Abstract
Whole-cell patch clamp recordings were used to investigate the properties of a non-inactivating outward current observed in mouse cerebellar Purkinje neurones at a holding potential of −20 mV.
Increasing the external potassium (K+) concentration from 3 mM to 20 mM produced a rightward shift in the observed reversal potential of ∼30 mV or ∼40 mV for a K+-or a caesium (Cs+)-based intracellular solution respectively, indicating the outward current was a K+ current.
The outward current was partially inhibited by the K+ channel blocker, tetraethylammonium (TEA; IC50=0.15 mM). Subsequently, the background or TEA-insensitive current was measured in the presence of 1 mM TEA.
The background current was reversibly inhibited by barium (Ba2+; 300 μM, 50%) and potentiated by the application of arachidonic acid (AA; 1 mM, 62%).
The volatile anaesthetic, halothane (1 mM), and the neuroprotectant, riluzole (500 μM), both reversibly inhibited the background current by 54% and 36% respectively.
The background current was insensitive to changes in both intracellular and extracellular acidification.
The GABAB and μ-opioid receptor agonists, baclofen and [D-Ala2, N-MePhe4-Gly-ol5] enkephalin (DAMGO) both reversibly potentiated the outward current by 42% and 26% respectively. In contrast, the metabotropic glutamate receptor and acetylcholine receptor agonists, (S)-3,5-dihydroxyphenylglycine (DHPG) and muscarine both reversibly inhibited the outward current by 48% and 42% respectively.
These data suggest that cerebellar Purkinje neurones possess a background current which shares several properties with recently cloned two-pore K+ channels, particularly THIK-1.
Keywords: Cerebellum, Purkinje, non-inactivating current, two-pore potassium channel, G-protein, modulation
Introduction
Membrane potential and excitability in neuronal and non-neuronal cells is largely regulated by a large family of pore-forming proteins, which are selectively permeable to potassium (K+) ions, i.e. K+ channels (e.g. see Coetzee et al., 1999 for review). Cloning studies have revealed that the K+ channel superfamily can be divided into distinct subgroups depending on their structural basis. It is proposed that all K+ channels are made up of four pore forming (P) segments (Coetzee et al., 1999). However, the number of transmembrane domains associated with each P segment may vary. The inwardly rectifying subgroup of K+ channels are distinguished by their two transmembrane domains, whereas voltage-gated K+ channels have six transmembrane domains. More recently, a third subgroup of K+ channels with four transmembrane domains has been cloned which have been shown to have two-pore forming domains in each subunit (2PKs; Lesage & Lazdunski, 1999; North, 2000; Patel & Honoré, 2001).
Currently, fourteen mammalian 2PKs have been cloned (Patel & Honoré, 2001; Goldstein et al., 2001). In expression systems, these exhibit selective K+ conductance although their permeability and gating properties may differ. They have been named in relation to the first channel cloned, TWIK-1, i.e. Tandem of P domains in a Weak Inward rectifier K+ channel (Lesage et al., 1996). A 2PK with similar sequence homology and properties to TWIK-1 has been identified, namely TWIK-2 (Chavez et al., 1999; Patel et al., 2000). A number of 2PKs have been shown to be acid-sensitive and thus are termed TWIK related acid-sensitive K+ channels (TASK-1, -2, -3, -4 and 5; Duprat et al., 1997; Reyes et al., 1998; Kim et al., 2000; Rajan et al., 2000; Decher et al., 2001; Ashmole et al., 2001; Kim & Gnatenco, 2001). Closely related to TASK-2 are the recently cloned TWIK related alkaline pH activated K+ channels (TALK-1 and TALK-2), with TALK-2 being identical to the earlier cloned TASK-4 (Girard et al., 2001). In addition, TWIK related K+ channel (TREK-1 and TREK-2; Fink et al., 1996; Bang et al., 2000) and TWIK related arachidonic acid sensitive K+ channel (TRAAK; Fink et al., 1998) are potentiated by arachidonic acid (AA). A 2PK channel that is inhibited by halothane has been cloned, Tandem pore domain Halothane Inhibited K+ channel (THIK-1), as well as a structurally related homologue, THIK-2 (Rajan et al., 2001). Finally, a 2PK clone, KCNK7, shows sequence homology to the other 2PKs but to date does not form functional channels when heterologously expressed alone or in combination (Salinas et al., 1999). The 2PK channels may be partially distinguished by pharmacological means, although due to the absence of selective and specific blockers of the individual channels, their precise roles and functions in the control of neuronal excitability are still to be determined.
Several studies have shown that background currents in neurones are susceptible to modulation by various pharmacological agents, for example activation of G-protein coupled receptors (Fisher & Nistri, 1993; Takeshita et al., 1996, Rouse et al., 2000) and by volatile anaesthetics (Lopes et al., 1998). In addition, recent work has shown that the background current present in a variety of neurones was carried, at least in part, by a 2PK channel. The voltage dependence, sensitivity to various specific pharmacological agents and channel distribution studies suggested the background conductance is carried by a TASK-like channel (Buckler et al., 2000; Millar et al., 2000; Sirois et al., 2000; Talley et al., 2000).
Certain K+ conductances in Purkinje neurones are potentiated by AA, and by the activation of GABAB receptors (Premkumar et al., 1990a, 1990b). More recently, it has been shown that antibodies selective for the AA-sensitive 2PK channels, TREK-1, TRAAK and TASK-1 (Hervieu et al., 2001; Kindler et al., 2000; Reyes et al., 2000) stained heavily in the Purkinje cell layer.
The aim of the present study is therefore to investigate a non-inactivating outward current observed in mouse cerebellar Purkinje neurones. At a holding potential of −20 mV, our data suggest that the non-inactivating outward current is carried mainly by K+ ions. Further characterization of the non-inactivating outward current using a wide variety of K+ channel blockers indicates that the current comprises of at least two components, a TEA-sensitive and a TEA-insensitive background component. Further investigation into the TEA-insensitive background current reveals that it shares certain properties with 2PKs.
