Abstract
Constitutive activity of the recombinant and native rat and human H3 receptors (H3Rs) was studied using H3R-mediated [35S]GTPγ[S] binding and [3H]-arachidonic acid release.
Ciproxifan, an inverse agonist at the rat H3R (rH3R), decreased [3H]arachidonic acid release from CHO cells expressing moderate densities (∼200–300 fmol mg−1 protein) of the human H3R (hH3R). This effect occurred with the same magnitude than at the rH3R.
The expression of the hH3R was associated with an increase in [35S]GTPγ[S] binding to membranes of CHO cells. Ciproxifan decreased [35S]GTPγ[S] binding to membranes of CHO (hH3R) cells. Both effects were correlated to receptor density and revealed that constitutive activity of the hH3R, although lower than that of the rH3R in this assay, was again observed at physiological densities (<500 fmol mg−1 protein). Ciproxifan was less potent at the human than the rat receptor, not only as an antagonist (Ki=45 nM), but also as an inverse agonist (EC50=15 nM).
Constitutive activity of the hH3R was also evidenced using inhibition of [35S]GTPγ[S] binding by unlabelled GTPγS. The expression of the hH3R generated a high affinity binding for GTPγS which was increased by imetit, but partially decreased by ciproxifan, therefore acting as a partial inverse agonist.
[35S]GTPγ[S] binding to rat brain membranes was decreased in several regions by thioperamide, ciproxifan and FUB 465, three inverse agonists at the H3R, whose effects were blocked by proxyfan, a neutral antagonist. [35S]GTPγ[S] binding was also decreased by an A1-adenosine receptor inverse agonist, but remained unchanged in the presence of inverse agonists at D2/D3 dopamine, H1 and H2 histamine, α2-adrenergic and δ opioid receptors.
In conclusion, the present study shows that the recombinant rat and human H3 receptors expressed at physiological densities display constitutive activity and suggests that constitutive activity of native H3Rs is one of the highest among G-protein-coupled receptors present in rat brain.
Keywords: Histamine, H3 receptor, G-protein-coupled receptors, recombinant receptors, native receptors, rat brain, [35S]GTPγ[S] binding, constitutive activity, ciproxifan
Introduction
The histamine H3 receptor (H3R) was initially characterized as an autoreceptor regulating histamine release in brain (Arrang et al., 1987; 1988). Subsequently, it has been shown to modulate the release of other monoamines, glutamate, GABA and tachykinins in brain or peripheral tissues (Schlicker et al., 1994; Hill et al., 1997; Brown et al., 2001). The sensitivity of agonist binding and various H3R-mediated responses to guanylnucleotides and pertussis toxin suggested that it was a Gi/o protein-coupled heptahelical receptor (Clark & Hill, 1996; Takeshita et al., 1998). This proposal was confirmed with the recent cloning of H3R cDNAs from human (Lovenberg et al., 1999), guinea-pig (Tardivel-Lacombe et al., 2000) and rat (Morisset et al., 2000; Lovenberg et al., 2000; Drutel et al., 2001).
Data accumulated over the last years strongly suggested that G-protein-coupled receptors (GPCRs) could be spontaneously active even in the absence of an agonist. This constitutive activity was mainly evidenced for recombinant receptors overexpressed and/or mutated (Lefkowitz et al., 1993; Milligan et al., 1995). However, indirect indications suggested that it could occur for native receptors endogenously expressed in cells or tissues (de ligt et al., 2000). Consistent with the physiological relevance of the phenomenon, we recently demonstrated high constitutive activity of native rat H3Rs (rH3Rs) (Morisset et al., 2000). Using functional assays, we showed that several prototypic antagonists such as thioperamide or ciproxifan (Arrang et al., 1987; Ligneau et al., 1998) were, in fact, acting as inverse agonists not only at recombinant rH3Rs but also at H3Rs present in rodent brain. Moreover we showed that constitutive activity of H3 autoreceptors regulated histamine neurons (Morisset et al., 2000). In the present study, we have assessed whether the recombinant human H3R (hH3R) also displays the constitutive activity that we previously detected in rodents. We have also analysed constitutive activity of the native H3R in various rat brain regions using three inverse agonists.
Methods
Cloning of the rH3R and hH3R cDNAs
The rH3R and hH3R were cloned as described (Ligneau et al., 2000). Briefly, a rat striatal cDNA library was screened with a cDNA fragment (third transmembrane domain/third intracellular loop) amplified from rat cerebral cortex using primers based on the sequence of the hH3R (Lovenberg et al., 1999). Several clones exhibited a full-length open reading frame encoding a 445-amino acid protein corresponding to the rH3R (Morisset et al., 2000). A human striatum cDNA library was screened with the same probe. One clone exhibited a full-length cDNA sequence corresponding to the hH3R (Lovenberg et al., 1999).
Stable transfection of CHO-K1 cells
cDNA inserts corresponding to the full-length coding sequences of the rH3R and hH3R, were ligated into the mammalian expression vector pCIneo (Promega, Charbonnières, France). CHO-K1 cells were transfected using SuperFect (Qiagen, Courtaboeuf, France). Stable transfectants were selected with 2 mg ml−1 of G418 and tested for [125I]-iodoproxyfan binding (Ligneau et al., 1994). Several clones, named CHO(rH3R) and CHO(hH3R), expressing various receptor densities, were selected for further characterization and maintained in presence of 1 mg ml−1 of G418. Histamine levels present in the cell culture media were determined using an enzymoimmunoassay (Beckman Coulter, Roissy, France).
[125I]-iodoproxyfan binding assay
CHO(rH3R or hH3R) cells were washed and homogenized with a Polytron in ice-cold binding buffer (Na2HPO4/KH2PO4 50 mM, pH 6.8) and assays performed as described (Ligneau et al., 1994).
[35S]GTPγ[S] binding assay
[35S]GTPγ[S] binding assays were performed according to Clark & Hill (1996) with slight modifications. Brain tissues from male Wistar rats (160–200 g, Iffa-Credo, L'Arbresle, France) and CHO(rH3R or hH3R) cells were homogenized in ice-cold buffer (Tris HCl 50 mM, pH 7.4). Homogenates were centrifuged twice at 20,000×g for 10 min and the final pellet was resuspended in 50 volumes of buffer. Membranes (20–50 μg) were pretreated with adenosine deaminase (1 U ml−1 Roche, Meylan, France) and incubated for 60 min at 25°C with 0.1 nM [35S]-GTPγ[S] and, when required, the various drugs tested, in 1 ml of assay buffer (50 mM Tris HCl, 50 mM NaCl, 5 mM MgCl2, 10 μM GDP, 0.02% bovine serum albumin (BSA) pH 7.4). The nonspecific binding was determined using GTPγS (10 μM). Incubations were stopped by rapid filtration under vacuum through Whatman GF/B filters. Filters were washed twice with 4 ml ice-cold water and the radioactivity retained on the filters was measured by liquid scintillation spectrometry.
[3H]-arachidonic acid release
CHO (rH3R or hH3R) cells were incubated for 2 h at 37°C with 0.5 μCi of [3H]-arachidonic acid in DMEM-Nut mix F12 (Life Technologies, Cergy-Pontoise, France) containing 0.2% BSA. After washing, cells were incubated for 30 min with 2 μM A23187 (Roche) and, when required, the H3-receptor ligands and [3H]-arachidonic acid release was determined by liquid scintillation counting.
