Abstract
A direct effect of uraemic toxins in promoting progression of chronic renal disease has not been established. In this study, we investigated the toxic effects of organic anions which characteristically appeared in the patients with progressive renal disease on renal proximal tubular cells expressing human organic anion transporter (hOAT) 1.
A renal proximal tubular cell line, opossum kidney (OK) cells, was transformed with hOAT1. Among the organic anions examined, hippuric acid, para-hydroxyhippuric acid, ortho-hydroxyhippuric acid, indoxyl sulphate and indoleacetic acid showed a high affinity for hOAT1 expressed in the OK cells. Indoxyl sulphate and indoleacetic acid concentration-dependently inhibited proliferation of the hOAT1-transformed cells. The h.p.l.c. analysis demonstrated that cellular uptake of these organic anions was significantly elevated in hOAT1-transformed cells.
These organic anions also concentration-dependently stimulated cellular free radical production. The degrees of inhibition of cell proliferation and the stimulation of free radical production induced by the organic anions were significantly higher in the hOAT1-transformed cells than vector-transformed cells. The stimulatory effect of indoxyl sulphate on free radical production was abolished by anti-oxidants and probenecid.
Less free radical production was observed in the hOAT1-transformed cells treated with p-hydroxyhippuric acid, o-hydroxyhippuric acid compared with indoxyl sulphate and indoleacetic acid. Hippuric acid had little effect on free radical production.
Organic anions present in the serum of patients with progressive renal disease may cause proximal tubular injury via hOAT1-mediated uptake. The mechanism of cellular toxicity by these uraemic toxins involves free radical production. Thus, some uraemic toxins may directly promote progression of chronic renal disease.
Keywords: Organic anion transporter, uraemic toxins, renal proximal tubules, free radical, cell injury
Introduction
Circulating uraemic toxins have been proposed to facilitate progression of chronic renal failure. In our previous study, two experimental manoeuvre that remove uraemic toxins; peritoneal dialysis (PD) and oral charcoal adsorbent, attenuated the progression of glomerular sclerosis in an experimental model of subtotal nephrectomy (Motojima et al., 1991). Clinically, such procedures have also been reported to attenuate progression of chronic renal disease (Lysaght et al., 1991; Rottembourg, 1993; Sanaka et al., 1988; Niwa et al., 1997).
Serum level of indoxyl sulphate, an endogenous organic anion, was markedly elevated in the serum of patients with end-stage kidney diseases (Niwa et al., 1988; 1991). Administration of indoxyl sulphate or its precursor indole accelerated the progression of renal disease in rats (Niwa & Ise, 1994; Niwa et al., 1994). This molecule is removable by PD or oral adsorbent and has been considered as one of the uraemic toxins that facilitate renal injury (Niwa et al., 1996; 1997). Beside indoxyl sulphate, other uraemic toxins that are organic anions include hipurric acid, indoleacetic acid, guanidinosuccinic acid, aminoisobutylic acid, phenylacetic acid and uric acid (Geijo et al., 1977; Dhondt et al., 2000; Vanholder et al., 2001).
Recently, a transporter responsible for organic anion transport across renal proximal tubular cells has been cloned (Sekine et al., 1997; Sweet et al., 1997) and named organic anion transporter 1 (OAT1). OAT1 shows a high affinity for p-aminohippuric acid (PAH) and wide substrate specificity for organic anions such as antibiotics and biological metabolites. Rat OAT1 and human OAT1 (hOAT1) have been localized on the basal side of proximal tubular cells (Tojo et al., 1999; Hosoyamada et al., 1999), and are therefore proposed to mediate uptake of organic anions from blood across the basal membranes. This uptake step is rate limiting since energy-dependent uphill uptake is required. Nephrotoxic agents have been proposed to induce nephrotoxicity via OAT1-mediated uptake into renal proximal tubules. Cephalosporin antibiotics and ochratoxin A have been shown to damage OAT1-expressing proximal tubular cells via OAT1-mediated transport (Jariyawat et al., 1999; Takeda et al., 1999; Tsuda et al., 1999). Organic anions have been shown to accumulate in patients with uraemia. This study was designed to examine whether some of these uraemic molecules evoke a toxic effect on renal proximal tubular cells by OAT-mediated uptake.
In the present study, we examined the affinities of various uraemic toxins for hOAT1 to identify the uraemic toxins actively transported into renal proximal tubular cells, and also investigated the toxic effects of these organic anions on cellular function in a hOAT1-expressing renal proximal tubular cell line (opossum kidney, OK) that we established.
Methods
Reagents
Hippuric acid, probenecid, indoleacetic acid, uric acid, p-hydroxyphenylacetic acid, Dulbecco's phosphate buffered saline (D-PBS) and Medium 199 were purchased from Nacalai tesque (Kyoto, Japan). Cell counting kits and p-hydroxyhippuric acid were purchased from Wako (Osaka, Japan). Indoxyl sulphate, guanidinosuccinic acid, tetraethylammonium chloride, α-ketoglutaric acid, N-acetyl-cysteine and probucol were purchased from Sigma (St. Louis, MO, U.S.A.). Para-hydroxyhippuric acid was purchased from Bachem (Bubendorf, Switzerland). Beta-aminoisobutylic acid was purchased from Tokyo Kasei (Tokyo, Japan). Foetal bovine serum (FBS) and Geneticin were purchased from Life Technologies (Rockville, MD, U.S.A.). Original TA cloning kit and pcDNA3.1 (+) were purchased from Invitrogen (Carlsbad, CA, U.S.A.). Human kidney 5′-stretch plus cDNA library was purchased from Clontech (Palo Alto, CA, U.S.A.). Oligonucleotides were synthesized by Nihon Bio-service (Saitama, Japan). GeneAmp RNA PCR Kit was purchased from Perkin-Elmer (Emeryville, CA, U.S.A.). DC protein assay kit was purchased from Bio-Rad (Hercules, CA, U.S.A.). D-[3H]-mannitol and [14C]-PAH were purchased from NEN Life Science (Boston, MA, U.S.A.). Dihydrofluorescein diacetate was purchased from Molecular Probe (Eugene, OR, U.S.A.).
Cell culture
OK cells were obtained from the American Type Culture Collection (CRL-1840, Manassas, VA, U.S.A.) and were grown in Medium 199 containing 10% FBS in an atmosphere of 5% CO2-95% air at 37°C.
