Abstract
Methylenedioxymethamphetamine (MDMA, “ecstasy”), widely used as a recreational drug, can produce hyponatraemia. The possibility that this could result from stimulation of vasopressin by MDMA or one of its metabolites has been investigated in vitro.
Release of both oxytocin and vasopressin from isolated hypothalami obtained from male Wistar rats was determined under basal conditions and following potassium (40 mM) stimulation. The results were compared with those obtained for basal and stimulated release in the presence of MDMA or metabolites in the dose range 1 μM to 100 pM (n=5 – 8) using Student's t-test with Dunnett's correction for multiple comparisons.
All compounds tested affected neurohypophysial hormone release, HMMA (4-hydroxy-3-methoxymethamphetamine) and DHA (3,4-dihydroxyamphetamine) being more active than MDMA, and DHMA (3,4-dihydroxymethamphetamine) being the least active. The effect on vasopressin release was greater than that on oxytocin. In the presence of HMMA the ratio test:control for basal release increased for vasopressin from 1.1±0.16 to 2.7±0.44 (s.e.m., P<0.05) at 10 nM and for oxytocin from 1.0±0.05 to 1.6±0.12 in the same hypothalami. For MDMA the ratio increased to 1.5±0.27 for vasopressin and to 1.28±0.04 for oxytocin for 10 nM.
MDMA and its metabolites can stimulate both oxytocin and vasopressin release in vitro, the response being dose dependent for each drug with HMMA being the most potent.
Keywords: Oxytocin; vasopressin; hypothalamus; 3,4-methylenedioxymethamphetamine, 4-hydroxy-3-methoxymethamphetamine, 3,4-dihydroxyamphetamine; 3,4-dihydroxymethamphetamine; 4-hydroxy-3-methoxyamphetamine; 3,4-methylenedioxyamphetine
Introduction
Methylenedioxymethamphetamine (MDMA, “ecstasy”) is a phenethylamine with structural similarities to both mescaline and amphetamine and is a relatively widely used recreational drug. Its acute effects include rapid release of serotonin (5HT) from central neurones resulting in well-documented changes including behavioural excitation and hyperthermia (Green et al., 1995). Less well-documented are the neuroendocrine changes. Administration of the drug to rats results in an increase in the circulating concentrations of cortisol and prolactin (Nash et al., 1988). Cortisol and prolactin release are similarly stimulated in man, although higher doses of MDMA are required to stimulate prolactin (Mas et al., 1999; de la Torre et al., 2000a). No effect on growth hormone was observed. The release of cortisol is consistent with the activation of serotonergic pathways, but dopaminergic or adrenergic mechanisms could also be involved as effects on monoaminergic transmission have been described (White et al., 1996). Similarly, serotonin and the catecholamines are involved in the release of vasopressin (Kostoglou-Athanassiou & Forsling, 1998; Renaud & Bourque, 1991) and release of this hormone in man on ingestion of MDMA has been reported, although it is not known if this is a direct effect or secondary to other changes (Henry et al., 1998; Forsling et al., 2001).
Following oral administration of a low dose (40 mg) of racemic MDMA to eight healthy male volunteers, vasopressin release was stimulated in all subjects, but shortly after administration an inverse correlation between plasma MDMA and vasopressin concentrations was observed (Forsling et al., 2001). This could result from a number of factors including the formation of an active metabolite, with the lower MDMA concentrations being associated with greater metabolite formation and enhanced vasopressin release. A number of metabolites are formed from MDMA, with N-demethylation and demethylenation being important pathways in the formation of most of them (de boer et al., 1997; Ortuno et al., 1999; Kreth et al., 2000). Thus, as shown in Figure 1, the metabolism of MDMA involves N-demethylation to methylenedioxyamphetamine (MDA) and both undergo O-demethylenation to 3,4-dihydroxymethamphetamine (DHMA) and 3,4-dihydroxyamphetamine (DHA), respectively. The major cytochrome P-450 (CYP) mediating demethylenation is CYP2D6, with possible contributions from 1A2, 2B6 and 3A4 (Kreth et al., 2000). Both DHMA and DHA are subsequently methylated by catechol-O-methyl transferase (COMT) to 4-hydroxy-3-methoxymethamphetamine (HMMA) and 4-hydroxy-3-methoxyamphetamine (HMA) respectively. These four metabolites, in particular HMMA and HMA are excreted in the urine as conjugated glucuronide or sulphate metabolites. In order to examine the hypothesis that a metabolite, as well as, or in addition to, the parent compound could contribute to enhanced vasopressin release, the activity of MDMA and the five metabolites have been investigated. Release of oxytocin was also investigated to determine if any effects are specific for vasopressin. To exclude any systemic effects of the drugs, the observations were performed on the isolated hypothalamus in vitro using a previously validated method (Tsagarakis et al., 1988). This model was chosen for these investigations because the neurohypophysial hormone responses to centrally acting compounds have been demonstrated to reflect the physiological responses in vivo following administration of, for example, dopamine (Bridges et al., 1976) or melatonin (Yasin et al., 1993).
Figure 1.

MDMA and its metabolites.