Methods
Slice preparation
Male mice (3 – 5 weeks old) were humanely killed by cervical dislocation and decapitated according to U.K. Home Office guidelines. The brains were rapidly removed and placed in ice-cold sucrose-based artificial cerebrospinal fluid (ACSF). Parasaggital slices (300 μM) were then cut using a Campden vibroslice and placed in a holding chamber containing ACSF bubbled with 95% O2/5% CO2 at room temperature. The slices were then transferred to a submerged recording chamber, after at least a 1 h equilibration period at room temperature, and perfused with ACSF at a rate of 2 ml min−1 at room temperature (21 – 25°C). The ACSF contained (in mM): NaCl 124, KCl 3, NaHCO3 26, NaH2PO4 2.5, MgSO4 2, CaCl2 2 and D-Glucose 10 and bubbled with 95% O2/5% CO2, pH∼7.3. The sucrose-based ACSF was identical to the above except NaCl was substituted for sucrose to preserve isosmolarity. In experiments where the K+ concentration was increased to 20 mM, NaCl concentration was reduced accordingly.
Electrophysiology
Purkinje cells were visualized using water-immersion lens mounted on an Olympus BXW510 microscope and identified by their large soma size and distinctive distribution. Recordings were made using the whole-cell voltage clamp technique utilizing borosilicate glass electrodes (Clark Electromedical, U.K.) of 4 – 7 MΩ resistance, filled with an intracellular solution containing (in mM): CsCl or KCl 140, MgCl 1, CaCl2 1, EGTA 10, HEPES 10 and Na-ATP 3, pH 7.2. Recordings were made using an EPC-9 amplifier (HEKA Electronik, Lambrecht, Germany), with Pulse software running on a MacIntosh computer. Series resistance was <15 MΩ and compensated by 50 – 65%. The non-inactivating outward current was investigated by a 300 ms step from the holding potential of −20 mV to −80 mV every 30 s and monitored on-line by measuring the holding current at −20 mV. Voltage-ramp protocols consisted of 400 ms ramp between −110 mV and 40 mV from the holding potential of −20 mV. Step data was digitized at 10 kHz whereas ramp data was digitized at 5 kHz, both filtered at one third of the sampling frequency. Off-line data analysis was performed using Pulsefit software (HEKA). All data are expressed as mean±s.e.m., where n=number of cells. All statistics were performed on raw data using ANOVA and Tukey tests with P<0.05 considered significant.
Pharmacological agents
All drugs utilized were made as a minimum of 100X stock solutions and stored at −20°C. Prior to application they were diluted to the required concentration in the appropriate ACSF and applied via the perfusate. All salts and sucrose were obtained from BDH chemicals (Poole, U.K.). 4-aminopyridine (4-AP), tetraethylammonium (TEA), barium chloride (BaCl2), lactic acid (LA), muscarine, noradrenaline, baclofen and bicuculline methiodide were all obtained from Sigma (Poole, U.K.). (S)-3,5-Dihydroxyphenylglycine (DHPG), 2R, 4R-4-aminopyrrolidine-2, 4-dicarboxylate (2R, 4R-APDC), (S)-2-amino-4-phosphonobutyrate (L-AP4), 2,3-Dioxo-6-nitro-1, 2,3,4-tetrahydrobenzo [f] quinoxaline-7-) sulphonamide (NBQX) and [D-Ala2,NMePhe4,Gly-ol]-enkephalin (DAMGO) were obtained from Tocris (Bristol, U.K.). Tetrodotoxin (TTX) and iberiotoxin were from Alomone Laboratories (Jerusalem, Israel). Arachidonic acid and riluzole were from Calbiochem (Nottingham, U.K.). [D-Pen2,D-pen5)enkephalin (DPDPE) was obtained from Bachem (St. Helens, U.K.). WIN 55.212-2 and CP 55,940 were kind gifts from Dr Iain James at Novartis (London, UK). The volatile anaesthetic, halothane, was a gift from Professor N. Franks, Biophysics Section, Department of Biological Sciences, Imperial College, London. The stock solution for the anaesthetic was prepared as described previously (Lopes et al., 1998).
Results
Cerebellar Purkinje neurones exhibit a non-inactivating outward current carried by K+ ions
Experiments were carried out using a similar step protocol to that described previously when investigating a background current, IK(SO), in cerebellar granule cells (Watkins & Mathie, 1996). With a K+/ATP+ -based intracellular solution, a non-inactivating outward current was observed when the Purkinje cells were held at −20 mV. To investigate whether the non-inactivating outward current was carried by K+ ions, we increased the extracellular K+ concentration, [K+]o, to 20 mM from the standard ACSF concentration of 3 mM. We conducted the experiments in the presence of various ion channel blockers in an attempt to reduce the effects of increased neurotransmitter release. In the presence of the non-NMDA receptor antagonist (NBQX, 5 μM), the GABAA receptor antagonist (bicuculline, 10 μM), the Na+ channel blocker (TTX, 0.5 – 1 μM) and the Ca2+ channel blockers (cadmium, 100 μM and nickel, 50 μM), an increase in [K+]o, from 3 mM (EK=−97.1 mV) to 20 mM (EK=−49.2 mV) gave a rightward shift in the reversal potential of 26.7±0.9 mV (n=3; Figure 1). Whilst conducting these experiments, ‘rundown' of the outward current was observed in approximately 90% of the cells tested. These problems with ‘rundown' were overcome by using a Cs+ /ATP -based intracellular solution. Using the same protocols, non-inactivating outward currents were still observed and a rightward shift in the reversal potential of 43.8±1.6 mV (n=3; Figure 1B) was observed by increasing [K+]o to 20 mM. As expected, this increased in [K+]o also lead to a decrease in the outward current as shown in Figure 1C.
Figure 1.
