Abstract
The combination of classic non-steroidal antiinflammatory drugs (NSAIDs) with opiates induces more analgesia than the summed effect of each drug given separately. No studies have been performed using new generation NSAIDs and fentanyl nor on the duration of this effect.
We have studied the analgesic effect of fentanyl alone and after the administration of subeffective doses of dexketoprofen trometamol in rat nociceptive responses. The responses were evoked by noxious mechanical stimulation and were recorded as single motor units in male Wistar rats anaesthetized with α-chloralose.
The effective dose 50 (ED50) observed with fentanyl was 22.4±1.5 μg kg−1 and full recovery was apparent 20 min later. The administration of a total dose of 40 μg kg−1 of dexketoprofen trometamol did not induce any significant effect on the nociceptive responses. In the presence of dexketoprofen trometamol, the ED50 for fentanyl was 5 fold lower than before: 3.8±1.1 μg kg−1 and no significant recovery was observed 45 min later. The opioid antagonist naloxone (200 μg kg−1) did not reverse the effect, although in control experiments the same dose was able to prevent any action of fentanyl given alone.
We conclude that the combination of fentanyl and subeffective doses of dexketoprofen trometamol induces a more potent and longer lasting analgesic effect than that observed with fentanyl alone, and that this is not an opioid mediated action.
Keywords: Pain, analgesia, spinal cord, opioids, non-steroidal antiinflammatory drugs
Introduction
The combination of some NSAIDs like aspirin or ketorolac and opiates like morphine induces more analgesia than the summed effect of each drug given separately (Beaver, 1984; Malmberg & Yaksh, 1993). The mechanism proposed for this interaction involves an inhibition of GABA mediated synaptic transmission in the periaqueductal grey area by opioids which, in turn, are modulated by cyclooxygenase (COX)-mediated metabolites (Vaughan et al., 1997). However, it is not known whether NSAIDs also enhance the duration of the analgesic effect of opiates. Furthermore, most of these studies have been performed using morphine and classic NSAIDs whereas no studies have been performed, as far as we can tell, on the interaction between new generation NSAIDs and other opiates like fentanyl, more in use nowadays. Fentanyl and its derivatives are very effective in the treatment of pain and probably used more frequently in the clinic than morphine, especially since its proven efficacy by transdermal application (Allan et al., 2001). In fact, more patients prefer fentanyl than morphine (Allan et al., 2001). Fentanyl is more rapidly absorbed and much more potent than morphine when tested in similar experimental conditions (Herrero & Headley, 1991). On the other hand, fentanyl is metabolized very quickly and therefore its therapeutic effect is very short. The use of latest generation NSAIDs like dexketoprofen trometamol is increasing rapidly. It is absorbed more rapidly by the digestive mucosa, enters the blood-brain barrier easily and as a result its analgesic efficacy is higher, and the side effects lower, than those observed with classic NSAIDs (Mazario et al., 2001, and references within). An enhancement of fentanyl effects by dexketoprofen trometamol may lead to a reduction in the therapeutic analgesic doses required and therefore a reduction in the unwanted side effects. The aim of the present study has been therefore to study the analgesic effect of fentanyl and the duration of its effect in the absence and in the presence of subeffective doses of dexketoprofen trometamol. We have used the technique of recording withdrawal reflexes as single motor units (SMUs) in anaesthetized rats, in which the antinociceptive efficacy of either compound has been studied separately in previous experiments (Herrero & Headley, 1991; Mazario et al., 1999). Preliminary results have been published elsewhere in abstract format (Gaitan & Herrero, 2001).