Analysis of data
For determination of EC50 and IC50 values of imetit and ciproxifan on [35S]GTPγ[S] binding, the total curves were analysed with an iterative least-squares method derived from that of Parker & Waud (1971). The Ki value of ciproxifan acting as an antagonist was calculated from its IC50 value, assuming a competitive antagonism and by using the relationship (Cheng & Prussoff, 1973):
where S represents the concentration of imetit and EC50 the imetit concentration required for a half-maximal stimulation of [35S]GTPγ[S] binding. Protein contents were determined according to the method of Lowry et al. (1951), using BSA as the standard. Statistical evaluation of the results was performed by ANOVA followed by Newman–Keuls test.
Radiochemicals and drugs
[125I]-lodoproxyfan (2000 Ci mmol−1) was prepared as described (Krause et al., 1997). [35S]GTPγ[S] (1250 Ci mmol−1) was from NEN Life Science (Zaventem, Belgium). Ciproxifan and thioperamide were from Bioprojet (Paris, France). FUB 465 (ethyl-3-(1H-imidazol-4-yl)propyl ether) and proxyfan (3-(1H-imidazol-4-yl)propylphenylmethyl ether) were provided by Pr Schunack (Freie Universität Berlin, Germany). Imetit was provided by Pr Ganellin (University College, London, U.K.). Mepyramine was from Specia (Paris, France) and cimetidine from Smith Kline Beecham (London, U.K.). ICI-174,864 was obtained from Fisher Bioblock Scientific (Illkirch, France). Yohimbine, haloperidol and CPDPX (8-cyclopentyl-1,3-dipropylxanthine) were from Sigma (St-Quentin-Fallavier, France). All other chemicals were obtained from commercial sources and were of the highest purity available.
Results
Effects of H3-receptor ligands on two responses mediated by the recombinant rat or human H3 receptor expressed in CHO cells
The basal specific [35S]GTPγ[S] binding to membranes of wild type CHO cells incubated with 0.1 nM [35S]GTPγ[S] represented 22.2±0.3 fmol mg−1 protein. It was significantly (P<0.001) increased to membranes of CHO cells expressing ∼200–300 fmol mg−1 protein of rat or human H3R (39.5±1.1 and 26.7±0.1 fmol mg−1 protein, respectively). Imetit, a selective H3-receptor agonist (Garbarg et al., 1992) used at a maximal concentration (1 μM), induced a similar increase (by ∼100%) of [35S]GTPγ[S] binding to membranes of CHO(rH3R) and CHO(hH3R) cells which represented 71.2±1.3 and 54.4±0.5 fmol mg−1 protein, respectively (Figure 1A). In contrast, ciproxifan, a H3-receptor inverse agonist (Morisset et al., 2000), significantly decreased [35S]GTPγ[S] binding to membranes of CHO(rH3R) and CHO(hH3R) cells (by 44 and 15%, respectively). Both compounds did not modify specific [35S]GTPγ[S] binding to membranes of wild type CHO cells (Figure 1A).
Figure 1.
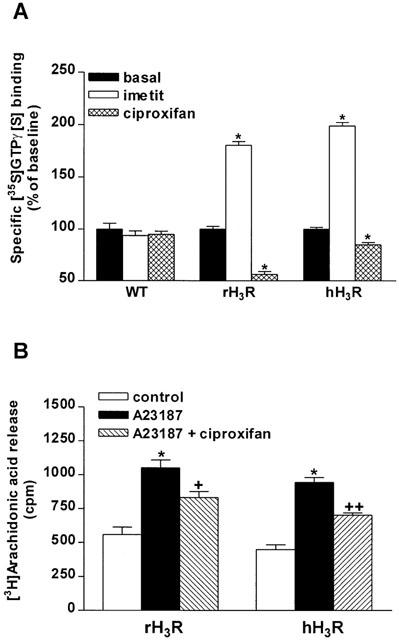
Effects of H3-receptor ligands on two responses mediated by the recombinant rat and human H3 receptors. (A) Effects of H3-receptor ligands on specific [35S]GTPγ[S] binding. Membranes of wild-type CHO cells (WT) or cells expressing ∼200–300 fmol mg−1 protein of rat (rH3R) or human (hH3R) receptors were incubated with 0.1 nM [35S]GTPγ[S] and, when required, 1 μM imetit or ciproxifan. Data represent means±s.e.mean of 11–16 determinations from two separate experiments. *P<0.001 vs the corresponding basal. (B) Effect of ciproxifan on A23187-evoked [3H]-arachidonic acid release. After incubation with 0.5 μCi of [3H]-arachidonic acid, CHO(rH3R) and CHO(hH3R) cells were incubated with 2 μM A23187 and, when required, 1 μM ciproxifan. Data are means±s.e.m. of 11–32 determinations from three to four experiments. *P<0.001 vs the corresponding control; +P<0.01, ++P<0.001 vs A23187.
Ciproxifan (1 μM) decreased significantly, and with a similar amplitude (by 44 and 49%, respectively), [3H]-arachidonic acid release evoked by the Ca2+-ionophore A23187 from CHO(rH3R) and CHO(hH3R) cells without affecting [3H]-arachidonic acid release alone (Figure 1B). All these effects were observed in total absence of histamine in the culture medium.
Effects of H3-receptor ligands on specific [35S]GTPγ[S] binding to membranes of CHO(hH3R) cells
Changes in the effect of imetit and ciproxifan associated with receptor expression were assessed on specific [35S]GTPγ[S] binding to membranes of CHO(hH3R) cells expressing increasing receptor densities. Following incubation with 0.1 nM [35S]GTPγ[S], the basal specific [35S]GTPγ[S] binding itself remained unchanged to membranes of cells expressing 100 fmol mg−1 protein (20.4±1.2 fmol mg−1 protein) and then progressively increased (from a receptor density of 170 fmol mg−1 protein) to reach 41.3±1.4 and 44.0±2.0 fmol mg−1 protein at a receptor density of ∼700 fmol mg−1 protein and 1 pmol mg−1 protein, respectively.
In the presence of imetit (1 μM), the basal [35S]GTPγ[S] binding significantly increased from a receptor density of 170 fmol mg−1 protein to reach 92.1±4.5 fmol mg−1 protein (+120±4%) and 124.4±3.9 fmol mg−1 protein (+183±6%) in membranes of cells expressing ∼700 fmol mg−1 protein and 1 pmol mg−1 protein, respectively (Figure 2). The decrease in basal binding induced by the inverse agonist ciproxifan (1 μM) was observed in membranes of cells expressing 250–400 fmol mg−1 protein of the hH3R and reached ∼20% at a density of 1 pmol mg−1 protein (Figure 2).
Figure 2.
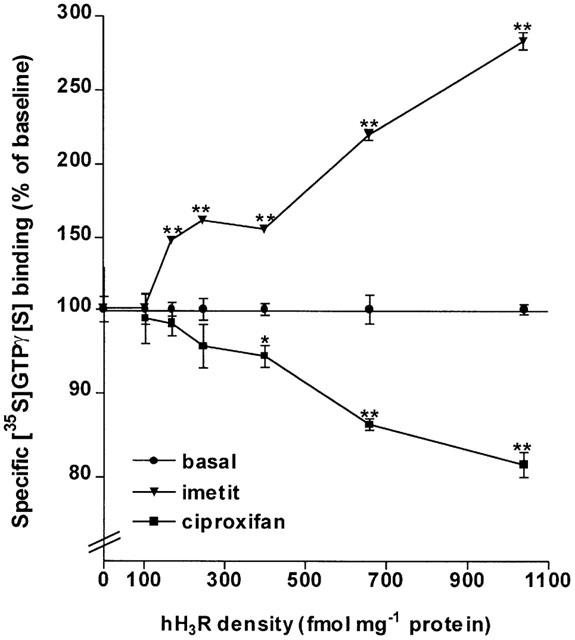
Effects of H3-receptor ligands on specific [35S]GTPγ[S] binding to membranes from CHO cells expressing various densities of the human H3 receptor (hH3R). Membranes of CHO(hH3R) cells expressing increasing densities of the human receptor (up to 1 pmol mg−1 protein) were incubated with 0.1 nM [35S]GTPγ[S] and, when required, imetit or ciproxifan (1 μM). hH3R densities were determined using [125I]-iodoproxyfan assay. Data represent means±s.e.mean of 8–14 determinations from two separate experiments. *P<0.01 and **P<0.001 vs the corresponding basal.