Establishment of proximal tubular cells stably expressing hOAT1
An 826 bp cDNA fragment of rat OAT1 spanning from bases 461 to 1286 was amplified from rat kidney total RNA by the GeneAmp RNA PCR Kit according to the sequence described by Sekine et al. (1997). The sequence of forward primer was 5′-GACAAGCAAGGACAACCCGAAT-3′ and that of reverse primer was 5′-GCATGGAGAGACAGAGGAAGAGG-3′. The PCR product was cloned into TA cloning vector and sequenced to confirm the sequence. The rat OAT1 cDNA fragment was used as a probe to screen human kidney cDNA library with low stringency wash. The positive clones isolated were analysed by restriction enzyme mapping and sequencing. A clone coding hOAT1-2 contained complete open reading frame. The cDNA was subcloned into pcDNA 3.1(+) to obtain an expression construct. The hOAT1 expression construct or pcDNA 3.1(+) with no insert (control) was used to transfect renal proximal tubular cells (OK) and stable transformants were isolated by selection with 400 μg ml−1 Geneticin. Stable transformants were screened for PAH transport and maintained in medium 199 containing 10% FBS and Geneticin.
PAH transport assay
The hOAT1-transformed cells were seeded in 24-well tissue culture plates at a cell density of 2×104 cells well−1. Two days after seeding, the cells were washed with Dulbecco's PBS and incubated in the same solution containing 5 mM D-glucose for 30 min at room temperature. Then, the cells were incubated in 200 μl of Dulbecco's PBS containing 5 mM D-glucose, 10 μM D-[3H]-mannitol and 10 μM [14C]-PAH, for 10 min at room temperature. The transport was terminated by washing with ice-cold Dulbecco's PBS (repeated three times). The cells in each well were dissolved in 300 μl of 0.2 N sodium hydroxide, and 250 μl of the cell lysate was used for radioactivity determination (Tri-Carb 2700TR, Packard, Meriden, CT, U.S.A.). Extracellular trapping and nonspecific uptake were obtained from the radioactivity of nontransportable D-[3H]-mannitol. Protein concentrations in the cell lysate were determined using a DC protein assay kit (Bio-Rad).
Cell proliferation assay
The hOAT1-transformed cells or vector-transformed control cells were seeded in a 96-well tissue culture plate at a density of 2×103 cells well−1. After incubation for 2 days, the culture medium was replaced by the same medium containing 2.5% FBS with/without uraemic toxins and incubated for another 24 h. Then, the cell number in each well was estimated using a cell counting kit.
Cellular uptake of indoxyl sulphate and indoleacetic acid
The hOAT1-transformed cells or vector-transformed control cells were seeded in a 6-well tissue culture plate at a density of 5×105 cells well−1. Two days after seeding, the cells were washed with Dulbecco's PBS and incubated in the same solution containing 5 mM D-glucose for 30 min at room temperature. Then, the cells were incubated in 1 ml of Dulbecco's PBS containing 5 mM D-glucose, 1 mM indoxyl sulphate or indoleacetic acid at 37°C for 1 h. The transport was terminated by washing with ice-cold Dulbecco's PBS (repeated three times). The cells in each well were extracted with 500 μl of methanol. The amount of indoxyl sulphate or indoleacetic acid in the cellular extracts (10 μl) was determined by h.p.l.c. analysis. The h.p.l.c. system consisted of CAPCELL PAK MF (ph) column (4.6×150 mm, Shiseido, Tokyo, Japan), 655A-12 pump (Hitachi, Tokyo, Japan), 655A-40 autosampler (Hitachi) and RF-550 fluorescent detector (Shimazu, Kyoto, Japan). The fluorescent detector was set at excitation of 295 nm and emission of 390 nm. The h.p.l.c. analysis was performed with a mobile phase (0.1 M KH2PO4 : THF=95 : 5) and flow rate of 1 ml min−1. Retention time of indoxyl sulphate was 7.9 min and that of indoleacetic acid was 3.5 min in this system.
Assessment of cellular oxidative stress
Cellular oxidative stress was assessed by the method described by Hampel et al. (1999). The hOAT1-transformed cells or vector-transformed control cells were seeded in a 96-well tissue culture plate at a density of 2×103 cells well−1. After incubation for 3 days, the cells were washed with Dulbecco's PBS. One hundred μl of Dulbecco's PBS containing 5 mM D-glucose, 20 μM dihydrofluorescein diacetate and indicated amount of uraemic toxins was added to each well. The fluorescence was measured immediately by a fluorescence plate reader (Spectromax GEMINI XS, Molecular Devices, Sunnyvale, CA, U.S.A.) at Exλ 485 nm and Emλ 538 nm to obtain a reference reading. The cells were then incubated for 60 min at 37°C and the fluorescence were again measured. The results were expressed as percentages of increase in intensity of fluorescence compared to the control experiment calculated by the following formula: (Fct60-Fct0)/(Fc0t60-Fc0t0)*100, where Fct60=fluorescence after 60 min incubation with organic anions, Fct0=fluorescence before incubation with organic anions (reference), Fc0t60=fluorescence after 60 min incubation without organic anions and Fc0t=fluorescence before incubation without organic anion (reference).
Data analysis
Results are presented as means±s.d. Student's t-test was used for comparisons. A P value less than 0.05 was considered statistically significant. Each experiment was performed at least four times.
Results
Characterization of proximal tubular cells stably expressing hOAT1
The hOAT1-transformed cells showed time-dependent intracellular accumulation of PAH to a level 75 fold higher than vector-transformed control cells (Figure 1). A concentration dependent transport of PAH was observed in hOAT1-transformed cells (Figure 2). Nonlinear regression analysis yielded Km value of 22 μM and Vmax of 690 pmol mg−1 protein 10 min−1. To characterize the hOAT1 expressed in OK cells, the effects of various substances on PAH transport were estimated (Figure 3A). Probenecid and α-ketoglutaric acid inhibited PAH transport, but an organic cation, tetraethylammonium, had no effect on the transport. To further characterize expressed hOAT1, the trans-stimulatory effect of α-ketoglutaric acid was examined. The hOAT1-transformed cells were pretreated with 1 mM of α-ketoglutaric acid for 2 h. Then, PAH uptake assay was performed. Pre-treatment with α-ketoglutaric acid increased PAH uptake (Figure 3B). These data indicate that the hOAT1 expressed showed characteristics of organic anion transport in the kidney.
Figure 1.