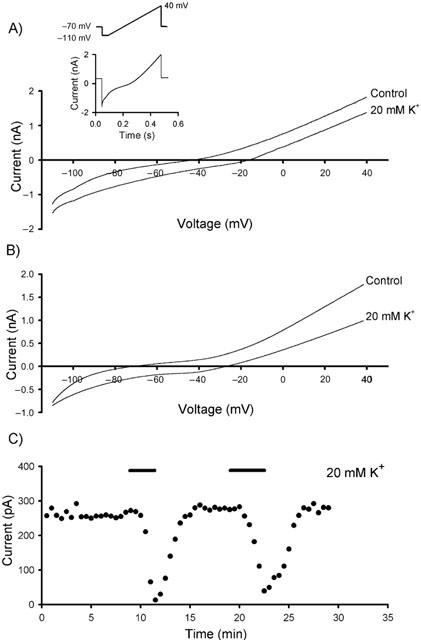
The non-inactivating outward current is carried by K+ ions. Increasing extracellular [K+]o to 20 mM from the control value of 3 mM produced a rightward shift of the reversal potential. (A) Voltage-ramp protocols in the presence of transmission blockers with a K+-based intracellular solution. The inset illustrates the voltage protocol used and the current versus time trace obtained prior to converting to a current versus voltage trace. (B) Using a Cs+-based intracellular solution, the rightward shift in reversal potential is in agreement with theoretical value of 47.2 mV. (C) Representative time course for the depression of the non-inactivating outward current at −20 mV by increasing extracellular [K+]o.
Comparison of the shift in the reversal potential with the theoretical value of 47.2 mV calculated from the Nernst equation, shows that K+ ions do indeed underlie the non-inactivating outward current. Differences between the theoretical and the observed shift in the reversal potentials using a K+-based intracellular solution could be explained by the observed ‘rundown' of the outward current. Experiments with the Cs+-based intracellular solution suggest there may be a finite permeability of the background current to other ions. In addition, a contribution from other activated, non-K+ mediated currents cannot be ruled out. With the increased stability of the outward current when using a Cs+-based intracellular solution, all experiments described below were carried out under these conditions.
TEA-sensitive K+ channels underlie part of the overall non-inactivating outward current
To investigate the molecular entities that underlie the non-inactivating outward current, initial experiments were carried out using the ‘classical' K+ channel blocker, TEA. To avoid increased neurotransmitter release, these experiments were performed with the cocktail of blockers described above. TEA (1 mM) inhibited the non-inactivating outward current by 60.5±3.4% (n=11; P<0.001; Figure 2A). A concentration-response curve revealed TEA had an IC50 of 0.15 mM (Figure 2B). Use of the earlier described voltage ramp protocol showed that TEA blocked with greater potency at more depolarized potentials. The TEA-sensitive current accounted for 35% of the total current at +40 mV which is reduced to 17% at −80 mV, suggesting that the TEA-sensitive component is voltage-activated (Figure 2C,D). BK channels were unlikely to be involved since iberiotoxin (100 nM; n=3; Figure 2E), was without effect on the outward current. The relatively low IC50 for TEA and voltage-dependence possibly suggests the functional activation of either Kv3-like subunits (Rudy et al., 1999) or KCNQ2-like subunits (Wang et al., 1998). In order to investigate whether KCNQ-like channels were involved, we applied a high concentration of the KCNQ inhibitor, linopirdine (Selyanko et al., 1999). Linopirdine (50 μM) only produced a small yet significant depression of the outward current, with a depression of 10.3±1.5% (n=3; P<0.05; Figure 2E). Furthermore, KCNQ channels are relatively insensitive to 1 mM 4-AP and we observed a depression of the non-inactivating current of 67.5±13.5% (n=4; P<0.01; Figure 2E), thereby discounting KCNQ-like channels and indicating the possible involvement of Kv3-like subunits. Thus, to isolate the background current, experiments described below were performed in the presence of 1 mM TEA (unless otherwise indicated).
Figure 2.
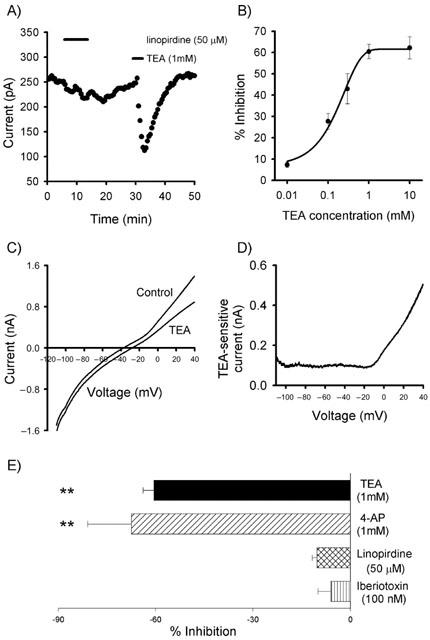
Kv3-like K+ channels partially underlie the non-inactivating outward current. (A) The total outward current was sensitive to the classical K+ channel blocker, TEA, whilst being relatively insensitive to the actions of the selective KCNQ channel blocker, linopirdine. (B) Concentration response curve for TEA on the total outward current. (C) Current versus voltage traces in the presence and absence of TEA. (D) Subtraction of the current remaining in the presence of TEA from the control current (from the traces in C) shows that TEA is more potent at depolarized potentials. (E) The action of various K+ channel blockers on non-inactivating outward current (**=P<0.01).
Ba2+ blocks the background current
Ba2+ and quinidine have been shown to inhibit a variety of K+ channels (Hille, 1992). In the presence of 1 mM TEA, Ba2+ (300 μM) produced an inhibition of the background current of 50.3±10.9% (n=4; P<0.05; Figures 3A,B) whereas quinidine (300 μM) was ineffective (17.3±3.7%; n= 5; P=0.133; Figure 3B). Application of Ba2+ (300 μM) also reversibly inhibited the outward current in the absence of TEA by 45.3±10.6% (n=6; P<0.01). Quinidine (300 μM) proved to be more effective in the absence of TEA (71.8±7.6%, n=5, P<0.01), indicating a greater potency on the TEA-sensitive component.
Figure 3.
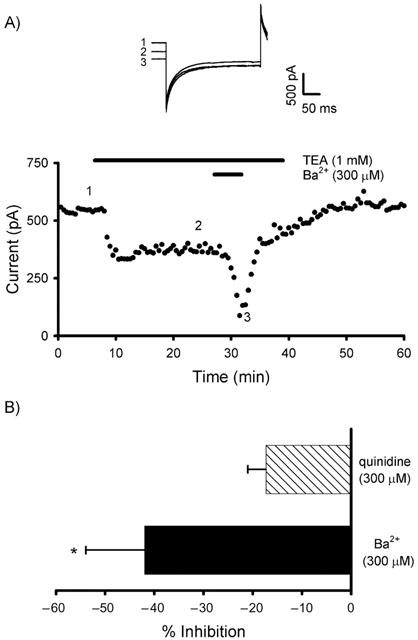
The background current is sensitive to block by Ba2+. (A) In the presence of TEA, Ba2+ produces a significant block of the TEA-insensitive current. Representative traces are illustrated from the time points indicated. (B) Inhibition by Ba2+ and quinidine in the presence of TEA (*P<0.05).