Methods
Animals and groups of experiments
The experiments were performed in 14 Wistar male rats weighing 280–350 g divided into three groups: control (n=3), treated with dexketoprofen trometamol (n=8) and naloxone control (n=3). The effect of the μ-opioid receptor agonist fentanyl was tested in the absence and in the presence of dexketoprofen trometamol following the same protocol in the two tests. Fentanyl (Sigma) was tested in log2 cumulative doses, starting with 1 μg kg−1, until responses fell below 20% of control response (maximal dose used of 32 μg kg−1). The test was performed twice in each animal, and in the same unit, leaving a gap of 1 h between each dose-response curve. Dexketoprofen trometamol (kindly supplied by Menarini, Spain) was administered, 1 h after the first dose-response curve of fentanyl, in cumulative doses from 10 to 40 μg kg−1 every 21 min, following the protocol used in previous studies (Mazario et al., 2001). The second dose-response curve of fentanyl was commenced 21 min after the last dose of the NSAID. In three of these experiments, the effect of fentanyl in the presence of dexketoprofen was challenged with the opioid antagonist naloxone (200 μg kg−1). Control experiments were performed to test whether or not the second dose-response curve of fentanyl might be influenced by the prior administration of the opioid, although similar studies had previously shown a lack of such an influence since fentanyl is fully metabolized 1 h after administration (Herrero & Solano, 1999 and references within). In these control experiments, fentanyl was tested twice following the same protocol, again leaving 1 h between the two dose-response curves. In order to check the effectiveness of the opioid receptor antagonist naloxone, three more experiments were performed injecting naloxone at a dose of 200 μg kg−1 6 min before a single dose of 32 μg kg−1 of fentanyl. This dose of naloxone was chosen since it is at least 10 times in excess of the dose required in similar experiments to reverse or prevent the effect of μ-opioid receptor agonists like morphine or fentanyl (Herrero & Headley, 1991; Thorn et al., 1994; Herrero & Solano, 1999).
Stimulus presentation and recording systems
The technique for recording single motor units (SMUs) has been published elsewhere in detail (see, for example, Herrero & Headley, 1991; Solano & Herrero, 1997; Herrero & Solano, 1999). Briefly, preparatory surgery was performed under halothane anaesthesia (5% for induction and 2.5% for maintenance in oxygen) and consisted in the cannulation of the trachea, two superficial jugular veins (for the administration of drugs and α-chloralose) and one carotid artery (to monitor blood pressure). After the surgery, the animal was transferred to a recording frame, the right hind limb was fixed in a semi-extended position using plaster of Paris, halothane was then discontinued and the anaesthesia maintained thereafter with α-chloralose i.v. (Sigma; 50 mg kg−1 initial dose and 20 mg kg−1 h−1 by perfusion pump for maintenance diluted in saline). The preparation was left to rest for at least 1 h before the experiment started. Blood pressure was continuously monitored (Presmeter, Cybertec) and the systolic level never fell below 100 mmHg, apart from a short time (first 2 min) during the administration of some doses of fentanyl (16 and 32 μg kg−1; see Herrero & Headley, 1991, for more details). Core temperature was also recorded and maintained at 37±0.5°C by means of a back controlled heating blanket. SMUs were activated by noxious mechanical stimulation (200 mN above threshold intensity) and recorded by means of bipolar tungsten electrodes inserted percutaneously into muscles of the right hind limb (usually peroneus longus and extensor digitorum longus; Schouenborg & Weng, 1994; Solano & Herrero, 1997). Pressure was applied by means of an electronic-controlled pincher device over a surface of 14 mm2. Threshold intensity was determined by the appliance of an increasing pressure ramp and was considered as the minimum intensity required to evoke a constant response (non-adapted firing rate) after at least 10 s of stimulation. Stimuli were applied in 3 min cycles on the most sensitive area of the SMU cutaneous receptive field. Figure 1 shows an original recording of responses evoked by noxious mechanical stimulation. The administration of the drugs only started when the responses were stable. Both fentanyl and dexketoprofen were dissolved in saline and injected intravenously in a constant volume of 0.3 ml. At the end of the experiment the animals were killed with an overdose of pentobarbitone (Euta Lender, Normon).
Figure 1.
