Abstract
The aims of this study were to develop a technique to measure blood flow in the mouse ear and to investigate the nature of the vasodilator mediator(s) involved in the response to capsaicin.
The response to capsaicin, applied topically, was investigated in anaesthetized CD1 or Sv129+C57BL/6 wild-type (+/+) or NK1 receptor knockout mice (−/−). Blood flow was assessed by laser Doppler flowmetry and oedema formation by 125I-albumin accumulation.
Capsaicin induced significant increases in blood flow (0.2–200 μg in 20 μl) and oedema (2–200 μg in 20 μl). The oedema response was absent in NK1−/− mice and NK1+/+mice treated with the selective NK1 receptor antagonist SR140333 (480 nmol kg−1) as expected. Furthermore, the capsaicin-evoked increase in blood flow was significantly potentiated in the knockout mice (203% of wild-type response, P<0.05) and wild-type mice treated with SR140333 (201%, P<0.05).
The CGRP receptor antagonist CGRP8–37 (400 nmol kg−1) had no effect on capsaicin-induced blood flow in NK1+/+mice but abolished the increased blood flow to capsaicin in NK1−/−, and NK1+/+wild-type mice pre-treated with SR140333.
The results indicate that neurogenic vasodilatation can be measured in the mouse ear. The capsaicin-induced increased blood flow involves activation of, and possible interactions between, both NK1 and CGRP1 receptors.
Keywords: Calcitonin gene-related peptide (CGRP), substance P, laser doppler flowmetry, neurogenic inflammation, blood flow, microvascular
Introduction
The acute neurogenic inflammatory response occurs as a result of neuropeptide release from stimulated primary afferent neurones. The major neuropeptides involved are considered to be substance P and CGRP. CGRP is often co-localized and released with the tachykinin substance P in sensory nerves (see Brain, 1997). Substance P acts to increase microvascular permeability, allowing the exudation of plasma proteins from the blood vessels into the tissues and the formation of inflammatory oedema (Lembeck & Holzer, 1979; Lembeck et al., 1992). CGRP is a potent vasodilator (Brain et al., 1985), affecting both major vessels and the microvasculature. CGRP is mainly considered to act on CGRP receptors, formed by co-expression of the calcitonin receptor-like receptor (CRLR) with receptor-accessory-modifying protein 1 (RAMP1; Mclatchie et al., 1998; Juaneda et al., 2000), and with other proteins (e.g. CGRP-Receptor Component Protein; Evans et al., 2000). There is good evidence for a major role for CGRP in mediating neurogenic vasodilatation, but this has been primarily obtained from studies in the rat. In this model, the CGRP antagonist CGRP8–37 is able to block the vasodilatation (Escott & Brain, 1993; Delay-Goyet et al., 1992) and substance P acts as a potent mediator of increased microvascular permeability, via tachykinin NK1 receptors. Furthermore, in the rat CGRP potentiates the oedema formation to substance P (Brain & Williams, 1985; Gamse & Saria, 1985), most probably as a consequence of its vasodilatory action.
The role of the NK1 receptor in mediating neurogenic oedema formation and pain sensation has been confirmed in recent years through the development and use of NK1 receptor knockout mice (Bozic et al., 1996; de felipe et al., 1998; Laird et al., 2000). Our group has demonstrated that oedema formation induced after intradermal injection of substance P and related agonists is totally dependent on the presence of the NK1 receptor, in that oedema formation is not observed in NK1 knockout mice (Cao et al., 1999). The use of the mouse ear as a model for measurement of oedema induction by capsaicin has been well characterized in several papers (Inoue et al., 1993; Gábor & Rázga, 1992). Capsaicin selectively activates sensory nerves via the VR1 receptor (Caterina et al., 1997) leading to neuropeptide release and inflammation. This neurogenic oedema model has now been used in studies with NK1 knockout mice to show that the oedema formation induced by capsaicin in the wild-type mouse ear is reduced in NK1 receptor knockout mice (Bozic et al., 1996; de felipe et al., 1998). Capsaicin ear oedema is also greatly reduced in mice in which the gene for preprotachykinin-A (PPT-A), the precursor of substance P, has been disrupted (Cao et al., 1998). These results support studies previously carried out using non-peptide NK1 receptor antagonists, such as CP-96,345 (Lembeck et al., 1992) or SR140333 (Emonds-Alt et al., 1993) in the rat. However, the contribution of CGRP to vasoactive responses in the capsaicin ear model of neurogenic inflammation in the mouse has not previously been investigated.
From the above observations, we hypothesized that NK1 receptor knockout mice would exhibit normal neurogenic vasodilatation in response to capsaicin application. The aim of the present study was to develop a protocol allowing simultaneous measurement of blood flow and oedema formation from a single mouse ear. This could then be developed to allow further characterization of the neurogenic inflammatory response to capsaicin by separating out the oedema and vasodilatation components. The relative importance of substance P and CGRP was examined using the selective NK1 receptor antagonist SR140333 and the CGRP antagonist CGRP8–37, and Sv129+C57BL/6+/+and NK1−/− mice.