Potentiation of the background current by arachidonic acid
We investigated the effects of AA on the background current. AA has been shown to modulate a number of K+ channels, for example some voltage-gated channels are activated (Zhu et al., 2000) or inhibited (Villarroel & Schwarz, 1996) whereas certain 2PK channels are activated (Fink et al., 1996; 1998; Bang et al., 2000). No significant effect of AA was observed when concentrations as used in expression systems were utilized, i.e. 10 μM (n=5). However, care needs to be taken when using AA as it can be rapidly oxidized, especially so in the highly aerated mixture required for CNS slice experiments. In order to overcome this, we increased the AA concentration to 100 μM and 1 mM. We still failed to see any potentiation with 100 μM AA (n=5), however, at 1 mM, AA potentiated the background current by 62.5±6.3% (n=4; P<0.01; Figure 4B,C). Similar potentiation was observed in the absence of TEA (59.7±7.3%; n=7; P<0.01).
Figure 4.
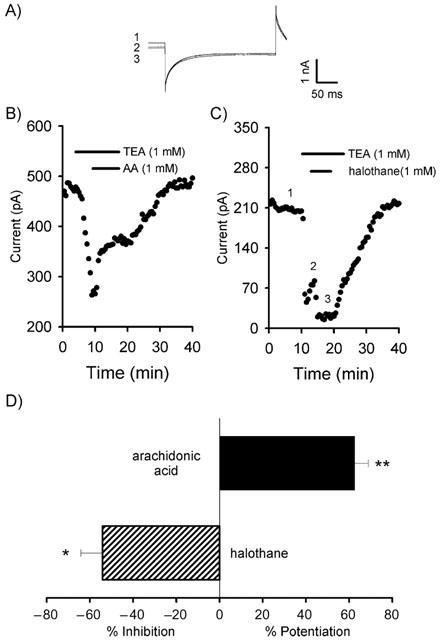
Arachidonic acid potentiates and halothane inhibits the background current. (A) Representative traces from the timepoints indicated in C. (B) Application of AA reversibly potentiates the background current. (C) Halothane inhibits the background current. (D) Summary of the actions of AA and halothane in the presence of TEA (*P<0.05; **P<0.01).
A volatile anaesthetic inhibits the background current
The exact mechanism by which volatile anaesthetics produce their characteristic clinical actions remains uncertain. Several volatile anaesthetics, for example, have been shown to lengthen the decay time constant of GABAA IPSCs (Gage & Robertson, 1985) and activate K+ currents (Lopes et al., 1998). Anaesthetics have been shown to produce either an activation (Patel et al., 1999) or an inhibition (Rajan et al., 2001) depending on the 2PK under investigation. Thus, we investigated the action of the volatile anaesthetic, halothane, on the Purkinje cell background current.
In order to verify that halothane was reaching the Purkinje cell, the decay time constant of spontaneous inhibitory postsynaptic currents (IPSCs) was analysed. In all cells studied, halothane significantly increased the decay time constant (control 5.9±0.3 ms v 15.4±1.0 ms in halothane (1 mM); n=20 IPSCs for each; n=three cells; P<0.001). Halothane produced a reversible inhibition of the TEA-insensitive background current of 54.0±10.1% (n=3; P<0.05; Figure 4C,D). Similar inhibitions were observed in the absence of TEA (48.0±10.3%; n=5; P<0.01).
The neuroprotective agent, riluzole, inhibits the background current
The neuroprotective agent, riluzole, has been shown to elicit complex effects on AA-sensitive 2PKs, potentially through either a direct interaction with the channel itself or through the activation of PKA (Duprat et al., 2000). Application of riluzole (500 μM) produced a reversible inhibition of 35.7±8.7% of the Purkinje cell background current (n=4; P<0.05; Figures 5A,C). Thus riluzole produces similar effects on the background current to those seen in AA-sensitive 2PKs in expression systems.
Figure 5.
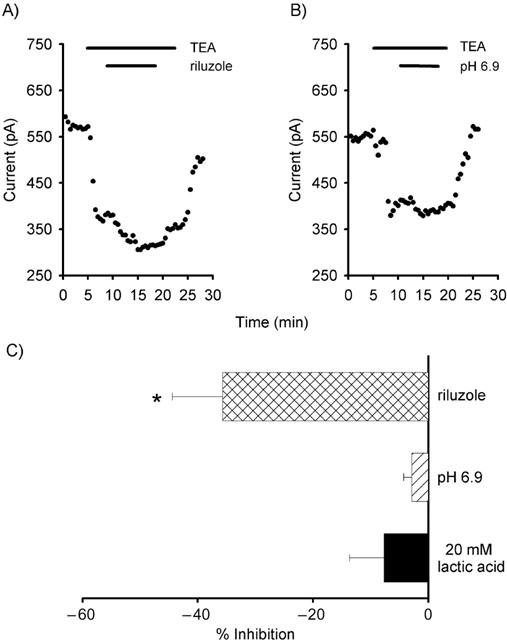
Riluzole inhibits, but small changes in pH have no effect on the background current. (A) The background current is inhibited by the application of the neuroprotective agent, riluzole. (B) Decreasing extracellular pH did not significantly modulate the background current. (C) Summary of the actions of riluzole and changes in intracellular and extracellular pH (*P<0.05).
The background current is insensitive to both extracellular and intracellular acidification
Both extracellular and intracellular acidification modulate a number of K+ channels (Duprat et al., 1997; Reyes et al., 1998; Steidl & Yool, 1999; Kim et al., 2000; Rajan et al., 2000), hence the effect of decreasing extracellular and intracellular pH on the background current was investigated.