Methods
Sources of compounds
(±)-(R,S)-MDMA hydrochloride, (±)-(R,S)-MDA hydrochloride were purchased from the Sigma Chemical Company Ltd, Poole, U.K.; (−)-(R)- and (+)-(S)-MDMA hydrochloride and (−)-(R)- and (+)-(S)-MDA hydrochloride were generously donated by the Research Technology Branch of the National Institute on Drug Abuse, Rockville, MD, U.S.A.
Syntheses of metabolites (see Figure 2).
The hydroxymethoxy derivatives, HMMA (2, Figure 2) and HMA (3) were prepared in good yield via reductive amination of the ketone 4-hydroxy-3-methoxyphenylacetone (1). Treatment of HMMA and HMA with aqueous hydrobromic acid under reflux yielded the corresponding catechol derivatives DHMA (4) and DHA (5) as their hydrobromides which were converted to hydrochloride salts by treatment with silver chloride under aqueous conditions (Beckett et al., 1965).
Figure 2.

Synthesis of HMMA, HMA and the corresponding catechol derivatives.
Preparation of (R,S)-4-hydroxy-3-methoxymethamphetamine hydrochloride, (R,S)-HMMA.HCl (2)
To a solution of methylamine hydrochloride (6.5 g, 96.4 mmol) in methanol (45 ml) were added 4-hydroxy-3-methoxyphenylacetone 1 (5.0 g, 27.8 mmol) followed by sodium cyanoborohydride (2.17 g, 34.5 mmol) and the solution stirred for 20 h at ambient temperature under a nitrogen atmosphere. The pH was maintained at 6 throughout by careful addition of concentrated hydrochloric acid. After the addition of water (100 ml) the solution was adjusted to pH 2 with concentrated hydrochloric acid (caution: HCN evolution). Volatiles were removed in vacuo and the remaining aqueous solution extracted with ethyl acetate (3–50 ml). The aqueous fraction was adjusted to pH 10 (solid sodium hydroxide), saturated with sodium chloride and extracted with ethyl acetate (3–50 ml). The combined extracts were dried (Na2SO4) and concentrated in vacuo to afford the crude product as a viscous oil (4.4 g). Further purification by flash chromatography (SiO2, methanol) afforded pure HMMA (free base, 3.4 g) that was converted to the hydrochloride salt to yield the title compound as a white powder (3.6 g, 56%); m.p. 209 – 210°C (m.p. 181 – 189°C; de boer et al., 1997); 1H NMR (400 MHz; DMSO-d6): δ, p.p.m.: 1.13 (d, 3H, J=6.5 Hz, CCH3), 2.55 (m, 4H, NCH3+Ar-CH), 3.11 (dd, 1H, JBX=4.2 Hz, JAB=13.2 Hz, Ar-CH), 3.32 (m, 1H, CH-CH3), 3.77 (s, 3H, OCH3), 6.62 (dd, 1H, Jo=8.0 Hz, Jm=1.7 Hz, H-6′), 6.76 (d, 1H, Jo=7.9 Hz, H-5′), 6.83 (d, 1H, Jm=1.7 Hz,H-2′), 8.93 (s, 1H, OH), 9.19 (bs, 2H, NH2+); 13C NMR (100 MHz; DMSO-d6): δ 14.9 (CH3), 29.5 (NCH3), 37.9 (CH2), 55.5 (CH), 55.5 (OCH3), 113.2 (C-5′), 115.4 (C-2′), 121.4 (C-6′), 127.2 (C-1′), 145.3 (C-4′), 147.5 (C-3′); Mass spectra (+FAB, HCl salt), m/z 196 (M+H, 100), 165 (30), 133 (12). Exact mass found: m/z 196.13319 (M+H), C11H18NO2 requires 196.13320 (difference −0.05 p.p.m.).
Preparation of (R,S)-4-hydroxy-3-methoxyamphetamine hydrochloride, (R,S)-HMA.HCl (3)
The title compound was prepared using an analogous procedure to the preparation of 2 using ammonium acetate in place of methylamine hydrochloride. HMA.HCl was isolated as a white powder (59% from 1); m.p. 260 – 261°C (m.p. 259 – 260.5°C; Glennon et al., 1980); 1H NMR (400 MHz; DMSO-d6): δ, p.p.m.: 1.15 (d, 3H, J=6.5 Hz, CCH3), 2.58 (dd, 1H, JBX=9.0 Hz, JAB=13.4 Hz, Ar-CH), 2.98 (dd, 1H, JAX=5.1 Hz, JAB=13.4 Hz, Ar-CH), 3.34 (m, 1H, CH-CH3), 3.77 (s, 3H, OCH3), 6.60 (dd, 1H, Jo=8.0 Hz, Jm=1.8 Hz, H-6′), 6.76 (d, 1H, Jo=8.0 Hz, H-5′), 6.80 (d, 1H, Jm=1.8 Hz, H-2′), 8.27 (bs, 3H, NH3+), 8.90 (s, 1H, OH); 13C NMR (100 MHz; DMSO-d6): 17.5 (CH3), 39.6 (CH2), 48.3 (CH), 55.5 (OCH3), 113.2 (C-5′), 115.4 (C-2′), 121.4 (C-6′), 127.3 (C-1′), 145.3 (C-4′), 147.4 (C-3′). Mass spectra (+FAB, HCl salt), m/z 182 (M+H, 97), 165 (100), 133 (75). Exact mass found: m/z 182.11748 (M+H), C10H16NO2 requires 182.11755 (difference −0.38 p.p.m.).
