Abstract
Nociceptin/orphanin FQ (N/OFQ), an endogenous opioid-like orphan receptor (NOP receptor, previously termed ORL1 receptor) agonist, has been found to inhibit capsaicin-induced bronchoconstriction in isolated guinea-pig lungs and in vivo. The underlying mechanisms are not clear. In the present studies, we tested the effect of N/OFQ on VR1 channel function in isolated guinea-pig nodose ganglia cells. Capsaicin increased intracellular Ca2+ concentration in these cells through activation of vanilloid receptors. Capsaicin-induced Ca2+ responses were attenuated by pretreatment of nodose neurons with N/OFQ (1 μM).
N/OFQ inhibitory effect on the Ca2+ response in nodose ganglia cells was antagonized by tertiapin (0.5 μM), an inhibitor of inward-rectifier K+ channels, but not by verapamil, a voltage gated Ca2+ channel blocker, indicating that an inward-rectifier K+ channel is involved in N/OFQ inhibitory effect.
In isolated guinea-pig bronchus, N/OFQ (1 μM) inhibited capsaicin-induced airway contraction. Tertiapin (0.5 μM) abolished the N/OFQ inhibition of capsaicin-induced bronchial contraction.
Capsaicin (10 μg) increased pulmonary inflation pressure in the isolated perfused guinea-pig lungs. This response was significantly attenuated by pretreatment with N/OFQ (1 μM). Tertiapin also abolished the N/OFQ inhibitory effect on capsaicin-induced bronchoconstriction in perfused lungs. Capsaicin increased the release of substance P and neurokinin A from isolated lungs. N/OFQ (1 μM) blocked the capsaicin-induced tachykinin release.
These results indicate that N/OFQ-induced hyperpolarization of tachykinin containing airway sensory nerves, through an inward-rectifier K+ channel activation, accounts for the inhibition of capsaicin-evoked broncoconstriction.
Keywords: Capsaicin, vanilloid receptor, nociceptin, opioid-like orphan receptor, intracellular calcium, tackykinin, airway, nodose ganglion
Introduction
Capsaicin-sensitive vanilloid receptor (VR) is expressed mainly in sensory neurons including those emanating from the nodose ganglion (visceral sensory neurons) and solitary tract region, the latter representing the central termination area for sensory neurons of the vagus nerve (Szallasi & Blumberg, 1999). VR has been detected in guinea-pig and human airways by receptor binding assay (Szallasi et al., 1995). VR is a cation channel with preference for Ca2+. Activation of VR in sensory nerves by vanilloids such as capsaicin, induces Ca2+ influx and membrane depolarization, leading to release of neurotransmitters from both peripheral and central endings, resulting in neurogenic inflammation, bronchoconstriction, cough, and the sensation of pain. Included among these neurotransmitters are substance P (SP), neurokinin A (NKA), and calcitonin gene-related peptide (CGRP, Holzer, 1988). In guinea-pig lungs, for example, capsaicin is known to induce bronchoconstriction mediated by a tachykinin-dependent mechanism, mainly through activation of NK2 receptors (Lou et al., 1993).
Opioid-like orphan receptors are widely expressed in mammalian central nervous system and several peripheral tissues including peripheral ganglia and the immune system (Mollereau & Mouledous, 2000). N/OFQ is an endogenous ligand for the NOP receptor (Meunier et al., 1995; Reinscheid et al., 1995). N/OFQ-containing nerve fibres have been detected in guinea-pig bronchus by immunohistochemical method (Fischer et al., 1998). NOP receptor activation induces inhibition of voltage-gated Ca2+ channel current (Knoflach et al., 1996; Abdulla and Smith, 1997; Connor & Christie, 1998; Moran et al., 2000; Larsson et al., 2000), and activation of inward-rectifier K+ channels in neurons (Lee et al., 1997; Ikeda et al., 1997; Wagner et al., 1998), either of which may lead to inhibition of neurotransmitter release. The interaction between NOP and VR1 receptors has presently not been studied.
In the airways, N/OFQ was found to inhibit the contractions of the guinea-pig isolated bronchus and tachykinin release induced by non-adrenergic non-cholinergic electrical field stimulation (EFS, Fischer et al., 1998; Shah et al., 1998). Recently, studies by Corboz et al. (2000; 2001) showed that N/OFQ also inhibited capsaicin-induced bronchoconstriction in isolated lungs and in vivo in guinea-pigs. We hypothesized that this effect of N/OFQ might be secondary to the inhibition of capsaicin-induced cell depolarization and tackykinin release from sensory neurons in the airway. In the current studies, we evaluated the interaction between NOP and VR1 receptors at the cellular level using fluorescence-based calcium imaging experiments performed on isolated nodose ganglion neurons. We also tested the mechanism of N/OFQ inhibitory effect on capsaicin-induced airway contraction in isolated guinea-pig bronchus and in isolated perfused lungs.