The effects of imetit and ciproxifan on specific [35S]GTPγ[S] binding to membranes of CHO(hH3R) cells expressing 400 fmol mg−1 protein were concentration dependent and occurred with EC50 values of 2.2±0.4 nM and 15.2±2.0 nM, respectively (Figure 3). In addition, ciproxifan progressively inhibited the effect of imetit (30 nM) with an IC50 value of 654±74 nM leading (Cheng & Prussoff, 1973) to a Ki value of 45±14 nM for the drug tested as an antagonist. At the highest concentrations tested against imetit, ciproxifan tended to decrease [35S]GTPγ[S] binding and the amplitude of this decrease was similar to that observed when the drug was added alone (Figure 3).
Figure 3.
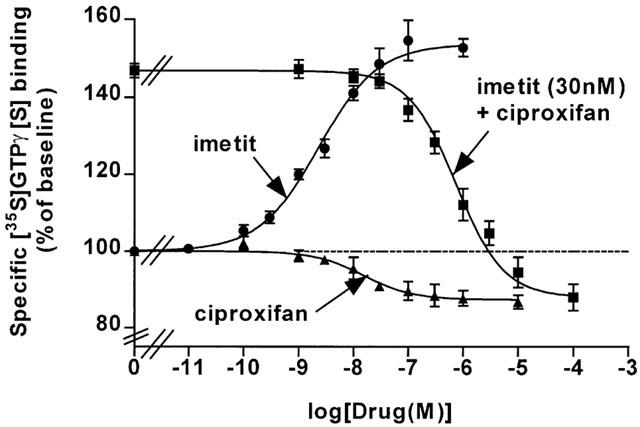
Effects of H3-receptor ligands on specific [35S]GTPγ[S] binding to membranes of CHO (hH3R) cells expressing 400 fmol mg−1 protein. Membranes were incubated with 0.1 nM [35S]GTPγ[S] in the presence, when required, of increasing concentrations of imetit, and ciproxifan alone or in the presence of 30 nM imetit. Means±s.e.mean of 7–16 determinations from two separate experiments.
The inhibitition curve of GTPγS on [35S]GTPγ[S] binding to membranes of CHO(WT) cells was found to be shallow, its pseudo-Hill coefficient calculated in a one-site model being nH=0.54±0.03. Nonlinear regression revealed that a two-site model analysis best accounted than a one-site model for the inhibition curve (c=0.9982 and 0.9684; χ2=2.60 and 41.95, respectively; P<0.0001 in F-test). The latter could be resolved in a medium-affinity population of sites, termed sites 2 (40% of maximal specific binding) and a low-affinity population of sites, termed sites 1, with pIC50 values of 7.3±0.1 and 5.5±0.1, respectively (Figure 4 and Table 1). Nonlinear regression analysis revealed that expression of the recombinant hH3R generated a third population of sites, termed sites 3, (c=0.9983) which was not observed in membranes of CHO(WT) cells, sites 1 and 2 being unchanged. This additional binding site displayed a higher affinity for GTPγS (pIC50=8.2±0.2) and represented 17% of specific binding in membranes of CHO(hH3R) cells (Figure 4). A three-site model analysis best accounted than a two-site model for the inhibition curve (χ2=0.67 and 2.37, respectively) and fitted the data significantly better in the presence of imetit (P<0.01 in F-test). Imetit (1 μM) significantly increased (by ∼300%) the capacity of the site 3 which, then, represented ∼50% of specific binding, but did not modify its affinity or the parameters of sites 1 and 2. In contrast, the inverse agonist ciproxifan significantly reduced (by 33%) the capacity of the high-affinity site 3 without changing its affinity, the low and medium affinity sites being also unaffected (Table 1 and Figure 4).
Figure 4.
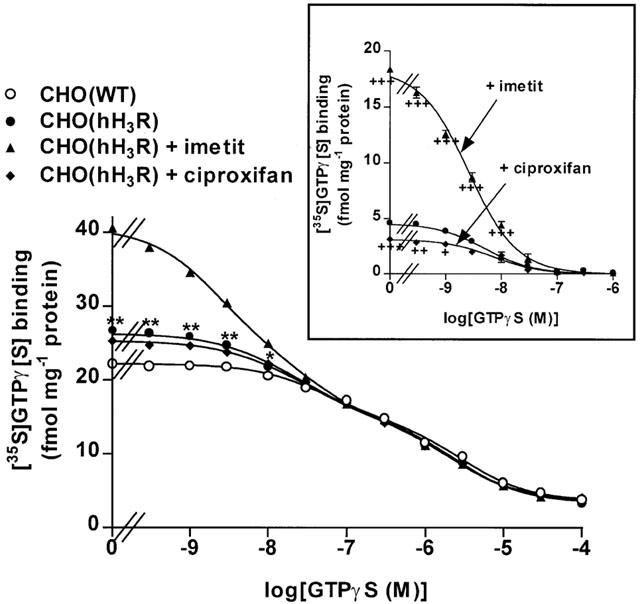
Inhibition of [35S]GTPγ[S] binding to membranes of CHO(WT) and CHO(hH3R) cells by GTPγS. Membranes of CHO(WT) or CHO(hH3R) cells expressing ∼300 fmol mg−1 protein, were incubated with 0.1 nM [35S]GTPγ[S] and increasing concentrations of GTPγS, in the presence, when required, of 1 μM imetit or ciproxifan. The inset shows the same data after subtraction of [35S]GTPγ[S] binding to membranes of CHO(WT) cells. Means±s.e.mean of 23–25 determinations from two independent experiments. *P<0.01; **P<0.001 vs CHO(WT) cells; +P<0.05; ++P<0.01; +++P<0.001 vs CHO(hH3R) cells in the absence of ligand.
Table 1.