Preparation of (R,S)-3,4-dihydroxymethamphetamine hydrobromide, (R,S)-DHMA.HBr (4a)
A solution of (R,S)-HMMA.HCl, 2, (1.0 g, 4.32 mmol) in 48% aq. HBr (40 ml) was refluxed under a nitrogen atmosphere for 5 h. The reaction mixture was cooled and concentrated in vacuo. The crude material (brown oil) (1.1 g, 97%) analysed as expected: 1H NMR (400 MHz; CD3OD): δ, p.p.m.: 1.23 (d, 3H, J=6.4 Hz, CCH3), 2.68 (m, 4H, NCH3+Ar-CH), 2.98 (dd, 1H, JBX=5.0 Hz, JAB=13.4 Hz, Ar-CH), 3.39 (m, 1H, CH-CH3), 4.71 (bs, 4H, 2–OH+NH2+), 6.57 (dd, 1H, Jo=8.0 Hz, Jm=1.6 Hz, H-6′), 6.70 (d, 1H, Jm=1.4 Hz, H-2′), 6.75 (d, 1H, Jo=8.0 Hz, H-5′).13C NMR (100 MHz; CD3OD): δ 15.9 (CH3), 30.8 (NCH3), 39.4 (CH2), 57.6 (CH), 116.6 (C-5′), 117.3 (C-2′), 121.5 (C-6′), 128.0 (C-1′), 145.4 (C-4′), 146.4 (C-3′); Mass spectra (+FAB, HBr salt), m/z 182 (M+H, 100), 152 (15), 124 (15). Exact mass found: m/z 182.11732 (M+H), C10H16NO2 requires 182.11755 (difference −1.3 p.p.m.).
This material (0.246 g, 0.94 mmol) was subsequently treated with silver chloride (0.324 g, 2.26 mmol) in water (4 ml) stirred at ambient temperature (overnight). The reaction mixture was filtered and the filtrate concentrated in vacuo to afford the hydrochloride salt, (R,S)-DHMA.HCl, 4b, isolated as an oil (83% from HBr salt).
Preparation of (R,S)-3,4-dihydroxyamphetamine hydrobromide, (R,S)-DHA.HBr (5a)
The title compound was prepared using an analogous procedure to the preparation of 4a, isolated as a pale brown solid (97%). m.p. 183°C (m.p. 180 – 182°C, Borgman, 1974); 1H NMR (400 MHz; CD3OD): δ, p.p.m.: 1.27 (d, 3H, J=6.5 Hz, CCH3), 2.68 (dd, 1H, JBX=8.2 Hz, JAB=13.6 Hz, Ar-CH), 2.88 (dd, 1H, JBX=6.2 Hz, JAB=13.6 Hz, Ar-CH), 3.46 (m, 1H, CH-CH3), 4.82 (bs, 5H, 2–OH+NH3+), 6.58 (dd, 1H, JBX=8.0 Hz, Jm=2.0 Hz, H-6′), 6.73 (d, 1H, Jm=2.0 Hz, H-2′), 6.77 (d, 1H, JBX=8.0 Hz, H-5′); 13C NMR (100 MHz; CD3OD): δ 18.3 (CH3), 41.0 (CH2), 50.4 (CH), 116.6 (C-5′), 117.2 (C-2′), 121.7 (C-6′), 128.6 (C-1′), 145.3 (C-4′), 146.3 (C-3′); Mass spectra (+FAB, HBr salt), m/z 168 (M+H, 100), 152 (60). Exact mass found: m/z 168.10208 (M+H), C9H14NO2 requires 168.10190 (difference +1.1 p.p.m.). Conversion to the hydrochloride salt (5b) as per method for 4b afforded (R,S)-DHA.HCl as a light grey solid (82% conversion from HBr salt). m.p. 193 – 194°C (m.p. 194°C, Borgman, 1974).
Animals
The studies were performed on male Wistar rats (Banting & Kingman Ltd., Aldeburgh, U.K.), specific pathogen free and weighing 225 – 275 g. They were given free access to food (Rat and Mouse no 1 maintenance diet; Special Diet Services Ltd, Witham Essex, U.K.) and water and housed under conditions of fixed lighting (12 h light : 12 h dark) and constant temperature and humidity.
Dissection of hypothalamus
Groups of animals were decapitated between 0900 and 1000 h and the brain removed. A hypothalamic block was dissected within the following limits: anterior border of the optic chiasm, anterior border of the mamillary bodies, and lateral hypothalamic sulci. The depth of the dissection was approximately 3.0 mm. The blocks were then bisected longitudinally through the midsaggital plane and the two hypothalamic halves incubated in one vial. The total dissection time was less than 2 min from decapitation.
