Abstract
A hallmark for unstable bladder contractions is hyperexcitability and changes in the nature of spontaneous phasic activity of the bladder smooth muscle. In this study, we have characterized the spontaneous activity of the urinary bladder smooth muscle from the pig, a widely used model for studying human bladder function.
Our studies demonstrate that phasic activity of the pig detrusor is myogenic and is influenced by the presence of urothelium. Denuded strips exhibit robust spontaneous activity measured as mean area under the contraction curve (AUC=188.9±15.63 mNs) compared to intact strips (AUC=7.3±1.94 mNs).
Spontaneous phasic activity, particularly the amplitude, is dependent on both calcium entry through voltage-dependent calcium channels and release from ryanodine receptors as shown by inhibition of spontaneous activity by nifedipine and ryanodine respectively.
Inhibition of BKCa channels by iberiotoxin (100 nM) resulted in an increase in contraction amplitude (89.1±20.4%) and frequency (92.5±31.0%). The SKCa channel blocker apamin (100 nM) also increased contraction amplitude (69.1±24.3%) and frequency (53.5±13.6%) demonstrating that these mechanisms are critical to the regulation of phasic spontaneous activity.
Inhibition of KATP channels by glyburide (10 μM) did not significantly alter myogenic contractions (AUC=18.5±12.3%). However, KATP channel openers (KCOs) showed an exquisite sensitivity for suppression of spontaneous myogenic activity. KCOs were generally 15 fold more potent in suppressing spontaneous activity compared to contractions evoked by electrical field-stimulation. These studies suggest that potassium channel modulation, particularly KATP channels, may offer a unique mechanism for controlling spontaneous myogenic activity especially those associated with the hyperexcitability occurring in unstable bladders.
Keywords: Pig urinary bladder smooth muscle, spontaneous phasic activity, myogenic, potassium channels, overactive bladder, calcium-activated K+ channel, ATP-sensitive K+ channel, apamin, glyburide, iberiotoxin
Introduction
Urine storage and voluntary micturition are primary physiological functions of the normal bladder controlled by complex integration of signals from neural pathways (neurologic) from the brain and spinal cord and coordination of smooth muscle contraction from the bladder wall (myogenic) (de groat, 1997; Brading, 1997). Alterations in neural and/or myogenic mechanisms may underlie dysfunction of bladder smooth muscle contractility precipitating in bladder control disorders such as overactive bladder. Muscarinic antagonists, the current mainstay of pharmacotherapy for overactive bladder, interfere with the neurogenic stimulus for physiologic bladder contraction, viz., acetylcholine-induced stimulation of post-ganglionic parasympathetic muscarinic receptors in bladder smooth muscle (Eglen et al., 1999; Wein, 2001).
It is well known that smooth muscle from the detrusor exhibits spontaneous action potentials (Levin et al., 1986; Brading, 1992), a phenomenon thought to underlie spontaneous phasic activity. It has been reasoned that urinary bladder smooth muscle tone is a manifestation of the spontaneous activity seen in vitro in detrusor strips and that poor electrical coupling of detrusor smooth muscle facilitates muscle bundles to adjust their length to achieve the minimum surface area/volume ratio during bladder filling with no contraction or rise in intravesicular pressure (Levin et al., 1986; Kinder & Mundy, 1987; Brading, 1994). However, a disorder of this spontaneous activity may result in synchronous activation of the bladder wall leading to bladder instability (Mills et al., 2000). Spontaneous phasic activity has been suggested to be myogenic in nature as evidenced from studies in isolated in vitro bladder preparations where atropine and tetrodotoxin have no effect on electrical and mechanical activity of the bladder smooth muscle (Liu et al., 1998).
The pig detrusor has been considered an appropriate model for investigating the physiology of bladder function, as the pig has comparable urodynamic characteristics to that of humans (Crowe & Burnstock, 1989). Indeed, previous studies from our laboratory have shown that the pig detrusor could serve as a suitable in vitro model for the evaluation of ATP-sensitive potassium channel openers, a class of compounds with potential for the management of overactive bladder (Buckner et al., 2000). Despite the fundamental importance of these spontaneous phasic contractions to bladder (dys)function, little is known about the mechanisms and regulation of this contractile activity in the pig detrusor. Fujii et al. (1990) and more recently, Herrera et al. (2000) have shown that different types of K+ channels are present in the guinea-pig detrusor, whose modulation offers potential mechanism(s) for affecting bladder contractility and function. In particular, openers of various K+ channel subtypes that dampen bladder excitability continue to be investigated for the potential treatment of bladder overactivity (Andersson, 1992; Coghlan et al., 2001). The observation that the isolated pig detrusor strips exhibit phasic spontaneous activity has enabled a detailed characterization of the nature and ion channel mechanisms underlying these contractions.
Methods
Isolated tissue studies
Studies were carried out in accordance with guidelines outlined by the Institutional Animal Care and Use Committee of Abbott Laboratories. Female Landrace pigs (9 – 25 kg; 9 – 12 weeks old) were euthanized with an intraperitoneal injection of pentobarbitone (Somlethol®) at a lethal dose of 150 – 200 mg/kg. The urinary bladder was removed, placed in Kreb's Ringer bicarbonate solution (Composition (mM): NaCl 120, NaHCO3 20, dextrose 11, KCl 4.7, CaCl2 2.5, MgSO4 1.5, KH2PO4 1.2, equilibrated with 5% CO2 : 95% O2 (pH=7.4 at 37°C) and tissue strips prepared as described previously (Buckner et al., 2000). Detrusor strips 5 mm above the trigone and away from the dome area were used; trigone and dome proper were discarded. Tissue strips were sliced transversely into approximately 4×10 mm strips. The urinary epithelium that lines the bladder (urothelial layer) was removed unless otherwise indicated and the tissues mounted in 10 ml tissue baths maintained at 37°C with one end fixed to a stationary rod and the other to a FT03 transducer (Grass Instruments, Rhode Island, U.S.A.). Preload tension was set to 9.8 milli-Newtons (mN). Tissues were rinsed at 15-min intervals and allowed to equilibrate for at least 60 min prior to data collection using a Power-Lab/800 data acquisition system (Castle Hill, Australia).
Experimental protocol
To examine concentration-dependent effects on spontaneous phasic activity, test compounds were introduced and their effects assessed as area under the contractile curve (AUC) for a 15-min period before rinsing and exposure to the next concentration of the agent. Compounds were tested using concentrations in half log increments generally from 1 nM to 300 μM. In preliminary studies, it was found that the area of the detrusor away from the dome region of the bladder provided the most consistent spontaneous responses. It was also observed that tissues with the urothelium intact demonstrated a significantly reduced signal to noise ratio compared to adjacent strips where the urothelium was removed. Therefore, denuded tissue strips from the area adjacent to the trigone region (away-dome) were used throughout the study. Wherever concentration response curves were generated, each bladder strip was used for only one test compound.