Methods
Nodose ganglia cell isolation
Male Hartley guinea-pigs (400 – 700 g, Charles River, Bloomington, MA, U.S.A.) were euthanized with CO2. Nodose ganglia were removed under aseptic conditions. Individual ganglia were collected, washed in Hank's balanced salt solution (HBSS) and then transferred to HBSS containing collagenase (type IA, 1 mg ml−1) for 45 min at 37°C in a water bath. The enzyme solution was aspirated from the tissues, after which they were rinsed with HBSS and then incubated in HBSS containing DNAse IV (0.1 mg ml−1) for 15 min at 37°C in a water bath. Tissues were washed and subjected to gentle trituration using a Pasteur pipette. This was sufficient to dissociate the ganglia. The resulting cell suspension was filtered through a sterile nylon mesh (Becton Dickinson Labware MA, U.S.A.), plated into poly-lysine coated black walled clear-based 96-well plates (∼10000 cells/well, Becton Dickinson Labware MA, U.S.A.). Cells were incubated for 3 h at 37°C prior to the intracellular Ca2+ measurements.
Measurements of intracellular Ca2+ concentration using the FLIPR
Intracellular Ca2+ ([Ca2+]i) in nodose ganglia cells was measured using fluorometric imaging plate reader (FLIPR, Molecular Devices Corp., CA, U.S.A.) technique. Briefly, cells were incubated with a calcium sensitive fluoresce dye, Fluo-4-AM (5 μg ml−1, Molecular Probes, OR, U.S.A.), in HBSS containing 0.4% bovine serum albumin (BSA) for 45 min at 37°C. The dye-loading solution was removed and the cells were washed twice with HBSS containing 0.4% BSA. The cells were pre-incubated with various antagonists and the plates were than placed into a FLIPR. Fluorescence change due to the change of [Ca2+]i was measured by FLIPR. Capsaicin responses were elicited by direct additions to an individual culture well during real-time recording (10 s after commencing recording). We made no attempt to calibrate the capsaicin response in terms of intracellular calcium concentration changes.
Isometric force measurement in isolated guinea-pig bronchus
Male Hartley guinea-pigs (400 – 700 g) were euthanized with CO2 and their main bronchus were immediately excised and cut into rings. Only the rings from the upper end of the bronchus were used to measure the mechanical responses. Bronchial rings were mounted on hooks, connected to force transducers (model FT03, Grass, Quincy, MA, U.S.A.), and incubated in a physiological saline solution containing (in mM): NaCl 118, CaCl2 2.55, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 24.9, Glucose 11.1 and indomethacin 2 μM (pH=7.4) bubbled with 95% O2 – 5% CO2 in 25-ml organ baths at 37°C. The passive tension was set at 1 g, and the tissue was equilibrated for 60 min in the presence of 1 μM phosphoramidon. Bronchus was then preincubated with or without tertiapin (40 min), verapamil (1 μM, 40 min) and/or N/OFQ (20 min). Isometric force of the bronchial rings in response to cumulative concentration of capsaicin was recorded. Only one concentration-response curve for capsaicin was performed in each bronchus.
Pulmonary inflation pressure in isolated guinea-pig lungs
The isolated lungs from guinea-pigs were used to test the effect of nociceptin on capsaicin-induced broncoconstriction and tachykinin release. Male Hartley guinea-pigs (400 – 700 g), were euthanized with an intraperitoneal overdose of sodium pentobarbital. The lungs were isolated and perfused as described previously (Vemulapalli et al., 1992). Briefly, a thoracotomy was rapidly performed and the lungs and the heart, with exception of the left atria, were removed en bloc. The trachea and pulmonary artery were rapidly cannulated. The lungs were then suspended inside a glass chamber (37°C) and perfused with a Tyrode's solution (mM: NaCl 137.0, KCl 2.7, CaCl2 0.4, MgCl2·6H2O 1.0, NaHCO3 11.9, NaH2 PO4 0.4 and dextrose 5.5) maintained at 37°C. They were mechanically ventilated with room air using a small rodent ventilator (Harvard). The respiratory rate was set at 60 strokes min−1 with a volume of 2.0 ml stroke−1. Pulmonary inflation pressure (PIP) was continuously monitored with a pressure transducer (Gould P231D) connected to a side arm of the tracheal cannula. Perfusion pressure was maintained with a peristaltic pump (Cole-Palmer 7553-20) at a rate of 4.5 – 5.0 ml min−1 to produce a baseline pulmonary artery pressure between 6 – 14 cm H2O. Pulmonary artery pressure was continuously monitored using a pressure transducer (Stathun P23XL) connected to the side arm of the pulmonary artery cannula. Transducers were connected to a polygraph (Grass Model no.7) for continuous monitoring of variables.