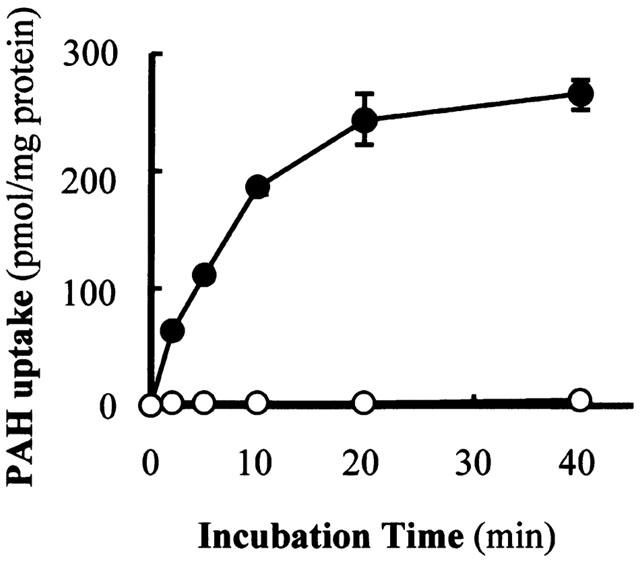
Time course of PAH uptake in hOAT1-transformed and vector-transformed OK cells. The hOAT1-transformed (closed circles) and vector-transformed control (open circles) cells were incubated with 200 μl of Dulbecco's PBS containing 5 mM D-glucose, 10 μM D-[3H]-mannitol and 10 μM [14C]-PAH for various time periods at room temperature. Radioactivity in the cell lysate was measured. Extracellular trapping and nonspecific uptake were obtained from the radioactivity of nontransportable D-[3H]-mannitol. Values are mean±s.d. (n=4).
Figure 2.
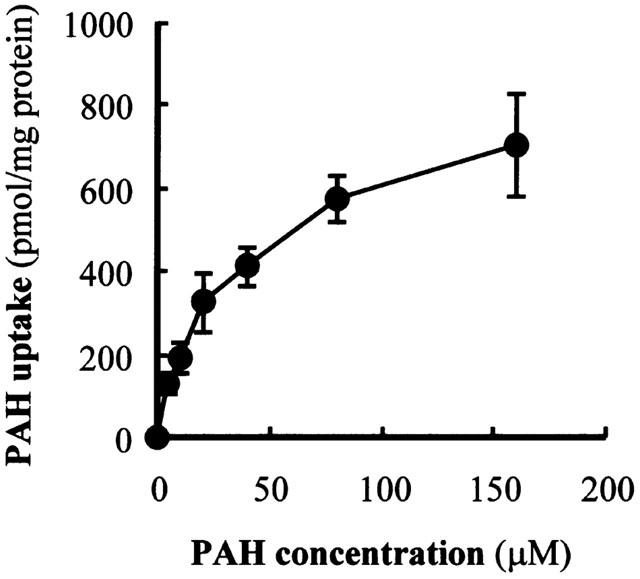
Concentration dependence of PAH uptake in hOAT1-transformed OK cells. The hOAT1-transformed cells were incubated with 200 μl of Dulbecco's PBS containing 5 mM D-glucose, 10 μM D-[3H]-mannitol and various concentrations of [14C]-PAH for 10 min at room temperature. Radioactivity in the cell lysate was measured. Extracellular trapping and nonspecific uptake were obtained from the radioactivity of nontransportable D-[3H]-mannitol. Values are mean±s.d. (n=4).
Figure 3.
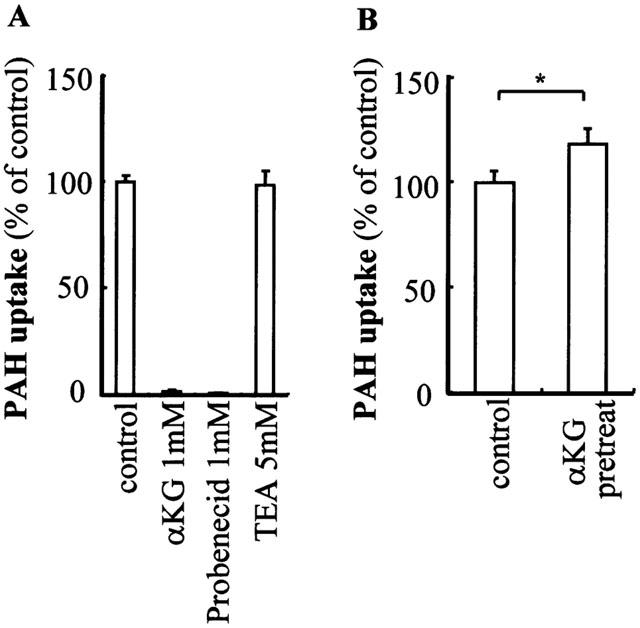
Characterization of hOAT1 expressed in OK cells. (A) Sis-inhibitory effect of various compounds on PAH uptake. The hOAT1-transformed cells were incubated with 200 μl of Dulbecco's PBS containing 5 mM D-glucose, 10 μM D-[3H]-mannitol, 10 μM of [14C]-PAH and indicated concentrations of competitors for 10 min at room temperature. Extracellular trapping and nonspecific uptake were obtained from the radioactivity of nontransportable D-[3H]-mannitol. Values are mean±s.d. (n=4). Abbreviations are α-ketoglutaric acid (αKG) and tetraethylammmonium (TEA). (B) Trans-stimulatory effect of α-ketoglutaric acid on expressed hOAT1. The hOAT1-transformed cells were pretreated with 1 mM of α-ketoglutaric acid for 2 h and PAH uptake assay was performed. Values are mean±s.d. (n=4).
Affinities of uraemic toxins for hOAT1
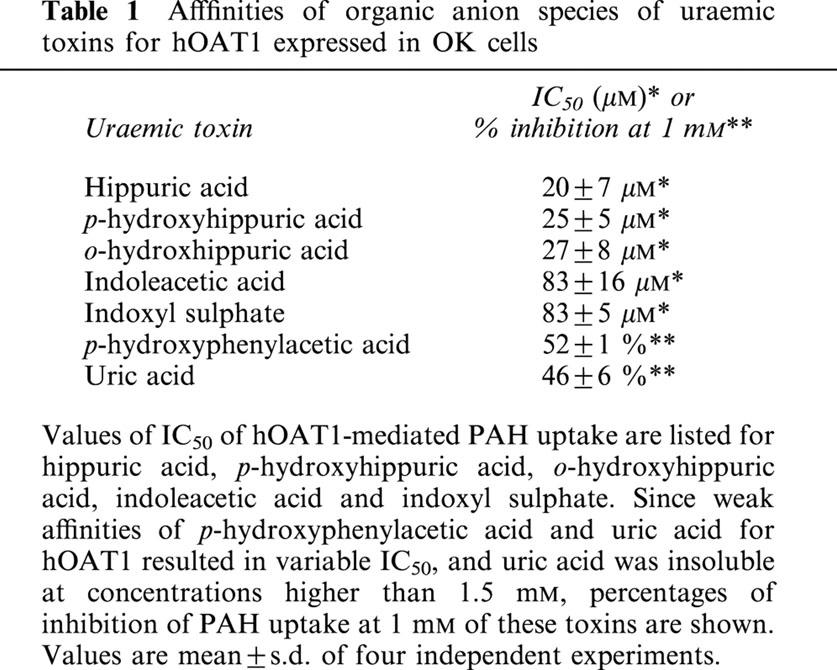
To identify the uraemic toxins that injure the proximal tubular cells via organic anion transporter, we first performed competition experiments of PAH transport by organic anion species of uraemic toxins. For this purpose, concentration-related cis-inhibitory effects of uraemic toxins on PAH transport were measured in hOAT1-transformed OK cells. A dose-dependent inhibition was observed in the cells treated with hippuric acid, p-hydroxyhippuric acid, o-hydroxyhippuric acid, indoxyl sulphate and indoleacetic acid at low concentrations (Figure 4A). The IC50 of these substances ranged from 20 to 85 μM (Table 1). Uric acid and p-hydroxyphenylacetic acid showed weak inhibitory effects (IC50=1 mM) (Figure 4B, Table 1). Guanidinosuccinic acid and β-aminoisobutylic acid had no effect on the transport up to 5 mM. These results indicate that the uraemic toxins examined can be categorized into high, low and no affinity for hOAT1.