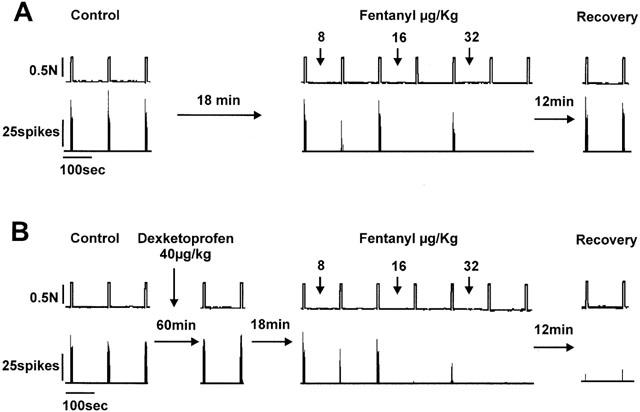
Original recording of a single motor unit activity evoked by noxious mechanical stimulation in the absence (A) and in the presence (B) of dexketoprofen trometamol. (A) Shows the three control responses previous to the administration of cumulative doses of fentanyl (initial dose of 1 μg kg−1), the effect observed after the administration of fentanyl and the full recovery of responses 18 and 21 min after the administration of the last dose. (B) Shows the responses observed with the same unit 1 h later. In this case, the control responses were similar to those observed in the first study (A) and the administration of a total cumulative dose of 40 μg kg−1 of dexketoprofen trometamol did not induce any significant change. In the presence of dexketoprofen however, the administration of fentanyl induced a more potent reduction of nociceptive responses and the effect did not recover 21 min later.
Collection and analysis of data
Data from different experiments were pooled and analysed together as raw data and expressed as the mean±s.e.m. The responses observed after the administration of each drug were compared to the control response, control being the mean of the three cycles of responses previous to the administration of the first dose. Data used for comparison were the averaged responses obtained after each dose of fentanyl (two cycles of stimulation) and mean responses observed in the last four cycles of stimulation for each dose of dexketoprofen trometamol (seven cycles between doses). Statistical analysis were performed using one-way analysis of variance (ANOVA) for repeated measures with the non-parametric post-hoc Dunn test (GraphPad-Prism and GraphPad-Instat for Windows) to calculate the significant level of results in dose-response curves, and the t-test for comparison of ED50s. All the experiments in this study were carried out in accordance with European Union legislation regarding the use of animals for experimental protocols and all efforts were made to reduce the number of animals used.
Results
Effect of fentanyl in the absence and in the presence of dexketoprofen trometamol
The possible interaction between dexketoprofen trometamol and fentanyl was studied by comparing the effect of fentanyl alone and in the presence of subeffective doses of dexketoprofen trometamol under closely comparable conditions, i.e. following the same protocol and in the same animal and unit. An example of one of these experiments is shown in Figure 1. In the first test performed (Figure 1A) the administration of fentanyl caused total inhibition of the responses evoked by noxious mechanical stimulation with a cumulative dose of 32 μg kg−1. A full recovery was observed 18 min after the administration of the last dose. One hour later, the responses recorded were similar to those observed before the administration of fentanyl and after the full recovery of its effect (Figure 1B). The administration of dexketoprofen in three cumulative doses did not induce a significant reduction of responses 21 min after the administration of each dose (see below and Figures 1B and 3). The administration of fentanyl in the presence of dexketoprofen, however, was more potent (Figure 1B) than in the previous dose-response curve and the effect did not recover at all 21 min after the administration of the last dose. Figure 2A shows pooled data of the results observed with the two dose-response curves of fentanyl previous to and after the administration of dexketoprofen. The μ opiate fentanyl was very effective in the two tests performed: maximum effect of 21±10 in the absence of dexketoprofen and 2±1.6% of control response in the presence of dexketoprofen (n=8). Previous to the administration of dexketoprofen, the minimum significant effective dose (MED) was 32 μg kg−1 and the ED50 was 22.4±1.5 μg kg−1. In all cases the effect induced by the administration of fentanyl fully recovered between 15 to 20 min after the last dose used (see below), and control responses previous to the administration of the second dose-response curve of fentanyl were similar to those obtained in the first study (see example in Figure 1B). Fentanyl, tested a second time in the presence of dexketoprofen, was much more potent in reducing nociceptive responses when compared to the first dose-response curve (Figure 2A). In this case, the MED was 8 μg kg−1, and the ED50 was more than 5 fold lower than that observed in the first test: 3.8±1.1 μg kg−1 (P<0.001, n=8). The administration of dexketoprofen trometamol at cumulative doses of 40 μg kg−1, 1 h after the first test performed with fentanyl, did not cause any significant reduction in SMU responses to mechanical stimulation. Figure 3 shows pooled data of the responses recorded 21 min after the administration of dexketoprofen and, as can be observed, the maximum reduction of responses was of 91±8% of control response with the last dose administered.