Methods
The experiments were carried out under the Animals (Scientific Procedures) Act, 1986, and after completion of experiments the animals were killed by cervical dislocation. Normal male CD1 mice (30–35 g) were obtained from Charles River, U.K. Wild-type and NK1 receptor knockout Sv129+C57BL/6 mice were obtained from Perlmutter Laboratory (Children's Hospital, Boston, MA, U.S.A.) then bred in house. Mice of both sexes (25–40 g) were used in this study. All were maintained on normal diet, with free access to food and water, in a climatically controlled environment. Both strains displayed normal growth and behavioural characteristics.
Measurement of capsaicin-induced vasodilatation
The mice were anaesthetized by intraperitoneal (i.p.) injection of urethane (7 μg g−1). A laser Doppler probe (P1 probe, Moor Instruments, U.K.) was placed on each ear and blood flow recorded for a 5 min period to ensure stability. Capsaicin solution (10 μl of 10 mg ml−1, except in dose-response study) was applied externally to both surfaces of one ear (i.e. 20 μl per ear in total) and ethanol (vehicle control) to both surfaces of the contralateral ear. This dose was chosen as it was a similar concentration to those used in previous studies (e.g. Gábor & Rázga, 1992; Inoue et al., 1993). Blood flow was then assessed for a period of 1 h (as Lawrence & Brain, 1992).
Measurement of capsaicin-induced oedema formation
Oedema formation was measured by the extravascular accumulation of intravenously injected [125I]-labelled bovine serum albumin (BSA) (Cao et al., 1999). [125I]-BSA (90 kBq) was injected with saline (0.1 ml i.v into tail vein) and then flushed through with 0.1 ml saline. After 5 min, capsaicin or vehicle control was applied topically to the ears as described above. Oedema development was allowed for a 1 h period, during which time blood flow measurements were made. A blood sample (0.3–0.7 ml) was then taken by cardiac puncture, and the animal killed by cervical dislocation. The blood samples were centrifuged at 10,000×g for 4 min, after which plasma was taken for measurement of plasma radioactivity in a gamma counter. The ears were removed and weighed, and their radioactivity measured. Ear oedema was expressed as microlitres of plasma per milligram of tissue.
Effect of test compounds on capsaicin-induced vasodilatation and oedema formation
Wild-type mice were anaesthetized by i.p. injection of urethane, as above. SR140333 (480 nmol kg−1, as Cao et al., 2000) was administered in saline, along with the 125I-labelled BSA solution (0.1 ml total volume i.v. into tail vein) and then oedema and blood flow measurements carried out as above. The effects of CGRP8–37 (400 nmol kg−1, as Escott & Brain, 1993), and a combination of CGRP8–37 and SR140333, were assessed using the same protocol
Materials
The following drugs were used: [125I]-bovine serum albumin was purchased from ICN, U.K. Capsaicin, BSA and urethane were obtained from Sigma Chemicals. Capsaicin was dissolved in ethanol and urethane in saline. SR140333 ((S)1-(2-[3-(3,4-dichlorophenyl)-1-(3-isopropoxyphenylacetyl)piperidin-3-yl]ethyl)-4-phenyl-1-azoniabicyclo[2.2.2]octane chloride) was a kind gift from Dr X. Emonds-Alt, Sanofi, Toulouse, France. SR140333 was dissolved in a minimum amount of ethanol, then made up to the final volume with saline. CGRP8–37 was obtained from Phoenix Pharmaceuticals, U.S.A and dissolved in 0.01% BSA solution.
Analysis of data and statistical analysis
The time course of the blood flow response to capsaicin (Figure 1) was expressed as the mean percentage increase over the basal value. For the other figures where blood flow was assessed, the areas under the recorded flux vs time were measured for the entire recording period, then an average of all the areas from a particular protocol was taken for comparison, and expressed as mean area under the curve (mm2). Plasma extravasation was expressed as μl of fluid per mg of tissue for all figures. Statistical analyses were carried out by analysis of variance (ANOVA) followed by Dunnett's test for the blood flow and dose-response oedema data. Comparisons between treated and untreated mice were carried out by unpaired t-tests, and by paired t-tests when comparing treated and control ears on one animal. Results were all expressed as mean±s.e.mean.
Figure 1.
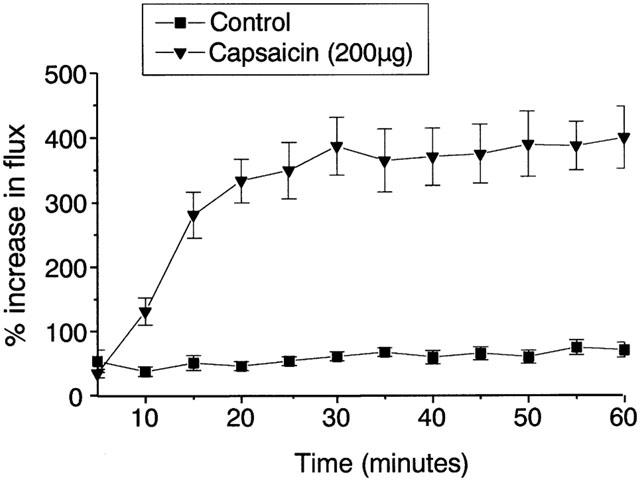
Effect of topically applied capsaicin (200 μg) on blood flow to the CD1 mouse ear, measured over 60 min. Results are expressed as percentage increase over the minimum measured flux, mean±s.e.mean, n=10. **P<0.01 compared to ethanol-treated control values.