Effect of H3-receptor ligands on the inhibition by GTPγS of [35S]GTPγ[S] binding to membranes of CHO (WT) and CHO(hH3R) cells
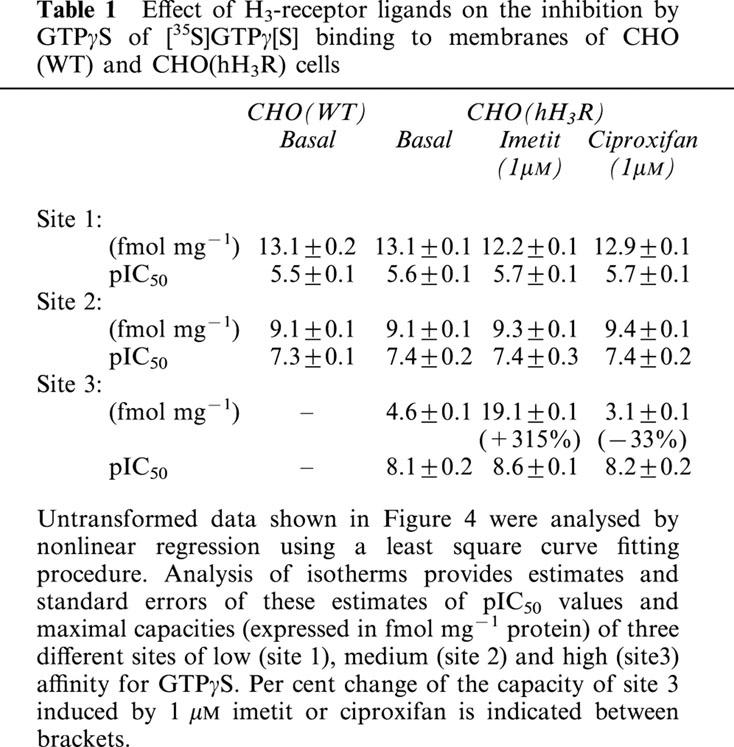
Effects of H3-receptor ligands on specific [35S]GTPγ[S] binding to membranes from various rat brain regions
Following incubation with 0.1 nM [35S]GTPγ[S], specific [35S]GTPγ[S] binding to rat brain membranes represented ∼5,000–10,000 d.p.m., i.e., 169±25 fmol mg−1 protein (hippocampus) to 261±41 fmol mg−1 protein (hypothalamus). It was increased significantly (by 10–20%) by imetit (10 nM) in all the regions studied, the effect of the agonist being stronger in the cerebral cortex and striatum, however (Figure 5). In all regions, the increase in binding elicited by imetit was blocked by the antagonist proxyfan (1 μM) (Morisset et al., 2000). In contrast, FUB 465, ciproxifan and thioperamide (10 nM), three compounds acting as inverse agonists (Morisset et al., 2000), reduced significantly [35S]GTPγ[S] binding, and their effect was also blocked by 1 μM proxyfan. Proxyfan did not itself significantly affect binding in all brain regions studied (Figure 5).
Figure 5.
Effects of H3-receptor ligands on specific [35S]GTPγ[S] binding to membranes from various rat brain regions. The effects of imetit, a selective agonist, and FUB 465, ciproxifan and thioperamide (10 nM), three inverse agonists, were studied in the absence (open bars) or presence (solid bars) of 1 μM proxyfan. The H3-receptor density was measured using [125I]-iodoproxyfan binding assay (Ligneau et al., 1994) and represented 130±1, 136±6, 108±2, 82±3, 103±4 and 99±6 fmol mg−1 protein in the cerebral cortex, striatum, hypothalamus, thalamus, hippocampus and midbrain respectively. Data are means±s.e.mean of 10–38 determinations from four to six separate experiments. *P<0.05, **P<0.001 vs basal; +P<0.05, ++P<0.001 vs without proxyfan.
Effects of inverse agonists at various G protein coupled-receptors on specific [35S]GTPγ[S] binding to membranes from rat cerebral cortex and striatum
The effects of various ligands described previously as inverse agonists in responses mediated by recombinant GPCRs ([35S]GTPγ[S] binding, GTPase assay, cyclic AMP formation, prolactin release, [3H]-thymidine incorporation, [3H]-inositolphosphates accumulation), were assessed on specific [35S]GTPγ[S] binding to membranes of rat cerebral cortex and striatum. Mepyramine, cimetidine, ICI-174,864, yohimbine and haloperidol that were reported in cell culture systems to act as inverse agonists at recombinant or endogenously expressed histamine H1- (Bakker et al., 2000), histamine H2- (Smit et al., 1996; Alewijnse et al., 1998), δ opioid (Costa & Herz, 1989; Milligan et al., 1997), α2- adrenergic (Tian et al., 1994; Pauwels et al., 2000) and dopamine D2/D3- (Nilsson & Eriksson, 1993; Griffon et al., 1996) receptors, respectively, did not affect specific [35S]GTPγ[S] binding to membranes of rat cerebral cortex (Figure 6) and striatum (not shown). In contrast, ciproxifan and CPDPX, an inverse agonist at recombinant adenosine A1-receptors (Shryock et al., 1998), reduced significantly specific [35S]GTPγ[S] binding to membranes from cerebral cortical (Figure 6) and striatal (not shown) membranes.
Figure 6.
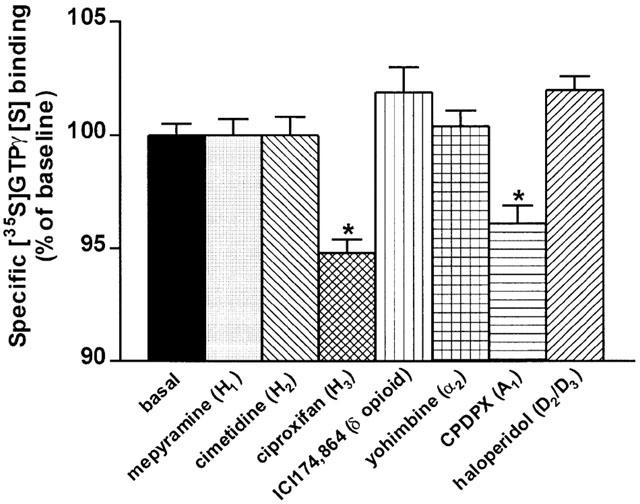
Effects of inverse agonists at various G protein coupled-receptors on specific [35S]GTPγ[S] binding to rat cerebral cortical membranes. Rat cerebral cortical membranes were incubated with 0.1 nM [35S]GTPγ[S] in the presence of the drugs at a 1 μM (mepyramine, cimetidine, ciproxifan, ICI-174,864 and CPDPX) or 0.1 μM (yohimbine and haloperidol) final concentration. Data represent means±s.e.mean of 8–39 determinations from two to four separate experiments. *P<0.001 vs basal.
Discussion
The present findings based upon two H3-receptor-mediated responses, i.e., phospholipase A2 activation (Morisset et al., 2000) and specific [35S]GTPγ[S] binding (Clark & Hill, 1996), provide the first direct evidence that the human histamine H3R displays constitutive activity. Ciproxifan, behaving as a potent inverse agonist (Morisset et al., 2000), decreased [3H]-arachidonic acid release from CHO cells expressing moderate densities of the human receptor. Moreover, its effect occurred with a magnitude similar to that observed with the rat receptor, thereby suggesting that the H3R displays the same high level of constitutive activity for this signalling pathway in both species. The carboxy-terminal portion of the third intracellular loop is critical for constitutive activity of GPCRs (Kjelsberg et al., 1992; Parma et al., 1993; Ren et al., 1993; Samama et al., 1993). The rH3R contains in this region a motif (SRDKKVAK) that is maintained in the sequence of the hH3R, with seven identical and one conserved amino acids (SRDRKVAK) (Lovenberg et al., 1999). As we recently noticed (Morisset et al., 2000), this motif is highly similar to the corresponding sequence of a mutated human β2-adrenergic receptor in which the mutations confer constitutive activity (Samama et al., 1993). This conserved motif may therefore account for constitutive activity of rat and human H3Rs.
