Abstract
Atherosclerosis is a focal inflammatory disease of the arterial wall. It starts with the formation of fatty streaks on the arterial wall that evolve to form a raised plaque made of smooth muscle cells (SMCs), and infiltrating leukocytes surrounding a necrotic core. The pathogenesis of the atherosclerotic lesion is incompletely understood, but it is clear that a dysfunction of the endothelium, recruitment and activation of inflammatory cells and SMC proliferation have a pivotal role. Over recent years receptors for extracellular nucleotides, the P2 receptors, have been recognized as fundamental modulators of leukocytes, platelets, SMCs and endothelial cells. P2 receptors mediate chemotaxis, cytokine secretion, NO generation, platelet aggregation and cell proliferation in response to accumulation of nucleotides into the extracellular milieu. Clinical trials have shown the benefit of antagonists of the ADP platelet receptor(s) in the prevention of vascular accidents in patients with atherosclerosis. Therefore, we anticipate that a deeper understanding of the involvement of P2 receptors in atheroma formation will open new avenues for drug design and therapeutic intervention.
Keywords: Atherosclerosis, endothelial dysfunction, fatty streaks, cytokines, metalloproteases, P2 receptors, extracellular ATP
Introduction
Atherosclerosis is a leading cause of morbidity and morbility in the western world. Although it was previously thought to be mainly a degenerative disease, it is now well ascertained that its pathogenesis is inflammatory. While retention of atherogenic lipoproteins and foam cell accumulation into the arterial intima are the main morphological stigma of atherosclerosis (Stary, 1989; Libby et al., 1996), more subtle changes in the microenvironment of the arterial wall caused by infiltration of inflammatory cells and local release of cytokines and other inflammatory mediators are increasingly recognized as additional key factors in the initiation and progression of the atherosclerotic lesion (Dong et al., 1998; Gu et al., 1998b; Phipps, 2000). The ‘inflammatory hypothesis' is not only one of the most stimulating recent advances in the understanding of atherogenesis, but also a conceptual breakthrough susceptible of far reaching therapeutical developments, given the high prevalence of inflammation as a risk factor in the population and the potential availability of preventive measures.
In the context of the ‘inflammatory hypothesis' the endothelium has a central role (Figure 1). Under physiological conditions the endothelial lining is crucial for haemostasis, platelet activation, coagulation, fibrinolysis, modulation of vascular tone and smooth muscle cell proliferation (Enderle et al., 2000; Bryan et al., 2001) In response to various challenges, endothelial cells may change their functions and shift from an anti-coagulant to a pro-coagulant status, or initiate synthesis and release of vasoactive factors, cytokines or growth factors. These modifications as a whole are recognized as key features of the so called ‘endothelial dysfunction' (or ‘endothelial instability') that is thought to participate in plaque formation and to precipitate the most severe consequences of this lesion (Forgione et al., 2000; Caillaud et al., 2001), even in the absence of an overt rupture of the plaque.
Figure 1.
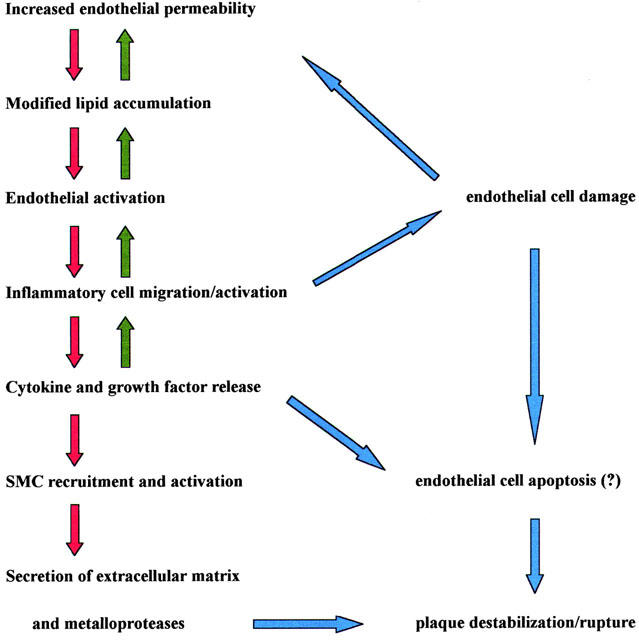
Steps in atheroma formation and progression.
