Abstract
Unfractionated heparin (UH) has been shown to possess a wide range of properties which are potentially anti-inflammatory. Many of these studies, including effects of heparin on adhesion of inflammatory cells to endothelium, have been carried out in vitro. In the present study, we have used radioisotopic techniques to study the effect of UH, and related molecules, on in vivo inflammatory responses (plasma exudation (PE) and PMN accumulation) in rabbit skin induced by cationic proteins, mediators and antigen.
Intradermal (i.d.) pretreatment with UH dose-dependently inhibited poly-L-lysine (PLL)-induced responses. The same treatment had no effect on antigen (extract of Alternaria tenuis, AT)-, formyl-methionyl-leucyl-phenylalanine (fMLP)- or leukotriene (LT) B4-induced responses, although i.d. dextran sulphate (DS) significantly inhibited responses to all of these mediators.
High dose (10,000 u kg−1) intravenous UH significantly decreased cutaneous responses to fMLP and LTB4. By comparison, the selectin inhibitor, fucoidin, and DS, were very effective inhibitors of these responses, and of responses to AT and PLL.
In contrast to the weak effect in the in vivo studies, UH significantly inhibited in vitro homotypic aggregation of rabbit PMNs, showing that it can modify PMN function.
Our data with i.d. UH confirm the important ability of this molecule to interact with and neutralize polycationic peptides in vivo, suggesting that this is a prime role of endogenous heparin. The lack of effect of exogenous heparin on acute inflammatory responses induced by allergen, suggests that cationic proteins are unlikely to be primary mediators of the allergen-induced PE or PMN accumulation.
Keywords: Glycosaminoglycan, heparin, trafficking, PMNs, plasma exudation, cutaneous, inflammation
Introduction
In an inflammatory response, leukocytes must leave the vascular lumen and enter the tissue where they perform effector functions. This process consists of three stages; rolling, triggering and firm adhesion and is orchestrated by cell adhesion molecules, namely, selectins (P-, E- and L-), β2 integrins (CD11b/CD18, on PMNs) and immunoglobulin-like cell adhesion molecules (such as intercellular cell adhesion molecule-1, ICAM-1; Pilewski & Albelda, 1993). Leukocytes cause damage within the tissue by release of polycationic molecules, cytotoxic enzymes and, through release of chemotactic factors, cause further leukocyte recruitment, thus increasing the potential for damage. Therefore, substances with the ability to inhibit leukocyte accumulation or to inhibit the activity of cytotoxic mediators have potential for use in the treatment of diseases where there is a large inflammatory exudate.
Heparin is a glycosaminoglycan (GAG), a member of a family of linear anionic heteropolysaccharides, comprised of repeating disaccharide units of either D-glucosamine or D-galactosamine and uronic acid (Tyrrell et al., 1995) and has long been used as an anticoagulant and an antithrombotic agent. Recently, there has been considerable interest in the potential anti-inflammatory properties of this drug including inhibition of leukocyte recruitment (reviewed by Tyrrell et al., 1999). It has been suggested that heparin may inhibit leukocyte accumulation by interfering with the function of cell adhesion molecules. For example, heparin chains have been shown to inhibit both P- and L- selectin binding to bovine serum albumin (BSA) sialyl Lewis X (sLex) in an enzyme-linked immunosorbent assay, and to inhibit binding of neutrophils to Chinese hamster ovary cells expressing P-selectin, but not to those expressing E-selectin (Nelson et al., 1993). Heparin has also been shown to inhibit P-selectin adhesion to neutrophils and also P- and L- selectin mediated binding to sLex or P-selectin glycoprotein ligand-1 (PSGL-1) on human leukaemic cell line (HL-60) cells (Skinner et al., 1991).
In vitro studies have shown heparin to inhibit homotypic aggregation of neutrophils, the production of superoxide and activation of platelets by neutrophils (Bazzoni et al., 1993), neutrophil chemotaxis (Matzner et al., 1984), adhesion of fMLP-stimulated neutrophils to resting endothelium (Bazzoni et al., 1993) and adhesion of non-activated neutrophils to platelet activating factor (PAF)-stimulated endothelial cells (Silvestro et al., 1994). Of particular relevance to allergic responses, heparin has been reported to inhibit mast cell degranulation induced by anti-immunoglobulin (Ig) E, compound 48/80 or inositol triphosphate (IP3) (Lucio et al., 1992; Ahmed et al., 1994).
There are many reports of the ability of heparin to inhibit neutrophil rolling in vivo, for example, in the mesenteric circulation in the rabbit (Ley et al., 1991; Tangelder & Arfors, 1991). In addition, Xie et al. (1997) reported that local administration of heparin inhibited firm adhesion of leukocytes to rat mesenteric endothelium. Intravenous heparin has been reported to inhibit PAF- induced airways hyperresponsiveness and pulmonary cell infiltration in the rabbit (Sasaki et al., 1993), although heparin, in the same species, failed to affect antigen-induced pulmonary leukocyte accumulation, whilst successfully inhibiting hyperresponsiveness (Preuss & Page, 2000). In the guinea-pig, intravenous heparin inhibited PAF- and antigen-induced eosinophil accumulation in the lung (Seeds et al., 1993) and aerosolized heparin inhibited antigen-induced eosinophil (but not neutrophil) pulmonary accumulation in the same model (Seeds & Page, 2001). In addition, heparin has been demonstrated to inhibit antigen-induced airway and cutaneous responses in allergic sheep undergoing isolated acute allergic responses (Ahmed et al., 1993; 1994; 1996), although no measurement of leukocyte accumulation was made in these studies. In fact, very little research has been conducted on the effect of heparin on leukocyte transmigration in the skin in vivo. Intradermal heparin has been shown to inhibit eosinophil accumulation induced by a range of inflammatory stimuli in guinea-pig skin (Teixeira & Hellewell, 1993).
Heparin has been shown to be released following mast cell degranulation (Green et al., 1993) and increased circulating levels of heparin-like material have been reported in patients with allergic asthma (Lasser et al., 1987). However, the role of endogenous heparin remains obscure. In an attempt to clarify the effects of heparin in vivo, we have investigated the ability of unfractionated heparin (UH) and related molecules, administered via the intradermal and intravenous routes, to inhibit plasma exudation (PE) and neutrophil accumulation (PMNA) in a range of inflammatory responses in rabbit skin. We examined polycation, mediator and antigen dependent inflammation. Poly-L-lysine (PLL), a synthetic polycation, has been used as a paradigm for cationic proteins released from inflammatory cells (Vehaskari et al., 1984; Needham et al., 1988; Antunes et al., 1990). The chemoattractants, f-methionyl-leucyl-phenylalanine (fMLP) and leukotriene (LT) B4 were included as they induce PMN dependent PE in rabbit skin (Wedmore & Williams, 1981). Platelet activating factor (PAF) causes increased permeability independently of the presence of neutrophils. We have also used a well characterized allergic rabbit model (Minshall et al., 1993) to investigate the action of UH on an allergen (Alternaria tenuis extract) -induced response.
Methods
Animals
All experiments were carried out on New Zealand White (NZW) rabbits. Animals were fed a normal diet and received water ad libitum. All naïve rabbits used were male and sensitized rabbits were used litter by litter.