Figure 3.
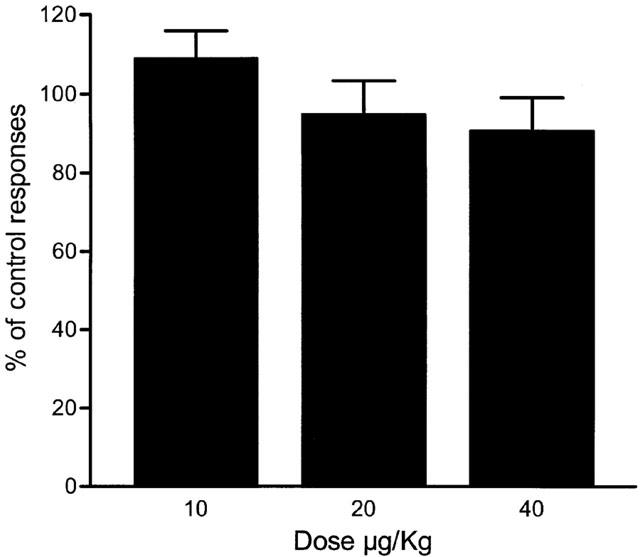
Lack of effect of dexketoprofen trometamol. The administration of three cumulative doses of dexketoprofen trometamol did not induce any significant reduction of single motor unit responses to noxious mechanical stimulation. Each dose of dexketoprofen trometamol was administered every 21 min following the same protocol as that in other studies (Mazario et al., 2001).
Figure 2.
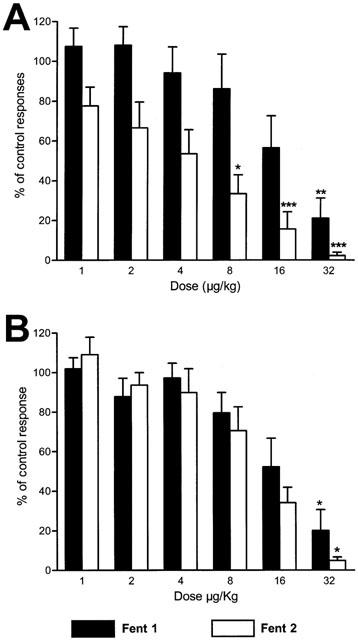
(A) Pooled data of the antinociceptive effects obtained with cumulative i.v. administrations of the μ-opioid agonist fentanyl previous to (Fent 1) and after the administration of 40 μg kg−1 of the NSAID dexketoprofen trometamol (Fent 2). The diagram represents the percentage of reduction of SMU responses to noxious mechanical stimulation. The potency of fentanyl was enhanced 5 fold by the NSAID whereas the administration of dexketoprofen trometamol on its own did not induce any significant reduction of responses (see Figure 3). (B) Shows the reduction of responses induced by fentanyl in control experiments when tested twice after an interval of 1 h. No significant differences were observed in this case between the two tests performed (statistical comparison was performed using the one-way analysis of variance, ANOVA, for repeated measures with the non-parametric post-hoc Dunn test, *P<0.05, **P<0.01, ***P<0.001).
Effect of fentanyl in control experiments
In order to test that the administration of fentanyl a second time was not influenced by the previous test, control experiments were performed studying the effect of fentanyl twice in the absence of dexketoprofen (Figure 2B), following the same protocol as that in the previous group of experiments. The reduction of SMU nociceptive responses was, in this case, similar in the two tests performed. No significant differences were observed either in the potency or efficacy of fentanyl or when individual doses were compared between the two tests. The mean ED50 in all the experiments performed was 20±1.3 μg kg−1 (n=3), not significantly different to that obtained with fentanyl previous to the administration of dexketoprofen in the first group of experiments (22.4±1.5 μg kg−1, n=8).