Hypothalamic incubation
The hypothalamic tissue was incubated in polyethylene vials containing 400 ml Earle's balanced salt solution (EBBS, Gibco, Biocult, Paisley U.K.) supplemented with 0.2% human serum albumin, 60 mg ml−1 ascorbic acid and 40 kallikrein-inhibiting units (KIU) ml−1 aprotinin. Incubation vials were placed in a shaking water bath at 37°C and gassed with 95% O2 and 5% CO2. As described previously (Yasin et al., 1993), an 80 min pre-incubation period was chosen during which time the medium was removed and the tissue carefully washed every 20 min. After pre-incubation the hypothalami were bathed in fresh medium for a control period of 20 min (B1), after which there was a further 20 min incubation (B2) either in medium alone (control groups) or in medium containing drugs (test groups). Each drug was dissolved in the medium described above at concentrations of 0.1, 10 and 1000 nM. The enantiomers of MDMA and MDA were also employed at a concentration of 500 nM. Additionally MDMA was re-examined in the presence of HMMA. The hypothalami were also exposed to 40 mM KCl before (S1) and after (S2) the addition of the drug under test. Again a control series in the absence of the drug was performed. Basal and stimulated release were monitored for each explant but, with the exception of the combined dosing of MDMA and HMMA, only one drug was tested per explant. At the end of the experiment, the maximum time of which was 3 h, the viability of the tissue was tested by incubation with 56 mM KCl. Greater than 90% of the hypothalami responded to 56 mM stimulation and only data obtained from these responding tissues was used for statistical analysis. Media from incubations were stored at −20°C until assay for vasopressin and oxytocin.
Hormone assay
The concentration of vasopressin was measured in the incubation medium at two dilutions in duplicate by the method of Forsling & Peysner (1988) using the first International Standard for vasopressin (77/501). The lower limit of detection was 0.8 pmol l−1 and the intra- and interassay coefficients of variation were 5.0% and 8.9% at 2.5 pmol l−1 respectively. The cross-reactivity of the vasopressin antiserum with oxytocin was less than 1%. The concentrations of drugs used did not interfere with the assay. Oxytocin concentrations were determined in two dilutions in duplicate as described by Windle & Forsling (1993) against the fourth International Standard for oxytocin (76/575). The lower limit of detection was 0.8 pmol l−1 and the intra- and interassay coefficients of variation were 5.1% and 7.8% at 2.5 pmol l−1, respectively. The cross-reactivity of the vasopressin antiserum with oxytocin was less than 0.1%. The concentrations of drugs used did not interfere with the assay.
Statistical analysis
As previously reported, the basal release of vasopressin and oxytocin varied between vials, so that results are expressed in terms of the ratio of hormone release in the test period (B2) with that in the preceding control period (B1). Similarly release on potassium stimulation is expressed as S2 : S. The data were analysed by an overall one way analysis of variance to establish a possible drug effect for a given series of observations. If this was statistically significant, then sets of data were compared using Student's t-test with Dunnett's correction for multiple comparisons. Data are given as means±s.e.m. and significance taken as P<0.05.
Results
Basal neurohypophysial hormone release was 8 to 14 fmol per hypothalamus per 20 min incubation period. The parent compound MDMA produced a dose-dependent increase in release of the neurohypophysial hormones oxytocin and vasopressin, although the oxytocin response was less marked. The metabolites were also effective in increasing release with HMMA being the most and DHMA the least potent.
MDMA
Basal release of vasopressin from the isolated rat hypothalamus was increased significantly by MDMA at concentrations of 10 and 1000 nM (P<0.01), the higher concentration producing a doubling of release (Figure 3a). Both enantiomers produced a similar degree of stimulation (P<0.05), but that in response to 500 nM (−)-(R)-MDMA was smaller (P<0.05) in comparison to (+)-(S)-MDMA. The effect of 1000 nM MDMA was similar to that produced by 40 mM KCl, while the response to potassium stimulation was significantly enhanced in the presence of MDMA (P<0.05). The presence of HMMA in the incubate did not affect the response in the presence of the higher concentration. Oxytocin release was also stimulated by MDMA at concentrations of 10 nM (P<0.01) and 1000 nM (P<0.05) (Figure 3b). However only stimulation by the (+)-S-enantiomer was statistically significant when compared with control (P<0.01). The oxytocin responses induced by the drug were smaller than those seen with 40 mM KCl, while an enhanced response was seen in the presence of HMMA (P<0.01).
Figure 3.

The effect of MDMA on (a) vasopressin and (b) oxytocin release from the isolated rat hypothalamus in vitro. Bars represent means±s.e.m.; *P<0.05 and **P<0.01 compared to control (Cont) or 40 mM potassium alone (K40) for the single drug. The vasopressin response to 500 nM (−)-MDMA was significantly less than that to 500 nM (+)- MDMA (P<0.05). There was a significant difference in the oxytocin response to the two enantiomers of MDMA (indicated by+ and- signs) and the response in the presence of HMMA was significantly greater than that seen with MDMA alone (P<0.01).