Contractions evoked by electrical field-stimulation were examined as described previously (Buckner et al., 2000). Briefly, bladder strips were prepared as above and stimulated by a pair of platinum electrodes included in the glass support rod. Square wave electrical pulses were delivered by a Grass model S88 stimulator set at a frequency of 0.05 Hz, duration of 0.5 ms and 20 V (supramaximal). Cumulative concentration response curves were generated allowing a minimum of 10 min exposure time or until plateau responses were obtained. Data were analysed as changes in maximum peak response in mN.
In experiments where carbachol was used to evoke a contractile response, the protocol was non-cumulative with rinse cycles between each concentration of test compound. Tissues were exposed to the test compound for 15 min, and contractions evoked with 100 nM carbachol until the maximum tension developed. The tissues were then rinsed for 15 min, repeating the cycle for each concentration of test compound. Data were analysed as changes in maximum peak response in mN.
Data analysis
Due to the phasic pattern of the spontaneous contractile responses, data were analysed as average changes in area under the curve (AUC) over a 15-min period / min (ADI Instruments, CB Sciences, Inc., Dover, NH, U.S.A.) and expressed as milli-Newtons×sec (mNs). The data were also analysed for changes in frequency, amplitude and duration over a 15-min interval. Frequency is expressed as the number of phasic contractions per min. Amplitude is expressed as the change in maximum response in mN. Duration is the time in seconds from beginning of phasic contraction to return to baseline. For electrical field-stimulated and carbachol-stimulated protocols, data were analysed as the peak maximum response in mN.
The concentration response curves were analysed using a four-parameter curve fitting routine “AGANTG” (Zielinski & Buckner, 1998) and EC50 or IC50 data expressed as the geometric mean±standard error of the mean (s.e.mean). Significant differences between multiple groups were calculated by ANOVA, followed by a Duncan's test of significance (StatView 5.0.1, SAS Institute, Cary, NC, U.S.A.).
Compounds
Glyburide, diazoxide, pinacidil, nifedipine, tetrodotoxin and (±)-propranolol hydrochloride were purchased from Research Biochemicals International (Natick, MA, U.S.A.). Other KCOs including P1075 [N-cyano-N′-(1,1-dimethylpropyl)-N′′-3-pyridylguanidine], ZD6169 [N-(4-benzoyl phenyl)-3,3,3-trifluro-2-hydroxy-2-methylpropionamine], (−)-cromakalim, ZM244085 [9-(3-cyanophenyl)-3,4,6,7,9,10-hexahydro-1,8-(2H,5H)-acridinedione], WAY133537 [1,2-diaminocyclobutene-3,4-dione ((R)-4-[3,4-dioxo-2-(1,2,2-trimethyl-propylamino)-cyclobut-1-enylamino]-3-ethyl-benzonitrile] and nicorandil were synthesized in house or available from the Abbott compound library (Abbott Park, IL, U.S.A.). Somlethol was obtained from J.A. Webster, Inc. (Sterling, MA, U.S.A.). All other chemicals and reagents were purchased from Sigma Chemical Co (St. Louis, MO, U.S.A.). All solutions were freshly prepared using either distilled water or DMSO as solvent. At the concentrations tested, DMSO had no effect on the frequency, duration or amplitude of the contractions.
Results
Characteristics of spontaneous phasic activity and regional differences
The pig detrusor smooth muscle exhibited spontaneous phasic activity that was irregular in amplitude, frequency and duration in the absence of external stimuli. A distinct regional variation in the contractile profile was noted where strips taken from the away-dome region exhibited spontaneous phasic activity that were significantly greater in frequency duration and amplitude compared to strips taken from the near-dome area, consistent to the findings of Sibley (1984; Table 1).
Table 1.
Role of the urothelium in modulating the phasic activity of the pig detrusor
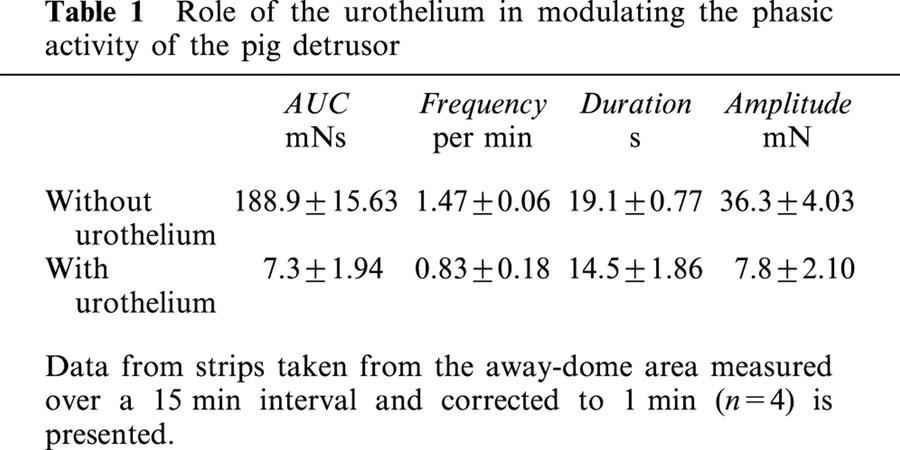
Role of the urothelium and geometry of section
Using matched away-dome bladder strips from the same bladder source, it was observed that tissue strips with urothelium removed (Figure 1A, Table 1) exhibited robust spontaneous phasic activity (AUC of the force integral=188.9±15.6 mNs; n=4), whereas intact strips exhibited modest spontaneous activity as reflected in a 26 fold reduction in the area under the curve (AUC=7.3±1.94 mNs; Figure 1B, Table 1). This overall reduction (AUC) in spontaneous phasic activity was manifested as reductions in amplitude (5 fold), frequency (2 fold) and duration (1.3 fold; Table 1). In this study, transverse tissue strips were taken from the area between the trigone and the dome (away-dome) with the urothelium removed. It has previously been reported that rabbit bladder strips sectioned longitudinally, exhibited spikes that were greater in amplitude than from strips sectioned in a transverse fashion (Potjer & Constantinou, 1989). Therefore, longitudinal strips from the pig bladder were also evaluated and found to behave similar to transverse sections both in spontaneous phasic contractions as well as in terms of inhibition of spontaneous activity by the potassium channel opener P1075 (data not shown).
Figure 1.
Functional role of the urothelium in modulating spontaneous phasic activity of pig bladder. Response of isolated strips taken from away-dome area without urothelium (A) and with urothelium (B). Data shown as the means±s.e.mean (n⩾4).