Figure 4.
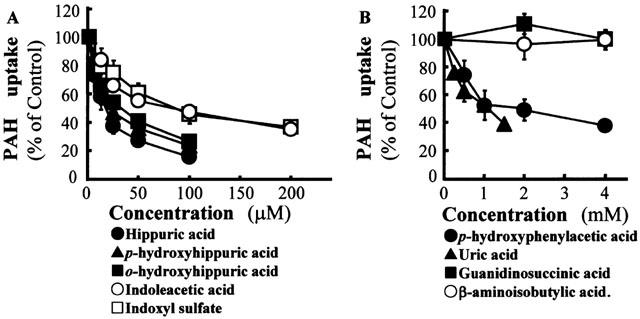
Effects of uraemic toxins in the form of organic anions on PAH uptake in OK cells expressing hOAT1. The hOAT1-transformed cells were incubated with 200 μl of Dulbecco's PBS containing 5 mM D-glucose, 10 μM D-[3H]-mannitol, 10 μM of [14C]-PAH and indicated concentrations of competitors for 10 min at room temperature. Extracellular trapping and nonspecific uptake were obtained from the radioactivity of nontransportable D-[3H]-mannitol. Values are mean±s.d. (n=4). (A) High affinity group uremic toxins. (B) Low and no affinity group uremic toxins.
Table 1.
Afffinities of organic anion species of uraemic toxins for hOAT1 expressed in OK cells
Effects of uraemic toxins on proliferation of hOAT1-transformed cells
To determine the cytotoxicity of organic anions showing high affinity for hOAT1, the effects of these organic anions on cellular proliferation of hOAT1-transformed tubular cells were studied. Indoxyl sulphate and indoleacetic acid inhibited cell proliferation at concentrations of 1–2 mM (Figure 5A,B). Cell proliferation was inhibited by these uraemic toxins, at least in part, via hOAT1-mediated transport because vector-transformed control cell proliferation was inhibited to a significantly less extent by these toxins. Hippuric acid and p-hydroxyhippuric acid had almost no effect on cell proliferation up to 10 mM (Figure 5C,D). Ortho-hydroxyhippuric acid showed an inhibitory effect at higher concentrations (10 mM) (Figure 5E), but no significant difference was observed between hOAT1-transformed and vector-transformed control cells.
Figure 5.
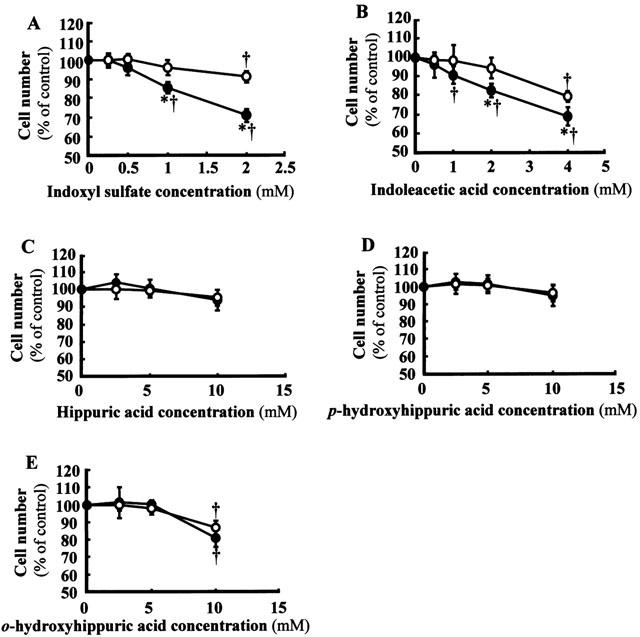
Effects of uraemic toxins on cellular proliferation of hOAT1-transformed OK cells. The hOAT1-transformed cells (closed circles) or vector-transformed control cells (open circles) were incubated in a culture medium containing 2.5% FBS and indicated concentrations of uraemic toxins for 24 h. Then, the cell number in each well was estimated by a cell counting kit. Values are mean±s.d. (n=4 to 8). (A), indoxyl sulphate. (B) indoleacetic acid. (C) Hippuric acid. (D) p-hydroxyhippuric acid. (E) o-hydroxyhippuric acid. *P<0.05 vs vector-transformed cells. †P<0.05 vs non-treated control.
Cellular uptake of indoxyl sulphate and indoleacetic acid
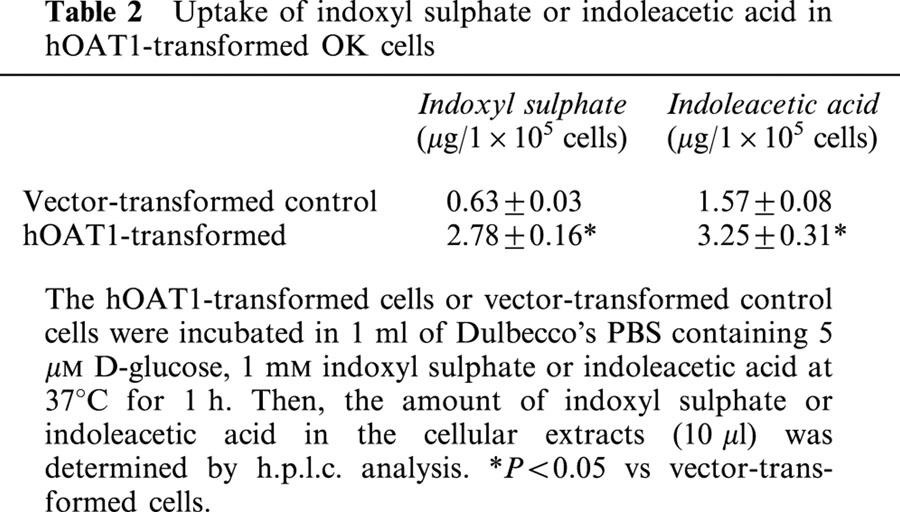
To investigate cellular uptake of indoxyl sulphate and indoleacetic acid, we measured cellular content of these organic anions by h.p.l.c. analysis. When the hOAT1-transformed tubular cells were incubated with 1 mM indoxyl sulphate, cellular content of indoxyl sulphate was significantly higher in these cells than that of vector-transformed control cells (Table 2). Similar results were obtained with cells incubated with indoleacetic acid. Thus, the uptake of these organic anions was substantially enhanced by expression of hOAT1, although there was some uptake of these organic anions in vector-transformed control cells.