Sensitization protocol
Sensitization of NZW rabbits was performed using a protocol similar to that reported by Shampain et al. (1982). Rabbits were injected intraperitoneally on the day of birth with 0.5 ml of a mixture of Alternaria tenuis extract (40,000 PNU ml−1) and aluminium hydroxide (Al(OH)3) moist gel diluted 1 : 1 with 0.9% sterile saline, in the ratio 2 : 1 : 1 by volume. Intraperitoneal allergen injection was repeated weekly for the first month and then biweekly for the next 2 months.
Isolation and radiolabelling of rabbit PMNs.
Rabbit PMNs were prepared using the method described by Jones et al. (2001). Briefly, arterial blood was taken from pentobarbitone anaesthetized rabbits into acid citrate dextrose (ACD) and red blood cells were removed by sedimentation using Hespan, followed by hypotonic lysis and PMNs isolated by centrifugation through Ficoll. The leukocyte pellets were combined and resuspended in 2 ml platelet poor plasma (PPP), and 10 μl taken for counting. The cell preparation was >95% neutrophils of which >95% were viable (Jones et al., 2001). Cells were incubated at room temperature with mercaptopyridine (5 min) followed by 111In Cl3 (11.1 MBq, 30 min). At the end of this period, 3×10 μl samples were removed for counting to obtain a measurement of total radioactivity. Excess Indium was removed by washing and the cells were resuspended in 2 ml PPP, and 10 μl removed for counting. The PMN preparation was injected at a concentration of between 0.7 and 1×107 cells kg−1, depending on the number of cells available and the weight of the recipient rabbits.
Skin tests
Recipient NZW rabbits (2.3–3.0 kg) were anaesthetized with sodium pentobarbitone (30 mg kg−1) and the dorsal and lateral fur was shaved at least 1 h before intradermal injections. The radiolabelled cells were injected i.v. and, 1 h later, 0.18 MBq of 125I-BSA mixed with ∼2 ml Evans Blue solution, (25 mg ml−1 in 0.9% sterile NaCl) was injected i.v. into each animal. Mediators were injected (0.1 ml site−1), using 27G needles, into marked intradermal sites according to a balanced Latin square design which allowed for inter site variation. Saline controls were included in each experiment. UH, other GAGs, sulphated dextrans (DS) or poly-L-glutamic acid (PGA) were injected intradermally or intravenously, at doses specified in the text, 10 min prior to the mediator injection.
Preparation of agents for injection
All agents were diluted to the desired concentration in 0.9% sterile, pyrogen free saline immediately before use. Stimuli were injected at the following doses: fMLP, 10−11 moles site−1, LTB4, 10−10 moles site−1, PAF, 10−9 moles site−1. These were mixed with PGE2 (3×10−10 moles site−1) in accordance with the two mediator hypothesis (Williams & Peck, 1977; Williams et al., 1983). Extract of Alternaria tenuis was used at a dose of 200 p.n.u. site−1, and PLL, at 100 μg site−1. These doses were determined from earlier dose response experiments (Jones et al., 2001). Although earlier experiments showed that it was not necessary to mix PLL with PGE2 to obtain adequate PE and PMNA responses (Jones et al., 2001), in the present study PLL was, in some instances, mixed with PGE2, and these are specified in the text and Figure legends. This was performed in order to rule out the possibility that the inhibitory effect of UH on PLL (an effect which was not observed on fMLP- or LTB4-induced responses) might have been due to the fact that PLL was the only stimulus not to be mixed with PGE2. A wide range of doses of UH was used due to earlier reports that heparin can exhibit bell-shaped dose-response curves (for example, Gorski et al., 1991; Itoh et al., 1995).
Preparation of blood and plasma samples
At the end of the allocated time period, animals were anaesthetized as described above, 10 ml blood collected by cardiac puncture into heparinized tubes and the animals sacrificed by sodium pentobarbitone overdose. Whole blood samples (2×1 ml) were taken and the remainder centrifuged to obtain plasma (2×1 ml).
Preparation of skin samples
The skin was removed and the treatment sites excised with a 17 mm diameter punch. Skin sites were counted along with cell, blood and plasma samples in an automated gamma spectrometer (LKB, Wallac 1282 Compugamma, CS) with windows set for 111In and 125I. Correction for the spillover of radioisotope emissions from the 111In channel to the 125I channel was performed on the data.
Measurement of precipitation of UH and related molecules with PLL or antigen
Equal volumes (100 μl) of PLL (1 mg ml−1) or Alternaria tenuis (2000 p.n.u. ml−1) and various solutions (at concentrations equivalent to the doses used in the skin, see text) of UH, Fragmin (F), dextran sulphate (DS), N-desulphated heparin (NDSH), O-desulphated heparin (ODSH) or poly-l-glutamic acid (PGA) were mixed in a 96 well plate and the interaction measured by the light absorbance at 450 nm on a plate reader (ELx808, Bio-tek Instruments Inc.). All values were converted to change in absorbance by subtracting the absorbance values of PLL or AT mixed with saline.
In vitro aggregation of rabbit PMNs
Rabbit PMNs were isolated as described above. The leukocyte pellet was resuspended at a concentration of 5×106 ml−1. Two hundred and fifty μl samples were placed in 1 ml cuvettes, in a Payton aggregometer and were heated to 37°C and constantly stirred. After 1 min fMLP or PAF (10−9–10−5 M) were added in a volume of 2.5 μl saline. Homotypic aggregation of PMNs was measured as the change in transmittance of light passing through the cuvette over a 3 min period, against cell free plasma. For inhibitory experiments, UH was placed in the cuvette at the same time as the cell sample.
Calculation of results
Blood and plasma samples were counted to determine 125I-counts / μl plasma and the level of free 111In in plasma (which was below 15% in all cases). PE was expressed as equivalent μl plasma skin site−1 as calculated by skin sample 125I count / 125I count in 1 μl plasma. PMN accumulation was expressed as the number of cells site−1 as calculated by (total 111In counts in skin site–111In-counts accounted for by the plasma volume in the site)/111In counts per PMN.
Materials
Dextran (MW10,000, MW500,000), dextran sulphate (MW10,000), poly-L-lysine hydrobromide (150–300 kD), poly-L-glutamic acid, N-desulphated heparin, Evans Blue dye, sodium pentobarbitone, Ficoll Histopaque-1077, sterile water, Trizma base, citric acid, EDTA, fMLP, LTB4, PAF, PGE2, fucoidin, 10× HANKS solution and mercaptopyridine were purchased from Sigma, Poole, Dorset, U.K. Hespan (6% hydroxyethylcellulose) was purchased from Dupont Pharmaceuticals, Letchworth, Herts, U.K. D-glucose was purchased from Fisher, Loughborough, U.K. and trisodium citrate from BDH Chemicals, Lutterworth, U.K. Unfractionated heparin (Multiparin) was purchased from CP Pharmaceuticals, Wrexham, U.K. and Fragmin from Pharmacia Laboratories, Milton Keynes, U.K. O-desulphated heparin was a gift from Dr T. Kennedy, University of North Carolina, Rayleigh, NC, U.S.A. Lignavet (lignocaine hydrochloride) was purchased from Veterinary Sciences, U.K. Sterile saline was purchased from Baxter Healthcare Ltd., Thetford, Norfolk, U.K. 111Indium chloride was purchased from Amersham International plc., Buckinghamshire, U.K. 125Iodinated-bovine serum albumin was purchased from ICN Pharmaceuticals. Alternaria tenuis was obtained from Greer Laboratories, Illinois, U.S.A.