Tachykinin measurement
Lung perfusate was collected before and 6 min after capsaicin application. All the samples were collected for 3 min. Protease inhibitors (aprotonin 500 KIU ml−1, captopril 1 μM, phosphoramidon 1 μM, bestanin 1 μM, and thiorphan 1 μM) were added to all samples immediately after sample collection. Samples were centrifuged at 17,000×g for 15 min at 4°C in the equal volume of 1% trifluoacetic acid. Supernatant was passed through a 200 mg C18 column (Waters MA, U.S.A.) and eluted with 2 ml of solution containing acetonitrile (60%) and 1% TFA (40%). Eluent was dried using a speed vacuum system and reconstituted to a desired volume with assay buffer. SP and NKA levels were determined by enzyme immunoassay (ELISA) method using Enzyme Immunoassay Kit from Peninsula Laboratories Inc.
Data analysis
All results are expressed as means±standard error of the mean (s.e.mean). An analysis of variance was performed on the different treatment groups to determine significant effects of the treatments. Post-hoc analysis between the different groups was performed with a Dunnett's t-test. Mann – Whitney Rank Sum Test was used to analyse non-normal distributed data (Figure 3B). A value of P<0.05 was accepted as the level of statistical significance.
Figure 3.
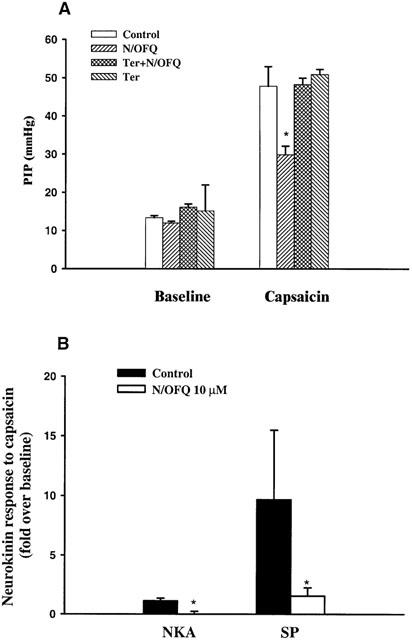
Effect of N/OFQ on capsaicin-induced bronchoconstriction and neurokinin release in isolated guinea-pig lungs. (A) The PIP response to capsaicin was measured in isolated perfused guinea-pig lungs. The results are means±s.e.mean. *P<0.005 as compared with the control group in the absence of nociceptin. (B) Lung perfusate was collected before and after the bolus injection of capsaicin (10 μg). NKA (n=6) and substance P (n=5) concentration in lung perfusate was measured. Capsaicin responses were expressed as fold over baseline. The results are means±s.e.mean. *P<0.05 as compared with N/OFQ group.
Materials
Capsaicin, tertiapin, DNAse IV, collagenase (type IA), aprotinin, captopril, bestanin and thiorphan were purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.), nociceptin/orphanin FQ from Phoenix Pharmaceuticals (Mountain View, CA, U.S.A.), [Phe1φ(CH2-NH)Gly2]Nociceptin(1-13)NH from Tocris (Avonmouth Bristol, U.K.), Verapamil from ICN Biomedicals Inc. (Aroro, Ohio, U.S.A.), and substance P and NKA ELISA kits from Peninsula Labs (Belmont, CA, U.S.A.). HBSS was obtained from Gibco (NY, U.S.A.). Indomethacin was synthesized by Schering-Plough Corp.
Results
Ca2+ response in isolated guinea-pig nodose ganglia cells
Capsaicin (0.1 μM) consistently increased [Ca2+]i in nodose ganglia cells as determined by Fluo-4 fluorescence change (Figure 1A). The concentration of capsaicin studied in these experiments was selected so as to be sub-maximal to avoid VR1 receptor saturation. The capsaicin response was inhibited by 88±6% when cells were pretreated with capsazepine (1 μM), a VR1 antagonist (Figure 1A,C), indicating that the capsaicin-induced Ca2+ response was VR1 receptor-mediated. Pre-treatment with N/OFQ (1 μM) attenuated the subsequent capsaicin-evoked response by 55±5% (Figure 1B,C). This inhibitory effect was abolished by [Phe1φ(CH2-NH)Gly2]Nociceptin(1-13)NH, a NOP receptor antagonist (Figure 1C). The inhibitory effect of N/OFQ was also significantly antagonized (to 20±4%) by prior blockade of inward rectifier potassium channels with tertiapin (0.5 μM, Jin & Lu, 1998; Kitamura et al., 2000), whereas tertiapin itself had no effect on capsaicin-induced response (Figure 1B,C). Similar effects were also observed using single cell Fluo-4 imaging (unpublished observation). Verapamil, a voltage-gated Ca2+ channel blocker, had no effect on N/OFQ inhibitory effect (Figure 1C).
Figure 1.