Table 2.
Uptake of indoxyl sulphate or indoleacetic acid in hOAT1-transformed OK cells
Effects of uraemic toxins on cellular free radical production
Since nephrotoxic agents such as cephaloridine, gentamicin and cyclosporine A have been shown to induce nephrotoxicity by increasing generation of free radicals (Takeda et al., 1999; Baliga et al., 1999), we investigated the effects of uraemic toxins with high affinity for hOAT1 on cellular free radical production. There was a time-dependent increase in cellular free radical production determined by the fluorescent probe when 2 mM indoxyl sulphate was added to the hOAT1-transformed cells (Figure 6). Indoxyl sulphate and indoleacetic acid dose-dependently increased free radical production (Figure 7A,B). When treated with indoxyl sulphate and indoleacetic acid, a significantly larger amount of free radical was produced in cells transformed with hOAT1 than in those transformed with control vector. In contrast, hippuric acid showed no effect (Figure 7C). Ortho-hydroxyhippuric acid and p-hydroxyhippuric acid increased cellular free radical production to a less extent than that induced by indoxyl sulphate and indoleacetic acid (Figure 7D,E). Since a considerable amount of free radical production was observed in the cells transfected with control vector, we investigated the effects of low and no affinity uraemic toxins on free radical production (Figure 8). Addition of uric acid to the assay system resulted in a marked induction of free radical production. However, no significant difference was observed between hOAT1-transfected and vector-transfected cells. Weak inducible effects of free radical production were observed with p-hydroxyphenylacetic acid and guanidinosuccinic acid. Beta-aminoisobutylic acid had little effect on the free radical production.
Figure 6.
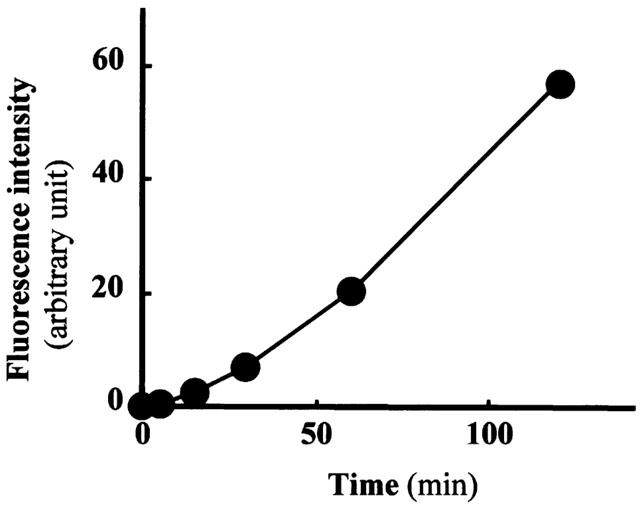
Time course of cellular free radical production in hOAT1-transformed OK cells stimulated by indoxyl sulphate. The hOAT1-transformed cells were incubated in Dulbecco's PBS containing 5 mM D-glucose, 20 μM dihydrofluorescein diacetate and 1 mM indoxyl sulphate for the indicated time at 37°C, and fluorescence intensities were measured. Values are mean±s.d. (n=4).
Figure 7.
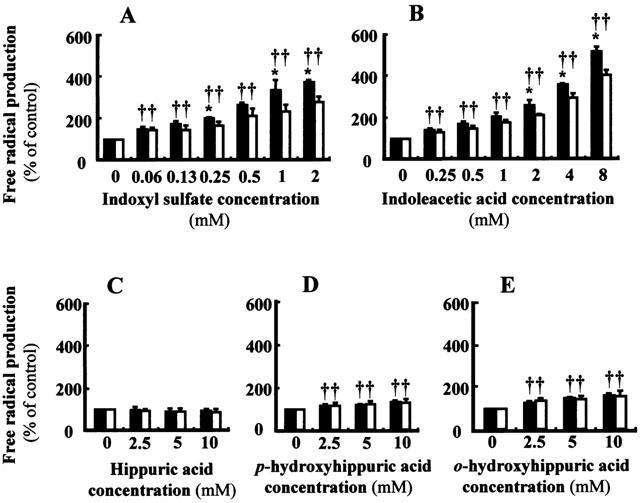
Effects of uraemic toxins on cellular free radical production of hOAT1-transformed OK cells. The hOAT1-transformed cells (closed columns) or vector-transformed control cells (open columns) were incubated in Dulbecco's PBS containing 5 mM D-glucose, 20 μM dihydrofluorescein diacetate and indicated amount of uraemic toxins for 60 min at 37°C, and fluorescence intensities were measured. Values are mean±s.d. (n=4). (A) Indoxyl sulphate. (B) Indoleacetic acid. (C) Hippuric acid. (D) p-hydroxyhippuric acid. (E) o-hydroxyhippuric acid. *P<0.05 vs vector-transformed cells. †P<0.05 vs non-treated control.
Figure 8.
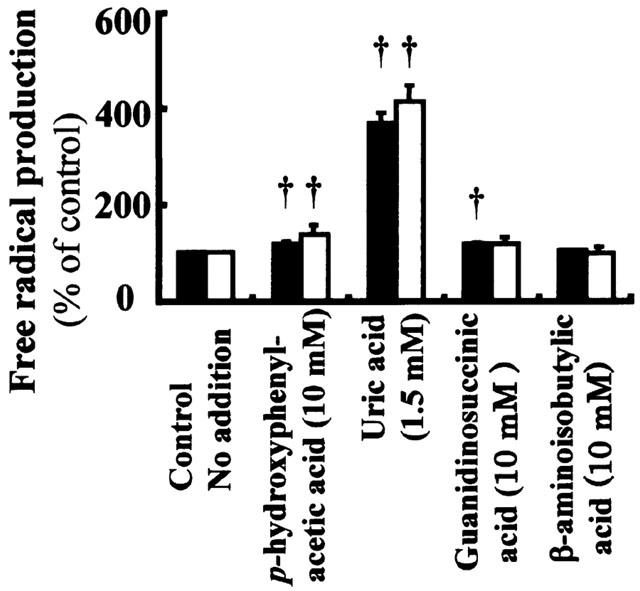
Effects of uraemic toxins with low and no affinity for hOAT1 on cellular free radical production of OK cells. The hOAT1-transformed cells (closed columns) or vector-transformed control cells (open columns) were incubated in Dulbecco's PBS containing 5 mM D-glucose, 20 μM dihydrofluorescein diacetate and indicated amount of uraemic toxins for 60 min at 37°C, and fluorescence intensities were measured. Values are mean±s.d. (n=4). †P<0.05 vs non-treated control.