Decreasing the extracellular pH from 7.3 to 6.9 did not significantly alter the background current (5.5±2.2% of control; n=3; Figures 5B,C). In addition, intracellular acidification with minimal change in extracellular pH, achieved by the application of lactic acid (LA; Church et al., 1998), did not significantly affect the background current (7.7±6.0%; n=3; Figure 5C).
Modulation of the non-inactivating outward current by G-protein coupled receptors
G-protein modulation of background conductances has been reported in a number of neuronal preparations such as the caudate putamen, hippocampus and trigeminal motorneurons (e.g. Takeshita et al., 1996; Rouse et al., 2000).
We found that the activation of various G-protein coupled receptors could indeed modulate the non-inactivating outward current. The selective GABAB agonist, baclofen (25 μM), and the selective μ-opioid receptor agonist, DAMGO (5 μM) both reversibly potentiated the current by 42.4±10.9% (n=11; P<0.01; Figure 6A) and 26.3±8.7 (n=6; P<0.05; Figure 6A) respectively. In contrast, the selective group I metabotropic glutamate receptor agonist, DHPG (50 μM) and the muscarinic acetylcholine receptor agonist, muscarine (10 μM), inhibited the current by 48.0±10.8% (n=5; P<0.01; Figure 6B) and 42.0±12.7% (n=4; P<0.05; Figure 6B) respectively. Agonists for other G-protein coupled receptors were ineffective at modulating the current. The group II and III selective metabotropic glutamate receptor agonists, APDC (10 μM; n=4) and L-AP4 (25 μM; n=6), were ineffective. Additionally, the cannabinoid receptor agonists, CP 55,940 (10 μM; n=5) and WIN 55.212-2 (10 μM; n=5), and agonists at other opioid and orphan opioid receptors DPDPE (2 μM; n=6), dynorphin (1 μM; n=4) and orphanin FQ (500 nM; n=4) and the adrenergic agonist, noradrenaline (50 μM; n=5), did not cause any significant modulation (Figure 6C).
Figure 6.
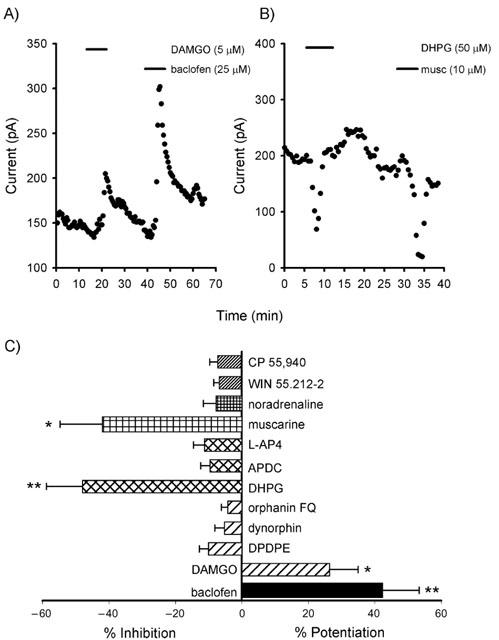
Potentiation and inhibition of the total outward current by the activation of G-protein coupled receptors. (A) Potentiation of outward current by activation of μ-opioid and GABAB receptors. (B) Inhibition of the outward current by activation of group I metabotropic glutamate and muscarinic acetylcholine receptors. (C) The actions of G-protein coupled receptor agonists on the total outward current (*P<0.05, **P< 0.01).
Discussion
Background conductances are suggested to be involved in the control of resting membrane potential and their modulation may therefore alter neuronal excitability (Hille, 1992). The molecular entities that underlie these potentially crucial neuronal background conductances still remain unclear. However, the recent cloning of 2PK channels has led to one of these being proposed to underlie the leak conductance in a variety of neurones (Millar et al., 2000; Sirois et al., 2000; Talley et al., 2000). Here we have carried out experiments designed to elucidate the nature of the non-inactivating outward current in mouse cerebellar Purkinje neurones.
The TEA-sensitive component has similarities withKv3-like K+ channels
We have investigated the non-inactivating current observed in Purkinje neurones at the depolarized holding potential of −20 mV. We have shown that the current is carried by K+ ions and consists of two components: a TEA-sensitive and a TEA-insensitive component. The TEA-sensitive component shares several characteristics with the Kv3 sub-family of K+ channels, a conclusion reached from the current's sensitivity to TEA and 4-AP and its voltage-dependence, together with its relative lack of sensitivity to iberiotoxin and linopirdine. We also show that the sensitivity to TEA decreases at more hyperpolarized potentials. The channels underlying this conductance do not deactivate or inactivate fully at this holding potential and, significantly, are not blocked by intracellular Cs+. The current that we describe here is similar to those described in a recent report of K+ currents in rat subthalamic nucleus neurones (Wigmore & Lacey, 2000), which possess a ‘persistent outward' current which was not inhibited by intracellular Cs+ and also shared several characteristics with Kv3-like K+ channels. In addition, the TEA sensitivity (IC50=0.15 mM) is in agreement with a previous study from our laboratory (Southan & Robertson, 2000), for the inhibition (IC50=0.17 mM) of the Purkinje somatic voltage-gated K+ current by TEA. Hence, Kv3-like subunits are the likeliest candidates for the observed TEA-sensitive current.