Frequency, duration and amplitude of the spontaneous phasic activity
In contrast to the rapid twitch responses evoked by low frequency electrical-stimuli (Buckner et al., 2000), the spontaneous contractions typically varied in frequency, duration and amplitude. The mean frequency of these spontaneous spikes was 1.83±0.69 contractions/min (n=20). The mean duration was 20.80±1.53 s whereas the mean maximum amplitude was 25.58±3.04 mN. Due to the variable pattern in the contractile responses, data were analysed by calculating the mean area under the contractile curve (AUC) relative to baseline for a 15-min interval, corrected to 1 min. No substantial changes in baseline were noted during the period over which AUC was calculated. The mean overall AUC response was 155.92±51.35 mNs (n=20). Within a set of tissue strips, the AUC values were stable for several hours when exposed to the DMSO vehicle.
Myogenic basis of spontaneous phasic activity
The muscarinic receptor antagonists examined, atropine, tolterodine and oxybutynin, were essentially inactive in suppressing spontaneous phasic activity of the pig detrusor. Over the concentration range tested, 1 nM to 30 μM, only about 12 – 13% inhibition of the AUC at the maximal concentration was noted and accordingly IC50 values or analysis of frequency, duration and maximum amplitude could not be performed. However, these agents suppressed both electrical field- and carbachol-stimulated contractions of the pig detrusor (Table 2). The maximal efficacy of these agents in suppressing electrical field stimulated contractions ranged from 43 to 56%; the residual component could be attributable to contribution from stimulus-evoked release of ATP (Buckner et al., 2000). When electrical field-stimulation was used to evoke contractions, oxybutynin demonstrated a biphasic response curve with IC50 values of 252±29.3 and 84,722±7,960 nM respectively. When carbachol was used to evoke contractions, both atropine and tolterodine totally suppressed contractions with IC50 values of 21.9±3.6 and 7.1±2.6 nM respectively. Oxybutynin, however, demonstrated a shallower, biphasic concentration response curve with IC50 values of 8.7±2.2 and 225±17.3 nM for the high and low affinity sites respectively.
Table 2.
Pharmacological differentiation of spontaneous phasic activity of the pig detrusor
As previously noted (Buckner et al., 2000), treatment of detrusor strips with tetrodotoxin (100 nM, 30 min exposure) suppressed contractions evoked by electrical field-stimulation, which was readily reversed upon 15 min of rinsing. However, a similar treatment protocol using a 10-fold higher concentration of tetrodotoxin (1 μM) did not affect overall spontaneous phasic activity AUC (decrease from control, 4.0±0.39%, n=4). The α1-adrenoceptor-antagonist prazosin, the β-adrenoceptor antagonist propranolol, the opioid receptor antagonist naloxone, and the cyclo-oxygenase inhibitor indomethacin (0.1 nM to 30 μM) had no significant effect on components of spontaneous phasic activity. Similarly, the purinoceptor antagonist PPADS (1 mM) was ineffective in inhibiting spontaneous phasic activity. Since none of these agents affected phasic spontaneous activity in the pig detrusor, these agents were not included in the assay as a cocktail for further studies.
Effect of extra- and intracellular calcium modulation
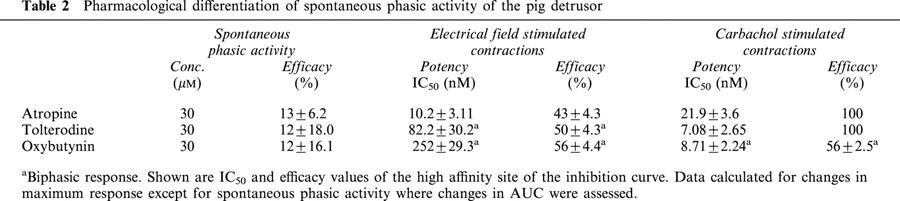
The L-type Ca2+ channel antagonist nifedipine completely inhibited spontaneous activity (AUC) in a concentration-dependent fashion with an IC50 value of 158.5±74.2 nM (n=4). As shown in Figure 2, nifedipine (300 nM) reduced the amplitude by 70.2±3.1% as well as the frequency (67.3±16.9%) of contractions. In contrast, blockers of other calcium channel classes such as, ω-conotoxin GVIA, ω-agatoxin TK and ω-conotoxin MVIIC that inhibit N, P, and Q-type Ca2+ channels respectively were ineffective in suppressing spontaneous phasic activity at a concentration of 300 nM.
Figure 2.
The effect of L-type Ca2+ channel inhibition on spontaneous phasic activity in the pig bladder. (A) Concentration response curve for nifedipine. Shown is a representative tracing. (B) Mean reductions in spontaneous phasic activity by 300 nM nifedipine expressed as a percentage change from baseline response. Data were analysed for changes in overall contractions (AUC), frequency, duration and amplitude. *Represents significant differences from baseline responses (P<0.05; n=4).
To assess the role of Ca2+ induced Ca2+ release via ryanodine-sensitive channels, tissues were exposed to 10 μM ryanodine (Figure 3A) that inhibits sarcoplasmic reticulum calcium release via ryanodine receptors. Application of ryanodine, 10 μM, suppressed myogenic activity as reflected by a significant decrease (46.5±8.3%) in the amplitude of contractions (Figure 3B). The latter suggests that ryanodine-mediated calcium release from the sarcoplasmic reticulum plays a key role in modulating spontaneous phasic activity of the bladder.
Figure 3.
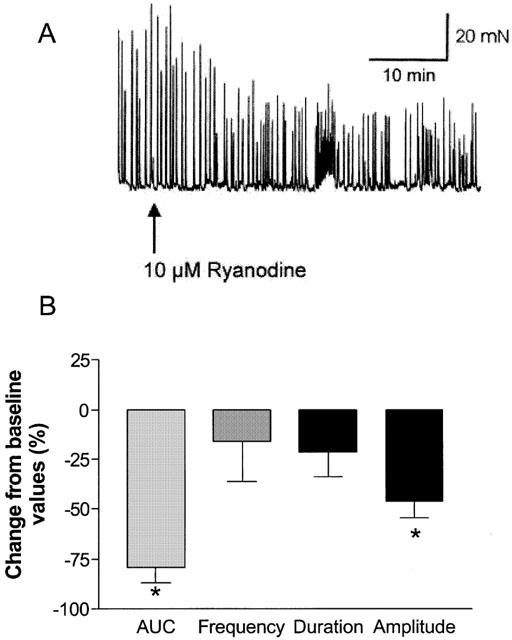
Effect of ryanodine on the spontaneous phasic activity of the pig bladder. (A) Representative tracing showing reduction in spontaneous phasic activity by 10 μM ryanodine. (B) Mean reductions in spontaneous activity expressed as a percentage change from baseline response. *Represents significant differences from baseline responses (P<0.05; n=4).
