Abstract
We have examined the role of ATP-dependent P2X1 receptors in megakaryocytes (MKs) and platelets using receptor-deficient mice and selective agonists.
α,β-meATP- and ATP- evoked ionotropic inward currents were absent in whole-cell recordings from MKs of P2X1−/− mice, demonstrating that the P2X receptor phenotype in MKs, and by inference, platelets, is due to expression of homomeric P2X1 receptors.
P2X1 receptor deficiency had no effect on MK (CD 41) numbers or size distribution, showing that it is not essential for normal MK development.
P2Y receptor-stimulated [Ca2+]i responses were unaffected in MKs from P2X1−/− mice, however the inward cation current associated with Ca2+ release was reduced by ∼50%, suggesting an interaction between the membrane conductances activated by P2X1 and P2Y receptors.
Interaction between P2X1 and P2Y receptors in human platelets was also examined using [Ca2+]i recordings from cell suspensions. α,β-meATP (10 μM) evoked a rapid transient P2X1 receptor-mediated increase in [Ca2+]i, whereas ADP-(10 μM) evoked P2Y receptor responses were slower, peaked at a higher level and remained elevated for longer periods. Co-application of α,β-meATP and ADP resulted in marked acceleration and amplification of the peak [Ca2+]i response.
We conclude that ionotropic P2X1 receptors may play a priming role in the subsequent activation of metabotropic P2Y receptors during platelet stimulation.
Keywords: P2X1, P2Y, P2 receptor, platelets, megakaryocytes, [Ca2+]i, knock-out mice, synergy, Ca2+ influx, CD 41
Introduction
Megakaryocytes (MKs) are platelet progenitor cells, whose principal role is to maintain the normal blood platelet count (Kaushansky, 1999). Platelets have little or no capacity to manufacture proteins and consequently MKs must express most, if not all, platelet proteins. Therefore, ion channels and receptors expressed in MKs may exist solely for later use in hemostatic functions, or alternatively, could play a role in megakaryocyte development. Purine nucleotides, acting through P2 receptors, are known to play important roles in fundamental platelet responses (see Kunapuli, 1998 for review). It is not known, however, at which stage of MK development P2 purinoceptors are expressed and whether they also have a role in megakaryocytopoiesis.
P2 receptors can be divided into ligand-gated ionotropic P2X receptors and metabotropic G-protein-coupled P2Y receptors (Burnstock, 1997). Seven distinct isoforms of the P2X receptor (P2X1–7), with a range of physiological and pharmacological properties, have been isolated at the molecular level (Burnstock, 1997). They have a novel molecular architecture with two transmembrane domains, intracellular amino and carboxy termini, and a large extracellular ligand binding loop (Surprenant et al., 1995) and form homo-multimeric channels with at least three subunits (Nicke et al., 1998). P2X receptor subunits can also co-assemble to form heteromeric channels, often with composite phenotypes, e.g. P2X1/5, P2X2/3, P2X2/6 and P2X4/6 receptors (King et al., 2000; Le et al., 1998; 1999; Lewis et al., 1995; Torres et al., 1998). P2X1 receptors have been cloned from human platelets (Clifford et al., 1998; Scase et al., 1998; Sun et al., 1998; Vial et al., 1997) and are expressed by smooth muscle, neurons (Collo et al., 1996; Vulchanova et al., 1996) and a range of hematopoietic cells including megakaryoblastic cell lines (Vial et al., 1997), monocyte/macrophage lineage-HL60 and granulocyte-rat basophilic leukaemia cells (Buell et al., 1996).
The properties of MK and platelet P2X receptors, α,β-methylene ATP- (α,β-meATP) evoked rapidly desensitizing responses, correspond closely to those of recombinant P2X1 receptors (Mackenzie et al., 1996; Mahaut-Smith et al., 2000). Recently a P2X1 receptor deletion mutant (P2X1del) that is ADP-sensitive and α,β-meATP-insensitive has been isolated from MK cell lines and platelets (Greco et al., 2001), however the functional role of this mutant receptor was not determined. In our studies on human platelets, α,β-meATP always evoked a rapid transient increase in [Ca2+]i and purified ADP only evoked a slower P2Y mediated calcium increase (platelets from 11 donors; Mahaut-Smith et al., 2000). Thus we have no evidence for an ADP sensitive P2X1 receptor-mediated response in human platelets, suggesting that if the P2X1del mutant is functionally expressed in human platelets it forms a heteromeric receptor dominated by the properties of full-length P2X1 receptors. However, it remains unclear whether other cloned or yet to be identified P2X receptor isoforms are also expressed by MKs and contribute to the P2X receptor phenotype. Furthermore, the role of P2X1 receptors in platelet function is unclear. P2X receptors are Ca2+ permeable (Benham & Tsien, 1987; Evans et al., 1996) and their activation results in Ca2+ influx (Mackenzie et al., 1996; Vial et al., 1997). Two groups failed to detect a functional response linked to P2X receptor activation (Jin & Kunapuli, 1998; Savi et al., 1998), however, it has recently been shown that the transient Ca2+ influx following selective P2X1 receptor activation can stimulate a shape change response in human platelets in vitro if receptor desensitization is limited (Rolf et al., 2001). A dominant negative P2X1 receptor mutation has been reported in platelets from a patient with a severe bleeding disorder (Oury et al., 2000), however the relative contribution of this mutation to hemostatic function is not known.
It has been suggested that P2X1 receptors contribute to the apoptosis of thymocytes but not peripheral T cells (Chvatchko et al., 1996). In addition, P2X1 receptor expression was markedly upregulated when promyelocytes (HL60 cells) were treated with dibutyryl cyclic AMP to induce a neutrophil-like phenotype (Buell et al., 1996) In contrast, P2X1 receptor expression may be repressed during phagocyte differentiation (Clifford et al., 1998). Thus, P2X1 receptors may play an important role in the development of various hematopoietic cell lineages including MKs.
At least two types of G-protein coupled P2Y receptors are also expressed by platelets (Kunapuli, 1998). P2Y1 receptors activate phospholipase-C, leading to Ca2+ mobilization, via Gαq proteins, and also stimulate Rho/Rho kinase via Gα12/13 (Leon et al., 1997; Paul et al., 1999). These two intracellular signalling pathways can account for the ADP-evoked platelet shape change response, with the more important contribution arising from the increase in [Ca2+]i (Paul et al., 1999). In addition, platelets express a P2Y receptor, the P2YAC receptor (also referred to as the P2cyc and P2TAC), that couples to the inhibition of adenylyl cyclase and activation of PI 3-kinase (Kunapuli, 1998; Trumel et al., 1999). An ADP receptor has been cloned recently from platelets that corresponds to this receptor and has been designated P2Y12 (Hollopeter et al., 2001). Co-activation of P2Y1 and P2TAC (P2Y12) receptors is required for normal ADP-evoked aggregation (Hechler et al., 1998; Kunapuli, 1998; Savi et al., 1998), although some degree of aggregation can occur via either P2Y1 or P2TAC (P2Y12) receptors (Fabre et al., 1999; Jarvis et al., 2000; Leon et al., 1999). Megakaryocytes most likely express both P2Y receptors found in the platelet, although their identity in the progenitor cell has not been reported in molecular studies. P2Y receptor activation in rat and mouse megakaryocytes evokes Ca2+ mobilization and a monovalent cation conductance (Somasundaram & Mahaut-Smith, 1994; Uneyama et al., 1993). Given the variety of P2 receptors expressed on MKs and platelets, it is possible that P2X and P2Y receptors interact in the control of these cells.