Results
Vasodilatation and oedema responses to capsaicin in CD1 mice
Topical application of capsaicin solution (10 μl) to the ears of male CD1 mice led to significantly increased blood flow, which was sustained from 15 min onwards throughout the 60 min measurement period (Figure 1). Simultaneous measurement of plasma extravasation caused by capsaicin within the ears demonstrated that a dose of 2 μg or above per ear was required to induce oedema formation (Figure 2a). However, doses of 0.2 μg and above were able to cause significant increased blood flow (Figure 2b). Neither the neurogenic vasodilatation nor the plasma extravasation response to capsaicin appeared to show dose-dependence, but instead the results suggest an ‘all-or-nothing' response. It is also noticeable that the effective dose for inducing plasma extravasation is 10 fold higher than that which triggers increased blood flow.
Figure 2.
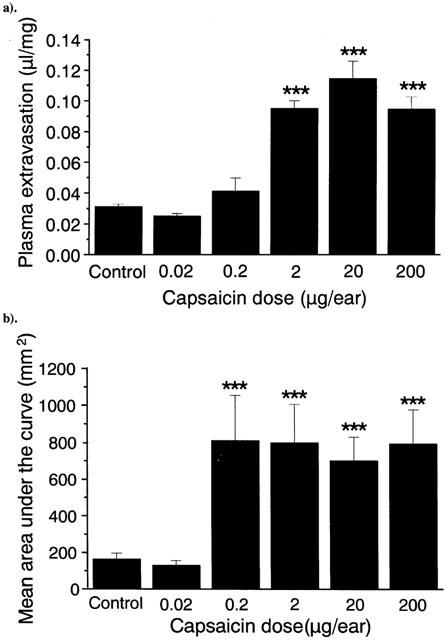
Effect of capsaicin (0.02–200 μg) on plasma extravasation (a) and blood flow (b) in the CD1 mouse ear, measured over 60 min. Results are expressed as (a) μl of fluid accumulated per mg of ear tissue and (b) area under the flux curve (mm2), n=10. Columns show the mean size of the response, and bars show the s.e.mean. ***P<0.001 compared to ethanol-treated control values.
Vasodilatation and oedema responses to capsaicin in Sv129+C57BL/6 mice
Topical application of capsaicin to the ears of SV129+C57BL/6 wild-type mice produced significant plasma extravasation, whereas the same dose had no effect on plasma extravasation in the ears of NK1 receptor knockout mice (Figure 3a).
Figure 3.
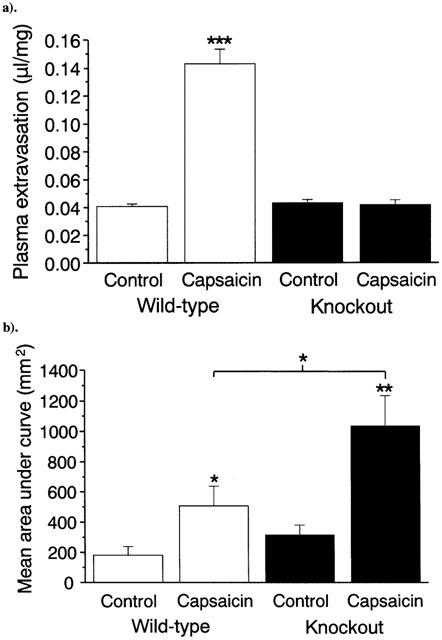
Comparison of effects of topical capsaicin (200 μg) on (a) plasma extravasation and (b) blood flow in the ears of wild-type (unfilled bars) and NK1 receptor knockout (black bars) Sv129+C57BL/6 mice. Results are expressed as (a) μl of fluid accumulated per mg of ear tissue and (b) area under the flux curve, n=10. Columns show the mean size of the response, and bars show the s.e.mean. *P<0.05, **P<0.01, ***P<0.001 compared to ethanol-treated control. #P<0.05 compared to capsaicin-treated wild-type.
Concurrent measurement of the increased blood flow produced by capsaicin showed that a significant increase was produced in both wild-type and knockout mice (Figure 3b). Interestingly, the increase in blood flow in knockout mice was double that produced in wild-type mice.
Effect of CGRP8–37 and SR140333 on neurogenic inflammation
Pre-treatment of Sv129+C57BL/6 wild-type or NK1 knockout mice with the CGRP receptor antagonist CGRP8–37 (400 nmol kg−1) had no effect on the plasma extravasation induced by capsaicin, compared to its vehicle control (Figure 4a). Significant plasma extravasation still occurred in wild-type mice, but was absent in the NK1 receptor knockout mice. Treatment with CGRP8–37 also failed to reduce the vasodilatation produced by capsaicin in wild-type mice, as shown in Figure 4b. Interestingly, treatment with the same dose of CGRP8–37 significantly attenuated the increased blood flow response to the same dose of capsaicin in knockout mice, reducing it to a similar level as observed in wild-type mice (561.3±100.2 vs 509.2±96.1mm2; mean area under curve).
Figure 4.