Effect of calcium-activated potassium channel modulators
Various types of calcium-activated K+ channels, regulated by membrane depolarization and/or changes in intracellular Ca2+ levels play critical roles in the regulation of phasic contractions in bladder smooth muscle (Suarez-Kurtz et al., 1991; Herrera et al., 2000). Inhibitors of various classes of calcium-activated K+ channels were evaluated for effects on spontaneous phasic contractions of isolated bladder strips. The increase was measured as the percentage increase in contractions above control and was calculated from the area under the contraction curve (AUC). The large conductance calcium-activated K+ channel inhibitor, iberiotoxin (100 nM) produced increases in spontaneous phasic activity with a maximal increase in AUC of 232.3±91.0% (n=4; Figure 4A). The predominant effect of iberiotoxin was to increase the frequency and the amplitude, but not the duration of contractions (Figure 4B, Table 3). Similarly, apamin (100 nM), an inhibitor of the small conductance calcium-activated K+ channel, also increased spontaneous phasic activity (Figure 5A). Although the per cent maximal increase observed with apamin was lower than observed with iberiotoxin, the predominant effect was again, an increase in frequency (53.5±13.6%) and amplitude (69.1±24.3%), with no change in duration of contractions (Figure 5B, Table 3). The effects of iberiotoxin and apamin were concentration-dependent. Although iberiotoxin evoked a concentration-dependent effect, saturable responses were unattained up to the highest concentration (100 nM) tested. The EC50 of apamin was found to be 1.66±0.69 nM (n=4) with an increase in maximum spontaneous activity of 61.6±11.7%. A similar increase in activity was observed with another SKCa blocker, scyallotoxin (see Table 3).
Figure 4.
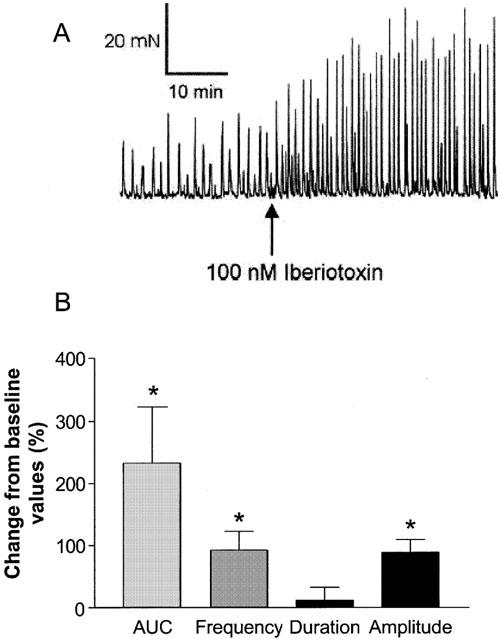
Effects of iberiotoxin on the spontaneous phasic activity of the pig bladder. (A) Iberiotoxin, (100 nM) increases the spontaneous phasic activity in the pig bladder. Shown is a representative tracing. (B) Mean increases in spontaneous contractions expressed as a percentage change from baseline response. *Represents significant differences from baseline responses (P<0.05; n=4).
Table 3.
Effects of K+ channel blockers on spontaneous phasic activity of the pig detrusor
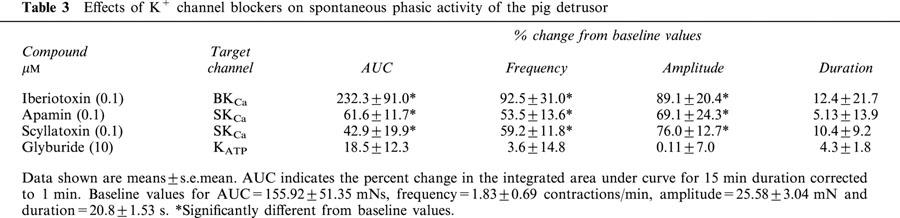
Figure 5.
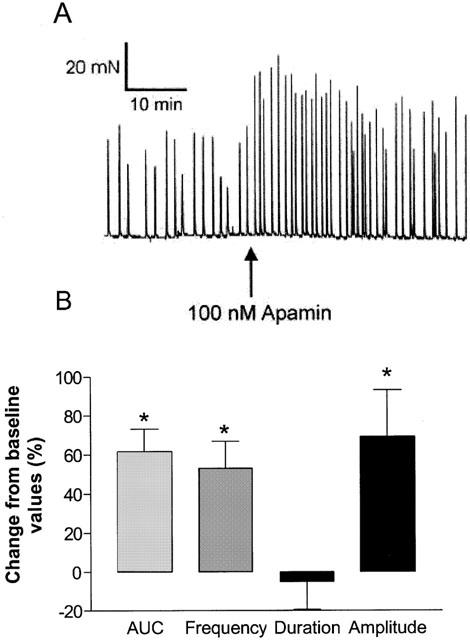
Effects of apamin on the spontaneous phasic activity of the pig bladder. (A) Apamin, (100 nM) increases the spontaneous phasic activity in the pig bladder. Shown is a representative tracing. (B) Mean increases in spontaneous contractions expressed as a percentage change from baseline response. *Represents significant differences from baseline responses (P<0.05; n=4).
Effect of ATP-sensitive potassium channel modulators
The KATP channel inhibitor glyburide did not evoke substantial increases in spontaneous activity (10 μM; n=4) suggesting that under basal conditions, the KATP channels may not participate in the regulation of phasic contractions (Figure 6A, B, Table 3). To further examine the contribution of KATP channels, compounds that are known to open these channels were examined for their effects on spontaneous phasic activity. P1075, a cyanoguanidine analogue was efficacious in suppressing spontaneous activity in a concentration-dependent manner (IC50=25.1±11.7 nM (n=8). Figure 7A shows a representative tracing following exposure of detrusor strips to varying concentrations of P1075. Figure 7B shows the mean response of P1075 and DMSO expressed as a percentage of maximum response. It was observed that the overall reduction in spontaneous activity primarily involves reductions in the frequency of contractions, with no substantial effects on amplitude and duration of the response (Figure 7C). The inhibition of spontaneous activity was restored by the addition of 10 μM glyburide, consistent with an interaction with the KATP channel. In addition to P1075, a variety of other chemotypes known to activate KATP channels evoked concentration-dependent suppression of spontaneous contractile activity. The rank order of potencies (nM±s.e.mean) of the various KCOs examined were, P1075 (25.1±11.7)=(−)-cromakalim (35.5±23.0)>WAY133537 (102.3±16.1)=ZD6169 (295.1±50.9)=pinacidil (338.8±86.0)=ZM244085 (407.4±127>nicorandil (5206±2767)>diazoxide (8913±2290) (n⩾4; Figure 8).