Physiological P2X receptor agonists include ATP and diadenosine polyphosphates (Ap4A, Ap5A, and Ap6A) (Evans et al., 1995; Wildman et al., 1999). Megakaryocyte and platelet P2Y receptors are principally activated by ADP (Kunapuli, 1998; Somasundaram & Mahaut-Smith, 1994; Uneyama et al., 1993), although ATP is a weak agonist at the MK P2Y receptors coupled to Ca2+ mobilization. On the other hand, ATP can act as an antagonist of ADP-dependent activation of P2Y1 and P2TAC (P2Y12) receptors in the platelet (Hechler et al., 1998; Hourani, 2000). This apparent discrepancy can be accounted for by a low efficacy of ATP at P2Y1 receptors and a low receptor density in the platelet compared to the MK (Hechler et al., 1998; Palmer et al., 1998). Although it had previously been thought that ADP was an agonist at both P2X and P2Y receptors (Mackenzie et al., 1996; Mahaut-Smith et al., 1992; Sage et al., 2000) it has recently been shown that the action of ADP at P2X receptors results from low levels of ATP contamination of commercially available ADP (Mahaut-Smith et al., 2000). During hemostasis, large amounts of ATP will be released from damaged cells at the site of vascular injury and ADP, ATP and diadenosine polyphosphates will be released from the dense granules of activated platelets. Thus, under physiological conditions, it is likely that platelet P2X and P2Y receptors will be activated simultaneously, although by separate agonists, and thus interactions between these two receptor subtypes could have important functional consequences for hemostasis and thrombosis.
In this study we have compared the properties of MKs from normal and P2X1 receptor-deficient mice (Mulryan et al., 2000) to determine (1) whether P2X1 receptor expression is essential for normal MK development and (2) the role of P2X1 receptor subunits in activation of mouse MKs by extracellular purines. We have also used selective agonists for ionotropic and metabotropic platelet purinoceptors to examine the interaction of P2X and P2Y receptors during receptor-operated Ca2+ mobilization in human platelets.
Methods
Marrow preparation and antibody staining
Adult male P2X1 receptor deficient (−/−), heterozygote (+/−) or wild type (+/+) mice (Mulryan et al., 2000) were killed by cervical dislocation and exsanguinated. For immunohistochemical studies the spleen and bone marrow (from femur and tibia) were dissected and rapidly frozen in Tissue-Tek® (Sakuna, The Netherlands). 12 μm sections were cut, thaw-mounted on slides coated with 3-aminopropyltriethoxysilane (Sigma-Aldrich, U.K.) and fixed in 2% paraformaldehyde (Sigma-Aldrich, U.K.), 80 mM Na2HPO4, 20 mM NaH2PO4 for 10 min and washed in PBS (phosphate buffered saline). The sections were incubated in 0.5% Triton X-100 (Sigma-Aldrich, U.K.), and 10% normal donkey serum (Jackson ImmunoResearch Laboratories, U.S.A.) in PBS for 30 min at room temperature, washed in PBS and incubated with rabbit anti-rat P2X1 polyclonal antibody (Alomone Labs, Israel) at a dilution of 1 : 200 with 10% normal donkey serum in PBS at 4°C overnight. The sections were subsequently washed in PBS, incubated with Texas Red® dye-conjugated donkey anti-rabbit IgG (dilution of 1 : 100) (Jackson ImmunoResearch Laboratories, U.S.A.) and for double labelling studies FITC-conjugated rat anti-mouse CD41 monoclonal antibody (dilution of 1 : 500) (Pharmingen International, U.S.A.) was added in the presence of 10% normal donkey serum in PBS for 2 h at room temperature, washed in PBS and then mounted in Citifluor (Citifluor, U.K.). Images were captured using Scion Imaging v 4.0.2 software (a modified version of the public domain program, NIH Image) and the intensity of P2X1 receptor immunofluorescence was determined.
For determination of the size distribution of MKs, bone marrow from a total of 3 (+/+) and 4 P2X1 receptor deficient (−/−) mice were collected and frozen in Tissue-Tek®. For each mouse, two different sections were cut and fixed as described above, incubated in 10% normal donkey serum in PBS for 30 min at room temperature, rinsed, incubated for 2 h at room temperature in FITC-conjugated rat anti-mouse CD41 monoclonal antibody (dilution of 1 : 500), then washed and mounted with Citifluor. The sections were photographed with an epifluorescence microscope and the size distribution of CD41 immunoreactive MKs was determined using NIH Image as described previously (Chopra et al., 2000).
Electrophysiological and [Ca2+]i measurements from single megakaryocytes
For these studies mouse MKs were isolated by gentle trituration from the femoral and tibial marrow and conventional whole cell patch clamp and intracellular Ca2+ recordings were made as described previously (Mahaut-Smith et al., 2000). To isolate the P2X receptor-mediated current, short pulses (<2 s) of α,β-meATP (Sigma-Aldrich, U.K.) were applied to cells held at −60 mV. The pipette filling solution contained (in mM) Kgluconate 140, NaCl 5, EGTA 9, HEPES 10, pH 7.3 (KOH) and the external solution contained (in mM) NaCl 150, HEPES 10, KCl 2.5, MgCl2 1, CaCl2 2.5, pH 7.3 (NaOH). For dual measurement of ATP-activated P2 receptor inward currents and changes in intracellular Ca2+, the holding potential was −70 mV, the pipette filling solution contained (in mM) CsCl 150, MgCl2 2, EGTA 0.1, K5fura-2 0.05, Na2GTP 0.05, HEPES 10, pH 7.2 (CsOH) and the external solution contained (in mM) NaCl 150, HEPES 10, CaCl2 1, MgCl2 1, glucose 10, pH 7.35 (NaOH). All megakaryocyte electrophysiological and [Ca2+]i studies were conducted at room temperature (20–24°C).
Platelet [Ca2+]i measurements
Cuvette fluorescence measurements of [Ca2+]i in fura-2-loaded human platelets were performed essentially as described previously (Mahaut-Smith et al., 2000; Rolf et al., 2001) at a temperature of either 13°C or 37°C. The saline contained (in mM), NaCl 145, KCl 5, CaCl2 1, MgCl2 1, HEPES 10, glucose 10, pH 7.35 (NaOH) and 0.32 U ml−1 apyrase. For data collected at 13°C, background-corrected 340/380 nm ratios are used to indicate [Ca2+]i due to problems associated with accurately calibrating fura-2 fluorescence at the reduced temperature (Mahaut-Smith et al., 2000). ATP-free ADP was prepared by incubation of a stock of 10 mM ADP (Sigma-Aldrich, U.K.) in a high glucose saline (composition, in mM: NaCl 150, CaCl2 2.5, HEPES 10, KCl 2.5, MgCl2 1, glucose 22, pH 8 (NaOH), with 3 units/ml hexokinase for 1 h at 37°C (Mahaut-Smith et al., 2000).
Data analysis
Data are presented throughout as mean±s.e.mean, n=number of observations. Differences between means were determined by the appropriate Student's t-test and were considered significant when P<0.05. Differences between the size distribution of MKs were tested using two-way analysis of variance.