The TEA-insensitive background current shares properties with 2PKs
Unlike the TEA-sensitive component, the TEA-insensitive background current is active at all potentials. The use of various pharmacological agents that have been shown to modulate 2PKs would indicate a potential role for these channels underlying the background current. In the present study, the background current is sensitive to block by Ba2+, potentiated by arachidonic acid, inhibited by halothane and insensitive to small changes in pH. These pharmacological characteristics are similar to those shown by the recently cloned 2PK channel, THIK-1 when expressed in Xenopus oocytes (Rajan et al., 2001). Our electrophysiological data would suggest, therefore, that either THIK-1, or a closely related, yet to be cloned, channel, might underlie the background current in Purkinje neurones. Distribution studies for THIK-1, revealed by in situ hybridization, indicate strong expression of THIK-1 in only a restricted number of regions of the rat brain. The cerebellum is not one of these. However, the closely related homologue, THIK-2, is expressed strongly in the cerebellum but as yet has proved to be non-functional in oocyte expression systems (Girard et al., 2001; Rajan et al., 2001). Distribution studies would also seem to rule out other anaesthetic-inhibited 2PKs (TALK-1, -2), which are only found in the periphery (Girard et al., 2001). The immunohistochemical data available reveals that Purkinje neurones express TASK-1 (Kindler et al., 2000) and TRAAK (Reyes et al., 2000) channels whilst mRNA localization studies have revealed the presence of TREK-1 (Fink et al., 1996), TREK-2 (Bang et al., 2000) and TWIK-1 (Talley et al., 2001). However, volatile anaesthetics activate rather than inhibit TREK 1 and TREK-2 and little is known about the action of anaesthetics on TWIK-1. Taken together, our data show that the background conductance in cerebellar Purkinje neurons shares many, but not all, of the characteristics of the cloned 2PK channel, THIK-1. This might suggest that the molecular entity underlying the TEA-insensitive non-inactivating outward current may be related to THIK-1, which has yet to be cloned or functionally characterized.
The potential role of the TEA-insensitive background current
We have shown that the background conductance present in mouse cerebellar Purkinje neurones is susceptible to modulation by a number of neurotransmitters that act through G-protein coupled receptors. The increase in the background conductance by the activation of GABAB receptors is similar to that seen by Premkumar and colleagues (Premkumar et al., 1990a, 1990b) who reported the activation of K+ channels by arachidonic acid and through the activation of GABAB receptors. Furthermore, the activation of μ-opioid receptors can potentiate the leak conductance, which may also be mediated through the production of arachidonic acid. This subtype of opioid receptor has been shown to increase levels of arachidonic acid in expression systems (Fukuda et al., 1996) and this transduction pathway has been suggested for the inhibition of GABA-mediated transmission in the periaqueductal grey region of the midbrain (Vaughan et al., 1997). Activation of these two distinct neurotransmitter receptors would decrease the excitability of the Purkinje neurones through hyperpolarization of the membrane potential and decreasing the neuronal input resistance. In contrast, the background conductance is inhibited by the selective group I mGluR agonist, DHPG and the acetylcholine receptor agonist, muscarine. Activation of these receptors would lead to an increase in the excitability of these neurones, an effect already suggested for muscarinic action on TASK-1 in cerebellar granule cells (Millar et al., 2000) and for thyrotropin-releasing hormone in rat motoneurons (Fisher & Nistri, 1993; Talley et al., 2000).
In conclusion, the conductance investigated in the present study comprises of two individual components, a TEA-sensitive component, probably Kv3-like, and a TEA-insensitive background current with many properties in common with the 2PK channel, THIK-1. Manipulation of the TEA-insensitive component could provide an important mechanism for altering the neuronal excitability of Purkinje neurones.
Acknowledgments
This work was supported by a Wellcome International Travelling Fellowship to T. Bushell, the B.B.S.R.C. to C. Clarke and by the Medical Research Council (U.K.) to A. Mathie and B. Robertson.
Abbreviations
- AA
arachidonic acid
- ACSF
artificial cerebrospinal fluid
- APDC
2R, 4R-4-aminopyrrolidine-2, 4-dicarboxylate
- DAMGO
[D-Ala2,NMePhe4,Gly-ol]-enkephalin
- DHPG
(S)-3,5-Dihydroxyphenylglycine
- DPDPE
[D-Pen2,D-pen5)enkephalin
- EK
equilibrium potential for potassium
- IPSC
inhibitory postsynaptic potential
- LAP4
(S)-2-amino-4-phosphonobutyrate
- NBQX
2,3-Dioxo-6-nitro-1, 2,3,4-tetrahydrobenzo [f] quinoxaline-7-) sulphonamide
- TEA
tetraethylammonium
- TTX
tetrodotoxin
- 2PK
two-pore potassium channel
- 4-AP
4-aminopyridine.
References
- ASHMOLE I., GOODWIN P.A., STANFIELD P.R. TASK-5, a novel member of the tandem pore K+ channel family. Pflügers Archiv. 2001;442:828–833. doi: 10.1007/s004240100620. [DOI] [PubMed] [Google Scholar]
- BANG H., KIM Y., KIM D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J. Biol. Chem. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- BUCKLER K.J., WILLIAMS B.A., HONORE E. An oxygen-, acid- and anaesthetic-sensitive TASK-like background potassium channel in rat arterial chemoreceptor cells. J. Physiol. 2000;525:135–142. doi: 10.1111/j.1469-7793.2000.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAVEZ R.A., GRAY A.T., ZHAO B.B., KINDLER C.H., MAZUREK M.J., MEHTA Y., FORSAYETH J.R., YOST C.S. TWIK-2, a new weak inward rectifying member of the tandem pore domain potassium channel family. J. Biol. Chem. 1999;274:7887–7892. doi: 10.1074/jbc.274.12.7887. [DOI] [PubMed] [Google Scholar]