Statistical analysis
All data are presented as mean±s.e.mean for the indicated number (n) of experiments. Data were analysed by two-way analysis of variance (ANOVA) and significant differences were determined by the Bonferroni post test. Where three groups were compared, a multiple comparison ANOVA followed by unpaired t-test was performed.
Results
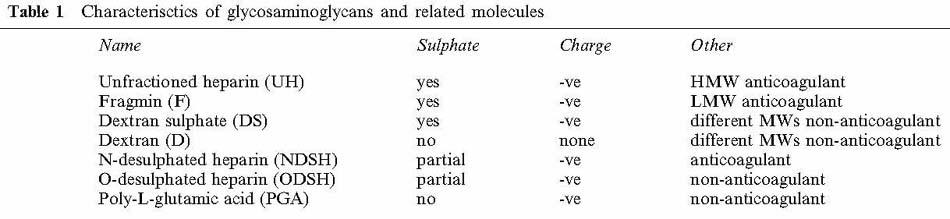
A list of the glycosaminoglycans and related molecules, and their properties is shown in Table 1.
Table 1.
Characterisctics of glycosaminoglycans and related molecules
Intradermal pretreatment
Effects of intradermal pretreatment with various GAGs and related molecules on poly-L-lysine (PLL)-induced PE and PMNA
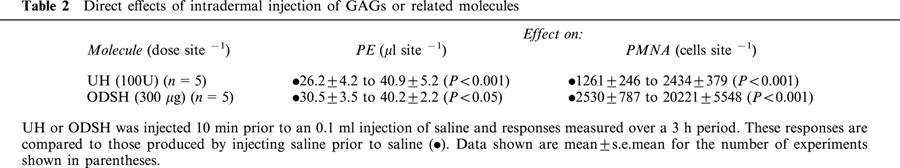
(i) Direct effects of intradermal injection of GAGs or related molecules. UH and ODSH caused significant increases in both PE and PMNA above those caused by injection of saline (Table 2). NDSH, PGA, Fragmin, dextran sulphate and dextran did not cause significant PE or PMNA compared to saline (data not shown).
Table 2.
Direct effects of intradermal injection of GAGs or related molecules
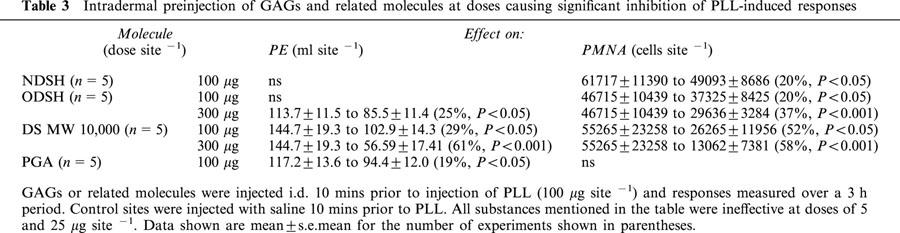
(ii) Effects of intradermal GAGs and related molecules on PLL- induced responses. We have previously shown that i.d. injection of PLL causes a dose and time dependent PE and PMNA response and that the PE response is PMN independent in the rabbit (Jones et al., 2001). Ten minute i.d. pre-injection of UH (Figure 1a) or the low molecular weight heparin, Fragmin or a sulphated dextran (Table 3), caused dose dependent inhibition of PLL-induced PE and PMNA responses. The partially sulphated derivatives, NDSH and ODSH, and the non-sulphated molecule, PGA, were less effective than UH, but any lack of effect was overcome by increasing the dose (Table 3). For example, at 500 μg site−1, NDSH and PGA significantly reduced PLL-induced PE and PMNA (Figure 1b). A non-charged dextran molecule, at MWs of 10,000 or 500,000 (up to 3 mg site−1) had no effect (n=5) on PLL-induced responses (data not shown).
Figure 1.
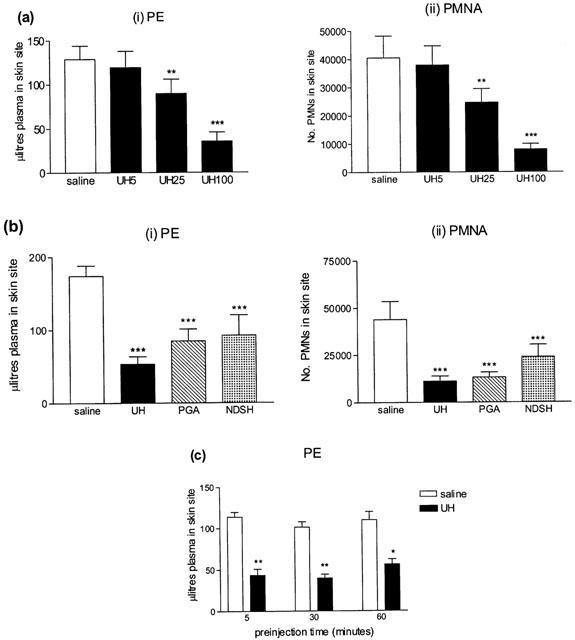
Effect of a 10 min intradermal preinjection of (a) unfractionated heparin (UH, 5–100 u site−1) or (b) unfractionated heparin (UH, 100 u site−1), poly-L-glutamic acid (PGA, 500 μg site−1) or N-desulphated heparin (NDSH, 500 μg site−1) on (i) plasma exudation (PE) and (ii) 111In-labelled PMN accumulation (PMNA) responses in rabbit skin to poly-L-lysine (PLL, 100 μg site−1) measured over 3 h. (c) Measurement of plasma exudation (PE) in rabbit skin 3 h after injection of PLL (100 μg site−1) following pretreatment, at different time intervals (5–60 min), with UH (100 u site−1) or saline. Mean±s.e.mean values (n=5) are shown.
Table 3.
Intradermal preinjection of GAGs and related molecules at doses causing significant inhibition of PLL-induced responses
(iii) Effect of intradermal preinjection with UH at different time intervals. UH significantly inhibited PLL-induced PE responses when injected 5 (P<0.01), 30 (P<0.01) or 60 (P<0.05) min prior to PLL (Figure 1c).
Effects of intradermal injection of various GAGs and related molecules on antigen-induced PE and PMNA
UH (5–100 u site−1, n=5), Fragmin (F, 10–250 u site−1, n=6) and PGA (140–3000 μg site−1, n=5) had no significant effect on antigen-induced PE or PMNA responses (data not shown). In the same animals, the highest dose of each of these substances significantly inhibited PLL-induced responses included as positive controls. Dextran sulphate (MW 10,000, 556 μg site−1), significantly inhibited antigen-induced PE and, at all doses tested, significantly inhibited PMNA (Figure 2a). At a high dose of 3000 μg site−1, DS inhibited both PE and PMNA in response to PLL (Figure 2a). The non-charged molecule, dextran (in the dose range 140–3000 μg site−1), at MWs of either 10,000 or 500,000 (both n=5) had no significant effect on antigen-induced responses (data not shown).
Figure 2.