Figure 6.
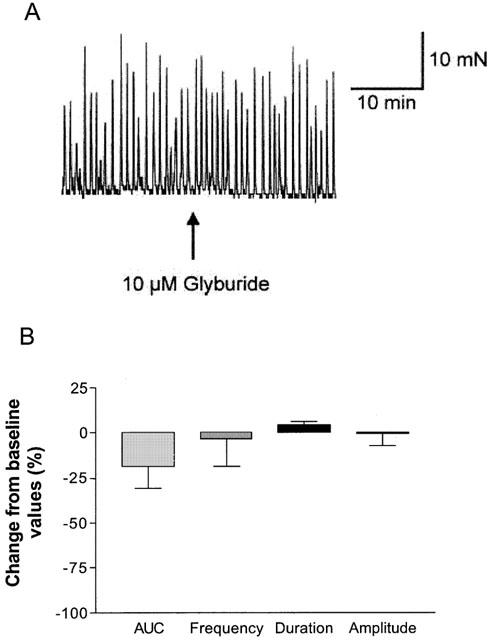
The effect of glyburide on the spontaneous phasic activity of the pig bladder. (A) Glyburide (10 μM) has no significant effect on the spontaneous phasic activity of the pig bladder. Shown is a representative tracing. (B) Mean responses in spontaneous contractions expressed as a percentage change from baseline response.
Figure 7.
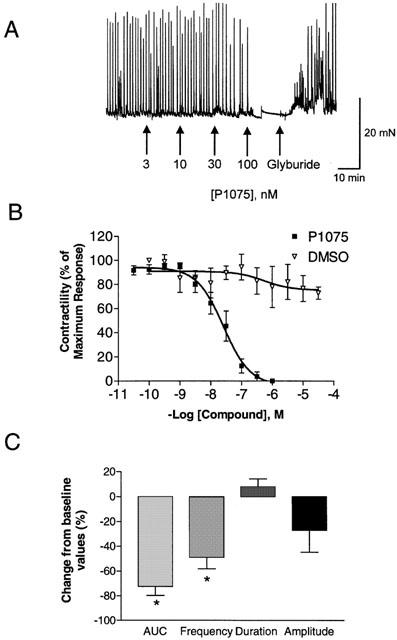
The inhibition of spontaneous phasic activity of the pig bladder by KATP channel openers. (A) Concentration response curve for the KATP channel opener, P1075 and its reversal by glyburide. Shown is a representative tracing. (B) Mean concentration response curves for P1075 (n=8) and DMSO (n=8). Data were analysed as a percentage of maximum response. (C) The overall inhibition caused by P1075 (30 nM) expressed as a percentage change from baseline was due to a reduction in frequency with no substantial change in duration or amplitude. *Represents significant differences from baseline responses (P<0.05; n=4).
Figure 8.
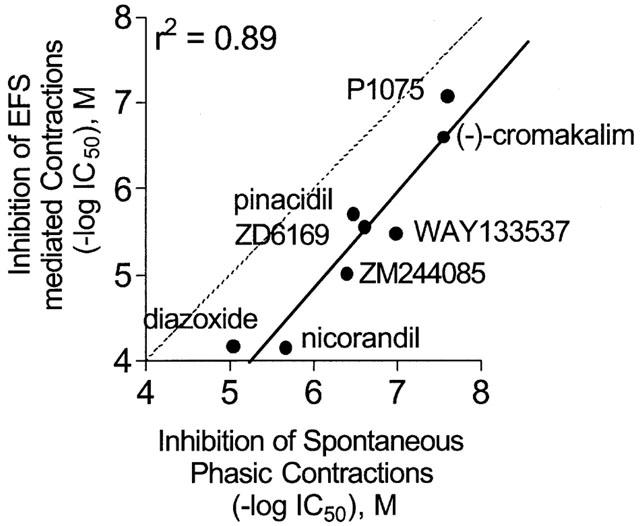
Comparison of the potencies of KATP channel openers for the inhibition of spontaneous phasic activity with potencies obtained for the suppression of electrical field-stimulated contractions. The correlation coefficient was r2=0.89 and slope=1.12. The dashed line represents 1 : 1 correlation. The KATP channel openers are 15 fold more potent in suppressing spontaneous phasic activity than contractions evoked by electrical field-stimulation. The −log IC50 data of KCOs for suppression of electrical field-stimulation taken from Buckner et al., 2000.
Unlike spontaneous phasic activity that varied in frequency, duration and amplitude, low frequency electrical stimulation (0.05 Hz, 0.5 ms, 20 V) thought to reflect presynaptic release principally of both acetylcholine and ATP, generally produced stable and reproducible maximum twitch responses (47.53±4.12 mN; n=8) (Buckner et al., 2000). It should be noted that analysis of KCO-evoked suppression of electrical field-stimulated contractions using either changes in AUC or the maximal amplitude of contractions yielded nearly identical potency values (for example, with P1075, AUC analysis pD2=6.95±0.06 and amplitude analysis, pD2=6.91±0.03). Comparison of the potencies of KATP channel openers for the inhibition of spontaneous contractions with those obtained for the suppression of electrically stimulated contractions showed a linear 1 : 1 correlation with r2 value of 0.89 (slope=1.12). Interestingly, the correlation line also showed a significant shift in potency with compounds some 15-fold more potent at inhibiting spontaneous phasic activity than contractions evoked by electrical field-stimulation (Figure 8).
Discussion
The results of the present study support the notion that spontaneous phasic activity of the detrusor tissue are myogenic in origin and that the urothelium plays a significant role in modulating the nature of these contractions. Both extracellular calcium entry through L-type Ca2+ channels and calcium release through ryanodine receptors play an important role. Furthermore, inhibition of both BKCa and SKCa channels, but not KATP channels, increased both frequency and amplitude of spontaneous activity. Openers of KATP channels showed a 15 fold increase in potencies to suppress spontaneous activity compared to electrical field-stimulated contractions, primarily via reduction in contraction frequency consistent with the idea that a small increase in K+ conductance evoked by KCOs is sufficient to suppress action potential firing and spontaneous myogenic activity.