Duration of the effect of fentanyl and lack of reversal by naloxone
The duration of the antinociceptive effect of fentanyl was different in the presence of dexketoprofen when compared to that in the first test or in control experiments. The inhibition of SMU responses induced by the administration of fentanyl fully recovered between 15 and 20 min after the last dose used (see example in Figure 1A). In the presence of dexketoprofen, however, no recovery of the effect induced by the administration of fentanyl was observed at this time (Figure 1B). Figure 4 shows the mean recovery observed 15 min after the last dose (68.6±11% in respect to the control response, n=8). When fentanyl was administered in the presence of dexketoprofen, no significant recovery was observed either 15 min (9.8±9% of control response) or 45 min (16.7±7%, n=8) after the administration of the last dose (Figure 4). In three experiments, the effect of fentanyl was challenged with 200 μg kg−1 of the opiate antagonist naloxone, but no recovery was observed (13.7±6%, Figure 4, n=3). To test the effectiveness of naloxone, three more experiments were performed injecting 200 μg kg−1 of naloxone 6 min before the administration of a single dose of 32 μg kg−1 of fentanyl (Figure 5). In this case, the administration of fentanyl did not induce any depression of SMU responses.
Figure 4.
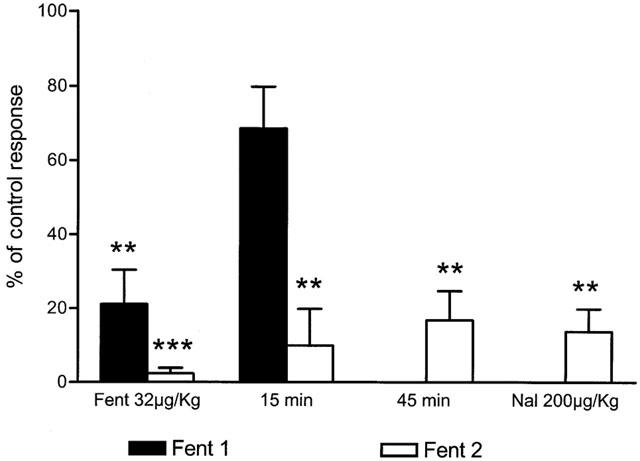
Duration and effect of naloxone on the antinociceptive effect of fentanyl previous to (Fent 1) and after the administration of 40 μg kg−1 of the NSAID dexketoprofen trometamol (Fent 2). The Figure illustrates the effect induced by the cumulative dose of 32 μg kg−1 of fentanyl and the recovery observed 15 min later. In presence of dexketoprofen trometamol, no significant recovery was observed 15 and 45 min after the last dose. No recovery was also observed after the i.v. administration of 200 μg kg−1 of the opiate antagonist naloxone (statistical significance and layout as for Figure 2).
Figure 5.
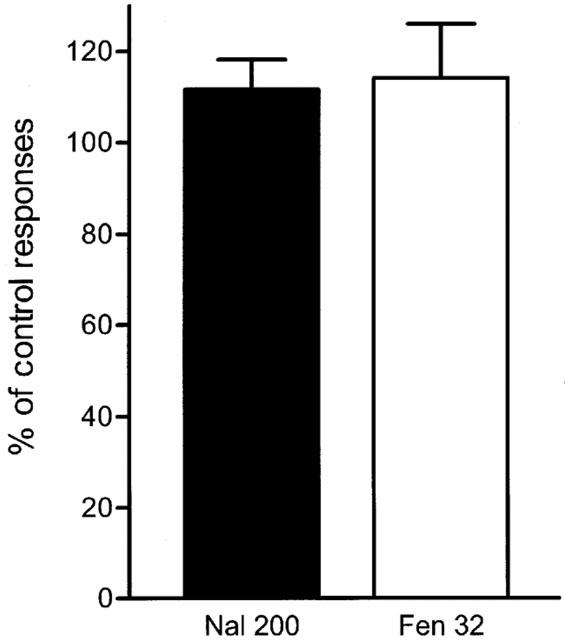
Control experiments performed with naloxone and fentanyl. In order to check the effectiveness of the dose used of naloxone, three experiments were performed injecting 200 μg kg−1 of naloxone 6 min before the administration of a single dose of 32 μg kg−1 of fentanyl. Pooled data show no effect of fentanyl in the presence of naloxone, confirming the effectiveness of the opioid receptor antagonist in the present experiments.