Results
P2X1 receptor expression in bone marrow and spleen
P2X1 receptor immunoreactivity was detected in sections of bone marrow (Figure 1) associated with large diameter hematopoietic cells. In addition, P2X1 receptor immunoreactivity was seen in arteries coursing through the marrow (not shown). This pattern of expression is consistent with P2X1 receptor expression in MKs and smooth muscle (Vial et al., 1997; Vulchanova et al., 1996). To confirm that the P2X1 receptor is expressed by MKs we looked for co-localization of immunoreactivity with the MK and platelet-specific marker glycoprotein-IIb (αIIb integrin or CD41) (Uzan et al., 1991). In dual labelling studies P2X1 receptor and CD41 immunoreactivity were co-localized in sections of bone marrow from wild type (+/+) mice (Figure 1) (excluding P2X1 receptor expression in vascular smooth muscle). This confirms that P2X1 receptors are expressed in MKs.
Figure 1.
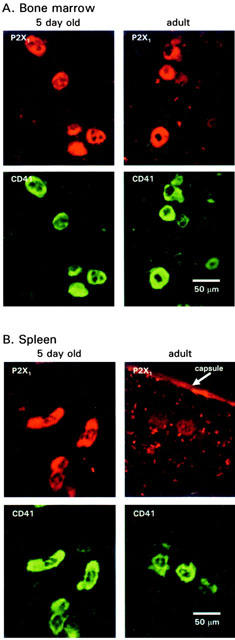
Immunoreactivity for P2X1 receptors and the MK lineage marker CD41 in bone marrow and spleen. P2X1 receptor immunoreactivity is co-localized with the MK marker CD41 in bone marrow. (A) Similar levels of immunoreactivity were detected in tissues from 5-day-old (left panel) and adult mice (right panel). P2X1 receptor immunoreactivity was also co-localized with CD41 immunoreactivity in the spleen from 5-day-old mice (B, left hand panels), in contrast P2X1 receptor immunoreactivity was barely detectable in CD41 immunoreactive cells from adult spleen.
In rodents, the major sites of megakaryocyte development and platelet production are the bone marrow and spleen (Davis et al., 1997). In 1–5 - day - old animals P2X1 receptor immunoreactivity (co-localized with CD41 immunoreactivity) was detected at similar levels of intensity in both the spleen and bone marrow (61.7±3.6 and 61.5±5.5 units of intensity respectively, n=34, 28 MKs) (Figure 1). Interestingly, in contrast to bone marrow where P2X1 receptor immunoreactivity was at similar levels in adults to that in 1–5-day-old animals (61.5±5.5 and 67.2±5.7 intensity units, n=28, 29 cells respectively), P2X1 receptor immunoreactivity was reduced significantly (P<0.001) in CD41 immunoreactive MKs in adult spleen (61.7±3.6 and 42.4±2.0 intensity units, n=34, 76 MKs from 1–5 day and adult respectively) (Figure 1). P2X1 receptor immunoreactivity was detected in the artery walls, the capsule layer of the spleen and in tuberculae of adult marrow. These results suggest the down regulation of P2X1 receptor expression in splenic MKs.
MK development in P2X1 receptor deficient mice
P2X1 receptor immunoreactivity was not detected in bone marrow from P2X1 receptor deficient (−/−) mice. These results confirm that the P2X1 receptor is not produced in these mice (as shown previously (Mulryan et al., 2000)). MKs are polyploid cells with different levels of ploidy reflected by the size of the cell (Levine et al., 1982). In order to test whether P2X1 receptor expression is essential for normal MK development we have determined the numbers and size distribution of megakaryocytes for +/+ and −/− mice using the MK marker CD41. The P2X1 receptor deficiency had no effect on the number or size distribution of MKs in bone marrow from adult mice (ANOVA p=0.8) (Figure 2). This finding is also supported by the electrophysiological studies in which the capacitance of the MKs (a measure of plasma membrane area) was not significantly different for +/+ and −/− mice (298±32 pF and 261±26 pF, respectively n=12–22). These results indicate that the P2X1 receptor is not essential for MK development.
Figure 2.
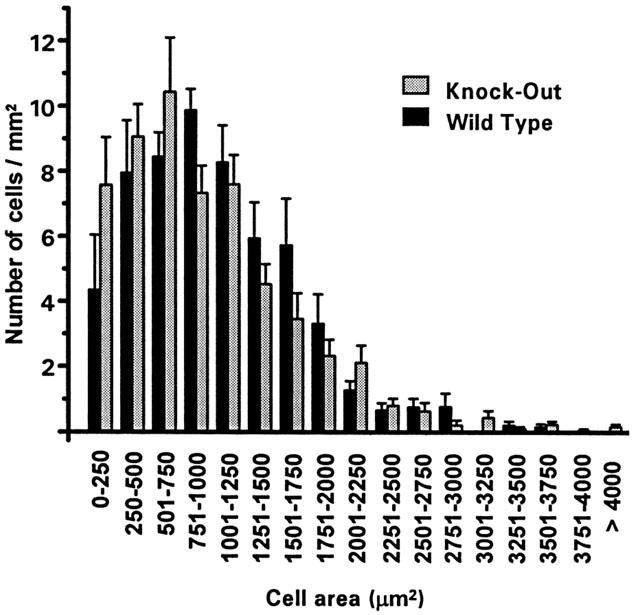
Size distribution of MKs from normal and P2X1 receptor deficient mice. Analysis of the area of CD41 immunoreactive cells in bone marrow. Two sections per mouse were analysed (n=3 and 4 mice for +/+ and −/− mice).
P2X1 receptors are essential for the production of functional MK P2X receptors
The P2X1, P2X1/5, P2X3 and P2X2/3 receptor agonist α,β-meATP (10 μM, a maximal concentration at these receptors) evoked rapid transient inward currents in +/+ and +/− MKs (707±116 and 680±200 pA respectively, n=15–16) but had no effect on MKs (n=12) from P2X1 receptor deficient mice (Figure 3). P2X1 receptors are non-selective cation channels; monovalent cations predominate ionic flow and calcium accounts for 5–10% of current flow under physiologcal conditions (Benham, 1989; Evans et al., 1996). ATP (30 μM), an effective agonist at all P2X receptors, also evoked rapid transient inward currents from +/+ but not −/− mice when P2Y receptor-mediated [Ca2+]i increases were prevented using the chelator EGTA (9 mM) in the recording pipette solution (not shown, but see responses to ATP with low Ca2+ buffering conditions, Figure 4). These results indicate that there is no residual P2X receptor mediated current in the MK in P2X1 −/− mice and demonstrates that the P2X1 receptor is essential for the expression of functional P2X receptor channels in this cell type.
Figure 3.
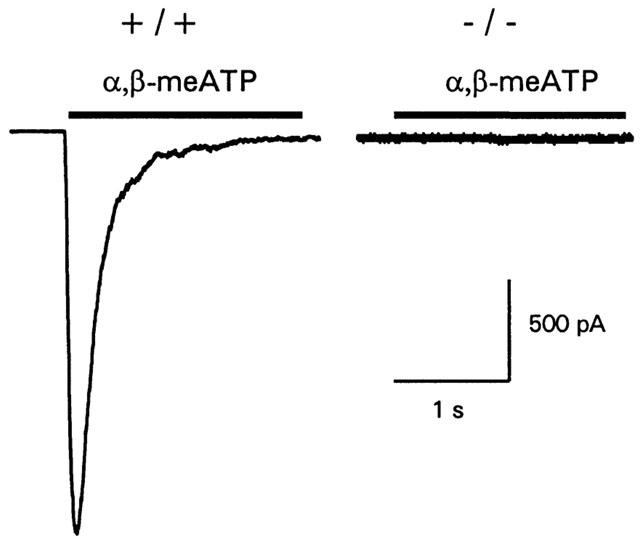
P2X receptor-mediated inward currents are absent in P2X1 receptor deficient mice MKs. α,β-meATP (10 μM) evoked rapid transient inward currents in +/+ MKs these were absent in MKs from P2X1 receptor deficient −/− mice. Bar indicates period of drug application.