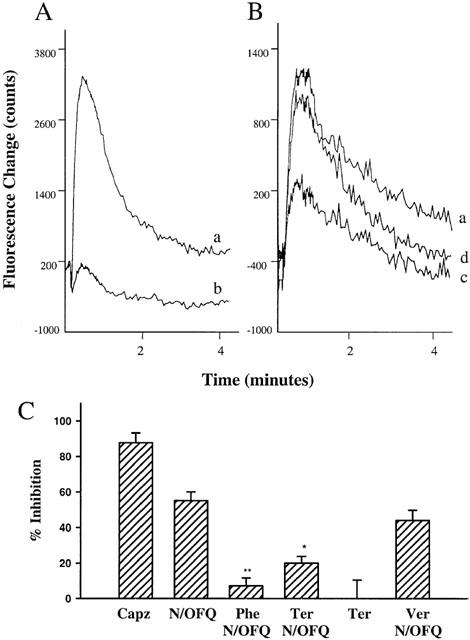
Effect of N/OFQ on capsaicin-induced [Ca2+]i increase in guinea-pig nodose ganglia cells. [Ca2+]i was determined by Fluo-4 fluorescence change and measured using FLIPR. Representative traces showing the Ca2+ response to capsaicin in nodose ganglia cells, in the presence of capsazepine (A), N/OFQ and tertiapin (B). Capsaicin 0.1 μM was added 10 s after commencing recording in all the experiments. a: control; b: cells preincubated with capsazepine (1 μM) for 10 min before the addition of capsaicin; c: cells pretreated with N/OFQ (1 μM) for 10 min before the addition of capsaicin; d: cells pretreated with tertiapin (0.5 μM) for 20 min and N/OFQ (1 μM) for 10 min before the addition of capsaicin. The recordings are representative examples from four experiments. (C) % inhibition of the capsaicin-induced fluorescence change by capsazepine (Capz, 1 μM), N/OFQ (1 μM), [Phe1φ(CH2-NH)Gly2]Nociceptin(1-13)NH (Phe, 10 μM)+N/OFQ (1 μM), tertiapin (Ter, 0.5 μM)+N/OFQ (1 μM), Tertiapin (0.5 μM), and verapamil (Ver, 1 μM)+N/OFQ (1 μM, P=0.13 compared with N/OFQ group). Data are means±s.e.mean. *P<0.001, **P<0.0001 compared with N/OFQ treatment.
Effect of tertiapin on N/OFQ inhibition of capsaicin response in isolated guinea-pig bronchus
Figure 2A shows the dose response curves of the capsaicin-induced bronchocontraction in the isolated guinea-pig bronchus. The response was expressed as the percentage of the maximal response induced by KCl (80 mM). N/OFQ (1 μM), when preincubated with bronchus, significantly inhibited the capsaicin-induced airway contraction. In tissues preincubated with tertiapin (0.5 μM), the N/OFQ inhibitory effect on the capsaicin-induced bronchocontraction was completely abolished. Tertiapin alone had no effect on capsaicin-induced response in isolated bronchus (Figure 2A). N/OFQ inhibitory effect was not affected by verapamil (1 μM, Figure 2B).
Figure 2.
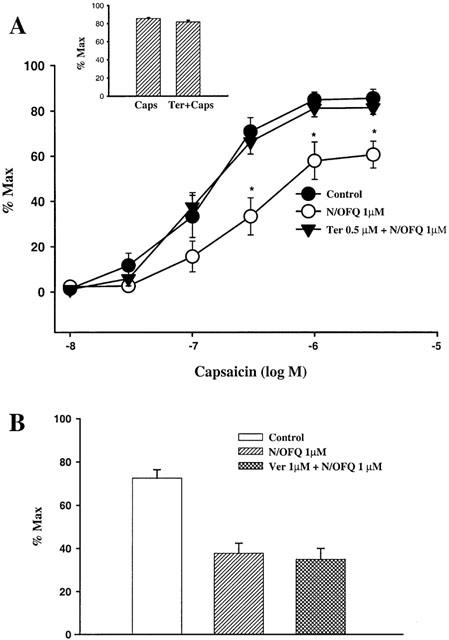
(A) Effect of tertiapin on N/OFQ inhibition of the capsaicin response in isolated guinea-pig bronchus. The results are means±s.e.mean and are expressed as percentage of the maximal response induced by KCl (80 mM). In this experiment, N/OFQ and tertiapin were always applied 20 min and 40 min, respectively, before the addition of capsaicin. *P<0.05 as compared with control or N/OFQ+tertiapin treatments. Insert bars: Effect of tertiapin (Ter, 0.5 μM) on capsaicin (1 μM)-induced contraction (n=7). (B) Lack of effect of verapamil (Ver, n=6) on N/OFQ inhibition of capsaicin (30 nM) response in isolated guinea-pig bronchus.