Role of urothelium in modulating phasic spontaneous contractions
The urothelium not only provides an important barrier function in minimizing alterations in the composition of urine during storage (Lewis, 2000), but also plays an active role in responding to stretch, contractile and relaxant agents and in afferent signalling processes. A substantial enhancement in spontaneous activity (∼26 fold increase in AUC values) was noted following removal of the urothelial layer. Replacing the previously removed urothelial layer to close proximity of the denuded strips under the same preload conditions did not suppress the spontaneous activity (unpublished observations). It could be speculated that even though the two strips were in contact, the surfaces remained disrupted, and accordingly, the inhibitory/relaxant potential of the urothelium could not be re-established. Levin et al. (1986) suggested that spontaneous activity might be related to the cut surfaces of the isolated strips. However, when a whole bladder preparation stretched longitudinally was utilized, the spontaneous activity developed in the absence of any cut surfaces (Levin et al., 1986). Hawthorn et al. (2000) have shown that the urothelium modifies contractile responses of the pig detrusor strips to carbachol, perhaps via release of an inhibitory factor from the urothelium itself. This putative factor remains unidentified, but evidence has been presented that it may not be nitric oxide, adenosine, GABA, catecholamine or a cyclo-oxygenase product (Hawthorn et al., 2000). The identity of this factor remains unknown, but the enhanced spontaneous activity following removal of the urothelium is consistent with an inhibitory effect of this layer. Tissue reactivity studies have shown that detrusor strips from obstructed bladders show increased spontaneous tetanic activity and enhanced electrical coupling between cells (Brading, 1994). More recent studies have shown that in idiopathic detrusor instability, a common cause of lower urinary tract storage symptoms, there is altered spontaneous contractile activity consistent with an enhanced electrical coupling of cells, a patchy denervation of the detrusor and potassium super-sensitivity (Mills et al., 2000). Although the urothelial layer plays a key role in excitability and afferent signalling processes in the bladder, it, however, remains to be determined whether and how disruption or loss of integrity of the urothelial layer could contribute to bladder instability arising from various etiologies in humans.
Regional differences in spontaneous phasic activity
The regional variation in spontaneous phasic activity seen in the pig detrusor is consistent with those reported previously in other species including man (Sibley, 1984). In this study, it was determined that the denuded pig detrusor strips taken away from the dome region and sectioned transversally offered the best protocol for characterizing spontaneous activity. It has previously been reported that rabbit bladder strips sectioned longitudinally, exhibited spikes that were greater in amplitude than from strips sectioned in a transverse fashion (Potjer & Constantinou, 1989). In the pig bladder, it was found that both longitudinal and transverse sections behaved similarly in spontaneous phasic activity as well as their inhibition by KATP channel openers. It was suggested that in rabbits, the bladder is not subjected to longitudinal stretch under normal conditions and therefore spontaneous contractions observed had little physiological significance although they might explain the pathophysiology of certain dysfunctions during the filling stage of micturition in other species (Levin et al., 1986). Although the present study was carried out using detrusor strips from young (9 – 12 weeks old) pigs, it should be pointed out that data generated from young pigs compared well with results obtained from fully mature animals. For example, examination of the relaxation potencies of a set of KATP channel openers in young versus mature pigs revealed a good correlation in both field-stimulated (r2=0.85, slope=1.22) and carbachol-stimulated (r2=0.85, slope=0.96) protocols (unpublished observations).
Myogenic basis of spontaneous phasic activity
Inhibition of muscarinic receptors, TTX-sensitive sodium channels and α and β-adrenoceptors were found to have no substantial effect on spontaneous phasic activity supporting the notion they are myogenic in nature (Brading, 1997). Atropine completely inhibited carbachol-evoked contractions whereas field-stimulated responses were partially resistant to inhibition with maximal observed efficacy of about 43% (Sibley, 1984; Buckner et al., 2000). The atropine resistant contractile signal has been attributed to stimulus-evoked release of ATP (Sibley, 1984; Burnstock et al., 1972; Hoyle et al., 1989; Dean & Downie, 1978). Both tolterodine and oxybutynin were found to be weaker in suppressing field-stimulated contractions than those evoked by carbachol. This could be attributed to the atropine resistance of the field-stimulated pig bladder at the lower frequencies (Sibley, 1984) and to the possible release of multiple transmitters in response to electrical stimulation (Hoyle et al., 1989). Additionally, oxybutynin has been shown to possess spasmolytic properties similar to papaverine (Nagy et al., 1990), which may help explain the biphasic inhibition response seen with this agent.
Effect of extra- and intracellular calcium modulation
Inhibition of L-, but not N-, P- and Q-, type calcium channels by nifedipine inhibited spontaneous activity by reducing both the amplitude and frequency. Although evidence for T-type calcium current has been reported in bladder smooth muscle (Sui et al., 2001), the precise role of these channels in modulating spontaneous phasic activity remains to be assessed, which is, in part, hampered by the lack of availability of selective T-type calcium channel blockers. Blockade of ryanodine receptors in the sarcoplasmic reticulum by ryanodine resulted in a suppression of spontaneous activity. The inhibition of phasic contractions by nifedipine supports the notion that contractility depends on the increase in calcium entry caused by membrane depolarization during action potentials as previously shown with guinea-pig bladder smooth muscle (Herrera et al., 2000). The suppression of spontaneous activity by ryanodine argues for a role for calcium release via the ryanodine receptor contributing to contractility, in agreement with the findings of Isenberg et al. (1992), Ganitkevich & Isenberg (1992) and Hashitani et al. (2001). This appears, however, in contrast to the observation of Herrera et al. (2000) in guinea-pig bladder smooth muscle bundles, where blockade of ryanodine receptors resulted in increased contractility and evidence was presented to suggest that these receptors function as a negative feedback regulator of contraction frequency and duration by influencing the activity of SKCa channels. It is possible that, besides the obvious difference in species, such as pig versus guinea-pig, that the lack of urothelial layer and/or the exclusion of a cocktail of antagonists in our studies may have contributed to the apparent discrepancy. Nonetheless, our results suggest that in pig urinary bladder smooth muscle, the maintenance of phasic spontaneous activity can be attributed to increase in cellular calcium levels via voltage-dependent calcium channels and through ryanodine receptors.