Figure 4.
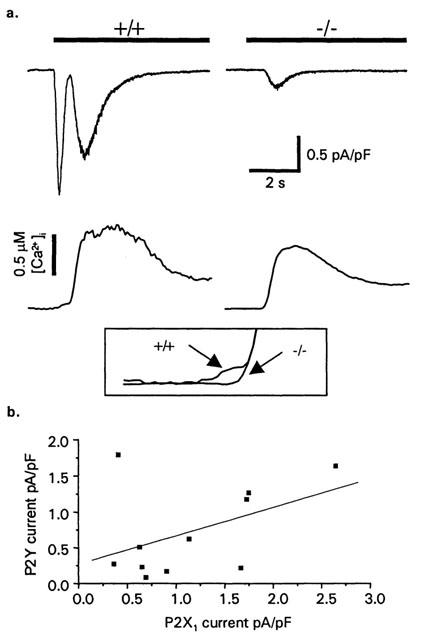
ATP-induced [Ca2+]i and inward current responses in murine megakaryocytes: effects of P2X1 receptor deficiency. (a) Whole-cell membrane currents (upper traces) and [Ca2+]i responses (lower traces) activated by ATP (30 μM, bar) in wild type (+/+) and P2X1 receptor-deficient (−/−) mice. Inset box shows the expanded region of the calcium trace showing the initial P2X1 receptor mediated increase in intracellular calcium in +/+ compared to P2X1 −/− MKs. Holding potential: −70 mV. Membrane currents have been normalized for whole-cell capacitance. (b) Correlation of the amplitude of P2X1 receptor and secondary currents recorded from mouse megakaryocytes.
At physiological levels of intracellular Ca2+ buffering, ATP application evokes a complex response consisting of multiple cationic currents and an increase in intracellular Ca2+ mediated by the activation of P2X and P2Y receptors (Uneyama et al., 1993; Somasundaram & Mahaut-Smith, 1994). The interaction of P2Y1 and P2YAC receptors is important in platelet activation (Jarvis et al., 2000), however the level of cross-talk between P2X receptors and P2Y receptors is unclear. The lack of functional P2X receptor-mediated responses in the P2X1 receptor deficient mouse allows assessment of P2X and P2Y receptor interaction in MKs. These experiments were conducted using Cs+-containing internal salines to fully resolve inward cationic currents including the P2X1 receptor current. In normal (+/+) mouse MKs, ATP (30 μM, a maximal concentration at P2X1 receptors (Valera et al., 1994) and P2Y receptors in MKs (Uneyama et al., 1993)), evoked a rapid initial transient P2X current (peak amplitude 1.31±0.32 pA/pF, n=11) and a slower P2Y receptor-coupled inward current (peak amplitude 0.62±0.17 pA/pF, n=11) (Figure 4a) as described in rat megakaryocytes (Somasundaram & Mahaut-Smith, 1994). The P2X receptor and slower P2Y receptor coupled currents from individual cells are shown in Figure 4b, the best fit correlation line is shown, however this is not significant (r=0.45, P=0.16). ATP application evoked a biphasic increase in intracellular Ca2+; an initial small but rapid rise (18±5 nM, n=12) associated with P2X receptor activation and a larger sustained P2Y receptor-mediated increase (peak increase 514±81 nM, n=12) (Figure 4).
In P2X1 receptor deficient mice, ATP failed to produce the fast transient inward P2X receptor current and intial increase in [Ca2+]i observed in normal MKs. In addition the amplitude of the slower secondary current was reduced significantly to 0.24±0.06 pA/pF (n=15; P<0.05). The amplitude of the ATP-evoked Ca2+ rise was not significantly affected by the P2X1 receptor deficiency (412±76 nM, n=13). Resting [Ca2+]i levels were slightly higher in P2X1 −/− megakaryocytes (169±25 nM, n=15) compared to +/+ mice (96±22 nM; n=15), however this difference was not significant. The above data suggest a role for interaction of P2X and P2Y receptors in the megakaryocytes by potentiation of the delayed inward current that is associated with P2Y1 receptor activation.
Interaction of P2X1 and P2Y receptors in the activation of human platelet [Ca2+]i responses
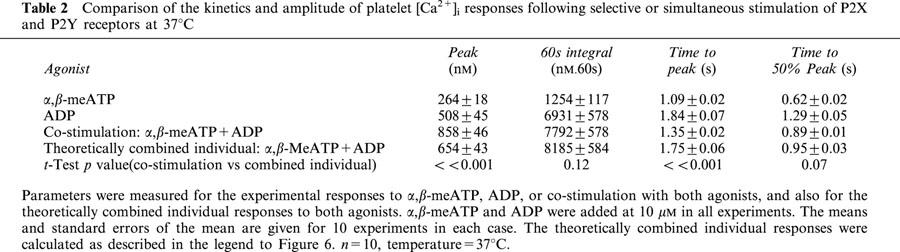
P2X1 receptors stimulate a much larger increase in [Ca2+]i in platelets compared to megakaryocytes (Rolf et al., 2001; Somasundaram & Mahaut-Smith, 1994), therefore we also investigated interactions between P2X1 and P2Y1 receptor Ca2+ signalling in human platelets using α,β-meATP and purified ADP to selectively activate ionotropic and metabotropic purinoceptors, respectively (Ennion et al., 2000). Initially, experiments were conducted at a reduced ambient temperature (13°C) in order to provide a clearer indication of the kinetics and interactions of P2X1 and P2Y1 receptor-evoked [Ca2+]i responses (see Mahaut-Smith et al., 2000). α,β-meATP (10 μM) evoked an immediate, transient increase in [Ca2+]i (Figure 5a). In contrast, the [Ca2+]i response to ADP (10 μM) had a characteristic lag, peaked at a higher level and remained elevated for longer periods. Average values for [Ca2+]i changes; the peak increase, integral over the first 60 s and two estimates of speed of the response, time to peak and time to 50% of the peak, are shown in Table 1. Co-application of α,β-meATP and ADP (both 10 μM) resulted in a marked acceleration of the peak response compared with ADP stimulation alone (Figure 5a; Table 1). Figure 5b compares the mathematically-derived combined [Ca2+]i response (dashed line) with the experimental effect of combined α,β-meATP and ADP stimulation. This demonstrates that the change in time-course of the peak response was not simply due to summation of the individual responses to the two agonists. The time to peak and time to 50% of the peak were both significantly reduced by co-stimulation (Table 1). However, there was no significant change in the peak Ca2+ response or the total Ca2+ increase, where the latter was measured as the integral of the Ca2+ response over the first 60 s (Table 1). At 37°C, the responses to ADP were considerably faster compared with 13°C, yet co-stimulation with α,β-MeATP still accelerated the overall response (Figure 6 and Table 2). The time to peak was significantly shorter following co-stimulation with α,β-MeATP and ADP compared to the combined individual responses. The time to 50% was slightly reduced for the co-stimulated compared to theoretically combined responses, however this difference was not significant. It is likely that the lack of significance results from the difficulty of accurately measuring responses with very short latencies. The integral of the Ca2+ response over the first 60 s remained unaltered by co-stimulation. Importantly, however, the peak [Ca2+]i increase was significantly increased by co-stimulation at 37°C. Taken together, these data suggest that platelet P2X1 and P2Y receptors display marked synergy by accelerating and amplifying the overall peak increase in [Ca2+]i.