HMMA
As shown in Figure 4a, vasopressin concentrations increased on the addition of HMMA at concentrations of 10 and 1000 nM, with ratios increasing from 1.05±0.08 to 2.71±1.35 and 3.41±0.62, respectively (P<0.01). These responses were significantly greater than produced by 40 mM KCl (P<0.05). The response in incubates containing equal amounts of HMMA and MDMA was less than the equivalent concentration of HMMA alone (P<0.01). Oxytocin concentrations also increased after the addition of HMMA with ratios increasing from 1.02±0.04 to 1.68±0.06 and 2.19±0.05 at concentrations of 10 and 1000 nM, respectively, as shown in Figure 4b. The response to 10 nM was not significantly different from that seen on potassium challenge, which was in turn augmented by HMMA (P<0.05). When MDMA in an equal concentration to HMMA was added to the incubate, the response to 10 nM “drug” was unaffected while that to 1000 nM “drug” was reduced as compared to HMMA alone (P<0.01) (in this case the “drug” concentration represents the sum of the concentrations of MDMA and HMMA).
Figure 4.

The effect of HMMA on (a) vasopressin and (b) oxytocin release from the isolated rat hypothalamus in vitro. Bars represent means±s.e.m.; *P<0.05 and **P<0.01 compared to control (Cont) or 40 mM potassium alone (K40) for the single drug. The vasopressin response to 1000 nM and 10 nM HMMA as well as the oxytocin response to 1000 nM HMMA was significantly less in the presence of MDMA (P<0.01).
MDA
MDA was found to stimulate release of vasopressin with ratios increasing from 0.95±0.05 to 1.5±0.07 and 1.91±0.1, respectively at the two higher concentrations (Figure 5a). This last increase was not statistically different from the response to potassium challenge. Both enantiomers were effective in stimulating release, but the response to (−)-(R)-MDA was significantly lower than that observed with the racemate (P<0.05). MDA also enhanced the response to potassium administration (P<0.01). Similarly MDA was found to stimulate the release of oxytocin from a ratio of 0.99±0.06 to 1.3±0.07 and 1.5±0.1 at a concentration of 10 and 1000 nM (P<0.01) (Figure 5b). Again both enantiomers were effective, the (−)-isomer being less active than (+)-MDA (P<0.05).
Figure 5.

The effect of MDA on (a) vasopressin and (b) oxytocin release from the isolated rat hypothalamus in vitro. Bars represent means±s.e.m. and *P<0.05 **P<0.01 compared to control (Cont) or 40 mM potassium alone (K40). The vasopressin response to 500 nM (−)-MDA was significantly less than that to 500 nM(+)-MDMA (P<0.05).
HMA, DHMA and DHA
Addition of HMA, DHMA or DHA to the incubation medium produced an increase in vasopressin, except at the lowest concentration of 0.1 nM (Figure 6), when only DHA produced a significant effect. Oxytocin release tended to increase, but the change was only significant with 10 nM DHA and HMA, DHMA or DHA at a concentration of 1000 nM (P<0.05). These three metabolites also tended to augment the vasopressin response to the potassium challenge but the change was only statistically significant for HMA and DHA at a concentration of 1000 nM (P<0.05) (Figure 7). There was a similar tendency for the oxytocin response to be enhanced but the change was only significant for DHA at a concentration of 1000 nM (P<0.01).
Figure 6.
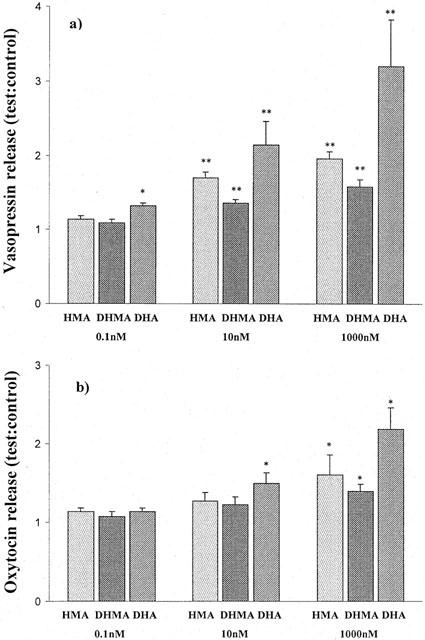
The effect of HMA, DHMA and DHA on (a) vasopressin and (b) oxytocin release from the isolated rat hypothalamus in vitro. Bars represent means±s.e.m.; *P<0.05 and **P<0.01 compared to the control values of 1.1±0.08.
Figure 7.

The effect of HMA, DHMA and DHA on 40 mM KCl stimulated release of (a) vasopressin and (b) oxytocin from the isolated rat hypothalamus. Bars represent means±s.e.m.; *P<0.05 and **P<0.01 compared to the response to potassium alone.
Discussion
The ratios reported above were similar to those observed in previous studies employing compounds known to stimulate hormone release, as were the responses to 40 mM KCl, see for example, Kostoglou-Athanassiou & Forsling (1998). The present studies confirm the observations in man (Henry et al., 1998) that MDMA can stimulate vasopressin release, also that the response is not confined to the vasopressinergic system as oxytocin release was also enhanced, but to a lesser extent. The concentrations employed span the range of concentrations of MDMA and MDA seen in man 1 h after administration of a low dose of MDMA (Fallon et al., 1999) and are similar to those estimated from the data presented by de la Torre et al (2000b). Furthermore the ratio of drug:metabolite when mixtures were employed were similar to those seen in vivo (de la Torre et al., 2000b). Release of vasopressin could be both from magnocellular and parvocellular neurones, although the concentrations of hormone achieved would indicate that the source was primarily magnocellular (Yasin et al., 1993). The results also confirm that MDMA acts directly on the hypothalamus. In man the release of vasopressin could represent a direct action or one produced by a variety of changes including hyperthermia (Dowling et al., 1987; Henry et al., 1992).