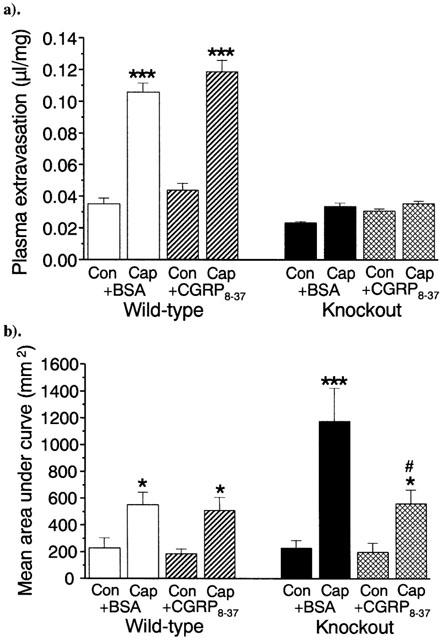
Comparison of the effects of treatment with capsaicin (20 μg ear−1) in the presence or absence of CGRP8–37 on (a) plasma extravasation and (b) blood flow in the ears of wild-type and NK1 receptor knockout mice. Results are expressed as (a) μl of fluid accumulated per mg of ear tissue and (b) area under in the ears of wild-type and NK1 receptor knockout mice, the flux curve, n=10. Columns show the mean size of the response, and bars show the s.e.mean. *P<0.05, ***P<0.001 compared to ethanol-treated controls. #P<0.05 compared to vehicle-treated capsaicin ear. Unfilled bars represent wild-type mice treated with BSA (CGRP8–37 vehicle control), hatched bars represent wild-type+CGRP8–37, black bars represent knockouts+BSA and cross-hatched bars knockouts+CGRP8–37.
To determine whether the efficacy of CGRP8–37 is related to the presence or absence of functional NK1 receptors the neurogenic inflammatory response to capsaicin in wild-type mice pre-treated with the selective NK1 receptor antagonist SR140333 (480 nmol kg−1) was examined. The oedema responses in the mice are shown in Figure 5a, after treatment with CGRP8–37 or its vehicle control. In Figure 5b, the blood flux changes in response to capsaicin are shown. Treatment with SR140333 alone is unable to block the increase in blood flow due to capsaicin, and, as observed in NK1 knockouts, actually causes an increase relative to the untreated wild-type mice (1025.2±226.8 vs 509.1±128.3 mm2; mean area under curve; P<0.05). However, in contrast to treatment with CGRP8–37 alone, a combination of CGRP8–37 and SR140333 significantly reduces the increased blood flow produced by capsaicin in wild-type mice (as shown in Figure 5b).
Figure 5.
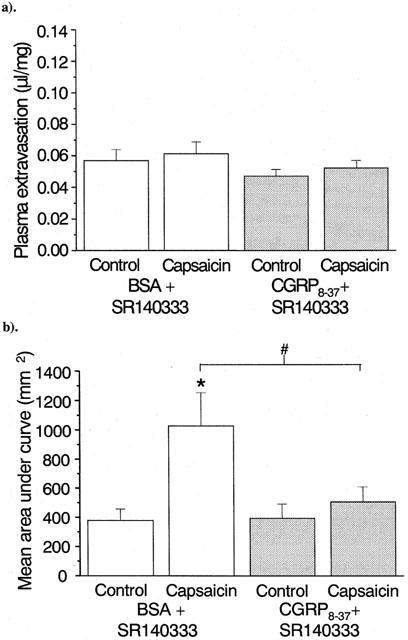
Comparison of the effects of treatment with SR140333 in the presence (hatched bars) or absence (unfilled bars) of CGRP8–37 on capsaicin-induced (a) plasma extravasation and (b) blood flow in the ears of wild-type mice. Results are expressed as (a) μl of fluid accumulated per mg of ear tissue and (b) area under the flux curve, n=9. Columns show the mean size of the response, and bars show the s.e.mean. *P<0.05 compared to ethanol-treated control, #P<0.05 compared to BSA-treated mouse.
Discussion
The mouse ear as a model for measurement of oedema induction by capsaicin has been well characterized in several papers (e.g. Inoue et al., 1993; Gábor & Rázga, 1992). However, it has not been used to assess vasodilatation in response to capsaicin. In fact, no previous studies have investigated the vasodilatory effects of topical capsaicin on murine skin. We have combined measurement of capsaicin-induced oedema by a I125-labelled BSA accumulation technique with measurement of the skin microvascular blood flow by laser Doppler flowmetry to produce simultaneous measurement of the two parameters for the first time in a murine model. The results show a clear and significant increase in blood flow that is observed in response to topical application of capsaicin. Surprisingly, the response in wild-type mice treated with the selective NK1 antagonist SR140333 or in NK1 receptor knockout mice was greater when compared with the response in untreated wild-type mice. Furthermore, the CGRP receptor antagonist CGRP8–37 was only able to block the responses in either the wild-type mouse treated with the NK1 antagonist SR140333 or in the NK1 knockout mouse. This suggests that both CGRP and NK1 receptor-mediated mechanisms are involved in neurogenic vasodilatation in these mice.