The expression of the human receptor in CHO cells was also associated with an increase in basal [35S]GTPγ[S] binding. This increment of agonist-independent [35S]GTPγ[S] binding above the basal level of the parent untransfected CHO cells, revealed again the constitutive activity of the transfected receptor. In agreement, ciproxifan alone significantly decreased the basal [35S]GTPγ[S] binding to membranes of cells expressing the human receptor. However, both the effect of the inverse agonist and the increase in [35S]GTPγ[S] binding were lower than those observed with the rat receptor. The increment of [35S]GTPγ[S] binding measured at ∼200–300 fmol mg−1 protein of hH3R represented ∼5 fmol mg−1 protein whereas that found at the same density of the rat receptor represented ∼20 fmol mg−1 protein, suggesting that constitutive activity of the human receptor was about four times lower than that of the rat receptor in this test system. This apparent lower constitutive activity may result from a lower coupling efficiency of the human receptor to some G-protein subtypes. In agreement, the propensity of a given GPCR to produce constitutive activity is known to be an inherent property of the receptor and has been shown to be dependent on the G-proteins and signalling pathways activated by the receptor (Perez et al., 1996; Pauwels et al., 2000; Chen et al., 2000). The H3-receptor-mediated stimulation of [35S]GTPγ[S] binding being sensitive to pertussis toxin (Clark & Hill, 1996), may involve distinct Gαi/o subunits inasmuch as the recombinant receptor couples to distinct signalling pathways involving Gi/Go proteins, i.e., adenylyl cyclase inhibition, phospholipase A2 and MAP kinase activation (Lovenberg et al., 1999; Morisset et al., 2000; Drutel et al., 2001; Héron et al., 2001).
As previously shown for other GPCRs (Chidiac et al., 1994; Pozvek et al., 1997; Claeysen et al., 1999; Newman-Tancredi et al., 2000), we reported that constitutive activity of the recombinant rH3R was positively correlated to receptor density and was favoured upon overexpression (Morisset et al., 2000). This observation, attributable to an increased receptor/G-protein stoichiometry (Kenakin, 1997), could be made with the hH3R. Indeed, the increment of [35S]GTPγ[S] binding due to the transfected receptor was 4 fold greater (∼20 and 5 fmol mg−1 protein, respectively) at ∼700 fmol mg−1 protein versus ∼300 fmol mg−1 protein of hH3R. The extended ternary complex model which describes two receptor states whereby the active state interacts with G proteins (Samama et al., 1993), predicts that the same maximal response that is produced by an agonist should be observed constitutively with high receptor expression levels. However, the increase in [35S]GTPγ[S] binding (i.e. constitutive activity) observed at the highest densities of hH3R was still substantially lower than the increase in binding induced by imetit (i.e. the agonist-induced maximal response), suggesting that the amount of receptor may be limiting for constitutive activity of the hH3R. Consistent with this proposal, the increment in [35S]GTPγ[S] binding observed at a receptor density of 1 pmol mg−1 protein was not significantly higher than that observed at ∼700 fmol mg−1 protein. However, it was already significant at moderate densities (<500 fmol mg−1 protein), i.e., consistent with natural cellular levels of receptors, indicating that the threshold expression level for constitutive activity, which varies with the different types of receptors (Chen et al., 2000), is rather small for the hH3R. This suggests that constitutive activity of the native H3R may be present not only in rodent brain (Morisset et al., 2000) but also in human brain.
We also analysed the inhibition of [35S]GTPγ[S] binding by unlabelled GTPγS to further investigate constitutive G-protein activation by the hH3R expressed at a moderate density (∼300 fmol mg−1 protein). This new approach was recently used successfully to examine directly constitutive activity of 5-HT receptors in CHO cells (Audinot et al., 2001). It shows that the increment in [35S]GTPγ[S] binding generated upon overexpression of the H3R corresponded to a high-affinity site able to bind the low concentration (0.1 nM) of [35S]GTPγ[S] used in the present study. In agreement with the low affinity binding previously reported (pIC50=6.2 to 6.6) (Newman-Tancredi et al., 2000; Audinot et al., 2001), GTPγS bound to low and medium affinity components (pIC50=5.5 and 7.3, respectively) in membranes of wild-type CHO cells. hH3R expression generated an additional high affinity binding site for GTPγS, similar to that associated with the expression of recombinant human serotonin 5-HT1B or 5-HT1D receptors in the same cells (pIC50=8.1 and 8.7, respectively) (Newman-Tancredi et al., 2000; Audinot et al., 2001). This high affinity site reflected the constitutive activity of the hH3R since it was increased by imetit, but decreased by ciproxifan, acting again as an inverse agonist abrogating the constitutive activation of G proteins. In contrast, the two ligands did not alter the density and affinity of the two lower affinity binding sites confirming that the latter are not related to H3 receptor/G-protein coupling events. The capacity of the high affinity site in the absence of agonist (∼5 fmol mg−1 protein at a receptor density of ∼300 fmol mg−1 protein) was similar to the total increment of binding generated by the transfected receptor. Moreover, both the KD value of [35S]GTPγ[S] to membranes from transfected CHO cells (Newman-Tancredi et al., 2000) and the pIC50 value of GTPγS for the high-affinity site (Table 1) were two orders of magnitude higher than the concentration of [35S]GTPγ[S] that we used (0.1 nM), indicating that the absolute increases in [35S]GTPγ[S] binding observed in the present study represented only a small fraction of G proteins activated per receptor. As discussed above, the capacity of the high affinity site was further increased by imetit. Since it represented ∼20 fmol mg−1 protein versus ∼5 fmol mg−1 protein in the presence and absence of imetit, respectively (at a receptor density of ∼300 fmol mg−1 protein), it can be concluded that approximately 25% of hH3Rs exist in a precoupled state. Similar values (29 and 20%, respectively) could be calculated from the constitutive increase in binding and from the binding induced by imetit at a receptor density of ∼700 fmol mg−1 protein (∼20 and ∼70 fmol mg−1 protein, respectively) or ∼1 pmol mg−1 protein (∼20 and ∼100 fmol mg−1 protein, respectively). These levels of precoupling are in the same range as those previously reported for other Gi-protein-coupled receptors (Neubig et al., 1988; Chen et al., 2000). Although it may be dependent on the receptor density and G-protein subtypes, the high affinity binding of GTPγS may allow to quantify the degree of constitutive activity and, therefore, the intrinsic activity of inverse agonists (Audinot et al., 2001). According to this model, ciproxifan, which did not abolish totally the high affinity component, would be acting as a partial inverse agonist at the hH3R.
Previous functional or binding studies revealed distinct pharmacological profiles of the rat and human H3Rs (Arrang et al., 1988; West et al., 1999; Lovenberg et al., 2000). Several agonists were found to be equipotent at both receptors but thioperamide and ciproxifan, tested as antagonists, displayed significantly higher potencies at the rat receptor when compared to the human receptor. These differences, ascribed to two amino acids in the third transmembrane domain (Ligneau et al., 2000), were confirmed here using another test. In agreement, whereas imetit increased [35S]GTPγ[S] binding to membranes expressing the human receptor, with a potency very similar to that displayed at the rat autoreceptor (Garbarg et al., 1992), this effect was blocked by ciproxifan with an antagonist potency (Ki=45 nM) consistent with that obtained using binding assays (Ki=46 nM) (Ligneau et al., 2000), i.e., lower than that displayed at the rat autoreceptor (Ki=0.5 nM) (Ligneau et al., 1998).
As expected from the extended ternary complex model (Samama et al., 1993; Lefkowitz et al., 1993; Weiss et al., 1996) and the higher affinity of inverse agonists for the inactive conformation of the receptor (Samama et al., 1994), ciproxifan was slightly more potent as an inverse agonist (EC50=15 nM) than as an antagonist (Ki=45 nM) at the human receptor. We recently reported a similar ratio between the inverse agonist and antagonist potencies of the drug at the rat receptor (EC50 and Ki values of 0.1 and 0.5 nM, respectively). It can be concluded that ciproxifan is less potent at the human than the rat receptor, not only as an antagonist, but also as an inverse agonist.