We then examined the effects of antioxidants and an inhibitor of transporters on the indoxyl sulphate-stimulated free radical production. Addition of an antioxidant; N-acetylcysteine or probucol, abolished the stimulatory effect of indoxyl sulphate on cellular free radical production (Figure 9A). Probenecid, a non-specific transporter inhibitor, reduced the indoxyl sulphate-stimulated radical production. There was little difference in the amount of free radical production between hOAT1-transformed and vector-transformed control cells when cells were treated with probenecid at higher concentrations (20 and 40 mM) (Figure 9B). These results indicate that indoxyl sulphate increases cellular free radical production via hOAT1-mediated cellular uptake of the molecule.
Figure 9.
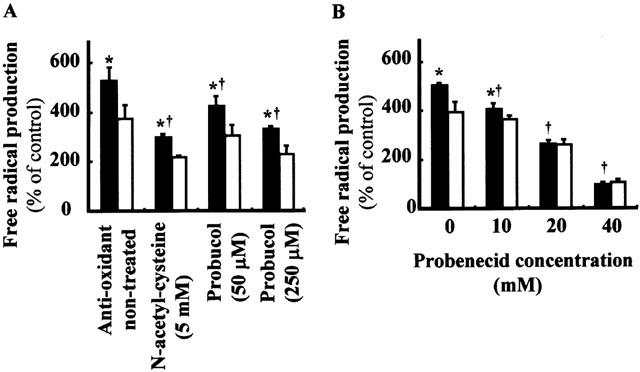
Characteristics of indoxyl sulphate-induced cellular free radical production. (A) Effects of anti-oxidants on indoxyl sulphate-induced cellular free radical production. The hOAT1-transformed cells (closed columns) or vector-transformed control cells (open columns) were incubated in Dulbecco's PBS containing 2 mM indoxyl sulphate, 5 mM D-glucose, 20 μM dihydrofluorescein diacetate and indicated amount of anti-oxidants for 60 min at 37°C, and fluorescence intensities were measured. Values are mean±s.d. (n=4). *P<0.05 vs vector-transformed cells. †P<0.05 vs 2 mM indoxyl sulphate-treated cells. (B) Effects of transporter inhibitor on indoxyl sulphate induced cellular free radical production. The hOAT1-transformed cells (closed columns) or control cells (open columns) were incubated in Dulbecco's PBS containing 2 mM indoxyl sulphate, 5 mM D-glucose, 20 μM dihydrofluorescein diacetate and indicated amount of probenecid for 60 min at 37°C, and fluorescence intensities were measured. Values are mean±s.d. (n=4). *P<0.05 vs vector-transformed cells. †P<0.05 vs 2 mM indoxyl sulphate-treated cells.
Discussion
In the present study, organic anions commonly present as uraemic toxins in the serum of patients with renal failure were studied for their toxic effect on renal cells. Since these molecules are actively taken up by specific transporters in proximal tubular cells, we established a proximal tubular cell line that expresses a high level of hOAT1. The hOAT1 expressed in OK cells showed properties of renal tubular transport of PAH (Figures 1, 2 and 3). The Km of 22 μM obtained from the hOAT1-transformed cells was close to the values obtained with xenopus oocytes (Sekine et al., 1997; Sweet et al., 1997; Hosoyamada et al., 1999) and mouse proximal tubular S3 cells (Takeda et al., 1999). PAH transport mediated by hOAT1 was inhibited by probenecid and α-ketoglutaric acid, and was trans-stimulated by pretreatment with α-ketoglutaric acid. Using this cell line, organic anion species of uraemic toxins were studied for their transport characteristics and cytotoxicity.
The organic anions tested in the present study showed variable characteristics with respect to hOAT1-mediated transport. Based on the inhibitory effects on the hOAT1-mediated transport of PAH, the organic anions were divided into three groups: high affinity, low affinity, and no affinity groups (Figure 4, Table 1). By competing for hOAT1, serum concentrations of uraemic toxins with high affinity for hOAT1 may be elevated in chronic renal disease in which the number of functional nephrons is reduced.
Among uraemic toxins with high affinity to hOAT1, indoxyl sulphate and indoleacetic acid concentration-dependently inhibited the proliferation of renal tubular cells, and the inhibition was also dependent on hOAT1 expression (Figure 5). Thus, these uraemic toxins transported by hOAT1 may induce cellular dysfunction leading to attenuated proliferation. The concentrations required for inhibition of proliferation were comparable to those required for stimulating free radical production (Figure 7). Indoxyl sulphate and indoleacetic acid showed higher stimulatory effect on free radical production than other organic anions. Para-hydroxyhippuric acid and o-hydroxyhippuric acid, also inhibited proliferation of the hOAT1-transformed cells and stimulated free radical production, however, the concentrations required for inhibition of cell proliferation and stimulation of free radical production were considerably higher compared with indoxyl sulphate and indoleacetic acid. Oxidative stress has been shown to inhibit or attenuate cell proliferation (Curcio & Ceriello, 1992; Harris et al., 2000). Therefore, the ability to inhibit cell proliferation was closely linked to the ability to induce free radical formation by the organic anions examined. Inconsistent with these observations, cellular uptake of indoxyl sulphate and indoleacetic acid was significantly higher in the cells transformed with hOAT1 than that in the cells transformed with the vector (Table 2). There was a considerable uptake of these organic anions in the vector-transformed control cells, which may inhibit cellular proliferation and induce free radical formation in these control cells. The cellular uptake of PAH in these cells was 1/75 of hOAT1-transformed cells. Taken together, OK cells may express other transport systems for these organic anions. With this regard, uraemic toxins in the form of organic anions with low affinity or no affinity for hOAT1 may be excreted by other transporters such as OAT3 or OATaps (Inui et al., 2000). Our results indicated that some of uraemic toxins with low and no affinity for hOAT1 also induced cellular free radical production (Figure 8). Thus, the oxidant stress in renal tubular cells in progressive renal disease may be induced by various uraemic toxins.