Effects of N/OFQ on capsaicin-induced bronchoconstriction in isolated guinea-pig lungs
The bronchoconstrictor response to capsaicin was measured in isolated guinea-pig lungs in the presence and absence of N/OFQ (Figure 3A). The PIP baseline was 13.4±0.5 mmHg in control group (n=6) and 12.0±0.5 mmHg in N/OFQ (1 μM) pretreatment group (n=6). Bolus injection of capsaicin (10 μg in 100 μl) to the pulmonary artery significantly increased PIP in both control (47.8±5.1 mmHg, P<0.01) and N/OFQ (29.8±2.3 mmHg, P<0.005) groups. However, the peak PIP response to capsaicin in N/OFQ group was significantly lower than that in control group (P<0.005, Figure 3A). Tertiapin (0.5 μM) reversed the N/OFQ inhibitory effect, but had no direct effect on capsaicin-induced broncoconstriction response (Figure 3A).
Effect of N/OFQ on capsaicin-induced NKA and substance P release from isolated guinea-pig lungs
NKA and substance P concentrations in lung perfusate were measured before and after bolus injection of capsaicin. Capsaicin induced a significant increase in tachykinin levels in the lung perfusate. NKA and substance P levels were increased by 2.1±0.3 and 10.7±5.8 fold of baseline respectively (P<0.05, Figure 3B). N/OFQ (1 μM, 20 min) induced a statistically significant inhibition of this stimulatory effect of capsaicin (P<0.05, Figure 3B). N/OFQ had no direct effect on baseline tachykinin release (data not shown).
Discussion
We recently found that N/OFQ inhibited capsaicin-induced bronchoconstriction in isolated guinea-pig lungs (Corboz et al., 2000) and in vivo (Corboz et al., 2001). The mechanism has not been previously elucidated. Clearly, a plausible mechanism might involve a direct NOP receptor-mediated inhibition of VR1 calcium influx, and/or an indirect effect via membrane hyperpolarization of sensory nerve terminals, leading to a decrease of tackykinin release. In the current studies, we tested the effect of N/OFQ on VR1 function in isolated guinea-pig nodose ganglia cells. We found that N/OFQ inhibited capsaicin-induced responses in guinea-pig nodose ganglia cells as well as in isolated bronchus and lungs. N/OFQ inhibitory effects were antagonized by the inward-rectifier potassium channel antagonist, tertiapin.
The nodose ganglion is a sensory ganglion whose axons run in the vagus nerve (vagal afferents) and provide sensory innervation to the viscera including airways. VR1 receptor has been detected using in situ hybridization (Michael & Priestley, 1999) and functionally (Bielefeldt, 2000) in nodose ganglia neurons. Our findings showing that capsaicin-induced Ca2+ responses in isolated guinea-pig nodose ganglia cells are blocked by capsazepine, a VR1 antagonist, confirmed the functional expression of VR1 receptors in these cells. In this study, we also found that N/OFQ inhibited capsaicin-induced increase in [Ca2+]i in nodose ganglia cells via an action on NOP receptors because the inhibitory effect of N/OFQ was antagonized by [Phe1φ(CH2-NH)Gly2]Nociceptin(1-13)NH, an NOP receptor antagonist. These observations are consistent with N/OFQ's effect on airway contraction in vitro and in vivo (Corboz et al., 2000; 2001). This finding also indicates that NOP receptors are functionally expressed in nodose ganglia neurons. Thus, these cells were ideal for the study of the mechanism by which N/OFQ inhibits VR1 function in the airways.
A prime candidate for the mechanism underlying the NOP/VR1 receptor interaction may be a change in membrane polarization. N/OFQ has been reported to produce a membrane hyperpolarization of β-endorphin neurons and other neurosecretory cells in the hypothalamic arcuate nucleus by activation of an inward-rectifier K+ conductance (Lee et al., 1997; Wagner et al., 1998). N/OFQ also induces hyperpolarizing currents via an inward-rectifier K+ channel in hippocampal pyramidal cells (Ikeda et al., 1997). To test whether these mechanisms are involved in the N/OFQ inhibition of bronchoconstriction induced by capsaicin, we tested the effect of tertiapin, a novel inhibitor for inward-rectifier K+ channels (Jin & Lu, 1998; Kitamura et al., 2000), on the N/OFQ inhibitory effect. The N/OFQ inhibitory effect on capsaicin-induced Ca2+ influx in guinea-pig nodose ganglia cells was reversed by tertiapin (Figure 1B,C). Consistently, N/OFQ's inhibition of capsaicin-induced bronchial contraction in isolated bronchus and perfused lungs were also abolished by tertiapin (Figures 2A, 3A). These results indicate that an inward-rectifier K+ channel is involved in the N/OFQ's inhibitory action. Activation of a K+ conductance by N/OFQ would lead to membrane hyperpolarization, a concomitant inhibition of neuronal firing and, presumably, attenuated neurotransmitter release. Indeed, we found that N/OFQ inhibited capsaicin-induced substance P and NKA release from isolated guinea-pig lungs, consistent with this proposed mechanism. Similar observations have also been reported in rats that N/OFQ inhibits capsaicin-induced SP and CGRP release in rat trachea (Nemeth et al., 1998).