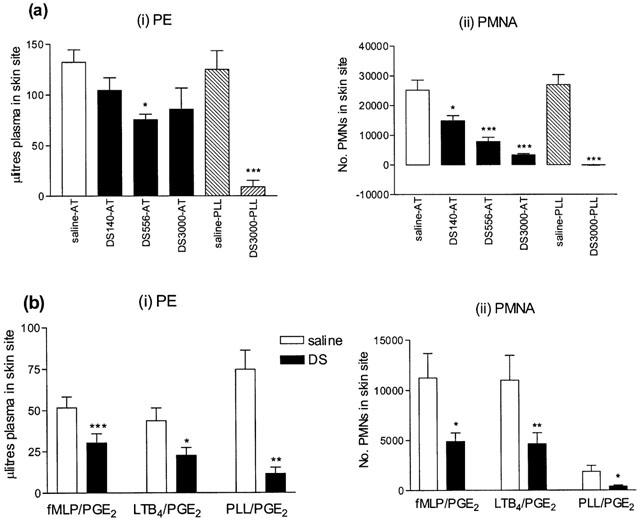
Effect of a 10 min intradermal preinjection of dextran sulphate (DS, 140–3000 μg site−1) on (i) plasma exudation (PE) and (ii) 111In-labelled PMN accumulation (PMNA) responses in rabbit skin to (a) antigen (AT, 200 p.n.u. site−1) measured at 3 h, and (b) f-met-leu-phe (fMLP, 10−11 moles site−1), leukotriene B4 (LTB4, 10−10 moles site−1) or poly-L-lysine (PLL, 100 μg site−1) all mixed with PGE2 (3×10−10 moles site−1) measured at 45 min. Mean±s.e.mean values (n=4, n=6 respectively) are shown.
Measurement of interaction of GAGs and related molecules with PLL or antigen
UH, Fragmin, dextran sulphate, PGA, NDSH and ODSH all caused precipitation of PLL (Figure 3a,b). Absorbance values for saline, AT and PLL ranged between 0.0318 and 0.0427. Upon mixture with PLL, UH gave the lowest maximum change in absorbance (0.28) and ODSH and NDSH the highest (0.89 and 0.81 respectively). Dextran sulphate, Fragmin and PGA produced Δ absorbance values of between 0.4 and 0.5. Peak absorbance measurements were found to occur at a concentration of 250 u ml−1 (∼1.4 mg ml−1) for UH, 1000 u ml−1 (∼5.6 mg ml−1) for Fragmin, 1 mg ml−1 for ODSH and PGA, 5 mg ml−1 for NDSH and dextran sulphate (Figure 3a,b). Neither UH nor dextran sulphate produced an observable precipitate when mixed with extract of Alternaria tenuis (AT) and absorbance measurements of these mixtures were not significantly different from those produced by antigen alone (Figure 3c).
Figure 3.
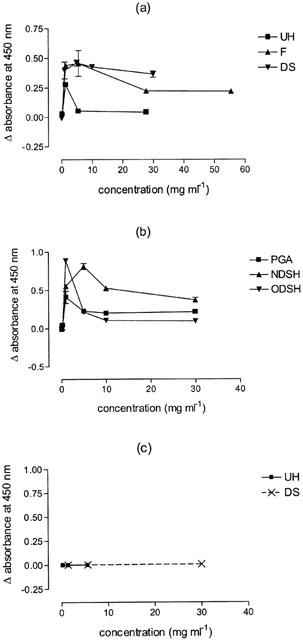
Precipitation measured as Δ light absorbance at 450 nm, of mixtures of 100 μl volumes of unfractionated heparin (UH), Fragmin (F), dextran sulphate (DS) (a), N-desulphated heparin (NDSH), O-desulphated heparin (ODSH) or poly-L-glutamic acid (PGA) (b), with 100 μl volumes of a 1 mg ml−1 solution of the polycation (PLL) and 100 μl volumes of UH or DS with 100 μl volumes of a 2000 p.n.u. ml−1 solution of Alternaria tenuis (c). Values are corrected for controls. Mean±s.e.mean values (n=4) are shown.
Effects of intradermal injection of various GAGs and related molecules on fMLP-and LTB4-induced PE and PMNA
I.d. injection of UH (100 u site−1) had no significant inhibitory effect on PE or PMNA responses, measured at 45 min or 3 h, induced by fMLP or LTB4, although PLL-induced responses were significantly reduced. Similarly, PGA (556 μg or 1.5 mg site−1) had no effect on fMLP- or LTB4-induced responses, but significantly reduced PLL-induced responses (data not shown). Dextran sulphate (556 μg site−1) significantly inhibited PMNA (30890±5990 to 24730±5915 cells site−1, P<0.05) but not PE induced by LTB4. PLL-induced responses were also significantly inhibited (PE: 88.2±8.5 to 10.0±2.6 μl site−1, P<0.05, PMNA: 8840±1269 to 1193±62 cells site−1, P<0.01). At 3 mg site−1 dextran sulphate significantly inhibited both PE and PMNA in response to i.d. injection of fMLP (P<0.001; P<0.05) and LTB4 (P<0.05; P<0.01, Figure 2b). No precipitate was formed upon mixture of the relevant concentrations of DS with fMLP or LTB4 (data not shown).
Intravenous pretreatment
Effects of intravenous injection of various GAGs and related molecules on responses measured at 45 min
UH at doses of 10, 100, 1000 (all n=5, data not shown) and 5000 u kg−1 (Figure 4a) had no significant inhibitory effect on PE or PMNA responses to fMLP, LTB4, PAF or PLL. In fact, with 5000 u kg−1, the PE response to PLL was significantly increased (Figure 4a), although we have no explanation for this observation, since no such effect was seen with a dose of 10,000 u kg−1. At 10,000 u kg−1 fMLP-induced PE (P<0.05) and PMNA (P<0.01) and LTB4-induced PMNA (P<0.001) were significantly inhibited, whilst PAF- and PLL-induced responses were unaffected (Figure 4b). I.v. pretreatment with DS at 25 mg kg−1 (which, by mass, approximates to 5000 u kg−1 of UH), significantly inhibited fMLP- and LTB4-induced PE (P<0.05, P<0.01) and PMNA (P<0.05) and PMN accumulation in response to PLL (P<0.01). However, PAF-induced responses were unaffected (Figure 4a).
Figure 4.
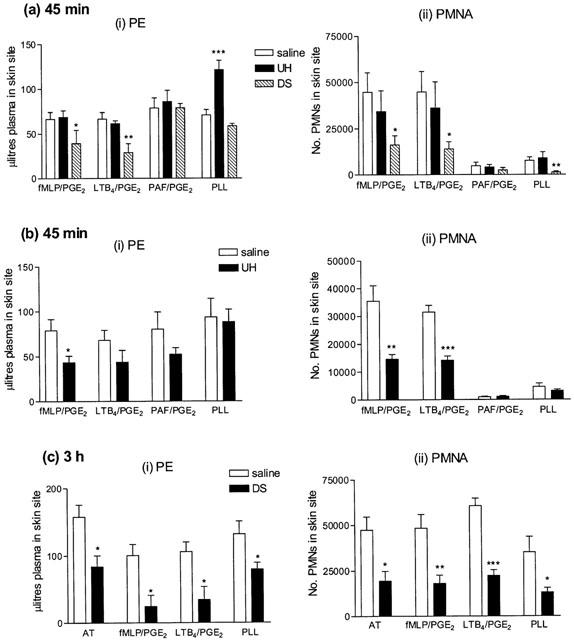
Effect of a 10 min intravenous preinjection of (a) unfractionated heparin (UH, 5000 u kg−1), dextran sulphate (DS 25 mg kg−1, n=6), (b) unfractionated heparin (UH, 10,000 u kg−1, n=5) measured at 45 min or (c) dextran sulphate (DS 25 mg kg−1, n=5) measured at 3 h on (i) plasma exudation (PE) and (ii) 111In-labelled PMN accumulation (PMNA) responses in rabbit skin sites to the neutrophil dependent mediators fMLP (10−11 moles site−1) and LTB4 (10−10 moles site−1), the neutrophil independent mediator PAF (10−9 moles site−1), all mixed with PGE2 (3×10−10 moles site−1), and poly-L-lysine (PLL, 100 μg site−1). Mean±s.e.mean values (n=6) are shown.