It is reported that serum concentrations of indoxyl sulphate in patients with chronic renal failure were approximately 80 μmol l−1 in undialyzed uraemic patients, 360 μmol l−1 in patients undergoing haemodialysis and 250 μmol l−1 in patients undergoing CAPD (Niwa et al., 1996). At these concentrations, significant increase in free radical formation was observed with cells transformed with hOAT-1 or vector (Figure 7). Thus, indoxyl sulphate is indicated to stimulate free radical formation at such pathophysiological conditions. Serum concentrations reported for indoleacetic acid were approximately 0.5 mg dl−1 (30 μmol l−1) in patients undergoing haemodialysis (Saito et al., 1980) and 4 μmol l−1 in patients undergoing CAPD (Qureshi & Baig, 1993). The concentrations in these pathophysiological conditions are less than those used in the present study. Indoleacetic acid may enhance toxic effects of indoxyl sulphate in pathophysiological conditions.
Previous reports have reported that oxygen radical production is increased in a remnant kidney model (Schrier et al., 1994; Alfrey, 1994). Increased oxygen radicals in nephron have been proposed to cause tubulo-interstitial injury and progression of chronic renal disease. Increased free radicals produced by organic anion species of uraemic toxins in proximal tubular cells may contribute to this pathophysiological process that lead to tubulo-interstitial damage, while the exact mechanism for inducing oxidative stress in hOAT1-transformed OK cells treated with indoxyl sulphate and other organic anions remains to be determined. Other nephrotoxic agents transported by OAT have been shown to produce free radicals via iron-dependent mechanisms and alterations of cellular anti-oxidant defenses (Baliga et al., 1999; Kiyomiya et al., 2000).
Indoxyl sulphate, indoleacetic acid and hippuric acid are metabolites of tryptophan and phenylalanine (Niwa et al., 1996; Dhondt et al., 2000; Qureshi & Baig, 1993). Low-protein diet has been shown to attenuate progression of the disease in experimental and clinical studies (Mitch, 2000). Low protein diet also has been reported to reduce the serum levels of indoxyl sulphate in progressive renal disease (Niwa & Ise, 1994). As mentioned, reduction of serum uraemic toxins by CAPD and oral adsorbent treatment has been shown to attenuate the progression of renal disease in a rat model of subtotal nephrectomy and clinical study. Further, protein restriction has been proposed to reduce oxidant stress in remnant kidney (Nath et al., 1990; Jarusiripipat et al., 1991). These circumstantial evidences suggest a pathophysiological significance of some uraemic toxins in the progression of chronic renal disease. The present study provided direct evidence that some organic anion species of uraemic toxins damage proximal tubular cells via OAT1-mediated uptake. Such mechanism may constitute important pathophysiology in progression of chronic renal disease.
Acknowledgments
Portions of this study were presented at the 74th Annual Meeting of the Japanese Pharmacological Society, Yokohama, 2001, and published in abstract form (2001, Jpn. J. Pharmacol. 85 (suppl. I), 202P). A portion of this study was supported by the Grant-in-Aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology (No.12670784).
Abbreviations
- FBS
foetal bovine serum
- OAT
organic anion transporter
- OK
opossum kidney
- PAH
p-aminohippuric acid
- PBS
phosphate buffered saline
- PD
peritoneal dialysis
References
- ALFREY A.C. Role of iron and oxygen radicals in the progression of chronic renal disease. Am. J. Kidney Dis. 1994;23:183–187. doi: 10.1016/s0272-6386(12)80969-3. [DOI] [PubMed] [Google Scholar]
- BALIGA R., UEDA N., WALKER P.D., SHAH S.V. Oxidant mechanisms in toxic acute renal failure. Drug Metab. Rev. 1999;31:971–997. doi: 10.1081/dmr-100101947. [DOI] [PubMed] [Google Scholar]
- CURCIO F., CERIELLO A. Decreased cultured endothelial cell proliferation in high glucose medium is reversed by antioxidants: New insights on the pathophysiological mechanisms of diabetic vascular complications. In Vitro Cell Dev. Biol. 1992;28A:787–790. doi: 10.1007/BF02631069. [DOI] [PubMed] [Google Scholar]
- DHONDT A., VANHOLDER R., BIESEN W.V., LAMEIRE N. The removal of uraemic toxins. Kidney Int. 2000;58 Suppl. 76:S47–S59. doi: 10.1046/j.1523-1755.2000.07606.x. [DOI] [PubMed] [Google Scholar]
- GEIJO F., KINOSHITA Y., IKENAKA T. Elevation of serum levels of β-aminoisobutyric acid in uraemic patients and the toxicity of the amino acid. Clin. Nephrol. 1977;8:520–525. [PubMed] [Google Scholar]
- HAMPEL S.L., BUETTNER G.R., O'MALLEY Y.Q., WESSELS D.A., FLAHERTY D.M. Dehydrofluorescein diacetate is superior for detecting intracellular oxidants: Comparison with 2′,7′-dechlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Rad. Biol. Med. 1999;27:146–159. doi: 10.1016/s0891-5849(99)00061-1. [DOI] [PubMed] [Google Scholar]
- HARRIS S.R., PANARO N.J., THORGEIRSSON U.P. Oxidative stress contributes to the anti-proliferative effects of flavone acetic acid on endothelial cells. Anticancer Res. 2000;20:2249–2254. [PubMed] [Google Scholar]
- HOSOYAMADA M., SEKINE T., KANAI Y., ENDOU H. Molecular cloning and functional expression of a multispecific organic anion transpoter from human kidney. Am. J. Physiol. 1999;276:F122–F128. doi: 10.1152/ajprenal.1999.276.1.F122. [DOI] [PubMed] [Google Scholar]
- INUI K., MASUDA S., SAITO H. Cellular and molecular aspects of drug transport in the kidney. Kidney Int. 2000;58:944–958. doi: 10.1046/j.1523-1755.2000.00251.x. [DOI] [PubMed] [Google Scholar]