Vasodilatation and oedema responses to capsaicin in CD1 mice
The initial experiments demonstrate that topical capsaicin produces a significant increase in measured flux compared to the vehicle treated ear, which correlates to vasodilatation. Interestingly, the measured flux reached a maximum within 30 min but, instead of decreasing back to baseline, remained high throughout the period of measurement. This was unexpected as previous studies have regularly demonstrated desensitization of the sensory nerves by prolonged exposure to capsaicin within this time (e.g. Baranowski et al., 1986), although none have investigated microvascular blood flow in the mouse. Desensitization can occur through a poorly defined receptor-dependent mechanism, in which calcineurin has been implicated (Docherty et al., 1996). It can also occur through depletion of substance P and CGRP, which require the presence of nerve growth factor for their synthesis, from the nerve terminals (Holzer, 1988). In a parallel series of experiments, the vasodilatation to capsaicin was measured over 3 h (data not shown), with blood flow remaining elevated throughout the measurement period. Even if enough neuropeptide was present to sustain release over 3 h it would be expected that the capsaicin VR1 receptor itself would desensitize within this time (Docherty et al., 1996; Liu & Simon, 1996). The elevated blood flow in response to capsaicin persisted for the entire recording period. CGRP has a half-life of only a few minutes in the circulation (Kraenzlin et al., 1985; Braslis et al., 1988), which may suggest that the sustained response is unlikely to be due to an initial release of neuropeptide which continues to act for 3 h. However, the intradermal injection of picomolar amounts of CGRP into human skin is associated with a prolonged (for several hours) increase in local erythema that is due to increased blood flow (Brain et al., 1985; 1986). Thus, the prolonged response may be due to a downstream consequence of CGRP receptor activation.
The dose response studies reveal that, at least over this range of doses, an ‘all-or-nothing' response appears to occur, with little dose related activity. This is probably caused because the dose of capsaicin will either be great enough to activate the entire nociceptive fibre, triggering maximal neuropeptide release, or it will have no effect on the fibre as a whole.
Vasodilatation and oedema responses to capsaicin in wild-type and NK1 knockout mice
Topical application of capsaicin induced oedema in Sv129+C57BL/6 wild-type mice. Removal of the NK1 receptor abolished this response, in confirmation of previous results with this strain of mice (Bozic et al., 1996; Cao et al., 1999) and with the selective non-peptide NK1 receptor antagonist SR140333 (e.g. Emonds-Alt et al., 1993) indicating that oedema is mediated by the NK1 receptor. Concurrent measurement of the increased blood flow induced by capsaicin revealed a significant increase in blood flow in both wild-type and knockout mice. However it was noted that the response to capsaicin in the knockout and SR140333-treated wild-type mice was significantly greater (approximately double) than that in the wild-type mice. This suggests the possibility that the vasodilatation induced by capsaicin may be influenced by the NK1 receptor, potentially by suppressing release of the vasodilator mediator(s) (e.g. by activation of inhibitory NK1 autoreceptors, either on the sensory fibres which release the substance P, or on adjacent fibres). NK1 receptors have been demonstrated on rat dorsal root ganglia cells in culture (von banchet & Schaible, 1999) and have been shown to inhibit substance P release from rat spinal cord (Malcangio & Bowery, 1994). Thus inhibitory NK1 receptors may have an important role in modulating neuropeptide release from sensory nerves. Alternatively, it is possible that there are intracellular interactions between the NK1 and CGRP signalling pathways, and evidence of similar interactions has been found in T cells (Levite, 1998) and smooth muscle cells (Ouyang et al., 1998). It is also possible that the NK1-mediated oedema formation opposes the increased blood flow in the ear. This is difficult to investigate in the ear, but evidence from rat skin does not support this hypothesis (Brain & Williams, 1989).
Neurogenic vasodilatation in wild-type and NK1 receptor knockout mice: identifying the CGRP component.
Pretreatment of wild-type mice with the CGRP receptor antagonist CGRP8–37 had no effect on the plasma extravasation induced by capsaicin. This is in contrast to previous studies in rat skin with exogenous (Brain & Williams, 1985; 1989) and endogenous CGRP (Escott & Brain, 1993) which show that it acts to potentiate oedema formation induced by mediators of increased microvascular permeability such as substance P. A similar phenomenon was also observed in mouse skin where intradermally-injected CGRP potentiated oedema formation by NK1 agonists (Cao et al., 1999). The inability of CGRP8–37 to attenuate plasma extravasation in this study is in keeping with the hypothesis that CGRP is not the primary neurogenic vasodilator substance.
The above findings are probably linked to the results which suggest a lack of involvement of CGRP in the capsaicin-induced neurogenic vasodilatation in wild-type mice. However, further experiments revealed that CGRP8–37 did act to attenuate the increased blood flow response to capsaicin in NK1 receptor knockout mice. In addition, after treatment with the selective NK1 receptor antagonist SR140333, CGRP8–37 was able to block the increased blood flow to capsaicin in wild-type mice. The mechanism for this remains unclear, but suggests that both substance P, acting via the NK1 receptor, and CGRP can contribute to neurogenic vasodilatation. We have learnt that similar responses have been obtained in human skin (Dr M. Schmelz, University of Erlangen, Germany), where an NK1 receptor antagonist acted to partially block the neurogenic vasodilatation observed in response to local electrical stimulation of human skin. This suggests that studies of neurogenic mechanisms in the mouse microvasculature are of direct relevance to those in the human microvasculature.
