. J. Pharmacol. 1996;117:1572–1578. doi: 10.1111/j.1476-5381.1996.tb15323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG S., CHEEK D.J., WESTFALL D.P., BUXTON I.L.O. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ. Res. 1994;74:401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- YEGUTKIN G.G., HENTTINEN T., JALKANEN S. Extracellular ATP formation on vascular endothelial cells is mediated by ecto-nucleotide kinase activities via phosphotransfer reactions. FASEB J. 2001;15:251–260. doi: 10.1096/fj.00-0268com. [DOI] [PubMed] [Google Scholar]