- JARIYAWAT S., SEKINE T., TAKEDA M., APIWATTANAKUL N., KANAI Y., SOPHASAN S., ENDOU H. The interaction and transport of β-lactam antibiotics with the cloned rat renal organic anion transporter 1. J. Pharmacol. Exp. Ther. 1999;290:672–677. [PubMed] [Google Scholar]
- JARUSIRIPIPAT C., SHAPIRO J.I., CHAN L., SCHRIER R.W. Reduction of remnant nephron hypermetabolism by protein restriction. Am. J. Kidney Dis. 1991;28:367–374. doi: 10.1016/s0272-6386(12)80097-7. [DOI] [PubMed] [Google Scholar]
- KIYOMIYA K., MATSUSHITA N., MATSUO S., KUREBE M. Roles of oxygen radical production and lipid peroxidation in the cytotoxicity of cephaloridine on cultured renal epithelial cells (LLC-PK1) J. Vet. Med. Sci. 2000;62:977–981. doi: 10.1292/jvms.62.977. [DOI] [PubMed] [Google Scholar]
- LYSAGHT M.J., VONESH E.F., GOTCH F., IBELS L., KEEN M., LINDHOLM B., NOLPH K.D., POLLOCK C.A., PROWANT B., FARRELL P.C. The influence of dialysis treatment modality on the decline of remaining renal function. ASAIO Trans. 1991;37:598–604. [PubMed] [Google Scholar]
- MITCH M.E. Dietary therapy in uremia: The impact on nutrition and progressive renal failure. Kidney Int. 2000;57 Suppl. 75:S38–S43. [PubMed] [Google Scholar]
- MOTOJIMA M., NISHIJIMA F., IKOMA M., KAWAMURA T., YOSHIOKA T., FOGO A.B., SAKAI T., ICHIKAWA I. Role for ‘uraemic toxin' in the progressive loss of intact nephrons in chronic renal failure. Kidney Int. 1991;40:461–469. doi: 10.1038/ki.1991.233. [DOI] [PubMed] [Google Scholar]
- NATH K.A., CROATT A.J., HOSTETTER T.H. Oxygen consumption and oxidant stress in surviving nephrons. Am. J. Physiol. 1990;258:F1354–F1362. doi: 10.1152/ajprenal.1990.258.5.F1354. [DOI] [PubMed] [Google Scholar]
- NIWA T., EMOTO Y., MAEDA K., UEHARA Y., YAMADA N., SHIBATA M. Oral sorbent suppresses accumulation of albumin bound indoxyl sulfate in serum of hemodialysis patients. Nephrol. Dial. Transplant. 1991;6:105–109. doi: 10.1093/ndt/6.2.105. [DOI] [PubMed] [Google Scholar]
- NIWA T., ISE M. Indoxyl sulfate, a circulating uraemic toxin, stimulates the progression of glomerular sclerosis. J. Lab. Clin. Med. 1994;124:96–104. [PubMed] [Google Scholar]
- NIWA T., ISE M., MIYAZAKI T. Progression of glomerular sclerosis in experimental uraemic rats by administration of indole, a precursor of indoxyl sulfate. Am. J. Nephrol. 1994;14:207–212. doi: 10.1159/000168716. [DOI] [PubMed] [Google Scholar]
- NIWA T., MIYAZAKI T., TSUKUSHI S., MAEDA K., TSUBAKIHARA Y., OWADA A., SHIIGAI T. Accumulation of indoxyl-β-D-glucuronide in uraemic serum: Suppression of its production by oral sorbent and efficient removal by hemodialysis. Nephron. 1996;74:72–78. doi: 10.1159/000189284. [DOI] [PubMed] [Google Scholar]
- NIWA T., NOMURA T., SUGIYAMA S., MIYAZAKI T., TSUKUSHI S., TSUTSUI S. The protein metabolite hypothesis, a model for the progression of renal failure: An oral adsorbent lowers indoxyl sulfate levels in undialyzed uraemic patients. Kidney Int. 1997;52 suppl. 62:S23–S28. [PubMed] [Google Scholar]
- NIWA T., TAKEDA N., TATEMATSU A., MAEDA K. Accumulation of indoxyl sulfate, an inhibitor of drug binding, in uraemic serum as demonstrated by internal-surface reversed-phase liquid chromatography. Clin. Chem. 1988;34:2264–2267. [PubMed] [Google Scholar]
- QURESHI G.A., BAIG S.M. The role of tryptophan, 5-hydroxy indole-3-acetic acid and their protein binding in uraemic patients. Biochem. Mol. Biol. Int. 1993;29:411–419. [PubMed] [Google Scholar]
- ROTTEMBOURG J. Residual renal function and recovery of renal function in patients treated by CAPD. Kidney Int. 1993;43 suppl. 40:S106–S110. [PubMed] [Google Scholar]
- SAITO A., NIWA T., MAEDA K., KOBAYASHI K., YAMAMOTO Y., OHTA K. Tryptophan and indolic tryptophan metabolites in chronic renal failure. Am. J. Clin. Nutr. 1980;33:1402–1406. doi: 10.1093/ajcn/33.7.1402. [DOI] [PubMed] [Google Scholar]
- SANAKA T., SUGINO N., TERAOKA S., OTA K. Therapeutic effects of oral sorbent in undialyzed uremia. Am. J. Kidney Dis. 1988;12:97–103. doi: 10.1016/s0272-6386(88)80002-7. [DOI] [PubMed] [Google Scholar]
- SCHRIER R.W., SHAPIRO J.I., CHAN L., HARRIS D.C. Increased nephron oxygen consumption: potential role in progression of chronic renal disease. Am. J. Kidney Dis. 1994;23:176–182. doi: 10.1016/s0272-6386(12)80968-1. [DOI] [PubMed] [Google Scholar]
- SEKINE T., WATANABE N., HOSOYAMADA M., KANAI Y., ENDOU H. Expression cloning and characterization of a novel multispecific organic anion transporter. J. Biol. Chem. 1997;272:18526–18529. doi: 10.1074/jbc.272.30.18526. [DOI] [PubMed] [Google Scholar]
- SWEET D.H., WOLFF N.A., PRITCHARD J.B. Expression cloning and characterization of rOAT1. J. Biol. Chem. 1997;272:30088–30095. doi: 10.1074/jbc.272.48.30088. [DOI] [PubMed] [Google Scholar]
- TAKEDA M., TOJO A., SEKINE T., HOSOYAMADA M., KANAI Y., ENDOU H. Role of organic anion transporter 1 (OAT1) in cephaloridine (CER)-induced nephrotoxicity. Kidney Int. 1999;56:2128–2136. doi: 10.1046/j.1523-1755.1999.00789.x. [DOI] [PubMed] [Google Scholar]
- TOJO A., SEKINE T., NAKAJIMA N., HOSOYAMADA M., KANAI Y., KIMURA K., ENDOU H. Immunohistochemical localization of multispecific renal organic anion transporter 1 in rat kidney. J. Am. Soc. Nephrol. 1999;10:464–471. doi: 10.1681/ASN.V103464. [DOI] [PubMed] [Google Scholar]
- TSUDA M., SEKINE T., TAKEDA M., CHA S.H., KANAI Y., KIMURA M., ENDOU H. Transport of ochratoxin A by renal multispecific organic anion transporter 1. J. Pharmacol. Exp. Ther. 1999;289:1301–1305. [PubMed] [Google Scholar]
- VANHOLDER R., SMET R.D., LAMEIRE N. Protein-bound uraemic solutes: The forgotten toxins. Kidney Int. 2001;59 suppl. 78:S266–S270. doi: 10.1046/j.1523-1755.2001.59780266.x. [DOI] [PubMed] [Google Scholar]