N/OFQ is also known to inhibit voltage-dependent Ca2+ channels in several neurons (Knoflach et al., 1996; Abdulla et al., 1997; Connor & Christie, 1998; Moran et al., 2000; Larsson et al., 2000). We considered the possibility that inhibition of voltage-dependent Ca2+ channels was also involved in the observed N/OFQ inhibitory effect, though this would be unlikely in view of the known capsaicin activation of Ca2+ influx via the VR1 cation channel. Accordingly, we found that blockade of voltage-activated Ca2+ channels by verapamil did not affect the N/OFQ inhibitory effect in either nodose ganglia cells or isolated bronchus. Likewise, capsaicin-induced SP release from rat trachea is independent of voltage-dependent Ca2+ channels (Szolcsanyi et al., 1998).
NOP receptors have been shown to inhibit neurotransmitter release from both central and peripheral neurons. In the respiratory system, N/OFQ has been shown to inhibit electrical field stimulation (EFS)-induced acetylcoline release from parasympathetic nerve terminals in guinea-pig trachea (Patel et al., 1997). EFS of guinea-pig isolated main bronchi induced a nonadrenergic non-cholinergic contractile response and release of substance P in guinea-pig airway (Fischer et al., 1998; Shah et al., 1998). N/OFQ inhibits the EFS induced bronchi contraction and substance P release (Shah et al., 1998). N/OFQ also inhibits EFS-induced substance P and CGRP release from the rat isolated trachea (Helyes et al., 1997; Shah et al., 1998). Our current findings provide the first evidence that N/OFQ also inhibits the tachykinin release in the guinea-pig lungs evoked by capsaicin. It is possible that the same mechanism involving the activation of inward-rectifier K+ channels also accounts for the N/OFQ inhibition of neurotransmitter release from central and other peripheral systems, as these channels are widely expressed in neurosecretory cells.
Although peptide ligands are generally highly specific for their respective receptors, we considered the remote possibility that inhibition of capsaicin-induced tachykinin release by N/OFQ may also involve a direct effect of the peptide on the VR1 channel. However, the present tertiapin data argue against this being the case, favouring membrane hyperpolarization as the causative mechanism linking the two receptor systems. Indeed, recent experiments using cloned rat VR1 receptors have demonstrated their sensitivity to membrane hyperpolarization (Gunthorpe et al., 2000). Current-voltage relationships for recombinant VR1 showed a region of negative-slope conductance consistent with channel function being compromised by membrane potentials more negative to -70 mV (Gunthorpe et al., 2000). These findings are consistent with the mechanism that N/OFQ inhibits capsaicin-induced VR1 activation through a hyperpolarizing K+ current.
In this study, we found that N/OFQ (1 μM) inhibited capsaicin-induced airway contraction in isolated guinea-pig bronchus. This finding is consistent with previous reports in perfused lungs (Corboz et al., 2000) and in vivo (Corboz et al., 2001). However, other groups reported that in the presence of N/OFQ, capsaicin-induced contraction in isolated guinea-pig bronchus was not affected (Fischer et al., 1998; Shah et al., 1998). In the present study, the isolated bronchus was preincubated with N/OFQ for 20 min before the addition of capsaicin, this may be important for N/OFQ inhibitory effect and account for the difference from other reports. In addition, in the study by Fischer et al. (1998), a 10 fold lower concentration of the peptide was used while in the study by Shah et al. (1998), (where the same concentration of N/OFQ was tested), a clear trend toward a peptide induced inhibitory effect was evident, although it did not reach the statistical level of significance. Therefore, we suggest that different experimental conditions and/or different assay sensitivities among laboratories may account for the apparent discrepancies.
The functional role of NOP activation on sensory airway reflexes has been recently investigated. Recent studies have been shown that selective activation of NOP receptors with N/OFQ produces antitussive activity in guinea-pigs (McLeod et al., 2001) and cats (Bolser et al., 2001). N/OFQ, when given by either a central or peripheral route, was found to inhibit capsaicin-induced cough in guinea-pigs. N/OFQ also inhibited cough induced by mechanical stimulation in cats. These findings are consistent with our current finding that NOP receptors are functional modulators of airway sensory responses. We propose that the antitussive activity of N/OFQ may also involve hyperpolarization of sensory neurons by activation of potassium channels.
In summary, our study has demonstrated that N/OFQ inhibits VR1 Ca2+ influx in isolated guinea-pig nodose ganglia cells and capsaicin-induced tackykinin release from isolated guinea-pig lungs. N/OFQ inhibition of capsaicin response is abolished by the inward-rectifier K+ channel inhibitor, tertiapin. Taken together, these findings indicate that the inhibitory effect of N/OFQ on capsaicin-induced bronchoconstriction involves the activation of an inward-rectifier K+ channel and, presumably, decreased terminal excitability at sensory nerve endings innervating lung tissue.