- CHURCH J., BAXTER K., MCLARNON J.G. pH modulation of Ca2+ responses and a Ca2+-dependent K+ channel in cultured rat hippocampal neurones. J. Physiol. 1998;511:119–132. doi: 10.1111/j.1469-7793.1998.119bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COETZEE W., AMARILLO Y., CHIU J., CHOW A., LAU D., MCCORMACK T., MORENO H., NADAL M.S., OZAITA A., POUNTNEY D., SAGANICH M., VEGA-SAENZ D.M., RUDY B. Molecular diversity of K+ channels. Ann. NY. Acad. Sci. 1999;868:233–285. doi: 10.1111/j.1749-6632.1999.tb11293.x. [DOI] [PubMed] [Google Scholar]
- DECHER N., MAIE M., DITTRICH W., GASSENHUBER J., BRUGGEMANN A., BUSCH A.E., STEINMEYER K. Characterization of TASK-4, a novel member of the pH-sensitive, two-pore domain potassium channel family. FEBS. Lett. 2001;492:84–89. doi: 10.1016/s0014-5793(01)02222-0. [DOI] [PubMed] [Google Scholar]
- DUPRAT F., LESAGE F., FINK M., REYES R., HEURTEAUX C., LAZDUNSKI M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO. J. 1997;16:5464–5471. doi: 10.1093/emboj/16.17.5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUPRAT F., LESAGE F., PATEL A.J., FINK M., ROMEY G., LAZDUNSKI M. The neuroprotective agent riluzole activates the two P domain K(+) channels TREK-1 and TRAAK. Mol. Pharmacol. 2000;57:906–912. [PubMed] [Google Scholar]
- FINK M., DUPRAT F., LESAGE F., REYES R., ROMEY G., HEURTEAUX C., LAZDUNSKI M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO. J. 1996;15:6854–6862. [PMC free article] [PubMed] [Google Scholar]
- FINK M., LESAGE F., DUPRAT F., HEURTEAUX C., REYES R., FOSSET M., LAZDUNSKI M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO. J. 1998;17:3297–3308. doi: 10.1093/emboj/17.12.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISHER N.D., NISTRI A. A study of the barium-sensitive and -insensitive components of the action of thyrotropin-releasing hormone on lumbar motoneurons of the rat isolated spinal cord. Eur. J. Neurosci. 1993;5:1360–1369. doi: 10.1111/j.1460-9568.1993.tb00922.x. [DOI] [PubMed] [Google Scholar]
- FUKUDA K., KATO S., MORIKAWA H., SHODA T., MORI K. Functional coupling of the delta-, mu-, and kappa-opioid receptors to mitogen-activated protein kinase and arachidonate release in Chinese hamster ovary cells. J. Neurochem. 1996;67:1309–1316. doi: 10.1046/j.1471-4159.1996.67031309.x. [DOI] [PubMed] [Google Scholar]
- GAGE P.W., ROBERTSON B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in CA1 pyramidal cells in rat hippocampus. Br. J. Pharmacol. 1985;85:675–681. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRARD C., DUPRAT F., TERRENOIRE C., TINEL N., FOSSET M., ROMEY G., LAZDUNSKI M., LESAGE F. Genomic and functional characteristics of novel human pancreatic 2P domain K(+) channels. Biochem. Biophys. Res. Commun. 2001;282:249–256. doi: 10.1006/bbrc.2001.4562. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN S.A., BOCKENHAUER D., O'KELLY I., ZILBERBERG N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat. Rev. Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- HERVIEU G.J., CLUDERAY J.E., GRAY C.W., GREEN P.J., RANSON J.L., RANDALL A.D., MEADOWS H.J. Distribution and expression of TREK-1, a two-pore-domain potassium channel, in the adult rat CNS. Neuroscience. 2001;103:899–919. doi: 10.1016/s0306-4522(01)00030-6. [DOI] [PubMed] [Google Scholar]
- HILLE B. Ion channels of excitable membranes 1992Sunderland, Massachusetts, U.S.A.: Sinauer Associates; 2nd edn [Google Scholar]
- KIM Y., BANG H., KIM D. TASK-3, a new member of the tandem pore K+ channel family. J. Biol. Chem. 2000;275:9340–9347. doi: 10.1074/jbc.275.13.9340. [DOI] [PubMed] [Google Scholar]
- KIM D., GNATENCO C. TASK-5, a new member of the tandem-pore K(+) channel family. Biochem. Biophys. Res. Commun. 2001;284:923–930. doi: 10.1006/bbrc.2001.5064. [DOI] [PubMed] [Google Scholar]
- KINDLER C.H., PIETRUCK C., YOST C.S., SAMPSON E.R., GRAY A.T. Localization of the tandem pore domain K+ channel TASK-1 in the rat central nervous system. Mol. Brain. Res. 2000;80:99–108. doi: 10.1016/s0169-328x(00)00136-4. [DOI] [PubMed] [Google Scholar]
- LESAGE F., GUILLEMARE E., FINK M., DUPRAT F., LAZDUNSKI M., ROMEY G., BARHANIN J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO. J. 1996;15:1004–1011. [PMC free article] [PubMed] [Google Scholar]
- LESAGE F., LAZDUNSKI M. Potassium channels with two P domains. Current Topics in Membranes. 1999;46:199–222. [Google Scholar]
- LOPES C.M., FRANKS N.P., LIEB W.R. Actions of general anaesthetics and arachidonic pathway inhibitors on K+ currents activated by volatile anaesthetics and FMRFamide in molluscan neurones. Br. J. Pharmacol. 1998;125:309–318. doi: 10.1038/sj.bjp.0702069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLAR J.A., BARRATT L., SOUTHAN A.P., PAGE K.M., FYFFE R.E.W., ROBERTSON B., MATHIE A. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc. Natl. Acad. Sci. 2000;97:3614–3618. doi: 10.1073/pnas.050012597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R.A. Potassium-channel closure taken to TASK. Trends. Neurosci. 2000;23:234–235. doi: 10.1016/s0166-2236(00)01592-7. [DOI] [PubMed] [Google Scholar]
- PATEL A.J., HONORÉ E. Properties and modulation of mammalian 2P domain K+ channels. Trends. Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- PATEL A.J., HONORE E., LESAGE F., FINK M., ROMEY G., LAZDUNSKI M. Inhalational anaesthetics activate two-pore-domain background K+ channels. Nat. Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- PATEL A.J., MAINGRET F., MAGNONE V., FOSSET M., LAZDUNSKI M., HONORE E. TWIK-2, an inactivating 2P domain K+ channel. J Biol Chem. 2000;275:28722–28730. doi: 10.1074/jbc.M003755200. [DOI] [PubMed] [Google Scholar]