Figure 5.
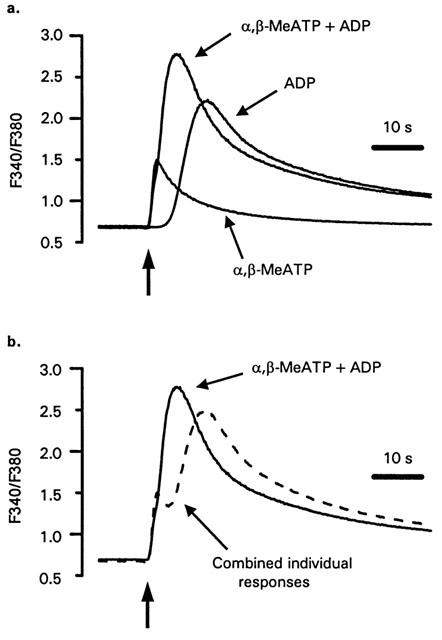
Low temperature studies of P2X and P2Y receptor interactions during platelet Ca2+ mobilization. (a) [Ca2+]i responses of human platelets in stirred suspension at 13°C to α,β-MeATP (10 μM), ADP (10 μM) or simultaneous addition of both agonists (10 μM). Background-corrected 340/380 nm ratios are used to indicate [Ca2+]i at the low temperature due to problems associated with accurately calibrating fura-2 fluorescence. (b) Comparison of the experimental trace following co-addition of α,β-MeATP and ADP with the result expected from summation of the responses to individual agonists (dashed line). The dashed line was derived by addition of the individual responses in panel a and subtraction of a F340/F380 nm ratio equal to the resting level prior to agonist stimulation. The vertical arrows indicate the point of agonist addition.
Table 1.
Comparison of the kinetics and amplitude of platelet [Ca2+]i responses following selective or simultaneous stimulation of P2X and P2Y receptors at 13°C
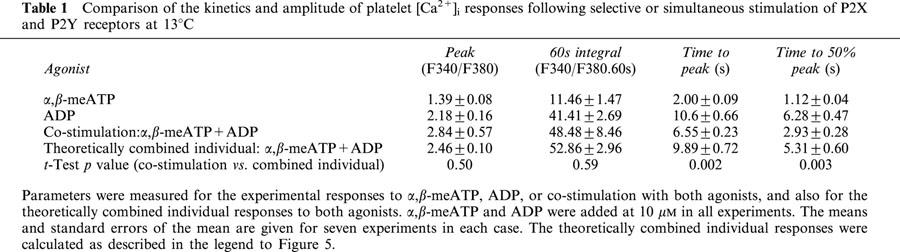
Figure 6.
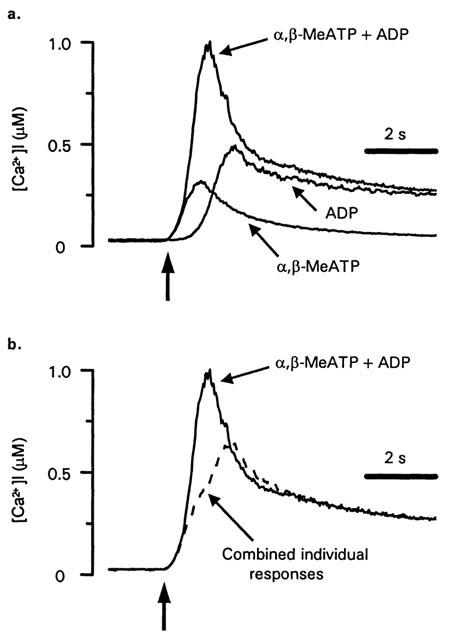
P2X and P2Y receptor interactions during platelet Ca2+ mobilization at 37°C. (a) [Ca2+]i responses of human platelets in stirred suspension at 37°C to α,β-MeATP (10 μM), ADP (10 μM) or simultaneous addition of both agonists (10 μM). (b) Comparison of the experimental trace following co-addition of α,β-MeATP and ADP with the result expected from summation of the responses to individual agonists (dashed line). The dashed line was derived by addition of the individual responses in panel a and subtraction of an [Ca2+]i equal to the resting level prior to agonist stimulation. The vertical arrows indicate the point of agonist addition.
Table 2.
Comparison of the kinetics and amplitude of platelet [Ca2+]i responses following selective or simultaneous stimulation of P2X and P2Y receptors at 37°C
Discussion
This study shows that the P2X1 receptor is expressed in MKs and is essential for the production of functional P2X receptors. The data also indicate that MK size or number is not affected by the P2X1 receptor deficiency demonstrating that these receptors do not play a central role in megakaryocytopoiesis. However, in electrophysiological and [Ca2+]i studies we have detected synergistic interactions between P2X and P2Y receptors in MKs and platelets and this may resolve a functional role for P2X1 receptors in these cells.
In bone marrow and spleen the co-expression of P2X1 and CD41 immunoreactivity in hematopoietic cells indicates significant expression of P2X1 receptors in MKs. This confirms previous studies showing that P2X1 receptors are expressed by megakaryoblastic cell lines and platelets (Mackenzie et al., 1996; Vial et al., 1997) and indicates that if P2X1 receptors are expressed by other hematopoietic cells in the marrow or spleen that they are below detection levels. In a variety of blood cell types changes in expression of P2X1 receptors have been associated with differentiation or apoptosis (Buell et al., 1996; Chvatchko et al., 1996; Clifford et al., 1998). The P2X1 receptor is expressed by MKs from 1 day old (the earliest time-point tested) as suggested by Clifford et al. (1998). The normal distribution of MKs in P2X1 receptor deficient mice demonstrates that the P2X1 receptor is not essential for MK development. In MKs derived from the long bones the level of P2X1 receptor immunoreactivity is maintained in the adult. In contrast the level of P2X1 receptor immunoreactivity declines with age in splenic MKs suggesting that in the adult the properties of long-bone and spleen derived MKs are different. The reason for this difference is unclear. We were unable to obtain high resistance giga-seals on adult splenic MKs due to what appeared to be a reduced membrane deformability compared to marrow megakaryocytes and thus could not further assess P2X1 current density in splenic MKs (R.J. Evans and M.P. Mahaut-Smith, unpublished observations). However, this property supports the conclusion that differences do exist between MKs from the two tissues.
The rapidly desensitizing α,β-meATP sensitive (and L-β,γ-methylene ATP sensitive, data not shown) P2X receptor mediated currents in the MKs bear the hallmark of the properties of recombinant P2X1 receptors (Valera et al., 1994) and are essentially the same as those recorded from platelets (Mackenzie et al., 1996). The lack of rapid P2X receptor mediated responses to α,β-meATP or ATP in MKs from P2X1 receptor deficient mice demonstrates that the P2X1 receptor is essential for the production of functional P2X receptors in MKs. The fact that there is no residual response indicates that MKs do not express other P2X receptor isoforms and confirms previous studies using a degenerate PCR screening approach which failed to detect RNA transcripts for P2X receptor isoforms other than the P2X1 subtype (Sun et al., 1998). It is therefore likely that MKs express homomeric P2X1 receptors, as shown in smooth muscle cells of the vas deferens and bladder (Mulryan et al., 2000; Vial & Evans, 2000).