Discussion
The main observation made in this study is the enhancement of the potency and duration of fentanyl-induced analgesia when tested in the presence of subeffective doses of the NSAID dexketoprofen trometamol. We have previously shown that dexketoprofen trometamol is an effective analgesic drug either in the normal situation or in monoarthritic animals (Mazario et al., 2001). Also, the analgesic efficacy of the μ-opiate fentanyl in similar experimental conditions is well known (Herrero & Headley, 1991; Thorn et al., 1994), and, although an increase in the analgesic potency of other opiates have been observed when combined with some NSAIDs (Malmberg & Yaksh, 1993; Maves et al., 1994; Vaughan et al., 1997; Melis et al., 2000), such a potent increase in the analgesic effect of an opiate by the administration of subeffective doses of an NSAID has not been shown. These results therefore show that it is not necessary for an NSAID to induce any significant analgesia to enhance the effects of μ-opiates, and that the interaction between these two types of drugs occurs at sub-therapeutic concentrations. The modulatory effect is probably due to an action at central sites, since dexketoprofen crosses the blood-brain barrier easily (see references in Mazario et al., 2001) and no obvious peripheral target is apparent since no inflammation was present. Furthermore, these data reveal a probable relationship between the endogenous opioid system and cyclooxygenase-1 (COX-1), since COX-2 should not be present in a high concentration due to the lack of inflammation.
More interestingly, the effect of fentanyl is not only increased but also its duration of action is augmented. This observation may be very useful in the treatment of pain with fentanyl and its derivatives since one of the main problems with the use of this opiate is its rapid metabolism, and therefore the need for a continuous administration.
The fact that fentanyl was tested twice in the same animal and unit permitted a direct comparison between the two tests under comparable conditions, but also raises the possibility of interactions between the two tests, although if anything, repetitive administration of the opiate would be expected to cause tolerance and therefore a reduction in potency (Herrero & Solano, 1999). Control experiments performed in this study also reveal that the potency of fentanyl was similar when the two tests performed in each experiment were compared. In other words, the second dose-response curve performed with fentanyl was not influenced by the previous administration of the opiate 1 h earlier. Recovery was also similar for each dose-response curve and comparable to that observed in other studies (Herrero & Headley, 1991; 1996).
Another important observation made in this study is the lack of recovery of the analgesic effect induced by fentanyl in the presence of dexketoprofen after the administration of the opioid receptor antagonist naloxone. A dose of 20 μg kg−1 of naloxone is sufficient to prevent the analgesic actions of fentanyl in experiments performed in similar conditions (Herrero & Headley, 1991). In the present study, we used a dose of 200 μg kg−1 of naloxone and no recovery was observed. In control experiments however the same dose was able to fully prevent any effect of the opiate. We therefore conclude that the enhancement of the opiate-analgesic actions induced by dexketoprofen trometamol was not a result of a direct interaction on opioid receptors. Since fentanyl is a very selective μ-opioid agonist, and therefore a mechanism of action apart from an interaction on μ-opioid receptors is not likely to occur, we interpret these results as a positive modulation of COX-mediated effects by the opiate. Further experiments to confirm this hypothesis are being performed in this laboratory.
In conclusion, an important enhancement of the potency and duration of the analgesic effects of fentanyl is induced by the previous administration of subeffective doses of the NSAID dexketoprofen trometamol. This enhancement is not reversed by the opiate antagonist naloxone.
Acknowledgments
This work was supported by Laboratorios Menarini S.A. (Barcelona, Spain) and by the Comunidad de Madrid, grant 08.5/0068/2000. We thank Mr K. Robinson for his help with the English version and Paula Mascias for her help in preliminary experiments.