Many factors may lead to endothelial dysfunction and to atherosclerosis, including an increased serum LDL concentration, presence of altered LDL, free radicals, hypertension, cigarette smoking, diabetes, high serum homocysteine levels, chronic infections (e.g. by Helicobacter pylorii, Streptococcus Pneumoniae, Herpes virus), endotoxaemia, and an unfavourable genetic background (Briner & Luscher, 1994; Heitzer et al., 1996; Nygard et al., 1997; Goldboult & Neufeld, 1988; Kullo et al., 2000; Shah, 2001). However, local conditions at the level of the arterial wall predisposing to this outcome are poorly known.
During the last few years the plasma membrane receptors for extracellular nucleotides have been recognized as important modulators of responses of blood cells (i.e. platelets, monocytes, granulocytes, lymphocytes) as wells as of cells of the vessel wall (i.e. endothelial cells, smooth muscle cells (SMCs) and fibroblasts) (Ralevic & Burnstock, 1998; Burnstock, 1999; Burnstock & Williams, 2000; Dubyak, 2000; di virgilio et al., 2001; Lewis & Evans, 2001). These receptors, named P2 receptors (P2R), are known to have an undisputed and crucial role in the modulation of vascular tone, and to be in perspective of similar importance as regulators of the inflammatory response (Di Virgilio, 1995; Brambilla et al., 1999; Souslova et al., 2000; Solle et al., 2001; John et al., 2001). Additionally, P2 receptors have a strong cardioregulatory activity that further emphasizes their potential in cardiovascular diseases (see Vassort, 2001, for a recent comprehensive review). Since the natural ligands for the P2R, ATP and ADP (and possibly UTP/UDP), are released into the blood under many circumstances (platelet aggregation, shear-stress or damage of the endothelial lining due to infections or surgical manoeuvres), it would not be surprising that the P2R were stimulated during plaque formation and participated actively in this process.
The atherogenic process
Changes in permeability of the endothelial barrier are crucial from the very beginning of atheroma formation. Recruitment of monocytes within the intima of large size artery occurs early during atherosclerosis, reflecting a peculiar pattern of expression of endothelial adhesion molecules such as endothelium leukocyte adhesion molecules (ELAM) and vascular cell adhesion molecule-1 (VCAM-1) (Cybulsky & Gimbrone, 1991). VCAM-1 bind the specific integrin receptor very late activating antigen 4 (VLA-4) expressed by monocytes and lymphocytes, but not granulocytes, thus probably explaining the selective recruitment of monocytes in early atherogenesis (Cybulsky et al., 1991; Bochner et al., 1991). Hypercholesterolemic diet appears to cause upregulation of VCAM-1 well before fatty streak formation takes place (Li et al., 1993). At the same time, soluble products such as macrophage chemotactic protein-1 (MCP-1) and macrophage colony stimulating factor (M-CSF) produced by endothelial cell, SMCs and the inflammatory cells themselves under the stimulation of locally released cytokines, accumulate in the arterial wall and support recruitment as well as activation-maturation of monocytes (Figure 2).
Figure 2.
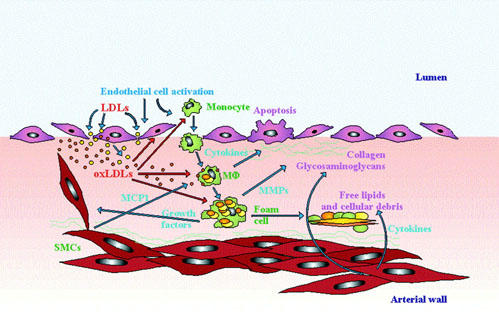
Events in atherosclerotic plaque formation. Injury or activation of endothelial cells allows penetration of molecules (e.g. LDL) from the arterial lumen into the subintimal space. Within this space such molecules may undergo modifications (e.g. oxidation) that unable them to further activate intimal cells, and also accelerate phagocytosis by macrophages (MΦ). Endothelial cell activation also facilitates migration of monocytes from the blood. Monocyte migration is sustained by release of chemotactic factors (e.g. MCP-1) by SMCs. Monocytes differentiate into macrophages and phagocytose oxLDLs and other extracellular lipids. During phagocytosis, macrophages become activated and release cytokines and growth factors. Lipid laden macrophages further differentiate into foam cells that eventually die, releasing their content into the necrotic core of the plaque. The plaque undergoes a continuous remodelling due on the one hand to deposition of extracellular matrix protein and on the other to secretion of MMPs that digest the extracellular matrix. Extracellular matrix proteins are secreted by SMCs. MMPs are secreted by SMCs and macrophages. SMCs under the stimulation with LDL, cytokines and growth factors actively proliferate thus forming the fibrous cap (not shown in the Figure). Some of the products released into the plaque may accelerate damage of the endothelial lining by causing apoptosis of endothelial cells.