Role of Ca2+-activated K+ channels in bladder smooth muscle activity
The BKCa channel inhibitor iberiotoxin is known to cause membrane depolarization and to increase action potential frequency, amplitude and duration in the bladder smooth muscle (Heppner et al., 1997; Suarez-Kurtz et al., 1991). In the present study, iberiotoxin evoked substantial increases in both amplitude and frequency, with no significant effect on the duration of contractions. Similarly, the SKCa channel blockers, apamin and scyallatoxin both increased amplitude and frequency of contractions, albeit of lesser magnitude compared to BKCa channel inhibition. In principle, the amplitude of spontaneous phasic activity could be dependent on changes in cellular Ca2+ levels, via either plasmalemmal voltage-dependent calcium channels and/or Ca2+ released from the ryanodine receptors of the sarcoplasmic reticulum, whereas the frequency of contractile activity reflects mechanisms that regulate smooth muscle action potential firing such as opening of calcium-activated K+ channels. The increase in amplitude of contractions following iberiotoxin and apamin exposure is consistent with their inhibition of large and small conductance calcium activated K+ channels and subsequent increase in cellular calcium levels. The lack of effect on duration suggests that these mechanisms do not affect the duration of bursts of smooth muscle action potentials per se. The integrated AUC value was some 5 fold lower following SKCa inhibition compared to BKCa inhibition with iberiotoxin. Apamin interacts with all subtypes of SK channels with SK2/SK3 channels (IC50=0.03 nM for rSK2 and 4 nM for rSK3) being more sensitive than SK1 (IC50=196 nM) (Grunnet et al., 2001). The potency of apamin (EC50=1.66 nM) in increasing spontaneous activity is comparable to the values for inhibition of SK2/SK3 channels suggesting that these subunits may contribute to the functional interaction of apamin in bladder smooth muscle.
Role of ATP-sensitive K+ channels and sensitivity to KATP channel openers
Several openers of the ATP-sensitive potassium channels caused complete suppression of spontaneous phasic activity in a concentration-dependent fashion under physiologic external K+ concentrations. As shown in Figure 7C, P1075 (30 nM) primarily reduced the frequency component without significant change in the amplitude or duration of contractions. This observation is consistent with the hypothesis that a small increase in K+ conductance could move the resting membrane potential away from the threshold for action potential firing, thus affecting initiation of phasic contractions and underlying action potential firing (Shieh et al., 2001; Petkov et al., 2001). Comparisons of the potencies to suppress spontaneous phasic activity versus electrical field-stimulated contractions show that the potencies of KCOs are generally 15-fold more potent in suppressing spontaneous activity compared to contractions evoked by electrical field-stimulation. Thus, under physiologic conditions, KCOs are significantly more potent in suppressing phasic activity compared to those evoked by electrical field-stimulation where contractions are triggered by evoked acetylcholine and ATP.
In summary, the present study provides a detailed characterization of contractions in the pig detrusor, a widely employed model for the human bladder. We demonstrate that the spontaneous phasic activity of the detrusor is myogenic and is influenced by the presence of urothelium. Phasic contractions, particularly the amplitude, are dependent on both calcium entry through voltage-dependent calcium channels and release from ryanodine receptors. Inhibition of BKCa, and to a lesser extent, SKCa, channels resulted in an overall increase in contraction amplitude and frequency demonstrating that these mechanisms are critical to the regulation of phasic spontaneous activity in the bladder. Although glyburide alone did not substantially influence myogenic activity, KATP channel openers showed an exquisite sensitivity for suppression of myogenic spontaneous activity. The latter suggests that KATP channel modulation by low concentrations of KCOs may offer a unique mechanism to control spontaneous myogenic activity, especially those associated with the hyperexcitability occurring in unstable bladders.
Acknowledgments
The authors thank Dr John Malysz for the critical reading of this manuscript and suggestions.
Abbreviations
- KATP channels
ATP-sensitive K+ channels
- KCOs
potassium channel openers
- mN
milliNewtons
- P1075
[N-cyano-N′-(1,1-dimethylpropyl)-N′′-3-pyridylguanidine]
- UUI
urge urinary incontinence
- WAY133537
[1,2-diaminocyclobutene-3,4-dione((R)-4-[3,4-dioxo-2-(1,2,2-trimethyl-propylamino)-cyclobut-1-enylamino]-3-ethyl-benzonitrile]
- ZD6169
[N-(4-benzoyl phenyl)-3,3,3-trifluro-2-hydroxy-2-methylpropionamine]
- ZM244085
[9-(3-cyanophenyl)-3,4,6,7,9,10-hexahydro-1,8-(2H,5H)-acridinedione]
References
- ANDERSSON K.-E. Clinical pharmacology of potassium channel openers. Pharmacol. Toxicol. 1992;70:244–254. doi: 10.1111/j.1600-0773.1992.tb00466.x. [DOI] [PubMed] [Google Scholar]
- BRADING A.F. Ion channels and control of contractile activity in urinary bladder smooth muscle. Jpn. J. Pharmacol. 1992;58 suppl 2:120–127. [PubMed] [Google Scholar]
- BRADING A.F. The pathophysiological changes in the bladder obstructed by benign prostatic hyperplasia. Br. J. Urol. 1994;74:133. [PubMed] [Google Scholar]
- BRADING A.F. A myogenic basis for the overactive bladder. Urol. 1997;50 suppl 6A:57–67. doi: 10.1016/s0090-4295(97)00591-8. [DOI] [PubMed] [Google Scholar]
- BUCKNER S.A., MILICIC I., DAZA A., DAVIS-TABER R., SCOTT V.E., SULLIVAN J.P., BRIONI J.D. Pharmacological and molecular analysis of ATP-sensitive K(+) channels in the pig and human detrusor. Eur. J. Pharmacol. 2000;400:287–295. doi: 10.1016/s0014-2999(00)00388-5. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., DUMSDAY B., SMYTHE A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br. J. Pharmacol. 1972;44:451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COGHLAN M.J., CARROLL W.A., GOPALAKRISHNAN M. Recent developments in the biology and medicinal chemistry of potassium channel modulators: update from a decade of progress. J. Med. Chem. 2001;44:1627–1653. doi: 10.1021/jm000484+. [DOI] [PubMed] [Google Scholar]