Abbreviations
- ANOVA
analysis of variance
- COX
cyclo-oxygenase
- ED50
effective dose 50
- MED
minimum effective dose
- NSAIDs
non-steroidal antiinflammatory drugs
- SMU
single motor unit
References
- ALLAN L., HAYS H., JENSEN N.H., DE WAROUX B.L., BOLT M., DONALD R., KALSO E. Randomised crossover trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. Br. Med. J. 2001;322:1154–1160. doi: 10.1136/bmj.322.7295.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVER W.T. Combination analgesics. Am. J. Med. 1984;77:38–53. doi: 10.1016/s0002-9343(84)80101-1. [DOI] [PubMed] [Google Scholar]
- GAITAN G., HERRERO J.F. Enhancement of the analgesic effect of the μ opiate fentanyl by the NSAID dexketoprofen trometamol. Inflamm. Res. 2001;50 S3:S179. [Google Scholar]
- HERRERO J.F., HEADLEY P.M. The effects of sham and full spinalization on the systemic potency of μ- and k-opioids on spinal nociceptive reflexes in rats. Br. J. Pharmacol. 1991;104:166–170. doi: 10.1111/j.1476-5381.1991.tb12402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRERO J.F., HEADLEY P.M. Reversal by naloxone of the spinal antinociceptive actions of a systemically-administered NSAID. Br. J. Pharmacol. 1996;118:968–972. doi: 10.1111/j.1476-5381.1996.tb15494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERRERO J.F., SOLANO R.E. The antinociceptive effect of the μ-opioid fentanyl is reduced in the presence of the α2-adrenergic antagonist idazoxan in inflammation. Brain Res. 1999;840:106–114. doi: 10.1016/s0006-8993(99)01780-1. [DOI] [PubMed] [Google Scholar]
- MALMBERG A.B., YAKSH T.L. Pharmacology of the spinal action of ketorolac, morphine, ST-91, U50488H, and L-PIA on the formalin test and an isobolographic analysis of the NSAID interaction. Anesthesiology. 1993;79:270–281. doi: 10.1097/00000542-199308000-00012. [DOI] [PubMed] [Google Scholar]
- MAVES T.J., PECHMAN P.S., MELLER S.T., GEBHART G.F. Ketorolak potentiates morphine antinociception during visceral nociception in the rat. Anesthesiology. 1994;80:1094–1101. doi: 10.1097/00000542-199405000-00018. [DOI] [PubMed] [Google Scholar]
- MAZARIO J., ROZA C., HERRERO J.F. The NSAID dexketoprofen trometamol is as potent as μ-opioids in the depression of wind-up and spinal cord nociceptive reflexes in normal rats. Brain Res. 1999;816:512–517. doi: 10.1016/s0006-8993(98)01203-7. [DOI] [PubMed] [Google Scholar]
- MAZARIO J., GAITAN G., HERRERO J.F. Cicloxygenase-1 versus Cicloxygenase-2 inhibitors in the induction of antinociception in rodent withdrawal reflexes. Neuropharmacology. 2001;40:937–945. doi: 10.1016/s0028-3908(01)00020-x. [DOI] [PubMed] [Google Scholar]
- MELIS M., DIANA M., GESSA G.L. Cyclo-oxygenase-inhibitors increase morphine effects on mesolimbic dopamine neurons. Eur. J. Pharmacol. 2000;387:R1–R3. doi: 10.1016/s0014-2999(99)00799-2. [DOI] [PubMed] [Google Scholar]
- SCHOUENBORG J., WENG H.R. Sensorimotor transformation in a spinal motor system. Exp. Brain Res. 1994;100:170–174. doi: 10.1007/BF00227291. [DOI] [PubMed] [Google Scholar]
- SOLANO R.E., HERRERO J.F. Cutaneous responsiveness of rat single motor units activated by natural stimulation. J. Neurosci. Methods. 1997;73:135–140. doi: 10.1016/s0165-0270(96)02220-0. [DOI] [PubMed] [Google Scholar]
- THORN S.A., HERRERO J.F., HEADLEY P.M. Stimulus intensity and the comparative efficacy of μ- and k-opioid agonists on nociceptive spinal reflexes in the rat. Brain Res. 1994;663:352–356. doi: 10.1016/0006-8993(94)91286-6. [DOI] [PubMed] [Google Scholar]
- VAUGHAN C.W., INGRAM S.L., CONNOR M.A., CHRISTIE M.J. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]