Migration of inflammatory cells into the intima is paralleled by infiltration of low density lipoproteins (LDL), to promote deposition of fatty streaks, a typical arterial lesion made of lipid-filled foam cells, T lymphocytes and a small amount of extracellular lipids. Fatty streak formation is now considered a pure inflammatory lesion. Mechanical forces acting on the arterial wall have a key role in facilitating plasma lipoprotein influx and in causing the adaptative intimal thickening typical of atherosclerosis (Gimbrone et al., 2000). If the offending agents are not removed, the inflammatory response can continue indefinitely, stimulating proliferation and migration of SMCs, and leading to formation of an intermediate lesion and to remodelling of the arterial wall. Fatty streaks undergo a progressive increase with intra and extracellular lipid deposition and enhanced extracellular matrix accumulation. The number of SMCs increases, either by in situ intimal proliferation or as a consequence of migration from the media (Gorski & Walsh, 1995).
Changes in the endothelial surface may contribute to plaque growth in several ways. In first place, a reduced ability to produce anti-thrombotic factors (e.g. prostacylins and NO) may promote platelet aggregation and mural thrombosis (Falk & Fernández-Ortiz, 1995; Diodati et al., 1998; Hasenstab et al., 2000). Secondly, the production of heparin-like glycoaminoglycans, thrombomodulin and plasminogen may also be decreased. Platelet deposition and activation on the endothelium generates products that further promote SMCs migration and plaque growth (e.g. platelet-derived growth factor, PDGF). SMCs are crucial in the atherogenic process, therefore understanding the physiology of factors that modulate their function, motility and growth is of the utmost importance.
Under normal physiological conditions, SMCs are confined to the medial layer of the artery wall, but early in the genesis of the atherosclerotic plaque they undergo mitogenic stimulation and phenotype change, losing contractile elements and acquiring the ability to replicate and migrate into the intima (Gorski & Walsh, 1995). Within the intima SMCs proliferate, start deposition a fibrotic connective tissue matrix and undergo an ‘inflammatory differentiation' that enables them to secrete pro-inflammatory agents (e.g. cytokines and metalloproteases). Secretion by SMCs of IL-8, MCP-1, TNFα, IL-1, TGFβ has been documented (Watson et al., 1998). Recently, it has been emphasized that a tight paracrine interaction between endothelial cells and SMCs might condition the progression of the plaque (Rainger & Nash, 2001). Endothelial cells, under the effect of soluble factors released by the SMCs, acquire an highly reactive inflammatory phenotype that on the one hand increases leukocyte adhesion and diapedesis, and on the other sensitizes them to cytokines released into systemic circulation. This could be an important factor in the increased susceptibility to atherosclerotic occlusive disease associated with chronic infections (Kiechl et al., 2001).
Production of extracellular matrix is another additional feature of the atherosclerotic lesion. The fibrous cap that is thus formed owes its genesis to several factors that operate a continuous remodelling of the plaque: deposition and degradation of extracellular matrix, SMCs proliferation, lipid deposition, macrophage and lymphocyte infiltration. Stable plaques often have a relatively thick fibrous cap protecting the lipid core from contact with blood. In the vulnerable, unstable, lesion we may observe a substantial lipid core and a relatively thin cap. Within these latter lesions, pro-inflammatory agents produced by T cells (e.g. IFNγ) seem to have a key role, on the one hand by decreasing SMC secretion of extracellular matrix proteins, and on the other by increasing production by macrophage (but also by SMCs) of metallo proteinases (MMPs) (Galis et al., 1995; Libby et al., 1996).