Acknowledgments
The authors are grateful to Dr R.L. McLeod, Dr. X-W Dong and Mr J. Crona for expert technical assistance and advice during the course of these experiments.
Abbreviations
- [Ca2+]i
intracellular Ca2+
- EFS
electrical field stimulation
- FLIPR
fluorometric imaging plate reader
- HBSS
Hank's balanced salt solution
- NKA
neurokinin A
- N/OFQ
nociceptin/orphanin FQ
- NOP receptor
opioid-like orphan receptor
- PIP
pulmonary inflation pressure
- VR
vanilloid receptor
References
- ABDULLA F.A., SMITH P.A. Nociceptin inhibits T-type Ca2+ channel current in rat sensory neurons by a G-protein-independent mechanism. J. Neurosci. 1997;17:8721–8728. doi: 10.1523/JNEUROSCI.17-22-08721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIELEFELDT K. Differential effects of capsaicin on rat visceral sensory neurons. Neuroscience. 2000;101:727–736. doi: 10.1016/s0306-4522(00)00375-4. [DOI] [PubMed] [Google Scholar]
- BOLSER D.C., MCLEOD R.L., TULSHIAN D.B., HEY J.A. Antitussive action of nociceptin in the cat. Eur. J. Pharmacol. 2001;430:107–111. doi: 10.1016/s0014-2999(01)01244-4. [DOI] [PubMed] [Google Scholar]
- CONNOR M., CHRISTIE M.J. Modulation of Ca2+ channel curents of acutely dissociated rat periaqueductal grey neurons. J. Physiol. 1998;509:47–58. doi: 10.1111/j.1469-7793.1998.047bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORBOZ M.R., FERNANDEZ X., EGAN R.W., HEY J.A. Inhibitory activity of nociceptin/orphanin FQ on capsaicin-induced bronchoconstriction in the guinea-pig. Life Sciences. 2001;69:1203–1211. doi: 10.1016/s0024-3205(01)01197-3. [DOI] [PubMed] [Google Scholar]
- CORBOZ M.R., RIVELLI M.A., EGAN R.B., TULSHIAN D., MATASI J., FAWZI A.B., BENBOW L., SMITH-TORHANTORHAN A., ZHANG H., HEY J.A. Nociceptin inhibits capsaicin-induced brochoconstriction in isolated guinea-pig lung. Eur. J. Pharmacol. 2000;402:171–179. doi: 10.1016/s0014-2999(00)00505-7. [DOI] [PubMed] [Google Scholar]
- FISCHER A., FORSSMANN W., UNDEM B.J. Nociceptin-induced inhibition of tachykinergic neurotransmission in guinea-pig bronchus. J. Pharmacol. Exp. Ther. 1998;285:902–907. [PubMed] [Google Scholar]
- GUNTHORPE M.J., HARRIES M.H., PROMJHA R.K., DAVIS J.B., RANDALL A. Voltage- and time-dependent properties of the recombinant rat vanilloid receptor (rVR1) J. Physiol. 2000;525:747–759. doi: 10.1111/j.1469-7793.2000.t01-1-00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELYES Z., NEMETH J., PINTER E., SZOLCSANYI J. Inhibition by nociceptin of neurogenic inflammation and the release of SP and CGRP from sensory verve terminals. Br. J. Pharmacol. 1997;121:613–615. doi: 10.1038/sj.bjp.0701209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZER P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988;24:739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- IKEDA K., KOBAYASHI K., KOBAYASHI T., ICHIKAWA T., KUMANISHI T., KISHID A.H., YANO R., MANABE T. Functional coupling of the nociceptin/orphanin FQ receptor with the G-protein-activated K+ (GIRK) channel. Mol. Brain Res. 1997;45:117–126. doi: 10.1016/s0169-328x(96)00252-5. [DOI] [PubMed] [Google Scholar]
- JIN W., LU Z. A novel high-affinity inhibitor for inward-rectifier K+ channels. Biochemistry. 1998;37:13291–13299. doi: 10.1021/bi981178p. [DOI] [PubMed] [Google Scholar]
- KITAMURA H., YOKOYAMA M., AKITA H., MATSUSHITA K., KURACHI Y., YAMADA M. Tertiapin potently and selectively blocks muscarinic K+ channels in rabbit cardiac myocytes. J. Pharmacol. Exp. Ther. 2000;293:196–205. [PubMed] [Google Scholar]
- KNOFLACH F., REINSCHEID R.K., CIVELLI O., KEMP J.A. Modulation of voltage-gated calcium channels by orphanin FQ in freshly dissociated hippocampal neurons. J. Neurosci.. 1996;16:6657–6664. doi: 10.1523/JNEUROSCI.16-21-06657.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARSSON K.P., OLSEN U.B., HANSEN A.J. N/OFQ is a potent inhibitor of N-type Ca2+ channels in rat sympathetic ganglion neurons. Neuroscience Letters. 2000;296:121–124. doi: 10.1016/s0304-3940(00)01640-2. [DOI] [PubMed] [Google Scholar]