Effect of intravenous injection of GAGs and related molecules on responses measured at 3 h
Experiments with intravenous UH were repeated in rabbits sensitized to Alternaria tenuis, using the same doses of fMLP, LTB4 and PLL, but also including an extract of Alternaria tenuis to elicit an allergic response. This response was, in earlier experiments, shown to cause PE and PMNA over at least a 3 h time period and the PE response was partially neutrophil dependent (Jones et al., 2001). UH or related substances were again injected i.v. 10 min before i.d. injection of the inflammatory stimuli.
UH, at doses of 100 (n=5) or 5000 (n=4) u kg−1 exhibited no significant inhibitory effect on PE or PMNA responses to antigen or any of the inflammatory mediators investigated (data not shown). Similarly, injection of the same dose of an uncharged dextran (n=4) or the non-sulphated, anionic molecule, PGA (n=5), had no effect on PE or PMNA in response to antigen, fMLP, LTB4 or PLL (data not shown). At 10,000 u kg−1, UH significantly inhibited only fMLP-induced PE (89.3±21.7 to 34.5±4.6 μl site−1, P<0.05, n=5). DS (25 mg kg−1) significantly inhibited PE and PMNA responses to all four mediators tested (Figure 5a). Fucoidin, a selectin inhibitor, at a dose of 5 mg kg−1, significantly inhibited PMNA responses to antigen, fMLP, LTB4 and PLL (P<0.05). It also significantly reduced PE responses to the neutrophil dependent mediators, fMLP and LTB4 (P<0.01), but not those to antigen or PLL (Figure 5b).
Figure 5.
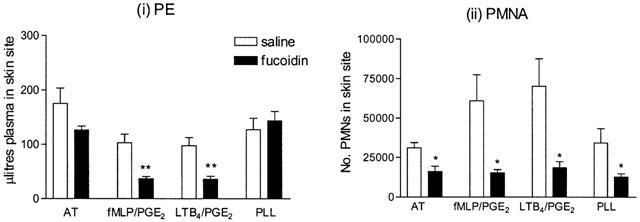
Effect of a 10 min intravenous preinjection of fucoidin (5 mg kg−1) on (i) plasma exudation (PE) and (ii) 111In-labelled PMN accumulation (PMNA) responses in rabbit skin sites to the neutrophil dependent mediators fMLP (10−11 moles site−1) and LTB4 (10−10 moles site−1), mixed with PGE2 (3×10−10 moles site−1), antigen (AT, 200 p.n.u. site−1) and poly-L-lysine (PLL, 100 μg site−1). Responses were measured at 3 h. Mean±s.e.mean values (n=5) are shown.
In vitro aggregation of rabbit neutrophils
To investigate the possible effects of UH on PMN homotypic interactions, an in vitro system for PMN aggregation was used. Isolated rabbit PMNs in vitro aggregated in a concentration-dependent manner to both fMLP and PAF, (Figure 6a). Responses to fMLP (10−6 M) or PAF (10−6 M) were significantly inhibited by UH (50–10,000 u ml−1, P<0.05, Figure 6b).
Figure 6.
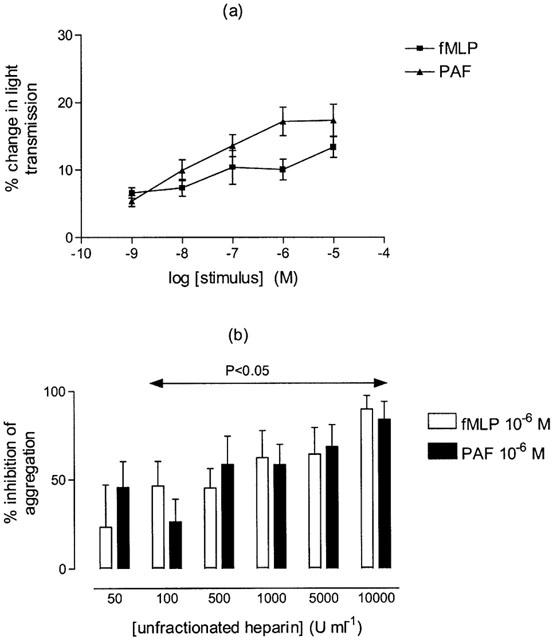
(a) Homotypic aggregation of rabbit polymorphonuclear cells (PMNs), at 37°C, under constant stirring, induced by formyl-methionyl-leucyl-phenylalannine (fMLP) or platelet activating factor (PAF) (10−9 m–10−5 M). (b) Inhibition by unfractionated heparin (UH, 50–10,000 u ml−1) of fMLP or PAF-induced homotypic aggregation of rabbit PMNs. Mean±s.e.mean values (n=6) are shown.
Discussion
We have previously reported that intradermal administration of PLL induces PE and PMNA in rabbit skin (Jones et al., 2001) and, in the present experiments we have demonstrated that 10 min intradermal preinjection of the highly sulphated molecules UH, Fragmin or dextran sulphate all significantly inhibited PLL-induced PE and PMNA. UH had direct effects on PE and PMNA, which were significantly greater than those caused by saline alone, but these increases were small in comparison to those caused by PLL (PE: 11%; PMNA: 3%). The partially sulphated molecules NDSH (which retains anticoagulant activity) and ODSH (which is devoid of anticoagulant activity, Fryer et al., 1997), also inhibited PLL-induced PMNA (although the latter had marked direct effects on PE and PMNA, which were markedly greater than those caused by UH, although the mechanism for this has yet to be investigated). These results indicate that the inhibitory action of UH on PLL-induced cutaneous responses is independent of its molecular weight and of its anticoagulant activity, supporting a considerable body of evidence showing that many of the biological effects of GAGs are independent of anticoagulant activity (Tyrrell et al., 1999; Lever et al., 2000). When the pretreatment dose of PGA was increased to 500 μg site−1, it significantly inhibited PLL-induced PE and PMNA, indicating that the ability of a molecule to inhibit PLL-induced inflammatory responses may, in part, be related to its anionic nature. This conclusion is supported by the lack of inhibition seen with the uncharged molecule, dextran, an inability which could not be overcome by substantially increasing the molecular weight of the dextran. Inhibition of PLL-induced responses is likely to be due to ability to interact with the polycation since the capacity to cause precipitation of PLL, measured by the change in light absorbance at 450 nm, correlated with activity as in vivo inhibitors of PLL-induced responses.
Indeed, Mulloy et al. (1996), using a circular dichroism method, reported that heparin interacts with PLL to produce an α-helical formation. Furthermore, heparin is known to inhibit, through interaction with critically positioned positively charged amino acids, both mitogen activated kinase (Ottlinger et al., 1993) and caesin kinase II (Hathaway et al., 1980).