P2 receptors in the vasculature
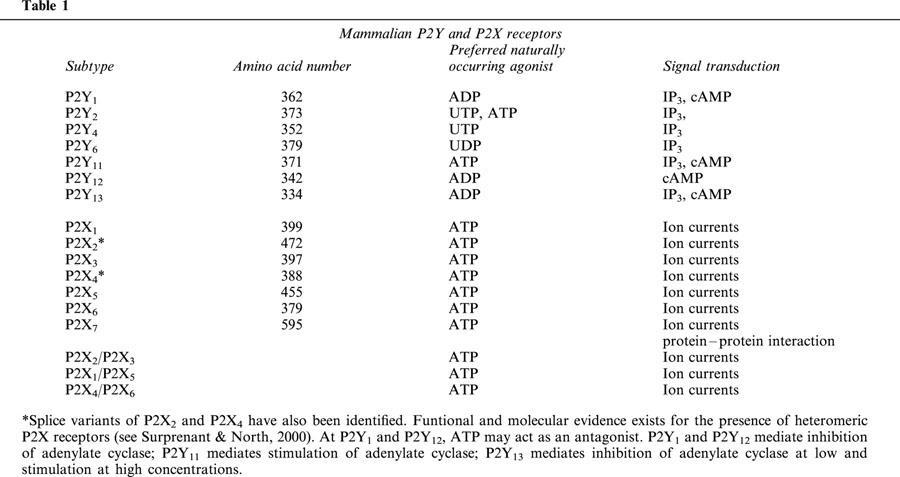
P2R are ubiquitously expressed throughout the human body, the vessel wall included. The P2YR are seven membrane spanning receptors coupled via G protein (Gi/o or Gq/11) to IP3 generation, Ca2+ release from intracellular stores or adenylate cyclase stimulation/inhibition (Cooper & Rodbell, 1979; von kugelgen & Wetter, 2000; Communi et al., 2001). P2XR are membrane ion channels made by the assembly of subunits of the same (homo oligomers) or different (hetero oligomers) subtype (Table 1) (North & Surprenant, 2000; Khakh et al., 2001). Among P2X receptors, P2X7 is endowed with the ability to generate a non-selective plasma membrane pore upon sustained stimulation by extracellular ATP and directly interacts with several cytoplasmic proteins (di virgilio, 1995; Kim et al., 2001). The principal P2R subtypes present in endothelial cells are P2Y1 and P2Y2, but mRNAs for P2Y4 and P2Y6 have also been identified (Jin et al., 1998). Although it is assumed that P2XR are expressed by endothelial cells to a very low level, it is likely that this belief will have to be re-evaluated as further studies are carried out. Functional evidence suggests that endothelial cells express the P2X7R (von Albertini et al., 1998; Goepfert et al., 2000), while molecular studies reveal expression of P2X4 (Yamamoto et al., 2000; Korenaga et al., 2001). There are so far no reports documenting expression by endothelial cells of other members of the P2 family (i.e. P2Y11, P2Y12, P2X1, P2X2, P2X3, P2X5 and P2X6).
Table 1.
Vascular smooth muscle cells express P2X1, P2X2, P2X4, P2X7, P2Y2, P2Y4, and P2Y6 (Kunapuli & Daniel, 1998; Cario-Toumaniantz et al., 1998). It is uncertain whether they also express the P2Y1R (Kunapuli & Daniel, 1998; Erlinge, 1998), and there are no reports on P2Y11 expression. While great emphasis is given to P2R present on the endothelium and the smooth muscle cell layer, expression of P2R by fibroblasts, another key cellular component of the vessel wall, should not be overlooked, especially in pathologic conditions. Human and rat fibroblasts are known to express P2Y1, P2Y2, P2Y4, P2Y6, P2X3, P2X4 and P2X7, likely in an activation-dependent fashion (Webb et al., 1996; Zheng et al., 1998; Solini et al., 1999; Solini & Morelli unpublished data). It is obvious that the final outcome of vascular stimulation by nucleotides depends on the integration of all the responses elicited by individual P2Y and P2X receptors expressed on all the cell elements of the vessel wall.
P2 receptors in blood cells
Blood cells express P2 receptors of both the P2Y and P2X subfamilies. In the P2YR subfamily, P2Y1, P2Y2, P2Y6 and P2Y11 were reported to be expressed by monocytes, B lymphocytes or polymorphonuclear granulocytes (di virgilio et al., 2001). Monocytes and macrophages also express P2X1, P2X4 and P2X7, while in granulocytes only expression of the P2X7 subtype has been reported (Suh et al., 2001). T lymphocytes express a P2X7-like receptor, while it is still controversial whether normal B lymphocytes express functional P2XR (Baricordi et al., 1996; Gu et al., 2000), although a recent confocal microscopy study has revealed expression of the PX1, P2X2, P2X4 and P2X7 proteins (Sluyter et al., 2001). Dendritic cells generated in vitro from monocyte precursors express P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2X1, P2X4, P2X5 and P2X7 (Liu et al., 1999; Berchtold et al., 1999; Ferrari et al., 2000b). Platelets express P2Y1, P2Y12 and P2X1 (Sun et al., 1998; Leon et al., 1997; Hollopeter et al., 2001). Erythrocytes are known to express a P2X7-like receptor and, at least in turkey, a P2Y1-like receptor (Parker & Snow, 1972; Boyer et al., 1989).