- PREMKUMAR L.S., CHUNG S.H., GAGE P.W. GABA-induced potassium channels in cultured neurons. Proc. R. Soc. Lond. B. Biol. Sci. 1990a;241:153–158. doi: 10.1098/rspb.1990.0079. [DOI] [PubMed] [Google Scholar]
- PREMKUMAR L.S., GAGE P.W., CHUNG S.H. Coupled potassium channels induced by arachidonic acid in cultured neurons. Proc. R. Soc. Lond. B. Biol. Sci. 1990b;242:17–22. doi: 10.1098/rspb.1990.0097. [DOI] [PubMed] [Google Scholar]
- RAJAN S., WISCHMEYER E., KARSCHIN C., PREISIG-MULLER R., GRZESCHIK K.H., DAUT J., KARSCHIN A., DERST C. THIK-1 and THIK-2, a novel subfamily of tandem pore domain K+ channels. J. Biol. Chem. 2001;276:7302–7311. doi: 10.1074/jbc.M008985200. [DOI] [PubMed] [Google Scholar]
- RAJAN S., WISCHMEYER E., LIU G.X., MULLER R.P., DAUT J., KARSCHIN A., DERST C. TASK-3, a novel tandem pore domain acid-sensitive K+ channel – An extracellular histidine as pH sensor. J. Biol. Chem. 2000;275:16650–16657. doi: 10.1074/jbc.M000030200. [DOI] [PubMed] [Google Scholar]
- REYES R., DUPRAT F., LESAGE F., FINK M., SALINAS M., FARMAN N., LAZDUNSKI M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J. Biol. Chem. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- REYES R., LAURITZEN I., LESAGE F., ETTAICHE M., FOSSET M., LAZDUNSKI M. Immunolocalization of the arachidonic acid and mechanosensitive baseline TRAAK potassium channel in the nervous system. Neuroscience. 2000;95:893–901. doi: 10.1016/s0306-4522(99)00484-4. [DOI] [PubMed] [Google Scholar]
- ROUSE S.T., HAMILTON S.E., POTTER L.T., NATHANSON N.M., CONN P.J. Muscarinic-induced modulation of potassium conductances is unchanged in mouse hippocampal pyramidal cells that lack functional M1 receptors. Neurosci. Lett. 2000;278:61–64. doi: 10.1016/s0304-3940(99)00914-3. [DOI] [PubMed] [Google Scholar]
- RUDY B., CHOW A., LAU D., AMARILLO Y., OZAITA A., SAGANICH M., MORENO H., NADAL M.S., HERNANDEZ-PINEDA R., HERNANDEZ-CRUZ A., ERISIR A., LEONARD C., VEGA-SAENZ D.M. Contributions of Kv3 channels to neuronal excitability. Ann. N.Y. Acad. Sci. 1999;868:304–343. doi: 10.1111/j.1749-6632.1999.tb11295.x. [DOI] [PubMed] [Google Scholar]
- SALINAS M., REYES R., LESAGE F., FOSSET M., HEURTEAUX C., ROMEY G., LAZDUNSKI M. Cloning of a new mouse two-P domain channel subunit and a human homologue with a unique pore structure. J. Biol. Chem. 1999;274:11751–11760. doi: 10.1074/jbc.274.17.11751. [DOI] [PubMed] [Google Scholar]
- SELYANKO A.A., HADLEY J.K., WOOD I.C., ABOGADIE F.C., DELMAS P., BUCKLEY N.J., LONDON B., BROWN D.A. Two types of K+ channel subunit, Erg1 and KCNQ2/3, contribute to the M-like current in a mammalian neuronal cell. J. Neurosci. 1999;19:7742–7756. doi: 10.1523/JNEUROSCI.19-18-07742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIROIS J.E., LEI Q.B., TALLEY E.M., LYNCH C., BAYLISS D.A. The TASK-1 two-pore domain K+ channel is a molecular substrate for neuronal effects of inhalation anaesthetics. J. Neurosci. 2000;20:6347–6354. doi: 10.1523/JNEUROSCI.20-17-06347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUTHAN A.P., ROBERTSON B. Electrophysiological characterization of voltage-gated K+ currents in cerebellar basket and purkinje cells: Kv1 and Kv3 channel subfamilies are present in basket cell nerve terminals. J. Neurosci. 2000;20:114–122. doi: 10.1523/JNEUROSCI.20-01-00114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEIDL J.V., YOOL A.J. Differential sensitivity of voltage-gated potassium channels Kv1.5 and Kv1.2 to acidic pH and molecular identification of pH sensor. Mol. Pharm. 1999;55:812–820. [PubMed] [Google Scholar]
- TAKESHITA Y., HARATA N., AKAIKE N. Suppression of K+ conductance by metabotropic glutamate receptor in acutely dissociated large cholinergic neurons of rat caudate putamen. J. Neurophysiol. 1996;76:1545–1558. doi: 10.1152/jn.1996.76.3.1545. [DOI] [PubMed] [Google Scholar]
- TALLEY E.M., LEI Q.B., SIROIS J.E., BAYLISS D.A. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- TALLEY E.M., SOLÓRZANO G., LEI Q., KIM D., BAYLISS D.A. CNS distribution of members of the two-pore-domain (KCNK) potassium channel family. J. Neurosci. 2001;21:7491–7505. doi: 10.1523/JNEUROSCI.21-19-07491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAUGHAN C.W., INGRAM S.L., CONNOR M.A., CHRISTIE M.J. How opioids inhibit GABA-mediated neurotransmission. Nature. 1997;390:611–614. doi: 10.1038/37610. [DOI] [PubMed] [Google Scholar]
- VILLARROEL A., SCHWARZ T.L. Inhibition of the Kv4 (Shal) family of transient K+ currents by arachidonic acid. J. Neurosci. 1996;16:2522–2532. doi: 10.1523/JNEUROSCI.16-08-02522.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG H.. , PAN Z., SHI W., BROWN B.S., WYMORE R.S., COHEN I.S., MCKINNON J.E., MCKINNON D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- WATKINS C.S., MATHIE A. A non-inactivating K+ current sensitive to muscarinic receptor activation in rat cultured cerebellar granule neurons. J. Physiol. 1996;491:401–412. doi: 10.1113/jphysiol.1996.sp021224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIGMORE M.A., LACEY M.G. A Kv3-like persistent, outwardly rectifying, Cs+-permeable, K+ current in rat subthalamic nucleus neurones. J. Physiol. 2000;527:493–506. doi: 10.1111/j.1469-7793.2000.t01-1-00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHU M., NATARAJAN R., NADLER J.L., MOORE J.M., GELBAND C.H., SUMNERS C. Angiotensin II increases neuronal delayed rectifier K+ current: role of 12-lipoxygenase metabolites of arachidonic acid. J. Neurophysiol. 2000;84:2494–2501. doi: 10.1152/jn.2000.84.5.2494. [DOI] [PubMed] [Google Scholar]