Stimulation of vasopressin release could account for the hyponatraemia reported following the use of MDMA (Satchell & Connaughton, 1994), which in some cases was severe enough to result in death (Parr et al., 1997; O'connor et al., 1999). Vasopressin levels were shown to be inappropriately high in cases of hyponatraemia following MDMA use (Ajaelo et al., 1998; Holden & Jackson, 1996). Inappropriate vasopressin alone would not cause hyponatraemia in these circumstances, there must also be unrestricted water intake. This may result from inappropriate stimulation of thirst or from the fact that those using MDMA have been advised to keep well hydrated (Matthai et al., 1996). All the recent UK reports of hyponatraemia after ingestion of MDMA refer to women (see for example Maxwell et al., 1993; Matthai et al., 1996) and this would be consistent with the observation in a group of hospital patients that, although men and women are equally likely to develop hyponatraemia, women are more likely to suffer serious side effects (Ayus et al., 1992). Oxytocin release could also contribute to the observed hyponatraemia. It has been suggested that oxytocin too may affect fluid balance and hyponatraemia has been observed following prolonged oxytocin infusion (Mwambingu, 1985). Moreover, increases in cortisol can affect salt and water balance but it is unclear whether the change in plasma cortisol associated with MDMA administration (de la Torre et al., 2000a; Forsling et al., 2001) would effect the renal response to AVP.
The drug(s) could be acting directly on the final common pathway, but the fact that thirst may be stimulated suggests that osmoreceptors might be involved. The response to MDMA could involve serotonergic and aminergic pathways. All the metabolites tested had some effect on neurohypophysial hormone release, HMMA and DHA being more effective than the parent compound. The least active was DHMA. Metabolites of MDMA have been shown to have a variety of actions. Both MDMA and MDA have been shown to increase locomotor activity in rats (Yeh & Hsu, 1991). There is also evidence that MDMA may not be the single causative agent for acute serotonin depletion in the rat but that a metabolite formed by CYP2D6 enzymes may also be involved (Chu et al., 1996), while studies on rat brain spheroids in culture demonstrate neural effects of DHMA (Walker et al., 1999).
The catechol derivative of the primary amine was found to be more potent than either the parent MDA or the mono methylated derivative i.e. HMA. Whereas in the case of the secondary amine HMMA which appears to be the major plasma metabolite in vivo is more potent than either the drug or the catechol derivative. Indeed, co-administration of HMMA with MDMA resulted in a reduction in the predicted response to the metabolite, presumably by blocking either a receptor or uptake mechanism. An alternative explanation could be that the presence of the added HMMA reduced the conversion to the active metabolite.
In conclusion, this investigation has shown that the major oxidative metabolites of MDMA could contribute to the observed effects on vasopressin release in man and support the hypothesis that a metabolite, possibly HMMA may be the cause of hyponatraemia and possibly other adverse effects.
Acknowledgments
We are grateful to Dr Dennis O'Shea for his contribution to the synthetic aspects of the work.
Abbreviations
- DHA
3,4-dihydroxyamphetamine
- DHMA
3,4-dihydroxymethamphetamine
- HMA
4-hydroxy-3methoxyamphetamine
- HMMA
4-hydroxy-3-methoxymethamphetamine
- MDA
3,4-methylenedioxyamphetamine
- MDMA
3,4-methylenedioxymethamphetamine
- δ
chemical shift, Jo and Jm coupling constant between ortho and meta aromatic protons respectively;
- +FAB
positive ion fast atom bombardment mass spectrometry
References
- AJAELO I., KOENIG K., SNOEY E. Severe hyponatremia and inappropriate antidiuretic hormone secretion following ecstasy use. Acad. Emerg. Med. 1998;5:839–840. doi: 10.1111/j.1553-2712.1998.tb02512.x. [DOI] [PubMed] [Google Scholar]
- AYUS J.C., WHEELER J.M., ARIEFF A.I. Postoperative hyponatremic encephalopathy in menstruant women. Ann. Intern. Med. 1992;117:891–897. doi: 10.7326/0003-4819-117-11-891. [DOI] [PubMed] [Google Scholar]