Following migration and/or stimulation with inflammatory mediators, pattern of expression in leukocytes may change substantially, leading to downmodulation of some subtypes (e.g. P2Y1) and upmodulation of others (e.g. P2X7) (Dubyak et al., 1996; Humphreys & Dubyak, 1996). The only known physiological activator of P2XR is ATP; on the contrary, the six P2YR subtypes are differentially sensitive to various nucleotides: at P2Y1, P2Y12 and P2Y13 the preferred agonist is ADP, at P2Y2 ATP and UTP are equipotent, at P2Y4 UTP is preferred, at P2Y6 UDP, while P2Y11 is the only P2YR selective for ATP (von kugelgen & Wetter, 2000; Communi et al., 2001). A relevant consequence of such a nucleotide selectivity is that even on the very same cell, P2Y receptors are differentially activated depending on the extracellular nucleotide milieu to which the cell is exposed. For example, where extensive conversion of extracellular ATP to ADP takes place, as it occurs at sites of platelet aggregation, P2Y1, P2Y12 and P2Y13 should be the main receptors activated, while on the contrary at sites exposed to shear-stress forces, where ATP is the principal nucleotide released, P2Y11 is likely to be the main receptor stimulated. It should also be pointed out that ATP may act as an antagonist at P2Y1 and P2Y12 (von kugelgen & Wetter, 2000). There is evidence that UTP is released via non-lytic pathways from a variety of cells (platelets, leukocytes, epithelia) (Lazarowski & Harden, 1999), and it has to be expected that cell membrane damage causes efflux into the pericellular milieu of both ATP and the other intracellular nucleotides, UTP and UDP included, thus it is possible that at sites of cell damage P2Y4 and P2Y6 are stimulated. An additional family of agonist molecules are the diadenosine polyphosphates, that are often co-released with ATP and ADP and stimulate P2 receptors, either directly of after breakdown to ATP (Rodriguez del Castillo et al., 1988; Miras-Portugal et al., 1999).
Another factor that modulates P2-mediated responses is the local nucleotide concentration: P2YR have usually high affinity for the ligand, and are therefore responsive to nanomolar concentrations (Ralevic & Burnstock, 1998), while on the contrary P2X1 – 6R have EC50 in the low micromolar range, and P2X7 in the hundred micromolar range (Khakh et al., 2001). This may suggest that P2 receptors that mediate release of pro-inflammatory factors or trigger a cytotoxic effect, such as P2X7, are not usually activated in conditions under which other members of the P2 family are fully active. This confers a great plasticity to a nucleotide-based extracellular signalling system.
An alternative, and as yet almost unexplored, pathway for increasing the extracellular ATP concentration is a transphosphorylation reaction whereby ATP is synthesized at the expenses of AMP or ADP. This mechanism has been reported to be active on the surface of cultured human umbilical vein endothelial cells, and might therefore contribute substantially to accumulation of extracellular ATP (and possibly other nucleotides) (Yegutkin et al., 2001).
Systems involved in the degradation of extracellular ATP
As it would be expected for a highly regulated signalling network, extracellular ATP is a substrate for powerful hydrolytic enzymes that shorten drastically its lifespan. A recent classification by Zimmermann identifies four families of enzymes involved in the hydrolysis of extracellular nucleotides: ecto-nucleoside triphosphate diphosphohydrolase (E-NTPDase), ecto-nucleotide pyrophosphatase/phosphodiesterase (E-NPP), alkaline phosphatase and ecto-5′-nucleotidase (Zimmermann & Braun, 1999; Zimmermann, 2000).