- CROWE R., BURNSTOCK G. A histochemical and immunohistochemical study of the autonomic innervation of the lower urinary tract of the female pig. Is the pig a good model for the human bladder and urethra. J. Urol. 1989;141:414–422. doi: 10.1016/s0022-5347(17)40785-3. [DOI] [PubMed] [Google Scholar]
- DEAN D.M., DOWNIE J.W. Contribution of adrenergic and “purinergic” neurotransmission to contraction in rabbit detrusor. J. Pharmacol. Exp. Ther. 1978;207:431–445. [PubMed] [Google Scholar]
- DE GROAT W.C. A neurologic basis for the overactive bladder. Urol. 1997;50 suppl 6A:36–52. doi: 10.1016/s0090-4295(97)00587-6. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M., CHOPPIN A., DILLON M.P., HEGDE S. Muscarinic receptor ligands and their therapeutic potential. Curr. Opin. Chem. Biol. 1999;3:426–432. doi: 10.1016/S1367-5931(99)80063-5. [DOI] [PubMed] [Google Scholar]
- FUJII K., FOSTER C.D., BRADING A.F., PAREKH A.B. Potassium channel blockers and the effects of cromakalim on the smooth muscle of the guinea pig bladder. Br. J. Pharmacol. 1990;99:779–785. doi: 10.1111/j.1476-5381.1990.tb13006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GANITKEVICH V.YA., ISENBERG G. Contribution of Ca2+ -induced Ca2+- release to the [Ca2+]I transients in myocytes from guinea pig urinary bladder. J. Physiol. 1992;458:119–137. doi: 10.1113/jphysiol.1992.sp019409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNNET M., JENSEN B.S., OLESEN S.P., KLAERKE D.A. Apamin interacts with all subtypes of cloned small-conductance Ca2+ activated K+ channels. Pflugers Arch. 2001;441 4:544–550. doi: 10.1007/s004240000447. [DOI] [PubMed] [Google Scholar]
- HASHITANI H., FUKUTA H., TAKANO H., KLEMM M.F., SUZUKI H. Origin and propagation of spontaneous excitation in smooth muscle of the guinea pig urinary bladder. J. Physiol. 2001;530:273–286. doi: 10.1111/j.1469-7793.2001.0273l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWTHORN M.H., CHAPPLE C.R., COCK M., CHESS-WILLIAMS R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility. Brit. J. Pharmacol. 2000;129:416–419. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEPPNER T.J., BONEV A.D., NELSON M.T. Ca(2+)-activated K+ channels regulate action potential repolarization in urinary bladder smooth muscle. Am. J. Physiol. 1997;273:C110–C117. doi: 10.1152/ajpcell.1997.273.1.C110. [DOI] [PubMed] [Google Scholar]
- HERRERA G.M., HEPPNER T.J., NELSON M.T. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:60–68. doi: 10.1152/ajpregu.2000.279.1.R60. [DOI] [PubMed] [Google Scholar]
- HOYLE C.H., CHAPPLE C., BURNSTOCK G. Isolated human bladder: Evidence for an adenine dinucleotide acting on P2x-purinoceptors and for purinergic transmission. Eur. J. Pharmacol. 1989;174:115–118. doi: 10.1016/0014-2999(89)90881-9. [DOI] [PubMed] [Google Scholar]
- ISENBERG G., WENDT-GALLITELLI M.F., GANITKEVICH V. Contribution of Ca2+ -induced Ca2+ release to depolarization-induced Ca2+ transients of myocytes from guinea pig urinary bladder myocytes. Jpn. J. Pharmacol. 1992;58:81–86. [PubMed] [Google Scholar]
- KINDER R.B., MUNDY A.R. Pathophysiology of idiopathic detrusor instability and detrusor hyperreflexia. An in vitro study of human detrusor muscle. Br. J. Urol. 1987;60:509–515. doi: 10.1111/j.1464-410x.1987.tb05031.x. [DOI] [PubMed] [Google Scholar]
- LEWIS S.A. Everything you wanted to know about the bladder epithelium but were afraid to ask. Amer. J. Physiol. Renal Physiol. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- LIU S.P., VOLFSON I., LEVIN R.M. Effects of hypoxia, calcium, carbachol, atropine and tetrodotoxin on the filling of the in-vitro rabbit whole bladder. J. Urol. 1998;160:913–919. doi: 10.1016/S0022-5347(01)62832-5. [DOI] [PubMed] [Google Scholar]
- LEVIN R.M., RUGGIERI M.R., VELAGAPUDI S., GORDON D., ALTMAN B., WEIN A.J. Relevance of spontaneous activity to urinary bladder function: An in vitro and in vivo study. J. Urol. 1986;136:517–521. doi: 10.1016/s0022-5347(17)44934-2. [DOI] [PubMed] [Google Scholar]
- MILLS I.W., GREENLAND J.E., MCMURRAY G., MCCOY R., HO K.M., NOBLE J.G., BRADING A.F. Studies of the pathophysiology of idiopathic detrusor instability: the physiological properties of the detrusor smooth muscle and its pattern of innervation. J. Urol. 2000;163:646–651. doi: 10.1016/s0022-5347(05)67951-7. [DOI] [PubMed] [Google Scholar]
- NAGY F., HAMVAS A., FRANG D. Idiopathic bladder hyperactivity treated with Ditropan (oxybutynin) Int. Urol. Nephrol. 1990;22:519–524. doi: 10.1007/BF02549739. [DOI] [PubMed] [Google Scholar]
- PETKOV G.V., HEPPNER T.J., BONEV A.D., HERRERA G.M., NELSON M.T. Low levels of K(ATP) channel activation decrease excitability and contractility of urinary bladder. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R1427–R1433. doi: 10.1152/ajpregu.2001.280.5.R1427. [DOI] [PubMed] [Google Scholar]
- POTJER R.M., CONSTANTINOU C.E. Frequency of spontaneous contractions in longitudinal and transverse bladder strips. Am. J. Physiol. 1989;257:781–787. doi: 10.1152/ajpregu.1989.257.4.R781. [DOI] [PubMed] [Google Scholar]
- SHIEH C., FENG J., BUCKNER S.A., BRIONI J.D., COGHLAN M.J., SULLIVAN J.P., GOPALAKRISHNAN M. Functional implication of spare ATP-sensitive K+ channels in bladder smooth muscle cells. J. Pharmacol. Exp. Ther. 2001;296:669–675. [PubMed] [Google Scholar]
- SIBLEY G.N.A. A comparison of spontaneous and nerve mediated activity in bladder muscle from man, pig and rabbit. J. Physiol. 1984;354:431–444. doi: 10.1113/jphysiol.1984.sp015386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUAREZ-KURTZ G., GARCIA M.L., KACZOROWSKI G.J. Effect of charybdotoxin on spontaneous motility and tonus of different guinea pig smooth muscle tissues. J. Pharmacol. Exp. Ther. 1991;259:439–443. [PubMed] [Google Scholar]
- SUI G.-P., WU C., FRY C.H. Inward calcium currents in cultured and freshly isolated detrusor muscle cells: Evidence of a T-type calcium current. J. Urol. 2001;165:621–626. doi: 10.1097/00005392-200102000-00084. [DOI] [PubMed] [Google Scholar]
- WEIN A.J. Pharmacological agents for the treatment of urinary bladder incontinence due to overactive bladder. Expert Opin. Investig. Drugs. 2001;10:65–83. doi: 10.1517/13543784.10.1.65. [DOI] [PubMed] [Google Scholar]
- ZIELINSKI P.J., BUCKNER S.A. AGANTG: A Microsoft Excel 5.0-visual basic routine for the analysis of dose-response data. Analyst. 1998;123:1661–1668. doi: 10.1039/a804438d. [DOI] [PubMed] [Google Scholar]