Intradermal UH (or Fragmin) had no effect on antigen-induced cutaneous responses, although PLL-induced responses, included as positive controls, were significantly inhibited. Intradermal dextran sulphate, however, significantly inhibited both antigen-induced PE and PMNA. Light absorbance measurements of mixtures of dextran sulphate and antigen suggested that inhibition of antigen-induced responses by dextran sulphate was not a result of direct interaction between these substances. This is further supported by the fact that DS appeared to have a more selective effect on PMNA than on PE responses. The anionic, but non-sulphated molecule, PGA, exhibited no inhibitory effect on antigen-induced responses, as was the case for uncharged dextran.
These results also demonstrate the dichotomy between antigen and PLL induced inflammation. We have discussed previously the similar, prolonged time course of active cutaneous anaphylactic (ACA) and PLL-induced responses, and also the differences between these two responses (Jones et al., 2001). Needham et al. (1988) investigated the possible endogeneous mediators of the PLL-induced response and found that prostaglandins and kinins contribute to the PLL-induced response, whereas inhibition of leukotriene, PAF or histamine had no effect. Hellewell et al. (1992) have published work regarding the passive cutaneous anaphlactic response which, like PLL, was determined to be unaffected by inhibition of histamine, PAF, 5-lipoxygenase, and also by bradykinin antagonists, kallikrein inhibitors and an anti C5a antibody. This response was inhibited by indomethacin, indicating a role for prostaglandins and some similarity with the PLL response. However, to the best of our knowledge, there currently exists no equivalent data for the ACA response. The ability of UH to inhibit PLL- but not antigen-induced responses suggests that cation release does not contribute greatly, if at all, to the ACA response.
Intravenous injection of UH (10, 100, 1000, and 5000 u kg−1) had no significant effect on PE or PMNA (measured at 45 min) to any of the stimuli used. However, at a dose of 10,000 u kg−1, UH significantly inhibited fMLP-induced PE and PMNA, and LTB4-induced PMNA. A sulphated dextran, at a dose of 25 mg kg−1, significantly inhibited both PE and PMNA to fMLP and LTB4, and also PLL-induced PMNA. Although both UH and dextran sulphate are highly sulphated, whilst dextran sulphate significantly inhibited cutaneous inflammatory responses, UH, administered at a similar dose (5000 u kg−1), was without effect. As the experiments with both of these substances were performed over the same time period, it is possible that the former may either remain in the bloodstream, or bound to the endothelium, for a longer period of time. Indeed, Ley et al. (1991), when testing a number of natural and synthetic polysaccharides, found that dextran sulphate was the most potent inhibitor of leukocyte rolling in rabbit mesentery venules. This group suggested that heparin and other sulphated polysaccharides interacted with the leukocyte rather than the endothelium. Our own in vitro results on homotypic aggregation support this concept since we have shown UH to have an inhibitory effect on PMNs.
The lack of effect of intravenous UH on antigen-induced responses supports the work of others who have reported that UH does not significantly inhibit the mast cell mediated early asthmatic response in subjects with atopic asthma (Diamant et al., 1996) or acute bronchospasm in the airways of allergic guinea-pigs (Howell & Woeppel, 1993) or rabbits (Preuss & Page, 2000). Furthermore, heparin does not inhibit acute inflammatory responses in the skin of allergic sheep exhibiting dual inflammatory responses, but does inhibit the acute allergic response in sheep exhibiting isolated early responses (Ahmed et al., 1996). In contrast to the lack of effect of UH, dextran sulphate at a dose of 25 mg kg−1 significantly inhibited PE and PMNA to antigen, fMLP, LTB4 and PLL measured at 3 h. Our results are in agreement with those published by Van Osselaer et al. (1993) showing the same dose of dextran sulphate to inhibit fMLP-induced PMNA and PE in rabbit skin. The same dose of dextran (which is uncharged) had no significant effect on any of the parameters investigated. Similarly, pretreatment with the negatively charged, but non-sulphated molecule, PGA (25 mg kg−1), failed to have any significant inhibitory effect. These results would suggest that inhibition of cutaneous responses by intravenous treatments is dependent upon sulphation. Nonetheless, UH had no effect on antigen-induced responses, although it did inhibit significantly, PE and PMNA responses induced by fMLP or LTB4 over 45 min.
In order to compare the actions of the GAGs tested with a known inhibitor of cell adhesion molecules, the selectin inhibitor, fucoidin, was investigated. Fucoidin is an algal polymer of fucose-4-sulphate, which binds to, and inhibits, both L- and P-selectin, but has very little effect on E-selectin (Skinner et al., 1991; Varki, 1994). It has been reported that systemic administration of fucoidin inhibits basal and stimulated leukocyte rolling in postcapillary venules (Ley et al., 1993) and eosinophil accumulation in the skin (Teixeira & Hellewell, 1997). In the model used here, i.v. fucoidin (5 mg kg−1), significantly inhibited PMNA responses to all three mediators and to antigen. PE responses to the neutrophil dependent mediators (fMLP and LTB4) were also significantly reduced. The discriminatory effect (significant effect on PMNA but not on PE) of fucoidin on antigen (partially neutrophil dependent PE) and PLL (neutrophil independent PE) responses (Jones et al., 2001), suggests that the mechanism of action was, indeed, inhibition of cell adhesion or trafficking. In view of the lack of effect of UH, our results suggest that it is unlikely that intravenous UH exhibits significant functional interaction with selectins in vivo.
In conclusion, in the rabbit, UH, by virtue of its high anionic charge, exerts an anti-inflammatory effect by neutralizing polycations. UH significantly inhibited PLL-induced PE when injected 60 min before injection of PLL, thus, intradermally administered UH is not rapidly removed from the injection site, suggesting that heparin released into tissues may stay active for a lengthy period of time. By comparison, in the rabbit, intravenous UH has a short plasma half life of 15–20 min (Palm & Mattsson, 1987) and is subject to uptake and catabolism by endothelial cells, probably serving to protect against haemorrhage, the result of which leaves the molecule devoid of biological activity. Together, these results suggest that inhibition of polycationic molecules released from activated inflammatory cells is a major role of endogenous heparin. Indeed, the cationic protein, heparin-binding protein (HBP) has been shown to be released from neutrophils upon adhesion to and, to induce cytoskeletal rearrangement and intercellular gap formation in endothelial monolayers in vitro (Gautam et al., 2001). Our data also suggest that the mechanisms of allergen-induced PE and PMNA is unlikely to be secondary to release of endogenous cationic proteins. A sulphated dextran was an effective inhibitor of cutaneous responses, whereas non-sulphated or non-charged molecules were ineffective. Although these results indicate a role for sulphation in the inhibition of PE and PMNA by GAGs, the effects of an endogenous sulphated molecule, UH, were variable. Therefore, the absolute role of sulphation in this context has not been resolved here and, indeed, the mechanism by which GAGs cause inhibition of PE and PMNA may not be common to all molecules, warranting further investigation.