We recently established inverse agonism at native H3Rs expressed at a normal level in mouse brain (Morisset et al., 2000). This observation brought direct evidence for the physiological relevance of constitutive activity of GPCRs (de ligt et al., 2000). In agreement, [35S]GTPγ[S] binding to mouse cerebral cortical membranes was decreased by FUB 465, thioperamide and ciproxifan, three inverse agonists whose effects were blocked by proxyfan, a neutral antagonist. Moreover, the constitutive activity of H3Rs, as determined by the [35S]GTPγ[S] binding assay, was further established for H3 autoreceptors regulating histamine release in vitro and in vivo (Morisset et al., 2000). A very similar pattern of [35S]GTPγ[S] binding was obtained in the present study using rat cerebral cortex and the same ligands. In addition, the magnitude of the decrease evoked by the three inverse agonists was similar in all rat brain regions studied, suggesting that constitutive activity of native H3Rs occurs at the same level in all cerebral areas. As expected, the overall regional distribution of the imetit-induced binding response paralleled the known distribution of H3Rs (Pollard et al., 1993). Consistent with autoradiographic studies (Laitinen & Jokinen, 1998), it was the highest in the striatum and cerebral cortex. All these findings show that [35S]GTPγ[S] binding is a useful assay to analyse the interactions of native H3 receptors with G proteins, inasmuch as the signaling pathways to which they couple in brain and peripheral tissues remain unclear (Hill et al., 1997).
Among authors suggesting an inverse agonism at native GPCRs (de ligt et al., 2000), Costa & Herz (1989) were pioneers by demonstrating for the first time that ICI-174864, an antagonist at the δ opioid receptor, behaved in fact as an inverse agonist at the δ opioid receptor endogenously expressed in NG 108-15 cells. However, the same authors reported that the inverse agonism induced by the drug occurred only in isolated membranes but not in intact cells (Costa et al., 1992). They suggested that constraints imposed by cytosolic factors such as GTP prevent constitutive activity, making inevitable its observation in membranes upon separation from cytosol leading to removal of these factors. However, the present findings show that, in contrast to H3 receptors, not all native receptors display constitutive activity in rat brain membranes. Indeed, in contrast to its inverse agonist effect on GTPase activity (Costa & Herz, 1989) and [35S]GTPγ[S] binding (Szekeres & Traynor, 1997) in membranes of NG108-15 cells, ICI-174864 did not decrease [35S]GTPγ[S] binding to membranes of rat cerebral cortex or striatum. These findings suggest that the threshold expression level for constitutive activity inherent to δ opioid receptors is not reached in the brain. The same interpretation may account for the apparent lack of effect of inverse agonists at D2/D3 dopamine, H1 and H2 histamine and α2-adrenergic receptors, that we report here in brain membranes, in spite of the inverse agonism that they induce in cells (Nilsson & Eriksson, 1993; Griffon et al., 1996; Bakker et al., 2000; Alewijnse et al., 1998; Pauwels et al., 2000). It should be pointed out, however, that an apparent lack of inverse agonism does not furnish definitive evidence for the absence of constitutive activity since the intrinsic activity of a given inverse agonist is dependent on receptor systems and experimental conditions (Newman-Tancredi et al., 1997; Szekeres & Traynor, 1997; Pauwels et al., 1997; 2000; Audinot et al., 2001). Besides H3Rs, A1 adenosine receptors display a high constitutive activity in rat brain. Constitutive inhibition of adenylyl cyclase by A1 adenosine receptors was previously shown using CPDPX. This drug known as a potent and highly selective antagonist, was acting as an inverse agonist not only at overexpressed receptors in CHO cells (Shryock et al., 1998) but also at endogenously expressed receptors of embryonic chick ventricular myocytes (Ma & Green, 1992). Although endogenous adenosine is present in high enough concentrations to stimulate [35S]GTPγ[S] binding in rat brain sections (Laitinen & Jokinen, 1998), the decrease evoked by CPDPX in membranes is more likely to reflect high constitutive activity of cerebral A1 receptors rather than antagonism of endogenous adenosine since it was observed after pretreatment of the membranes with adenosine deaminase.
In conclusion, the present study suggests that constitutive activity of native H3Rs is one of the highest among GPCRs present in rat brain. Recombinant hH3Rs also display high constitutive activity. The latter is easily detected at moderate concentrations, suggesting that it is present in human brain. Since we recently showed that constitutive activity of H3Rs regulates histamine neurons in brain, the present findings further suggest that inverse agonists should find therapeutic applications. Ciproxifan, which is known to display distinct affinities at the rat and human receptors, is less potent not only as an antagonist, but also as an inverse agonist at the human receptor when compared to its rat counterpart. The evaluation of inverse agonist potency at human receptors should facilitate the rational design of novel compounds to be used in therapeutics.
Acknowledgments
The authors would like to thank Pr W. Schunack and H. Stark for providing FUB 465 and proxyfan, and Pr C.R. Ganellin for providing imetit.
Abbreviations
- BSA
bovine serum albumin
- CPDPX
8-cyclopentyl-1,3-dipropylxanthine
- GPCR
G-protein-coupled receptor
- H3R
histamine H3 receptor
- rH3R
rat histamine H3 receptor
- hH3R
human histamine H3 receptor
References
- ALEWIJNSE A.E., SMIT M.J., HOFFMANN M., VERZIJL D., TIMMERMAN H., LEURS R. Constitutive activity and structural instability of the wild-type human H2 receptor. J. Neurochem. 1998;71:799–807. doi: 10.1046/j.1471-4159.1998.71020799.x. [DOI] [PubMed] [Google Scholar]
- ARRANG J.-M., DEVAUX B., CHODKIEWICZ J.-P., SCHWARTZ J.-C. H3-receptors control histamine release in human brain. J. Neurochem. 1988;51:105–108. doi: 10.1111/j.1471-4159.1988.tb04841.x. [DOI] [PubMed] [Google Scholar]
- ARRANG J.-M., GARBARG M., LANCELOT J.-C., LECOMTE J.-M., POLLARD H., ROBBA M., SCHUNACK W., SCHWARTZ J.-C. Highly potent and selective ligands for histamine H3-receptors. Nature. 1987;327:117–123. doi: 10.1038/327117a0. [DOI] [PubMed] [Google Scholar]