A role for P2X1 receptors in platelets has been disputed. Two groups have reported a lack of P2X receptor-evoked shape change or aggregation (Jin & Kunapuli, 1998; Savi et al., 1998). This probably results from desensitization of the receptors during the isolation procedures. For example it has been shown that HL60 cells have to be treated with the P2 receptor antagonist suramin and/or apyrase (to breakdown endogenous ATP) to protect the cells from endogenously released ATP in culture to reveal the P2X1-like phenotype (Buell et al., 1996). In human platelets, using conditions designed to minimize desensitization, Rolf et al. (2001) have demonstrated a P2X receptor-mediated shape change. Therefore release of ATP and diadenosine polyphosphates during phlebotomy and/or from spontaneously activated platelets most likely accounts for the apparent difficulty in detecting a functional platelet response linked to the ionotropic purinoceptor (Rolf et al., 2001). Apyrase is required in vitro to observe a platelet P2X1 response, which may reflect the situation in vivo where ectonucleotidases metabolise ATP and thus limit P2X receptor desensitisation. A functional role of P2X1 receptors on platelets has been suggested by the identification of a patient with a severe bleeding disorder that has a point deletion mutation in the second transmembrane domain resulting in the production of a dominant negative mutant (Oury et al., 2000). Recent studies have shown that the P2X1 receptor deficiency in mice leads to a significant ∼70% increase in bleeding time (Hechler et al., 2001) similar to that for the P2Y1 receptor deficient mouse (Leon et al., 1999). These results indicate that the P2X1 receptor does play a role in the regulation of hemostasis.
P2X and P2Y receptors will normally be co-activated under physiological conditions, thus crosstalk between their signalling pathways may have potentially important consequences. P2X1 receptor activation will result in Na+ and Ca2+ influx and membrane depolarization (Evans et al., 1996; Mackenzie et al., 1996; Mulryan et al., 2000). Activation of metabotropic P2Y1 and P2TAC (P2Y12) receptors will lead to a combination of signals including IP3-mediated release of Ca2+ from intracellular stores, the stimulation of a slow secondary inward current, inhibition of adenylyl cyclase activity and activation of PI 3-kinase (Somasundaram & Mahaut-Smith, 1994; Kunapuli, 1998; Trumel et al., 1999). Phospholipase-C and IP3 receptor activity are known to be potentiated by an increase in [Ca2+]i and therefore P2X1 receptors could play a priming role in subsequent P2Y receptor responses (Bezprozvanny et al., 1991; Eberhard & Holz, 1988). To investigate interactions between P2X1 and P2Y receptor signalling we looked at agonist-induced membrane currents and [Ca2+]i increases in MKs and platelets. In MKs the amplitude of the secondary current was significantly reduced in MKs from P2X1−/− mice. These results suggest a functional interaction of signalling through P2X1 and P2Y receptors.
An increase in cytosolic levels of both IP3 and Ca2+ has been proposed to activate the P2Y receptor conductance (Hussain & Mahaut-Smith, 1998) therefore Ca2+ influx through P2X1 receptors (Benham, 1989; Evans et al., 1996) may explain the potentiation of the P2Y receptor current. Although the global Ca2+ increase mediated via P2X1 receptors in MKs was extremely small (Figure 4a), a larger Ca2+ increase will occur immediately under the plasma membrane compared to further into the cell, and thus could act locally to enhance the subsequent secondary current. The functional relevance of P2 receptor-evoked [Ca2+]i increases and associated membrane conductances in the MK are unknown. The P2Y receptor current carries Na+ into the cell under normal conditions, however the ability to conduct Ca2+ is unclear. The fact that megakaryocytopoiesis was unaltered in mice lacking P2X1 receptors suggests that the reduction in the delayed P2Y receptor activated current does not have a major influence on MK development. However the data from the MKs clearly indicate that P2X1 receptors can potentiate P2Y receptor signalling and this may be functionally more important when when P2X1 receptor-evoked [Ca2+]i responses are larger.
The rapid influx of Ca2+ through P2X1 receptors causes a larger increase in [Ca2+]i in platelets compared to megakaryocytes, presumably due to the larger surface area to volume ratio of the former (Mackenzie et al., 1996; Somasundaram & Mahaut-Smith, 1994). In studies of human platelet [Ca2+]i signalling, a significant interaction was observed between P2X1 and P2Y receptors (Figures 5,6 and Tables 1,2). Co-activation of P2X1 and P2Y receptors caused both an acceleration and an enhancement of the [Ca2+]i increase compared with the result predicted from summation of the individual responses. Potentiation of P2Y1 receptor-stimulated phospholipase-C or IP3 receptor activity by P2X1 receptor-derived intracellular Ca2+ can explain the synergistic action between the two receptors (Bezprozvanny et al., 1991; Eberhard & Holz, 1988). Intracellular Na+ also increases in platelets following stimulation of P2X receptors (Sage et al., 1997), although there is less direct evidence for a Na-dependence to metabotropic receptor-dependent Ca2+ signalling. However, in the platelet suspension experiments P2X1 receptors will depolarize the cell which has been shown to stimulate Ca2+ release during P2Y receptor activation in the megakaryocyte (Mahaut-Smith et al., 1999). The ability of P2X receptors to potentiate metabotropic receptor events and thus accelerate platelet activation may play a part in determining the overall speed of the hemostatic response. Alternatively, this synergistic action of P2X1 receptors may make platelets more prone to unwanted activation during thrombosis.
In conclusion, our results in MKs demonstrate that P2X1 receptors mediate the initial transient inward current and Ca2+ influx triggered by ATP, but that this receptor is not essential for normal megakaryocytopoiesis. We also show a synergistic interaction between P2X1 and P2Y receptors in both megakaryocytes and platelets which suggests that P2X1 receptors could have a priming role in the activation of P2Y receptors. Since large amounts of ATP will be rapidly released from damaged cells at the site of vascular injury, P2X1 receptors may therefore act to accelerate platelet activation during hemostasis or thrombosis.
Acknowledgments
This work was conducted in the laboratories of R.J. Evans and M.P. Mahaut-Smith and was supported by the Wellcome Trust and British Heart Foundation. M.G. Rolf is the recipient of a BHF Studentship (FS/97052) and M.P. Mahaut-Smith the recipient of a BHF Science Lectureship (BS/10).