The reasons for the observed results are unclear at this stage, but it is tempting to speculate with regard to the mechanisms involved. The phenomenon of functional redundancy among mediators, including vasodilator mediators, has previously been described. For example, it has been demonstrated that in the gastro-intestinal microcirculation, where a lack of sufficient blood flow is associated with gastric ulcer formation, that more than one class of endogenous vasodilators have to be inhibited before injury occurs (Whittle & Lopez-Belmonte, 1993). This suggests a positive co-operation may occur in the microcirculation between vasodilator mediators. However, our results are complicated by the finding that a significantly greater blood flow, which is inhibited by CGRP8–37, is observed in the absence of functional NK1 receptors. It is possible, as discussed above, that technical considerations may be responsible for the measurement of the significantly increased blood flow in the absence of oedema formation, but these cannot account for the lack of ability of CGRP8–37 to block the quantitatively smaller increased blood flow in the wild-type mouse.
CGRP and substance P are co-localized in sensory nerves and there are many reports showing that they can be co-released (e.g Lundberg et al., 1985; Stjarne et al., 1989), indeed they have even been shown to be present in the same vesicles in neurons in the chicken ureter (Sann et al., 1997). Thus it is logical to suggest that activation of the NK1 receptor is influencing either the release or activity of CGRP. The possibility of prejunctional NK1 receptors modulating CGRP release has been mentioned above, however, to explain the present results the prejunctional receptors would have to be situated on CGRP-containing nerves. CGRP has been cited as the most abundant neuropeptide present in human skin and is to be found in both nerves that contain CGRP alone and those in which CGRP is co-localized with other neuropeptides (Gibbins et al., 1987; Dalsgaard et al., 1989). By comparison little is known about mouse skin, but a similar situation has been suggested to exist in mouse hind paw (Navarro et al., 1995); thus it may be possible that NK1 receptors can modulate release of CGRP from nerves that predominantly contain CGRP. Our knowledge of the composition of the CGRP receptors allows us to suggest another alternative mechanism that may influence the ability of CGRP to act as a primary mediator of neurogenic vasodilatation in the wild-type mouse. There is recent evidence that expression of RAMPs in tissues and levels can be altered, depending on situation (Frayon et al., 2000; Nagae et al., 2000). It is possible that NK1-mediated events can influence the functional compatibility of RAMP1 with CRLR at the cell surface. However, such a mechanism would require an acute alteration in receptor function to be feasible and the research in this area to date points to a more delayed response.
In conclusion, the results indicate that capsaicin induces a sustained increase in blood flow in the mouse ear that can be measured by laser Doppler flowmetry. The increased blood flow appears to be mediated by both NK1 and CGRP receptors in that the response is observed in NK1 knockout mice and in wild-type mice treated with a CGRP antagonist.
By comparison a CGRP antagonist is without effect in wild-type mice, but blocks the response in both NK1 knockout mice and in wild-type mice treated with an NK1 receptor antagonist. Thus an interaction between functional NK1 and CGRP receptors is suggested.
Acknowledgments
This study was funded by the British Heart Foundation.
Abbreviations
- CGRP
calcitonin gene-related peptide
- CRLR
calcitonin receptor-like receptor
- NK1
neurokinin-1
- PPT-A
preprotachykinin-A
- RAMP
receptor-accessory-modifying protein
- VR1
vanilloid receptor 1
References
- BARANOWSKI R., LYNN B., PINI A. The effects of locally applied capsaicin on conduction in cutaneous nerves in four mammalian species. Br. J. Pharmacol. 1986;89:167–176. doi: 10.1111/j.1476-5381.1986.tb10256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOZIC C., LU B., HOPKEN U.E., GERARD C., GERARD N.P. Neurogenic amplification of immune complex inflammation. Science. 1996;273:1722–1725. doi: 10.1126/science.273.5282.1722. [DOI] [PubMed] [Google Scholar]
- BRAIN S.D. Sensory neuropeptides: their role in inflammation and wound healing. Immunopharmacology. 1997;37:133–152. doi: 10.1016/s0162-3109(97)00055-6. [DOI] [PubMed] [Google Scholar]
- BRAIN S.D., TIPPINS J.R., MORRIS H.R., MACINTYRE I., WILLIAMS T.J. Potent vasodilator activity of calcitonin gene-related peptide in human skin. J. Invest. Dermatol. 1986;87:533–536. doi: 10.1111/1523-1747.ep12455620. [DOI] [PubMed] [Google Scholar]