- AUDINOT V., NEWMAN-TANCREDI A., MILLAN M.J. Constitutive activity at serotonin 5-HT1D receptors: detection by homologous GTPγS versus [35S]-GTPγS binding isotherms. Neuropharmacology. 2001;40:57–64. doi: 10.1016/s0028-3908(00)00104-0. [DOI] [PubMed] [Google Scholar]
- BAKKER R.A., WIELAND K., TIMMERMAN H., LEURS R. Constitutive activity of the histamine H1 receptor reveals inverse agonism of histamine H1 receptor antagonists. Eur. J. Pharmacol. 2000;387:R5–R7. doi: 10.1016/s0014-2999(99)00803-1. [DOI] [PubMed] [Google Scholar]
- BROWN R.E., STEVENS D.R., HAAS H.L. The physiology of brain histamine. Prog. Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- CHEN G., WAY J., ARMOUR S., WATSON C., QUEEN K., JAYAWICKREME C.K., CHEN W.-J., KENAKIN T. Use of constitutive G protein-coupled receptor activity for drug discovery. Mol. Pharmacol. 2000;57:125–134. [PubMed] [Google Scholar]
- CHENG Y.C., PRUSSOFF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- CHIDIAC P., HEBERT T.E., VALIQUETTE M., DENNIS M., BOUVIER M. Inverse agonism activity of β-adrenergic antagonists. Mol. Pharmacol. 1994;45:490–499. [PubMed] [Google Scholar]
- CLAEYSEN S., SEBBEN M., BECAMEL C., BOCKAERT J., DUMUIS A. Novel brain-specific 5-HT4 receptor splice variants show marked constitutive activity: role of the C-terminal intracellular domain. Mol. Pharmacol. 1999;55:910–920. [PubMed] [Google Scholar]
- CLARK E.A., HILL S.J. Sensitivity of histamine H3 receptor agonist-stimulated [35S]GTPγ[S] binding to pertussin toxin. Eur. J. Pharmacol. 1996;296:223–225. doi: 10.1016/0014-2999(95)00800-4. [DOI] [PubMed] [Google Scholar]
- COSTA T., HERZ A. Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins. Proc. Natl. Acad. Sci. U.S.A. 1989;86:7321–7325. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COSTA T., OGINO Y., MUNSON P.J., ONARAN O., RODBARD D. Drug efficacy at guanine nucleotide-binding regulatory protein-linked receptors: thermodynamic interpretation of negative antagonism and of receptor activity in the absence of ligand. Mol. Pharmacol. 1992;41:549–560. [PubMed] [Google Scholar]
- DE LIGT R.A.F., KOUROUNAKIS A.P., IJZERMAN A.P. Inverse agonism at G protein-coupled receptors: (patho)physiological relevance and implications for drug discovery. Br. J. Pharmacol. 2000;130:1–12. doi: 10.1038/sj.bjp.0703311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRUTEL G., PEITSARO N., KARLSTEDT K., WIELAND K., SMIT M.J., TIMMERMAN H., PANULA P., LEURS R. Identification of rat H3 receptor isoforms with different brain expression and signaling properties. Mol. Pharmacol. 2001;59:1–8. [PubMed] [Google Scholar]
- GARBARG M., ARRANG J.-M., ROULEAU A., LIGNEAU X., DAM TRUNG TUONG M., SCHWARTZ J.-C., GANELLIN C.R. S-[2-(4-imidazolyl)ethyl]isothiourea, a highly specific and potent histamine H3-receptor agonist. J. Pharmacol. Exp. Ther. 1992;263:304–310. [PubMed] [Google Scholar]
- GRIFFON N., PILON C., SAUTEL F., SCHWARTZ J.-C., SOKOLOFF P. Antipsychotics with inverse agonist activity at the dopamine D3 receptor. J. Neural Transm. 1996;103:1163–1175. doi: 10.1007/BF01271201. [DOI] [PubMed] [Google Scholar]
- HÉRON A., ROULEAU A., COCHOIS V., PILLOT C., SCHWARTZ J.-C., ARRANG J.-M. Expression analysis of the histamine H3 receptor in developing rat tissues. Mech. Dev. 2001;105:167–173. doi: 10.1016/s0925-4773(01)00389-6. [DOI] [PubMed] [Google Scholar]
- HILL S.J., GANELLIN C.R., TIMMERMAN H., SCHWARTZ J.-C., SHANKLEY N.P., YOUNG J.M., SCHUNACK W., LEVI R., HAAS H.L. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol. Rev. 1997;49:253–278. [PubMed] [Google Scholar]
- KENAKIN T.P. Pharmacological Analysis of Drug Receptor Interaction. New-York: Lippincott-Raven; 1997. [Google Scholar]
- KJELSBERG M.A., COTECCHIA S., OSTROWSKI J., CARON M.G., LEFKOWITZ R.J. Constitutive activation of the α1B-adrenergic receptor by all amino acid substitutions at a single site. J. Biol. Chem. 1992;267:1430–1433. [PubMed] [Google Scholar]
- KRAUSE M., STARK H., SCHUNACK W. Iododestannylation : an improved synthesis of [125I]iodoproxyfan, a specific radioligand of the histamine H3 receptor. J. Label. Comp. Radiopharm. 1997;39:601–606. [Google Scholar]
- LAITINEN J.T., JOKINEN M. Guanosine 5′-(γ-[35S]thio)triphosphate autoradiography allows selective detection of histamine H3 receptor-dependent G protein activation in rat brain tissue sections. J. Neurochem. 1998;71:808–816. doi: 10.1046/j.1471-4159.1998.71020808.x. [DOI] [PubMed] [Google Scholar]
- LEFKOWITZ R.J., COTECCHIA S., SAMAMA P., COSTA T. Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol. Sci. 1993;14:303–307. doi: 10.1016/0165-6147(93)90048-O. [DOI] [PubMed] [Google Scholar]
- LIGNEAU X., GARBARG M., VIZUETE M.L., DIAZ J., PURAND K., STARK H., SCHUNACK W., SCHWARTZ J.-C. [125I]lodoproxyfan, a new antagonist to label and visualize cerebral histamine H3 receptors. J. Pharmacol. Exp. Ther. 1994;271:452–459. [PubMed] [Google Scholar]
- LIGNEAU X., LIN J.-S., VANNI-MERCIER G., JOUVET M., MUIR J.L., GANELLIN C.R., STARK H., ELZ S., SCHUNACK W., SCHWARTZ J.-C. Neurochemical and behavioral effects of ciproxifan, a potent histamine H3-receptor antagonist. J. Pharmacol. Exp. Ther. 1998;287:658–666. [PubMed] [Google Scholar]
- LIGNEAU X., MORISSET S., TARDIVEL-LACOMBE J., GBAHOU F., GANELLIN C.R., STARK H., SCHUNACK W., SCHWARTZ J.-C., ARRANG J.-M. Distinct pharmacology of rat and human H3 receptors: role of two amino acids in the third transmembrane domain. Br. J. Pharmacol. 2000;131:1247–1250. doi: 10.1038/sj.bjp.0703712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVENBERG T.W., PYATI J., CHANG H., WILSON S.J., ERLANDER M.G. Cloning of rat histamine H3 receptor reveals distinct species pharmacological profiles. J. Pharmacol. Exp. Ther. 2000;293:771–778. [PubMed] [Google Scholar]
- LOVENBERG T.W., ROLAND B.L., WILSON S.J., JIANG X., PYATI J., HUVAR A., JACKSON M.R., ERLANDER M.G. Cloning and functional expression of the human histamine H3 receptor. Mol. Pharmacol. 1999;55:1101–1107. [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MA H., GREEN R.D. Modulation of cardiac cyclic AMP metabolism by adenosine receptor agonists and antagonists. Mol. Pharmacol. 1992;42:831–837. [PubMed] [Google Scholar]
- MILLIGAN G., BOND R., LEE M. Inverse agonism: pharmacological curiosity or potential therapeutic strategy. Trends Pharmacol. Sci. 1995;16:10–13. doi: 10.1016/s0165-6147(00)88963-4. [DOI] [PubMed] [Google Scholar]