E-NTPDase is a large family that can be subdivided into two subgroups according to the membrane topology: members of the first group are predicted to have two hydrophobic transmembrane domains, while members of the second group have only one transmembrane domain, with a large COOH residue facing the extracellular environment. E-NTPDases hydrolyze ATP, ADP, several other purine and pyrimidine nucleotides, and are also known as differentiation markers of lymphocytes (CD39). The E-NPP family includes three members (NPP1, NPP2 and NPP3) that are also known as plasma cell differentiation antigens, motility stimulating proteins (autotaxin) or neural differentiation and tumor surface markers (Zimmermann, 2000). These enzymes cleave 3′, 5′-cyclic AMP to AMP, ATP to AMP and PPi, ADP to AMP and Pi or NAD+ to AMP and nicotinamide mononucleotide. In addition, they also hydrolyze pyrimidin nucleotides as well as the phosphodiester bonds of nucleic acids and the pyrophosphate bond of nucleotide sugars. E-NPP have a single transmembrane domain with an extracellular COOH terminus. Alkaline phosphatases are a family of non-specific ecto-phosphomonoesterases with a broad substrate specificity. Besides degrading nucleoside 5′-tri, -di, and -monophosphates, they also degrade PPi and a large number of phosphorylated substrates. Alkaline phosphatases are glycosylphosphatidylinositol (GPI)-anchored membrane protein. Ecto-5′-nucleotidase is also a GPI-anchored molecule known as a lymphocyte maturation marker (CD73). This enzyme catalyzes the conversion of nucleoside 5′-monophosphates to the respective nucleosides an Pi, and is the main enzyme responsible for the generation of adenosine.
Extracellular nucleotide-hydrolyzing enzymes do not show a selective tissue distribution and are often found colocalized on the same cells. Soluble forms are also present. Functional role of enzymes involved in metabolism of extracellular ATP is not entirely understood, with the exception of a few examples where their activity appears crucial such as platelet aggregation (Enjyoji et al., 1999), inflammation (Kaczmarek et al., 1996; Robson et al., 1997), ischaemia (Braun et al., 1998), calcification (Okawa et al., 1998), cell motility (Murata et al., 1994) or adhesion (Airas et al., 1997). CD39 is a potent inhibitor of platelet aggregation since this enzyme converts pro-aggregatory ADP to anti-aggregatory adenosine (Kaczmarek et al., 1996; Marcus et al., 1997). Recombinant CD39 inhibits ADP and collagen-induced platelet aggregation, suggesting that soluble forms of this enzyme might be useful as anti-thrombotic drugs. Similarly, intravenous administration of apyrase might have anti-aggregant effects of potential therapeutic value.
P2 receptor-dependent release of inflammatory mediators from blood cells
Stimulation of P2 receptors is coupled to release of a wealth of inflammatory mediators (see di virgilio et al., 2001, for a recent review). Of obvious relevance for atherosclerotic plaque formation and progression, is release of the pro-inflammatory cytokines IL-1β, IL-1α, IL-6, IL-8, and TNFα (Perregaux & Gabel, 1994; 1998; Ferrari et al., 1997a; Solini et al., 1999; Ferrari et al., 2000b; Warny et al., 2001). Furthermore, nucleotide receptors may also mediate up-regulation of NO synthase and NO generation (Tonetti et al., 1994; Denlinger et al., 1996; Hu et al., 1998). In neutrophilic and eosinophilic polymorphonuclear granulocytes, P2 receptor stimulation triggers superoxide anion generation and exocytosis of both specific and azurophilic granules (Cockcroft & Stutchfield, 1989; Balazovich & Boxer, 1990; Ferrari et al., 2000a; Suh et al., 2001). Nucleotide stimulation also increases membrane expression of CD11b/CD18 and cell adhesion to albumin-coated latex beads (Freyer et al., 1988). Presence of the integrin-binding domain RGD in the first extracellular loop of P2Y2 (Erb et al., 2001) strengthens involvement of P2 receptors in cell adhesion. Platelets are a key target for nucleotides in the blood (Hourani & Hall, 1994). Three P2 receptors expressed on platelets (P2X1, P2Y1 and P2Y12) have a major role in the regulation of platelet aggregation and thrombus formation (Kunapuli, 2000; Savi & Herbert, 2000; Hollopeter et al., 2001). ADP, the main agonist nucleotide for platelets, causes shape changes, aggregation, thromboxane A2 formation and granule secretion.