Abbreviations
- α,β-meATP
α,β-methylene ATP
- MKs
megakaryocytes
- CD41
glycoprotein-IIb
References
- BENHAM C.D. ATP-activated channels gate calcium entry in single smooth muscle cells from rabbit ear artery. J. Physiol. 1989;419:689–701. doi: 10.1113/jphysiol.1989.sp017893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENHAM C.D., TSIEN R.W. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. doi: 10.1038/328275a0. [DOI] [PubMed] [Google Scholar]
- BEZPROZVANNY I., WATRAS J., EHRLICH B.E. Bell-shaped calcium response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- BUELL G., MICHEL A., LEWIS C., COLLO G., HUMPHREY P.P.A., SURPRENANT A. P2X1 receptor activation in HL60 cells. Blood. 1996;7:2659–2664. [PubMed] [Google Scholar]
- BURNSTOCK G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacol. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- CHOPRA B., GIBLETT S., LITTLE J.G., DONALDSON L.F., TATE S., EVANS R.J., GRUBB B.D. Cyclooxygenase-1 is a marker for a subpopulation of putative nociceptive neurons in rat dorsal root ganglia. Eur. J. Neurosci. 2000;12:911–920. doi: 10.1046/j.1460-9568.2000.00979.x. [DOI] [PubMed] [Google Scholar]
- CHVATCHKO Y., VALERA S., AUBRY J.P., RENNO T., BUELL G., BONNEFOY J.Y. The involvement of an ATP-gated ion channel, P2X1 in thymocyte apoptosis. Immunity. 1996;5:275–283. doi: 10.1016/s1074-7613(00)80322-2. [DOI] [PubMed] [Google Scholar]
- CLIFFORD E.E., PARKER K., HUMPHREYS B.D., KERTSEY S.B., DUBYAK G.R. The P2X1 receptor, an adenosine triphosphate-gated cation channel, is expressed in human platelets but not in human blood leucocytes. Blood. 1998;91:3172–3181. [PubMed] [Google Scholar]
- COLLO G., NORTH R.A., KAWASHIMA E., MERLO-PICH E., NEIDHART S., SURPRENANT A., BUELL G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. J. Neurosci. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS R.E., STENBERG P.E., LEVIN J., BECKSTEAD J.H. Localization of megakaryocytes in normal mice following administration of platelet antiserum, 5-fluorouracil, or radiostrontium: evidence for the site of platelet production. Exp. Haematol. 1997;25:638–648. [PubMed] [Google Scholar]
- EBERHARD D.A., HOLZ R.W. Intracellular Ca2+ activates phospholipase C. Trends Neurosci. 1988;12:517–520. doi: 10.1016/0166-2236(88)90174-9. [DOI] [PubMed] [Google Scholar]
- ENNION S., HAGAN S., EVANS R.J. The role of positively charged amino acids in ATP recognition by human P2X1 receptors. J. Biol. Chem. 2000;275:29361–29367. doi: 10.1074/jbc.M003637200. [DOI] [PubMed] [Google Scholar]
- EVANS R.J., LEWIS C., BUELL G., VALERA S., NORTH R.A., SURPRENANT A. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2X purinoceptors) Mol. Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- EVANS R.J., LEWIS C., VIRGINIO C., LUNDSTROM K., BUELL G., SURPRENANT A., NORTH R.A. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J. Physiol. 1996;497:413–422. doi: 10.1113/jphysiol.1996.sp021777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FABRE J.E., NGUYEN M., LATOUR A., KEIFFER J.A., AUDOLY L.P., COFFMAN T.M., KOLLER B.H. Decreased platelet aggregation, increased bleeding time and resistance to thromboembolism in P2Y1-deficient mice. Nature Medicine. 1999;5:1199–1202. doi: 10.1038/13522. [DOI] [PubMed] [Google Scholar]
- GRECO N.J., TONON G., CHEN W., LUO X., DALAL R., JAMIESON G.A. Novel structurally altered P2X1 receptor is preferentially activated by adenosine diphosphate in platelets and megakaryocytic cells. Blood. 2001;98:100–107. doi: 10.1182/blood.v98.1.100. [DOI] [PubMed] [Google Scholar]
- HECHLER B., LEON C., VIAL C., VIGNE P., FRELIN C., CAZENAVE J.P., GACHET C. The P2Y1 receptor is necessary for adenosine 5′-diphosphate -induced platelet aggregation. Blood. 1998;92:152–159. [PubMed] [Google Scholar]
- HECHLER B., VIAL C., FREUND M., CAZENAVE J.P., EVANS R.J., GACHET C.A role of the platelet P2X1 receptor ion channel in collagen-induced platelet activation. Studies in P2X1 knock-out mice Blood 2001. American Society of Haematology abstractin press
- HOLLOPETER G., JANTZEN H.-M., VINCENT D., LI G., ENGLAND L., RAMAKRISHNAN V., YANG R.-Y., NURDEN P., NURDEN A., JULIUS D., CONLEY P.B. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- HOURANI S.M.O. Pharmacology of the platelet ADP receptors: agonists and antagonists. Haematologica. 2000;85:58–65. [Google Scholar]
- HUSSAIN J.F., MAHAUT-SMITH M.P. ADP and inositol trisphosphate evoke oscillations of a monovalent cation conductance in rat megakaryocytes. J. Physiol. 1998;511:791–801. doi: 10.1111/j.1469-7793.1998.791bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARVIS G.E., HUMPHRIES R.G., ROBERTSON M.J., LEFF P. ADP can induce aggregation of human platelets via both P2Y1 and P2T receptors. Br. J. Pharmacol. 2000;129:275–282. doi: 10.1038/sj.bjp.0703046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN J., KUNAPULI S.P. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUSHANSKY K. The enigmatic megakaryocyte gradually reveals its secrets. Bioessays. 1999;21:353–360. doi: 10.1002/(SICI)1521-1878(199904)21:4<353::AID-BIES12>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- KING B.F., TOWNSEND-NICHOLSON A., WILDMAN S.S., THOMAS T., SPYER K.M., BURNSTOCK G. Coexpression of rat P2X2 and P2X6 subunits in Xenopus oocytes. J. Neurosci. 2000;20:4871–4877. doi: 10.1523/JNEUROSCI.20-13-04871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNAPULI S.P. Multiple P2 receptor subtypes on platelets: a new interpretation of their function. Trends Pharmacol. Sci. 1998;19:391–394. doi: 10.1016/s0165-6147(98)01248-6. [DOI] [PubMed] [Google Scholar]
- LE K.-T., BABINSKI K., SEGUELA P. Central P2X4 and P2X6 channel subunits coassemble into a novel heteromeric ATP receptor. J. Neurosci. 1998;18:7152–7159. doi: 10.1523/JNEUROSCI.18-18-07152.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE K.-T., BOUE-GRABOT E., ARCHAMBAULT V., SEGUELA P. Functional and biochemical evidence for heteromeric ATP-gated channels composed of P2X1 and P2X5 subunits. J. Biol. Chem. 1999;274:15415–15419. doi: 10.1074/jbc.274.22.15415. [DOI] [PubMed] [Google Scholar]
- LEON C., HECHLER B., FREUND M., ECKLY A., VIAL C., OHLMANN P., DIERICH A., LEMEUR M., CAZENAVE J.-P., GACHET C. Defective platelet aggregation and increased resistance to thrombosis in purinergic P2Y1 receptor-null mice. J. Clin.Invest. 1999;104:1731–1737. doi: 10.1172/JCI8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEON C., HECHLER B., VIAL C., LERAY C., CAZENAVE J.-P., GACHET C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Letters. 1997;403:26–30. doi: 10.1016/s0014-5793(97)00022-7. [DOI] [PubMed] [Google Scholar]