- BRAIN S.D., WILLIAMS T.J. Inflammatory oedema induced by synergism between calcitonin gene-related peptide (CGRP) and mediators of increased vascular permeability. Br. J. Pharmacol. 1985;86:855–860. doi: 10.1111/j.1476-5381.1985.tb11107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAIN S.D., WILLIAMS T.J. Interactions between the tachykinins and calcitonin gene-related peptide lead to the modulation of oedema formation and blood flow in rat skin. Br. J. Pharmacol. 1989;97:77–82. doi: 10.1111/j.1476-5381.1989.tb11926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAIN S.D., WILLIAMS T.J., TIPPINS J.R., MORRIS H.R., MACINTYRE I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- BRASLIS K.G., SHULKES A., FLETCHER D.R., HARDY K.J. Pharmacokinetics and organ-specific metabolism of calcitonin gene-related peptide in sheep. J. Endocrinol. 1988;118:25–31. doi: 10.1677/joe.0.1180025. [DOI] [PubMed] [Google Scholar]
- CAO Y.Q., MANTYH P.W., CARLSON E.J., GILLESPIE A-M., EPSTEIN C.J., BASBAUM A.I. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- CAO T., GERARD N.P., BRAIN S.D. Use of NK1 knockout mice to analyze substance P-induced edema formation. Am. J. Physiol. 1999;277:476–481. doi: 10.1152/ajpregu.1999.277.2.R476. [DOI] [PubMed] [Google Scholar]
- CAO T., PINTER E., AL-RASHED S., GERARD N.P., HOULT J.R., BRAIN S.D. Neurokinin-1 receptor agonists are involved in mediating neutrophil accumulation in the inflamed, but not normal, cutaneous microvasculature: An in vivo study using neurokinin-1 receptor knockout mice. J. Immunol. 2000;164:5424–5429. doi: 10.4049/jimmunol.164.10.5424. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- DALSGAARD C.J., JERNBECK J., STAINS W., KJARTANSSON J., HAEGERSTRAND A., HOKFELT T., BRODIN E., CUELLO A.C., BROWN J.C. Calcitonin gene-related peptide-like immunoreactivity in nerve fibers in the human skin. Relation to fibers containing substance P-, somatostatin- and vasoactive intestinal polypeptide-like immunoreactivity. Histochemistry. 1989;91:35–38. doi: 10.1007/BF00501907. [DOI] [PubMed] [Google Scholar]
- DE FELIPE C., HERRERO J.F., O'BRIEN J.A., PALMER J.A., DOYLE C.A., SMITH A.J., LAIRD J.M., BELMONTE C., CERVERO F., HUNT S.P. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392:394–397. doi: 10.1038/32904. [DOI] [PubMed] [Google Scholar]
- DELAY-GOYET P., SATOH H., LUNDBERG J.M. Relative involvement of substance P and CGRP mechanisms in antidromic vasodilatation in the rat skin. Acta. Physiol. Scand. 1992;146:537–538. doi: 10.1111/j.1748-1716.1992.tb09460.x. [DOI] [PubMed] [Google Scholar]
- DOCHERTY R.J., YEATS J.C., BEVAN S., BODDEKE H.W.G.M. Inhibition of calcineurin inhibits the desensitization of capsaicin evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflug. Arch. Eur. J. Phy. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- EMONDS-ALT X., DOUTREMEPUICH J.D., HEAULME M., NELIAT G., SANTUCCI V., STEINBERG R., VILAIN P., BICHON D., DUCOUX J.P., PROIETTO V., VANBROECK D., SOUBRIE P., LEFUR G., BRELIERE J.C. In vitro and in vivo biological activities of SR140333, a novel potent non-peptide tachykinin NK1 receptor antagonist. Eur. J. Pharmacol. 1993;250:403–413. doi: 10.1016/0014-2999(93)90027-f. [DOI] [PubMed] [Google Scholar]
- ESCOTT K.J., BRAIN S.D. Effect of a calcitonin gene-related peptide antagonist (CGRP8−37) on skin vasodilatation and oedema induced by stimulation of the rat saphenous nerve. Br. J. Pharmacol. 1993;110:772–776. doi: 10.1111/j.1476-5381.1993.tb13878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS B.N., ROSENBLATT M.I., MNAYER L.O., OLIVER K.R., DICKERSON I.M. CGRP-RCP: A novel protein required for signal transduction at CGRP and adrenomedullin receptors. J. Biol. Chem. 2000;275:31438–31443. doi: 10.1074/jbc.M005604200. [DOI] [PubMed] [Google Scholar]
- FRAYON S., CUEILLE C., GNIDEHOU S., DE VERNEJOUL M.C., GAREL J.M. Dexamethasone increases RAMP1 and CRLR mRNA expressions in human vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2000;270:1063–1067. doi: 10.1006/bbrc.2000.2552. [DOI] [PubMed] [Google Scholar]
- GÁBOR M., RÁZGA Z. Development and inhibition of mouse ear oedema induced with capsaicin. Agents Actions. 1992;36:83–86. doi: 10.1007/BF01991233. [DOI] [PubMed] [Google Scholar]
- GAMSE R., SARIA A. Potentiation of tachykinin-induced plasma protein extravasation by calcitonin gene-related peptide. Eur. J. Pharmacol. 1985;114:61–66. doi: 10.1016/0014-2999(85)90520-5. [DOI] [PubMed] [Google Scholar]
- GIBBINS I.L., WATTCHOW D., COVENTRY B. Two immunohistochemically identified populations of calcitonin gene-related peptide (CGRP)-immunoreactive axons in human skin. Brain Res. 1987;414:143–148. doi: 10.1016/0006-8993(87)91335-7. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- INOUE H., NAGATA N., KOSHIHARA Y. Profile of capsaicin-induced mouse ear oedema as neurogenic inflammatory model: comparison with arachidonic acid-induced ear oedema. Br. J. Pharmacol. 1993;110:1614–1620. doi: 10.1111/j.1476-5381.1993.tb14009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUANEDA C., DUMONT Y., QUIRION R. The molecular pharmacology of CGRP and related peptide receptor subtypes. Trends Pharmacol. Sci. 2000;21:432–438. doi: 10.1016/s0165-6147(00)01555-8. [DOI] [PubMed] [Google Scholar]