- BORGMAN R.J. α-Methyldopamine derivatives. Synthesis and pharmacology. J. Med. Chem. 1974;17:427–430. doi: 10.1021/jm00250a012. [DOI] [PubMed] [Google Scholar]
- BECKETT A.H., KIRK G., SHARPEN A.J. The configuration of α-methyldopamine. Tetrahedron. 1965;21:1489–1493. [Google Scholar]
- BRIDGES T.E., HILLHOUSE E.W., JONES M.T. The effect of dopamine on neurohypophysial release in vivo and from the rat neural lobe and hypothalamus in vitro. J. Physiol. 1976;260:647–666. doi: 10.1113/jphysiol.1976.sp011537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHU T., KUMAGAI Y., DISTEFANO E.W., CHO A.K. Disposition of methylenedioxymethamphetamine and three metabolites in the brains of different rat strains and their possible roles in acute serotonin depletion. Biochem. Pharmacol. 1996;51:789–796. doi: 10.1016/0006-2952(95)02397-6. [DOI] [PubMed] [Google Scholar]
- DE BOER D., TAN L.P., GORTER P., VAN DE WAL R.M.A., KETTENES-VAN DEN BOSCH J.J., DE BRUIJN E.A., MAES R.A.A. Gas chromatographic/mass spectrometric assay for profiling the enantiomers of 3,4-methylenedioxymethamphetamine and its chiral metabolites using positive chemical ionization ion trap mass spectrometry. J Mass Spectrom. 1997;32:1236–1246. doi: 10.1002/(SICI)1096-9888(199711)32:11<1236::AID-JMS584>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- DE LA TORRE R., FARRE M., ORTUNO J., MAS M., BRENNEISEN R., ROSET P.N., SEGURA J., CAMI J. Non-linear pharmacokinetics of MDMA (“ecstasy”) in humans. Br. J. Clin. Pharmacol. 2000b;49:104–109. doi: 10.1046/j.1365-2125.2000.00121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LA TORRE R., FARRE M., ROSET P.N., HERNANDEZ LOPEZ C., MAS M., ORTUNO J., MENOYO E, , PIZZARO N., SEGURA J., CAMI J. Pharmacology of MDMA in humans. Ann. N.Y. Acad. Sci. 2000a;914:225–237. doi: 10.1111/j.1749-6632.2000.tb05199.x. [DOI] [PubMed] [Google Scholar]
- DOWLING G.P., MCDONOUGH E.T., BOST R.O. “Eve and Ecstasy”. A report of five deaths associated with the use of MDEA and MDMA. JAMA. 1987;257:1615–1617. doi: 10.1001/jama.257.12.1615. [DOI] [PubMed] [Google Scholar]
- FALLON J.K., KICMAN A.T., HENRY J.A., MILLIGAN P.J., COWAN D.A., HUTT A.J. Stereospecific analysis and enantiomeric disposition of 3,4-methylenedioxymethamphetamine (Ecstasy) in humans. Clin. Chem. 1999;45:1058–1069. [PubMed] [Google Scholar]
- FORSLING M.L., PEYSNER K. Pituitary and plasma vasopressin concentrations and fluid balance over the oestrous cycle of the rat. J. Endocrinol. 1988;117:397–402. doi: 10.1677/joe.0.1170397. [DOI] [PubMed] [Google Scholar]
- FORSLING M.L., FALLON J.K., KICMAN A.T., HUTT A.J., COWAN D.A., HENRY J.A. Arginine vasopressin release in response to the administration of 3,4-methylenedioxymethamphetamine (ecstasy); is metabolism a contributory factor. J. Pharm. Pharmacol. 2001;53:1357–1363. doi: 10.1211/0022357011777855. [DOI] [PubMed] [Google Scholar]
- GLENNON R.A., LIEBOWITZ S.M., LEMING-DOET D. Demethyl analogues of psychoactive methoxyphenalkylamines: synthesis and serotonin receptor affinities. J. Med. Chem. 1980;23:990–994. doi: 10.1021/jm00183a006. [DOI] [PubMed] [Google Scholar]
- GREEN A.R., CROSS A.J., GOODWIN G.M. Review of the pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA or “Ecstasy”) Psychopharmacology. 1995;119:247–260. doi: 10.1007/BF02246288. [DOI] [PubMed] [Google Scholar]
- HENRY J.A., FALLON J.K., KICMAN A.T., HUTT A.J., COWAN D.A., FORSLING M.L. Low-dose MDMA (ecstasy) induces vasopressin secretion. Lancet. 1998;351:1784. doi: 10.1016/S0140-6736(05)78744-4. [DOI] [PubMed] [Google Scholar]
- HENRY J.A., JEFFREYS K.J., DAWLING S. Toxicity and deaths from 3,4-methylenedioxymethamphetamine (“ecstasy”) Lancet. 1992;340:384–387. doi: 10.1016/0140-6736(92)91469-o. [DOI] [PubMed] [Google Scholar]
- HOLDEN R., JACKSON M.A. Near-fatal hyponatraemic coma due to vasopressin-oversecretion after “ecstasy”. Lancet. 1996;347:1052. doi: 10.1016/s0140-6736(96)90196-8. [DOI] [PubMed] [Google Scholar]
- KOSTOGLOU-ATHANASSIOU I., FORSLING M.L. Effect of 5-hydroxytryptamine and pineal metabolites on the secretion of neurohypophysial hormones. Brain Res. Bull. 1998;46:417–422. doi: 10.1016/s0361-9230(98)00027-6. [DOI] [PubMed] [Google Scholar]