Abbreviations
- AT
Alternaria tenuis extract
- D
dextran
- DS
dextran sulphate
- F
Fragmin
- fMLP
formyl-methionyl-leucyl-phenylalanine
- GAG
glycosaminoglycan
- 125I-BSA
125Iodinated bovine serum albumin
- 111In
111Indium chloride
- LTB4
leukotriene B4
- NDSH
N-desulphated heparin
- ODSH
O-desulphated heparin
- PAF
platelet activating factor
- PE
plasma exudation
- PGA
poly-L-glutamic acid
- PGE2
prostaglandin E2
- PLL
poly-L-lysine
- PMNA
polymorphonuclear leukocyte accumulation
- u
units
- UH
unfractionated heparin
References
- AHMED T., D'BROT J., ABRAHAM W.M., LUCIO J., MENDELSSOHN R., ROBINSON M.J., SHAKIR S., SANPEDRO B. Heterogeneity of allergic airway responses in sheep. Differences in signal transduction. Am. J. Respir. Crit. Care Med. 1996;154:843–849. doi: 10.1164/ajrccm.154.4.8887573. [DOI] [PubMed] [Google Scholar]
- AHMED T., SYRISTE T., LUCIO J., ABRAHAM W., ROBINSON M., D'BROT J. Inhibition of antigen-induced airway and cutaneous responses by heparin: a pharmacodynamic study. J. Appl. Physiol. 1993;74:1492–1498. doi: 10.1152/jappl.1993.74.4.1492. [DOI] [PubMed] [Google Scholar]
- AHMED T., SYRISTE T., MENDELSSOHN R, , SORACE D, , MANSOUR E., LANSING M., ABRAHAM W.M., ROBINSON M.J. Heparin prevents antigen-induced airway hyperresponsiveness: interference with IP3-mediated mast cell degranulation. J. Appl. Physiol. 1994;76:893–901. doi: 10.1152/jappl.1994.76.2.893. [DOI] [PubMed] [Google Scholar]
- ANTUNES E., MARIANO M., CIRINO G., LEVI S., DE NUCCI G. Pharmacological characterization of polycation-induced rat hind-paw oedema. Br. J. Pharmacol. 1990;101:986–990. doi: 10.1111/j.1476-5381.1990.tb14193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAZZONI G., BELTRAN NUNEZ A., MASCELLANI G., BIANCHINI P., DEJANA E., DEL MASCHIO A. Effect of heparin, dermatan sulfate, and related oligo-derivatives on human polymorphonuclear leukocyte functions. J. Lab. Clin. Med. 1993;121:268–275. [PubMed] [Google Scholar]
- DIAMANT Z., TIMERS M.C., VAN DER WEEN F.I., PAGE C.P., VAN DER MEIR F.J., STERK P.J. Effects of inhaled heparin on allergen induced early and late asthmatic responses in patients with atopic asthma. Am. J. Respir. Crit. Care Med. 1996;153:1790–1795. doi: 10.1164/ajrccm.153.6.8665036. [DOI] [PubMed] [Google Scholar]
- FRYER A., HUANG Y.-C., RAO G., JCOBY D., MANCILLA E., WHORTON R., PIANTADOSI C.A., KENNEDY T., HOIDAL J. Selective O-desulphation produces nonanticoagulant heparin that retains pharmacological activity in the lung. J. Pharmacol. Exp. Ther. 1997;282:208–219. [PubMed] [Google Scholar]
- GAUTAM N., OLOFSSON A.M., HERWALD H., INVERSEN L.F., LUNDGREN-KERLUND E., HEDQVIST P., ARFORS K.-E., FLODGAARD H., LINDBOM L. Heparin-binding protein (HBP/CAP37): A missing link in neutrophil-evoked alteration of vascular permeability. Nature Med. 2001;7:1123–1127. doi: 10.1038/nm1001-1123. [DOI] [PubMed] [Google Scholar]
- GORSKI A., WASIK M., NOWACZYK M., KORCZAK-KOWALSKA G. Imunomodulating activity of heparin. FASEB J. 1991;5:2287–2291. doi: 10.1096/fasebj.5.9.1860620. [DOI] [PubMed] [Google Scholar]
- GREEN W.F., KONNARIS K., WOOLCOCK A.J. Effect of salbutamol, fenoterol and sodium cromoglycate on the release of heparin from sensitised human lung fragments challenged with Dermatophagoides pteronyssinus allergen. Am. J. Respir. Cell Mol. Biol. 1993;8:518–521. doi: 10.1165/ajrcmb/8.5.518. [DOI] [PubMed] [Google Scholar]
- HATHAWAY G.M., LUBBEN T.H., TRAUGH J.A. Inhibition of caesin kinase II by heparin. J. Biol. Chem. 1980;255:8038–8041. [PubMed] [Google Scholar]
- HELLEWELL P.G., JOSE P.J., WILLIAMS T.J. Inflammatory mechanisms in the passive cutaneous anaphylactic reaction in the rabbit: evidence that novel mediators are involved. Br. J. Pharmacol. 1992;107:1163–1172. doi: 10.1111/j.1476-5381.1992.tb13424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWELL R.E., WOEPPEL S.L. Potential species dependency for the pulmonary antiallergic effects of aerosolized heparin. Pulmon. Pharm. 1993;6:237–239. doi: 10.1006/pulp.1993.1031. [DOI] [PubMed] [Google Scholar]
- ITOH K., NAKAO A., KISHIMOTO W., TAKAGI H. Heparin effects on superoxide production by neutrophils. Eur. Surg. Res. 1995;27:184–188. doi: 10.1159/000129398. [DOI] [PubMed] [Google Scholar]
- JONES H., PAUL W., PAGE C.P. A comparison of allergen and polycation induced cutaneous responses in the rabbit. Br. J Pharmacol. 2001;133:1181–1189. doi: 10.1038/sj.bjp.0704172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASSER E.C., SIMON R.A., LYON S.G., HAMBLIN A.E., STEIN R. Heparin-like anticoagulants in asthma. Allergy. 1987;42:619–625. doi: 10.1111/j.1398-9995.1987.tb00393.x. [DOI] [PubMed] [Google Scholar]
- LEVER R., HOULT J.R.S., PAGE C.P. The effects of heparin and related molecules upon human polymorphonuclear leucocyte aggregation and adhesion in vitro. Br. J. Pharmacol. 2000;129:553–540. doi: 10.1038/sj.bjp.0703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEY K., CERRITO M., ARFORS K.E. Sulphated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am. J. Physiol. 1991;260:H1667–H1673. doi: 10.1152/ajpheart.1991.260.5.H1667. [DOI] [PubMed] [Google Scholar]
- LEY K., LINNERMANN G., MEINEN M., STOOLMAN L.M., GAEGHTGENS P. Fucoidin, but not yeast polyphosphomannan PPME, inhibits leukocyte rolling in venules of the rat mesentery. Blood. 1993;81:177–185. [PubMed] [Google Scholar]
- LUCIO J., D'BROT J., GUO C.-B., ABRAHAM W.M., LICHTENSTEIN L.M., KAGEY-SOBOTKA A., AHMED T. Immunologic mast cell-mediated responses and histamine release are attenuated by heparin. J. Appl. Physiol. 1992;73:1093–1101. doi: 10.1152/jappl.1992.73.3.1093. [DOI] [PubMed] [Google Scholar]
- MATZNER Y., MARX G., DREXLER R., ELDOR A. The inhibitory effect of heparin and related glycosaminoglycans on neutrophil chemotaxis. Thromb. Haemost. 1984;52:134–137. [PubMed] [Google Scholar]