- KRAENZLIN M.E., CH'NG J.L., MULDERRY P.K., GHATEI M.A., BLOOM S.R. Infusion of a novel peptide, calcitonin gene-related peptide (CGRP) in man. Pharmacokinetics and effects on gastric acid secretion and on gastrointestinal hormones. Regul. Pept. 1985;10:189–197. doi: 10.1016/0167-0115(85)90013-8. [DOI] [PubMed] [Google Scholar]
- LAIRD J.M., OLIVAR T., ROZA C., DE FELIPE C., HUNT S.P., CERVERO F. Deficits in visceral pain and hyperalgesia of mice with a disruption of the tachykinin NK1 receptor gene. Neuroscience. 2000;98:345–352. doi: 10.1016/s0306-4522(00)00148-2. [DOI] [PubMed] [Google Scholar]
- LAWRENCE E., BRAIN S.D. Responses to endothelins in the rat cutaneous microvasculature: a modulatory role of locally-produced nitric oxide. Br. J. Pharmacol. 1992;106:733–738. doi: 10.1111/j.1476-5381.1992.tb14402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMBECK F., DONNERER J., TSUCHIYA M., NAGAHISA A. The non-peptide tachykinin antagonist CP-96,345 is a potent inhibitor of neurogenic inflammation. Br. J. Pharmacol. 1992;105:527–530. doi: 10.1111/j.1476-5381.1992.tb09013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMBECK F., HOLZER P. Substance P as a mediator of antidromic vasodilatation and neurogenic plasma extravasation. Naunyn-Schmiedeberg's Arch. Pharmacol. 1979;310:175–183. doi: 10.1007/BF00500282. [DOI] [PubMed] [Google Scholar]
- LEVITE M. Neuropeptides, by direct interaction with T cells, induce cytokine secretion and break the commitment to a distinct T helper phenotype. Proc. Natl. Acad. Sci. 1998;95:12544–9. doi: 10.1073/pnas.95.21.12544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU L., SIMON S.A. Capsaicin-induced currents with distinct desensitization and Ca2+ dependence in rat trigeminal ganglion cells. J. Neurophysiol. 1996;25:1503–1514. doi: 10.1152/jn.1996.75.4.1503. [DOI] [PubMed] [Google Scholar]
- LUNDBERG J.M., FRANCO-CERECEDA A., HUA X., HOKFELT T., FISCHER J.A. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur. J. Pharmacol. 1985;108:315–319. doi: 10.1016/0014-2999(85)90456-x. [DOI] [PubMed] [Google Scholar]
- MALCANGIO M., BOWERY N.G. Effect of the tachykinin NK1 receptor antagonists, RP 67580 and SR 140333, on electrically-evoked substance P release from rat spinal cord. Br. J. Pharmacol. 1994;113:635–641. doi: 10.1111/j.1476-5381.1994.tb17037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLATCHIE L.M., FRASER N.J., MAIN M.J., WISE A., BROWN J., THOMPSON N., SOLARI R., LEE M.G., FOORD S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- NAGAE T., MUKOYAMA M., SUGAWARA A., MORI K., YAHATA K., KASAHARA M., SUGANAMI T., MAKINO H., FUJINAGA Y., YOSHIOKA T., TANAKA I., NAKAO K. Rat receptor-activity-modifying proteins (RAMPs) for adrenomedullin/CGRP receptor: cloning and upregulation in obstructive nephropathy. Biochem. Biophys. Res. Commun. 2000;270:89–93. doi: 10.1006/bbrc.2000.2390. [DOI] [PubMed] [Google Scholar]
- NAVARRO X., VERDU E., WENDELSCAFER-CRABB G., KENNEDY W.R. Innervation of cutaneous structures in the mouse hind paw: a confocal microscopy immunohistochemical study. J. Neurosci. Res. 1995;41:111–120. doi: 10.1002/jnr.490410113. [DOI] [PubMed] [Google Scholar]
- OUYANG A., BROUSSARD D.L., FENG H.S. Action of substance P and interaction of calcitonin gene-related peptide and substance P on the cat antral circular muscle. Regul. Pept. 1998;77:25–32. doi: 10.1016/s0167-0115(98)00033-0. [DOI] [PubMed] [Google Scholar]
- SANN H., HAMMER K., HILDESHEIM I.F., PIERAU F.K. Neurons in the chicken ureter are innervated by substance P- and calcitonin gene-related peptide-containing nerve fibres: immunohistochemical and electrophysiological evidence. J. Comp. Neurol. 1997;380:105–118. doi: 10.1002/(sici)1096-9861(19970331)380:1<105::aid-cne8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- STJARNE P., LUNDBLAD L., ANGGARD A., HOKFELT T., LUNDBERG J.M. Tachykinins and calcitonin gene-related peptide: co-existence in sensory nerves of the nasal mucosa and effects on blood flow. Cell. Tissue. Res. 1989;256:439–446. doi: 10.1007/BF00225591. [DOI] [PubMed] [Google Scholar]
- VON BANCHET G.S., SCHAIBLE H.G. Localization of the neurokinin 1 receptor on a subset of substance P-positive and isolectin B4-negative dorsal root ganglion neurons of the rat. Neurosci. Lett. 1999;274:175–178. doi: 10.1016/s0304-3940(99)00719-3. [DOI] [PubMed] [Google Scholar]
- WHITTLE B.J., LOPEZ-BELMONTE J. Actions and interactions of endothelins, prostacyclin and nitric oxide in the gastric mucosa. J. Physiol. Pharmacol. 1993;44:91–107. [PubMed] [Google Scholar]