On endothelial cells extracellular nucleotides have multiple effects, such as modulation of proliferation and angiogenesis (van daele et al., 1992; Rathbone et al., 1992), PGI2 and NO generation (Boeynaems & Galand, 1983; Mathie et al., 1991; Yagi et al., 1994), release of von Willebrand factor (Vischer & Wollheim, 1998) and tissue-type plasminogen activator (Hrafknelsdottir et al., 2001). Besides their action on endothelium, nucleotides also have a well documented action on two key components of the vessel wall: SMCs and fibroblasts (Wang et al., 1991; Erlinge, 1998; Harper et al., 1998; Solini et al., 1999; 2000 White et al., 2000). In smooth muscle cells, nucleotides act as mitogens or co-mitogens in synergism with polypeptide growth factors or neuropeptides. Of particular relevance is synergism with PDGF, a factor with a well defined role in atherogenesis. Furthermore, the potent pro-inflammatory cytokine IL-1β up-regulates the P2Y2 receptor subtype and increases the mitogenic response to UTP (Hou et al., 2000). This is of obvious relevance in view of the inflammatory etiology of the atherosclerotic plaque. Little attention has been paid to fibroblasts both as a possible target of extracellular nucleotides in the vessel wall, and as a possible source of inflammatory mediators. Fibroblasts are known to produce several cytokines and pro-inflammatory factors, besides being very active in the deposition of extracellular matrix. We have recently shown that human primary fibroblast cultures release substantial amount of IL-6 upon stimulation with extracellular ATP, likely via activation of P2X7, and this activity is potentiated in conditions mimicking hyperglycaemia (Solini et al., 1999; 2000). However, since human fibroblasts express several other P2 receptors, besides P2X7 (P2Y1, P2Y2, P2Y4, P2Y6, P2X3 and P2X4), a wider range of responses to nucleotide stimulation is anticipated. It would be of interest to investigate if and to what extent P2 receptor stimulation could also cause secretion of extracellular matrix constituents such as laminin, collagen or elastin. An additional aspect of interest would be the ability of P2 receptor agonist to stimulate fibroblast proliferation and/or differentiation into smooth muscle cells.
A hypothesis for a role for P2 receptors in atherosclerosis
Activation of endothelial cells and their interaction with blood cells is one of the key factors in the pathogenesis of atherosclerosis. Ample evidence supports the interpretation that local inflammatory changes that involve the endothelium, circulating cells as well as elements of the arterial wall have a crucial role in plaque progression and instability. It is increasingly appreciated that the luminal side of the endothelium is exposed to variable ATP levels, that are only grossly reflected by the ATP concentration, up to 20 μM, measured in bulk plasma (Born & Kratzer, 1984). The endothelial cells themselves are capable of ATP release under the effect of shear stress forces (Bodin et al., 1991), swelling (Oike et al., 2000), or stimulation of plasma membrane receptors (Yang et al., 1994) (Figure 3). In addition, since ATP is stored within platelet dense granules, massive amounts of ATP are released during platelet aggregation on the endothelial surface (Meyers et al., 1982; Lages & Weiss, 1999). The level of extracellular ATP measured at sites of massive platelet aggregation (20 – 50 μM) is clearly an underestimation of the concentration that could be reached if degranulation occurred in a protected compartment between the platelet and endothelial cell plasma membranes. Besides endothelial cells and platelets, leukocytes are an additional important source of extracellular ATP. Although it is still a debated issue (see for example Beigi & Dubyak, 2000), substantial evidence is accumulating to support the ability of inflammatory mediators (e.g. bacterial endotoxin, LPS) to cause ATP release from leukocytes and endothelial cells (Ferrari et al., 1997b; Sperlagh et al., 1998; Sikora et al., 1999; Imai et al., 2000; Warny et al., 2001). This suggests that purinergic stimulation can also occur in the vessel wall in response to transient or long lasting bacteraemia, or to the deposition of septic emboli. A purinergic loop could also be triggered by mechanical insults, especially if associated to the concomitant administration of high cholesterol diet, as suggested by experiments showing a dramatic redistribution of P2XR in rabbit aorta subjected to balloon injury (Pulvirenti et al., 2000). Finally, human primary fibroblast cultures in vitro spontaneously release ATP (A. Solini et al., submitted), therefore it can be assumed that ATP is also secreted by fibroblasts within the arterial wall.
Figure 3.
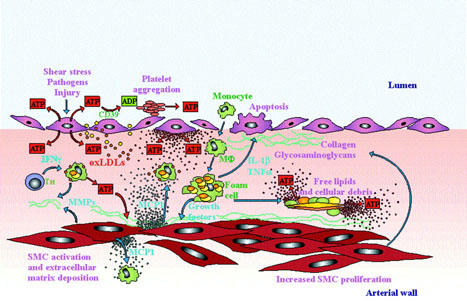
Hypothetical role of extracellular ATP and nucleotide receptors in atherosclerotic plaque formation. Endothelial damage or activation causes ATP release both into the blood and the arterial wall. On the endothelial surface ATP is hydrolized to ADP thus triggering platelet aggregation via P2Y1R and P2Y12R. Activated platelets release further ATP that feed-backs on the endothelium increasing its state of activation. In the subintimal space ATP synergyzes with other agents (e.g. oxLDL or IFNγ released by T helper lymphocytes) to promote macrophage activation. ATP acting at P2YR also contributes with MCP-1 to the formation of a chemoatctic gradient for monocytes and macrophages. Macrophages are recruited from circulation as well as from the arterial wall. Under stimulation with ATP, SMCs proliferate and release MMPs and extracellular matrix proteins. ATP, acting at P2X7R and presumably also at P2YR triggers IL-1β and TNFα release from activated macrophages. At the same time, a high level of extracellular nucleotide released by activated or damaged cells causes a sustained activation of the P2X7R, thus accelerating cell death, either by necrosis or apoptosis.