- MILLIGAN G., MACEWAN D.J., MERCOURIS M., MULLANEY I. Inverse agonism at adrenergic and opioid receptors: studies with wild type and constitutively active mutant receptors. Recep. Channels. 1997;5:209–213. [PubMed] [Google Scholar]
- MORISSET S., ROULEAU A., LIGNEAU X., GBAHOU F., TARDIVEL-LACOMBE J., STARK H., SCHUNACK W., GANELLIN C.R., SCHWARTZ J.-C., ARRANG J.-M. High constitutive activity of native H3 receptors regulates histamine neurons in brain. Nature. 2000;408:860–864. doi: 10.1038/35048583. [DOI] [PubMed] [Google Scholar]
- NEUBIG R.R., GANTZOS R.D., THOMSEN W.J. Mechanism of agonist and antagonist binding to α2-adrenergic receptors : evidence for a precoupled receptor-guanine nucleotide complex. Biochemistry. 1988;27:2374–2384. doi: 10.1021/bi00407a019. [DOI] [PubMed] [Google Scholar]
- NEWMAN-TANCREDI A., AUDINOT V., MOREIRA C., VERRIELE L., MILLAN M.J. Inverse agonism and constitutive activity as functional correlates of serotonin h5-HT1B receptor/G-protein stoichiometry. Mol. Pharmacol. 2000;58:1042–1049. doi: 10.1124/mol.58.5.1042. [DOI] [PubMed] [Google Scholar]
- NEWMAN-TANCREDI A., CONTE C., CHAPUT C., VERRIELE L., MILLAN M.J. Agonist and inverse agonist efficacy at human recombinant serotonin 5-HT1A receptors as a function of receptor: G-protein stoichiometry. Neuropharmacology. 1997;36:451–459. doi: 10.1016/s0028-3908(97)00022-1. [DOI] [PubMed] [Google Scholar]
- NILSSON C.L., ERIKSSON E. Haloperidol increases prolactin release and cyclic AMP formation in vitro: inverse agonism at dopamine D2 receptors. J. Neural. Transm. 1993;92:213–220. doi: 10.1007/BF01244880. [DOI] [PubMed] [Google Scholar]
- PARKER R.B., WAUD D.R. Pharmacological estimation of drug-receptor dissociation constants. Statistical evaluation I. Agonists. J. Pharmacol. Exp. Ther. 1971;177:1–24. [PubMed] [Google Scholar]
- PARMA J., DUPREZ L., VAN SANDE J., COCHAUX P., GERVY C., MOCKEL J., DUMONT J., VASSART G. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenoma. Nature. 1993;365:649–651. doi: 10.1038/365649a0. [DOI] [PubMed] [Google Scholar]
- PAUWELS P.J., TARDIF S., WURCH T., COLPAERT F.C. Stimulated [35S]-GTPγS binding by 5-HT1A receptor agonists in recombinant cell lines: modulation of apparent efficacy by G-protein activation state. Naunyn-Schmiedeb. Arch. Pharmacol. 1997;356:551–561. doi: 10.1007/pl00005090. [DOI] [PubMed] [Google Scholar]
- PAUWELS P.J., TARDIF S., WURCH T., COLPAERT F.C. Facilitation of constitutive α2A-adrenoceptor activity by both single amino acid mutation (thr373lys) and Gαo protein coexpression: evidence for inverse agonism. J. Pharmacol. Exp. Ther. 2000;292:654–663. [PubMed] [Google Scholar]
- PEREZ D.M., HWA J., GAIVIN R., MATHUR M., BROWN F., GRAHAM R. Constitutive activation of a single effector pathway : Evidence for multiple activation states of a G protein-coupled receptor. Mol. Pharmacol. 1996;49:112–122. [PubMed] [Google Scholar]
- POLLARD H., MOREAU J., ARRANG J.-M., SCHWARTZ J.-C. A detailed autoradiographic mapping of histamine H3 receptors in rat brain areas. Neuroscience. 1993;52:169–189. doi: 10.1016/0306-4522(93)90191-h. [DOI] [PubMed] [Google Scholar]
- POZVEK G., HILTON J.M., QUIZA M., HOUSSAMI S., SEXTON P.M. Structure/function relationships of calcitonin analogues as agonists, antagonists, or inverse agonists in a constitutively activated receptor cell system. Mol. Pharmacol. 1997;51:658–665. doi: 10.1124/mol.51.4.658. [DOI] [PubMed] [Google Scholar]
- REN Q., KUROSE H., LEFKOWITZ R.J., COTECCHIA S. Constitutively active mutants of the α2-adrenergic receptor. J. Biol. Chem. 1993;268:16483–16487. [PubMed] [Google Scholar]
- SAMAMA P., COTECCHIA S., COSTA T., LEFKOWITZ R.J. A mutation-induced activated state of the β2-adrenergic receptor. Extending the ternary complex model. J. Biol. Chem. 1993;268:4625–4636. [PubMed] [Google Scholar]
- SAMAMA P., PEI G., COSTA T., COTECCHIA S., LEFKOWITZ R.J. Negative antagonists promote an inactive conformation of the β2-adrenergic receptor. Mol. Pharmacol. 1994;45:390–394. [PubMed] [Google Scholar]
- SCHLICKER E., MALINOWSKA B., KATHMANN M., GOTHERT M. Modulation of neurotransmitter release via histamine H3 heteroreceptors. Fund. Clin. Pharmacol. 1994;8:128–137. doi: 10.1111/j.1472-8206.1994.tb00789.x. [DOI] [PubMed] [Google Scholar]
- SHRYOCK J.C., OZECK M.J., BELARDINELLI L. Inverse agonists and neutral antagonists of recombinant human A1 adenosine receptors stably expressed in chinese hamster ovary cells. Mol. Pharmacol. 1998;53:886–893. [PubMed] [Google Scholar]
- SMIT M.J., LEURS R., ALEWIJNSE A.E., BLAUW J., VAN NIEUW AMERONGEN G.P., VAN DE VREDE Y., ROOVERS E., TIMMERMAN H. Inverse agonism of histamine H2 antagonists accounts for upregulation of spontaneous active histamine H2 receptors. Proc. Natl. Acad. Sci. U.S.A. 1996;93:6802–6807. doi: 10.1073/pnas.93.13.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZEKERES P.G., TRAYNOR J.R. Delta opioid modulation of the binding of guanosine-5′-O-(3-[35S]thio)triphosphate to NG108-15 cell membranes: characterization of agonist and inverse agonist effects. J. Pharmacol. Exp. Ther. 1997;283:1276–1284. [PubMed] [Google Scholar]
- TAKESHITA Y., WATANABE T., SAKATA T., MUNAKATA M., ISHIBASHI H., AKAIKE N. Histamine modulates high-voltage-activated calcium channels in neurons dissociated from the rat tuberomammillary nucleus. Neuroscience. 1998;87:797–805. doi: 10.1016/s0306-4522(98)00152-3. [DOI] [PubMed] [Google Scholar]
- TARDIVEL-LACOMBE J., ROULEAU A., HERON A., MORISSET S., PILLOT C., COCHOIS V., SCHWARTZ J.-C., ARRANG J.-M. Cloning and cerebral expression of the guinea pig histamine H3 receptor: evidence for two isoforms. NeuroReport. 2000;11:755–759. doi: 10.1097/00001756-200003200-00020. [DOI] [PubMed] [Google Scholar]
- TIAN W.-N., DUZIC E., LANIER S.M., DETH R.C. Determinants of α2-adrenergic receptor activation of G proteins: evidence for a precoupled receptor/G protein state. Mol. Pharmacol. 1994;45:524–531. [PubMed] [Google Scholar]
- WEISS J.M., MORGAN P.H., LUTZ M.W., KENAKIN T.P. The cubic ternary complex receptor occupancy model. J. Theor. Biol. 1996;181:381–397. doi: 10.1006/jtbi.1996.0139. [DOI] [PubMed] [Google Scholar]
- WEST R.R., WU R.L., BILLAH M.M., EGAN R.W., ANTHES J.C. The profiles of human and primate [3H]Nα -methylhistamine binding differ from that of rodents. Eur. J. Pharmacol. 1999;377:233–239. doi: 10.1016/s0014-2999(99)00424-0. [DOI] [PubMed] [Google Scholar]