- MINSHALL E.M., RICCIO M.M., HERD C.M., DOUGLAS G.J., SEEDS E.A.M., MCKENNIFF M.G., SASAKI M., SPINA D., PAGE C.P. A novel animal model for investigating persistent airway hyperresponsiveness. J. Pharmacol. Toxicol. Methods. 1993;30:177–188. doi: 10.1016/1056-8719(93)90015-7. [DOI] [PubMed] [Google Scholar]
- MULLOY B., CRANE D.T., DRAKE A.F., DAVIES D.B. The interaction between heparin and poly-L-lysine: a circular dichroism and molecular modelling study. Braz. J. Med. Biol. Res. 1996;29:721–729. [PubMed] [Google Scholar]
- NEEDHAM L., HELLEWELL P.G., WILLIAMS T.J., GORDON J.L. Endothelial functional responses and increased vascular permeability induced by polycations. Lab. Invest. 1988;59:538–548. [PubMed] [Google Scholar]
- NELSON R.M., CECCONI O., ROBERTS W.G., ARUFFO A., LINDHART R.J. Heparin oligosaccharides bind L- and P- selectins and inhibit acute inflammation. Blood. 1993;82:3253–3258. [PubMed] [Google Scholar]
- OTTLINGER M.E., PUKAC L.A., KARNOVSKY M.J. Heparin inhibits mitogen activated protein kinase activation in intact rat vascular smooth muscle cells. J. Biol. Chem. 1993;268:19173–19176. [PubMed] [Google Scholar]
- PALM M., MATTSSON C.H. Pharmacokinetics of heparin and low molecular weight heparin fragment (Fragmin®) in rabbits with impaired renal or metabolic clearance. Thromb. Haem. 1987;58:932–935. [PubMed] [Google Scholar]
- PILEWSKI J.M., ALBELDA S.M. Adhesion molecules in the lung. An overview. Am. Rev. Respir. Dis. 1993;148:S31–S37. doi: 10.1164/ajrccm/148.6_Pt_2.S31. [DOI] [PubMed] [Google Scholar]
- PREUSS J.M.H., PAGE C.P. Effect of heparin on antigen-induced airway responses and pulmonary leukocyte accumulation in neonatally immunized rabbits. Br. J. Pharmacol. 2000;129:1585–1596. doi: 10.1038/sj.bjp.0703247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SASAKI M., HERD C.M., PAGE C.P. Effect of heparin and a LMW heparinoid on PAF induced airway responses in neonatally immunised rabbits. Br. J. Pharmacol. 1993;110:107–112. doi: 10.1111/j.1476-5381.1993.tb13778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEEDS E.A.M., PAGE C.P. Heparin inhibits allergen-induced eosinophil infiltration into guinea-pig lung via a mechanism unrelated to its anticoagulant activity. Pulm. Pharmacol. Ther. 2001;14:111–119. doi: 10.1006/pupt.2000.0277. [DOI] [PubMed] [Google Scholar]
- SEEDS E.A.M., HANSS J., PAGE C.P. The effect of heparin and related proteoglycans on allergen and PAF induced eosinophil infiltration. J. Lipid Med. 1993;7:269–278. [PubMed] [Google Scholar]
- SHAMPAIN M.P., BEHRENS B.L., LARSEN G.L., HENSON P.M. An animal model of late pulmonary responses to Alternaria challenge. Am. Rev. Respir. Dis. 1982;126:493–498. doi: 10.1164/arrd.1982.126.3.493. [DOI] [PubMed] [Google Scholar]
- SILVESTRO L., VIANO I., MACARIO M., COLANGELO D., MONTRUCCHIO G., PANICO S., FANTOZZI R. Effects of heparin and its desulfated derivatives on leukocyte-endothelial adhesion. Semin. Thromb. Hemost. 1994;20:254–258. doi: 10.1055/s-2007-1001910. [DOI] [PubMed] [Google Scholar]
- SKINNER M.P., LUCAS C.M., BURNS G.F., CHESTERMAN C.N., BERNDT M.C. GMP-140 binding to neutrophils is inhibited by sulfated glycans. J. Biol. Chem. 1991;266:5371–5374. [PubMed] [Google Scholar]
- TANGELDER G.J., ARFORS L.E. Inhibition of leukocyte rolling in venules by protamine and sulphated polysaccharides. Blood. 1991;77:1565–1571. [PubMed] [Google Scholar]
- TEIXEIRA M.M., HELLEWELL P.G. Suppression by intradermal administration of heparin of eosinophil accumulation but not oedema formation in inflammatory reactions in guinea-pig skin. Br. J. Pharmacol. 1993;110:1496–1500. doi: 10.1111/j.1476-5381.1993.tb13991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA M.M., HELLEWELL P.G. The effect of the selectin binding polysaccharide fucoidin on eosinophil recruitment in vivo. Br. J. Pharmacol. 1997;120:1059–1066. doi: 10.1038/sj.bjp.0701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TYRRELL D.J., PAGE C.P., KILFEATHER S. Therapeutic uses of heparin beyond its traditional role as an anticoagulant. Tr. Pharmacol. Sci. 1995;16:198–204. doi: 10.1016/s0165-6147(00)89022-7. [DOI] [PubMed] [Google Scholar]
- TYRRELL D.J., HORNE A.P., HOLME K.R., PREUSS J.M.H., PAGE C.P.Heparin in inflammation: Potential Therapeutic Applications beyond Anticoagulation Advances in Pharmacology 1999San Diego: Academic Press; 151–189.ed. August, J.T., Aanders M.W., Murad, F., Coyle, J.T. Vol. 46. pp [DOI] [PubMed] [Google Scholar]
- VAN OSSELAER N., HERMAN A.G., RAMPART M. Dextran sulphate inhibits neutrophil emigration and neutrophil-dependent plasma leakage in rabbit skin. Agents Actions. 1993;38:C51–C53. doi: 10.1007/BF01991134. [DOI] [PubMed] [Google Scholar]
- VARKI A. Selectin ligands. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7390–7397. doi: 10.1073/pnas.91.16.7390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEHASKARI V.M., CHANG C.T.-C., STEVENS J.K., ROBSON A.M. The effects of polycations on vascular permeability in the rat. A proposed role for charge sites. J. Clin. Invest. 1984;73:1053–1061. doi: 10.1172/JCI111290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEDMORE C.V., WILLIAMS T.J. Control of vascular permeability by polymorphonuclear leukocytes in inflammation. Nature. 1981;289:646–650. doi: 10.1038/289646a0. [DOI] [PubMed] [Google Scholar]
- WILLIAMS T.J., PECK M.J. Role of prostaglandin-mediated vasodilatation in inflammation. Nature. 1977;270:530–532. doi: 10.1038/270530a0. [DOI] [PubMed] [Google Scholar]
- WILLIAMS T.J., JOSE P.J., WEDMORE C.V., PECK M.J., FORREST M.J. Mechanisms underlying inflammatory edema: the importance of synergism between prostaglandins, leukotrienes and complement-derived peptides. Adv. Prostaglan. Thrombox. Leukot. Res. 1983;11:33–37. [PubMed] [Google Scholar]
- XIE X., THORLACIUS H., RAUD J., HEDQUVIST P., LINDBOM L. Inhibitory effect of locally administered heparin on leukocyte rolling and chemoattractant-induced firm adhesion in rat mesenteric venules in vivo. Br. J. Pharmacol. 1997;122:906–910. doi: 10.1038/sj.bjp.0701454. [DOI] [PMC free article] [PubMed] [Google Scholar]