- KRETH K.P., KOVAR K.A., SCHWAB M., ZANGER U.M. Identification of the human cytochromes P450 involved in the oxidative metabolism of “Ecstasy”-related designer drugs. Biochem. Pharmacol. 2000;59:1563–1571. doi: 10.1016/s0006-2952(00)00284-7. [DOI] [PubMed] [Google Scholar]
- MAS M, , FARRE M., DE LA TORRE R., ROSET P.N., ORTUNO J., SEGURA J., CAMI J. Cardiovascular and neuroendocrine effects and pharmacokinetics of 3,4-methylenedioxymethamphetamine in humans. J. Pharmacol. Exp. Ther. 1999;290:136–145. [PubMed] [Google Scholar]
- MATTHAI S.M., SILLS J.A., DAVIDSON D.C., ALEXANDROU D. Cerebral oedema after ingestion of MDMA (“ecstasy”) and unrestricted intake of water. BMJ. 1996;312:1359. doi: 10.1136/bmj.312.7042.1359b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAXWELL D.L., POLKEY M.I., HENRY J.A. Hyponatreamia and catatonic stupor after taking “ecstasy”. BMJ. 1993;307:1399. doi: 10.1136/bmj.307.6916.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MWAMBINGU F.T. Water intoxication on oxytocin. BMJ. 1985;290:113. doi: 10.1136/bmj.290.6462.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASH J.F., MELTZER H.Y., GUDELSKY G.A. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. J. Pharmacol. Exp. Ther. 1988;245:873–887. [PubMed] [Google Scholar]
- O'CONNOR A., CLUROE A., COUCH R., GALLER L, , LAWRENCE J., SYNEK B. Death from hyponatraemia-induced cerebral oedema associated with MDMA (“Ecstasy”) use. N.Z. Med. J. 1999;112:255–256. [PubMed] [Google Scholar]
- ORTUNO J, , PIZARRO N., FARRE M., MAS M., SEGURA J., CAMI J., BRENNEISEN R., DE LA TORRE R. Quantification of 3,4-methylenedioxymethamphetamine and its metabolites in plasma and urine by gas chromatography with nitrogen-phosphorous detection. J. Chromatogr. B. 1999;723:221–232. doi: 10.1016/s0378-4347(98)00506-4. [DOI] [PubMed] [Google Scholar]
- PARR M.J., LOW H.M., BOTTERILL P. Hyponatraemia and death after “ecstasy” ingestion. Med. J. Aust. 1997;166:136–137. doi: 10.5694/j.1326-5377.1997.tb140044.x. [DOI] [PubMed] [Google Scholar]
- RENAUD L.P., BOURQUE C.W. Neurophysiology and neuropharmacology of hypothalamic magnocellular neurons secreting vasopressin and oxytocin. Prog. Neurobiol. 1991;36:131–169. doi: 10.1016/0301-0082(91)90020-2. [DOI] [PubMed] [Google Scholar]
- SATCHELL S.C., CONNAUGHTON M. Inappropriate antidiuretic hormone secretion and extreme rises in serum creatinine kinase following MDMA ingestion. Br. J. Hosp. Med. 1994;51:495. [PubMed] [Google Scholar]
- TSAGARAKIS S., HOLLY J.M., REES L.H., BESSER G.M., GROSSMAN A. Acetylcholine and norepinephrine stimulate the release of corticotropin-releasing factor-41 from the rat hypothalamus in vitro. Endocrinology. 1988;123:1962–1969. doi: 10.1210/endo-123-4-1962. [DOI] [PubMed] [Google Scholar]
- WALKER T.M., DAVENPORT-JONES J.E., FOX R.M., ATTERWILL C.K. The neurotoxic effect of methylenedioxymethamphetamine (MDMA) and its metabolites on rat brain spheroids in culture. Cell. Biol. Toxicol. 1999;15:137–142. doi: 10.1023/a:1007658501306. [DOI] [PubMed] [Google Scholar]
- WHITE S.R., OBRAADOVIC T., IMEL K.M., WHEATON M.J. The effects of methylenedioxymethamphetamine (MDMA, “Ecstasy”) on monoaminergic neurotransmission in the central nervous system. Prog. Neurobiol. 1996;49:455–479. doi: 10.1016/0301-0082(96)00027-5. [DOI] [PubMed] [Google Scholar]
- WINDLE R.J., FORSLING M.L. Variations in oxytocin secretion during the 4-day oestrous cycle of the rat. J. Endocrinol. 1993;136:305–311. doi: 10.1677/joe.0.1360305. [DOI] [PubMed] [Google Scholar]
- YASIN S.A., COSTA A., BESSER G.M., HUCKS D., GROSSMAN A., FORSLING M.L. Melatonin and its analogs inhibit the basal and stimulated release of hypothalamic vasopressin and oxytocin in vitro. Endocrinology. 1993;132:1329–1336. doi: 10.1210/endo.132.3.8440190. [DOI] [PubMed] [Google Scholar]
- YEH S.Y., HSU F.L. The neurochemical and stimulatory effects of putative metabolites of 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine in rats. Pharmacol. Biochem. Behav. 1991;39:787–790. doi: 10.1016/0091-3057(91)90165-x. [DOI] [PubMed] [Google Scholar]