- LEE K., NICHOLSON J.R., MCKNIGHT A.T. Nociceptin hyperpolarizes neurons in the rat ventromedial hypothalamus. Neuroscience Letters. 1997;239:37–40. doi: 10.1016/s0304-3940(97)00875-6. [DOI] [PubMed] [Google Scholar]
- LOU Y.P., LEE L.Y., SATOH H., LUNDBERG J.M. Postjunctional inhibitory effect of the NK2 receptor antagonist, SR 48968, on sensory NANC bronchoconstriction in the guinea-pig. Br. J. Pharmacol. 1993;109:765–773. doi: 10.1111/j.1476-5381.1993.tb13640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCLEOD R.L., PARRA L.E., MUTTER J.C., ERICKSON C.H., CAREY G.J., TULSHIAN D.B., FAWZI A.B., SMITH-TORHAN A., EGAN R.W., CUSS F.M., HEY J.A. Nociceptin hihibits cough in the guinea-pig by activation of ORL1 receptors. Br. J. Pharmacol. 2001;132:1175–1178. doi: 10.1038/sj.bjp.0703954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEUNIER J.C., MOLLEREAU C., TOLL L., SUAUDEAY C., MOISAND C., ALVINERIE P., BUTOUR J.L., GUILLEMOT J.C., FERRARA P., MONSARRAT B., MAZARGUIL H., VASSART G., PARMENTIER M., COSTENTIN J. Isolation and structure of the endogenous agonist of opioid receptor like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- MICHAEL G.J., PRIESTLEY J.V. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J. Neurosci. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOLLEREAU C., MOULEDOUS L. Tissue distribution of opioid receptor-like (ORL1) receptor. Peptides. 2000;21:907–917. doi: 10.1016/s0196-9781(00)00227-8. [DOI] [PubMed] [Google Scholar]
- MORAN T.D., ABDULLA F.A., SMITH P.A. Cellular neurophysiological acrions of N/OFQ/orphanin FQ. Peptides. 2000;21:969–976. doi: 10.1016/s0196-9781(00)00235-7. [DOI] [PubMed] [Google Scholar]
- NEMETH J., HELYES Z., OROSZI G., THAN M., PINTER E., SZOLCSANYI J. Inhibition of N/OFQ on sensory neuropeptide release and mast cell-mediated plasma extravasation in rats. Eur. J. Pharm. 1998;347:101–104. doi: 10.1016/s0014-2999(98)00216-7. [DOI] [PubMed] [Google Scholar]
- PATEL H.J., GYEMBICZ M.A., SPICUZZA L., BARNES P.J., BELVISI M.G. Naloxone-insensitive inhibition of acetylcholine release from parasympathetic nerves innervating guinea-pig trachea by the novel opioid, nociceptin. Br. J. Pharmacol. 1997;120:735–736. doi: 10.1038/sj.bjp.0701013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REINSCHEID R.K., NOTHACKER H.P., BOURSON A., ARDATI A., HENNINGSEN R.A., BUNZOW J.R., GRANDY D.K., LANGEN H., MONSMA F.J., CIVELLI O. Orphanin FQ: a neuropeptide that activates an opioid like G-protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- SHAH S., PAGE C.P., SPINA D. Nociceptin inhibits non-adrenergic non-cholinergic contraction in guinea-pig airway. Br. J. Pharmacol. 1998;125:510–516. doi: 10.1038/sj.bjp.0702068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacological reviews. 1999;51:159–211. [PubMed] [Google Scholar]
- SZALLASI A., GOSO C., MANZINI S. Resiniferatoxin binding to vanilloid receptors in guinea-pig and human airways. Am. J. Respir. Cirt. Care Med. 1995;152:59–63. doi: 10.1164/ajrccm.152.1.7599863. [DOI] [PubMed] [Google Scholar]
- SZOLCSANYI J., NEMETH G., OROSZI G., HELYES Z., PINTER E. Effect of capsaicin and resiniferatoxin on the release of sensory neuropeptides in the rat isolated trachea. Br. J. Pharmacol. 1998;124 Suppl:8p. [Google Scholar]
- VEMULAPALLI S., RIVELLI CHIU P.J.S., DEL PRADO M., HEY J.A. Phosphoramidon abolishes the increases in endothelin-1 release induced by ischemia-hypoxia in isolated perfused guinea-pig lungs. J. Pharmacol. Exp. Ther. 1992;262:1062. [PubMed] [Google Scholar]
- WAGNER A.J., RONNEKLEIV P.K., GRANDY D.K., KELLY M.J. The peptide orphanin FQ inhibits β-endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology. 1998;67:73–82. doi: 10.1159/000054301. [DOI] [PubMed] [Google Scholar]