- LEVINE R.F., HAZZARD K.C., LAMBERG J.D. The significance of megakaryocyte size. Blood. 1982;60:1122–1131. [PubMed] [Google Scholar]
- LEWIS C., NEIDHART S., HOLY C., NORTH R.A., BUELL G., SURPRENANT A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- MACKENZIE A.B., MAHAUT-SMITH M.P., SAGE S.O. Activation of receptor-operated cation channels via P2X1 not P2T purinoceptors in human platelets. J. Biol. Chem. 1996;271:2879–2881. doi: 10.1074/jbc.271.6.2879. [DOI] [PubMed] [Google Scholar]
- MAHAUT-SMITH M.P., ENNION S.J., ROLF M.G., EVANS R.J. ADP is not an agonist at P2X1 receptors: evidence for separate receptors stimulated by ATP and ADP on human platelets. Br. J. Pharmacol. 2000;131:108–114. doi: 10.1038/sj.bjp.0703517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHAUT-SMITH M.P., HUSSAIN J.F., MASON M.J. Depolarisation-evoked Ca2+ release in a non-excitable cell, the rat megakaryocyte. J. Physiol. 1999;515:385–390. doi: 10.1111/j.1469-7793.1999.385ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHAUT-SMITH M.P., SAGE S.O., RINK T.J. Rapid ADP-evoked currents in human platelets recorded with the nystatin permeabilized patch technique. J. Biol. Chem. 1992;267:3060–3065. [PubMed] [Google Scholar]
- MULRYAN K., GITTERMAN D.P., LEWIS C.J., VIAL C., LECKIE B.J., COBB A.L., BROWN J.E., CONLEY E.C., BUELL G., PRITCHARD C.A., EVANS R.J. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- NICKE A., BAUMERT H., RETTINGER J., EICHELE A., LAMBRECHT G., MUTSCHLER E., SCHMALZING G. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OURY C., TOTH-ZSAMBOKI E., VAN GEET C., THYS C., WEI L., NILIUS B., VERMYLEN J., HOYLAERTS M.F. A natural dominant negative P2X1 receptor due to deletion of a single amino acid residue. J. Biol. Chem. 2000;275:22611–22614. doi: 10.1074/jbc.C000305200. [DOI] [PubMed] [Google Scholar]
- PALMER R.K., BOYER J.L., SCHACHTER J.B., NICHOLAS R.A., HARDEN T.K. Agonist action of adenosine triphosphate at the human P2Y1 receptor. Mol. Pharmacol. 1998;54:1118–1123. [PubMed] [Google Scholar]
- PAUL B.Z.S., DANIEL J.L., KUNAPULI S.P. Platelet shape change is mediated by both calcium-dependent and -independent signalling pathways; role of p160 Rho associated coiled-coil-controlling protein kinase in platelet shape change. J. Biol. Chem. 1999;274:28293–28300. doi: 10.1074/jbc.274.40.28293. [DOI] [PubMed] [Google Scholar]
- ROLF M.G., BREARLEY C.A., MAHAUT-SMITH M.P. Platelet shape change evoked by selective activation of P2X1 purinoceptors with α,β-methylene ATP. Thromb. Haemost. 2001;85:303–308. [PubMed] [Google Scholar]
- SAGE S.O., MACKENZIE A.B., JENNER S., MAHAUT-SMITH M.P. Purinoceptor-evoked calcium signalling in human platelets. Prostaglandins, Leukotrienes and Essential Fatty Acids. 1997;57:435–438. doi: 10.1016/s0952-3278(97)90424-5. [DOI] [PubMed] [Google Scholar]
- SAGE S.O., YAMOAH E.H., HEEMSKERK J.W.M. The roles of P2X1 and P2TAC receptors in ADP-evoked calcium signalling in human platelets. Cell Calcium. 2000;28:119–126. doi: 10.1054/ceca.2000.0139. [DOI] [PubMed] [Google Scholar]
- SAVI P., BEAUVERGER P., LABOURET C., DELFAUD M., SALEL V., KAGHAD M., HERBERT J.M. Role of P2Y1 purinoceptor in ADP-induced platelet activation. FEBS Lett. 1998;422:291–295. doi: 10.1016/s0014-5793(98)00025-8. [DOI] [PubMed] [Google Scholar]
- SCASE T.J., HEATH M.F., ALLEN J.M., SAGE S.O., EVANS R.J. Identification of a P2X1 receptor expressed on human platelets. Biochem. Biophys. Res. Comm. 1998;242:525–528. doi: 10.1006/bbrc.1997.8001. [DOI] [PubMed] [Google Scholar]
- SOMASUNDARAM B., MAHAUT-SMITH M.P. Three cation influx currents activated by purinergic receptor stimulation in rat megakaryocytes. J. Physiol. 1994;480:225–231. doi: 10.1113/jphysiol.1994.sp020355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN B., LI J., OKAHARA K., KAMBAYASHI J. P2X1 purinoceptor in human platelets. J. Biol. Chem. 1998;273:11544–11547. doi: 10.1074/jbc.273.19.11544. [DOI] [PubMed] [Google Scholar]
- SURPRENANT A., BUELL G., NORTH R.A. P2X receptors bring new structure to ligand-gated ion channels. Trends Neurosci. 1995;18:224–229. doi: 10.1016/0166-2236(95)93907-f. [DOI] [PubMed] [Google Scholar]
- TORRES G.E., HAINES W.R., EGAN T.M., VOIGT M.M. Co-expression of P2X1 and P2X5 receptor subunits reveals a novel ATP-gated ion channel. Mol. Pharmacol. 1998;54:989–993. doi: 10.1124/mol.54.6.989. [DOI] [PubMed] [Google Scholar]
- TRUMEL C., PAYRASTRE B., PLANTAVID M., HECHLER B., VIALA C., PRESEK P., MARTINSON E.A., CAZENAVE J.P., CHAP H., GACHET C. A key role of adenosine diphosphate in the irreversible platelet aggregation induced by PAR-1 activating peptide through the late activation of phosphoinositide-3-kinase. Blood. 1999;94:4156–4165. [PubMed] [Google Scholar]
- UNEYAMA C., UNEYAMA H., AKAIKE N. Cytoplasmic Ca2+ oscillation in rat megakaryocytes evoked by a novel type of purinoceptor. J. Physiol. 1993;470:731–749. doi: 10.1113/jphysiol.1993.sp019885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UZAN G., PRENANT M., PRANDINI M.-H., MARTIN F., MARGUERIE G. Tissue-specific expression of the platelet GPIIb gene. J. Biol. Chem. 1991;266:8932–8939. [PubMed] [Google Scholar]
- VALERA S., HUSSY N., EVANS R.J., ADAMI N., NORTH R.A., SURPRENANT A., BUELL G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- VIAL C., EVANS R.J. P2X receptor expression in mouse urnary bladder and the requirement of P2X1 receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br. J. Pharmacol. 2000;131:1489–1495. doi: 10.1038/sj.bjp.0703720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIAL C., HECHLER B., LEON C., CAZENAVE J.-P., GACHET C. Presence of P2X1 purinoceptors in human platelets and megakaryoblastic cell lines. Thromb. Haemost. 1997;78:1500–1504. [PubMed] [Google Scholar]
- VULCHANOVA L., ARVIDSSON U., RIEDL M., BUELL G., SURPRENANT A., NORTH R.A., ELDE R.P. Differential distribution of two ATP-gated ion channels (P2X receptors) determined by immunohistochemistry. Proc. Natl. Acad. Sci. USA. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDMAN S.S., BROWN S.G., KING B.F., BURNSTOCK G. Selectivity of diadenosine polyphosphates for rat P2X receptor subunits. Eur. J. Pharmacol. 1999;367:119–123. doi: 10.1016/s0014-2999(98)00976-5. [DOI] [PubMed] [Google Scholar]