It is evident that presence of ATP in the pericellular space must be considered the rule rather than the exception, although a rigorous quantitative determination of the actual concentration of this nucleotide has never been performed. In vitro measurements of ATP released from different cell types indicate a bulk solution concentration in the nanomolarlow micromolar range, however the local concentration of ATP in the vicinity of the plasma membrane might be substantially higher. Beigi et al. (1999) measured the local ATP concentration with a luciferase tethered to the platelet plasma membrane, obtaining a peak release, after stimulation with thrombin, in the 15 – 20 μM range. This concentration is sufficient to trigger on all P2Y and P2X receptor subtypes, with the exception of P2X7. However, it has been reported that P2X7R undergoes a progressive increase in current (current growth) in response to repeated stimulation with submaximal agonist (ATP or benzoyl ATP) doses, presumably due to either increase in pore size or in agonist potency (or both) (Surprenant et al., 1996; Rassendren et al., 1997; Chessell et al., 1997; Hibell et al., 2000). This observation suggests that in vivo even low but repeated pulses of ATP might be able to activate P2X7R. The requirement for a high ATP dose or repeated ATP pulses would be in keeping with the hypothesis that the P2X7R should be quiescent under normal physiological conditions and only be activated when extensive tissue damage or activation of the inflammatory system occur (di virgilio, 1995; di virgilio et al., 2001). Indirect evidence that ATP release in circulation occurs at a level at least sufficient to activate P2YR is provided by the surprising phenotype of the cd39/ATP diphosphohydrolase knockout (cd39−/−) mouse. In these animals the ability to degrade extracellular ATP and ADP is severely impaired, thus it was expected that they should exhibit a thrombotic diathesis due to pronounced platelet stimulation by ADP. On the contrary, the cd39−/− mouse shows a prolonged bleeding time and failure to undergo platelet aggregation. These defects are due to P2Y1R desensitization dependent on the increased accumulation of extracellular ATP, and are largely corrected by the administration of apyrase (Enjyoji et al., 1999). In addition, factors predisposing to atherosclerosis might contribute to prolong the half life of extracellular ATP in the vasculature, since it has been reported that activity of endothelial ATP diphophohydrolase is lost when endothelial cells are exposed to inflammatory mediators that cause generation of oxygen radicals (Robson et al., 1997).
Under normal physiological conditions, local ATP release from the endothelium in response to shear stress forces or locally released neuromediators has an important role in the regulation of blood flow. It is well documented that ATP is a potent NO and PGI2 releasing agent, and thus a vasodilatatory stimulus (Burnstock, 1999; Burnstock & Williams, 2000). However, prolonged stimulation of endothelial cells by haemodinamic stress, or platelet aggregation on the endothelial surface, may cause a large and sustained ATP release that may initiate an ‘inflammatory' activation of the endothelium. Nucleotides have been reported to cause upregulation of CD11b/CD18 on granulocytes and enhance adherence of leukocytes to latex beads and endothelial cells (Freyer et al., 1988; Oryu et al., 1996). Enhancement of the adhesive properties of the endothelium favours platelet aggregation and the release of platelet-derived pro-atherosclerotic factors. Release of ATP within the vessel wall may also participate in the generation of an inflammatory microenvironment. Besides endothelial cells, sources of ATP may be vascular smooth muscle cells or fibroblasts. Furthermore, as discussed above, inflammatory cells migrating into the nascent atherosclerotic plaque are another potentially important source of extracellular ATP. Accumulation of this nucleotide at this stage might contribute to plaque formation via several potential mechanisms: (a) by generating a chemotactic gradient for migration of monocytes into the nascent plaque; (b) by synergyzing with other growth factors to stimulate and sustain SMC growth; (c) by activating, alone or in concert with other stimuli released into the vessel wall, effector functions of inflammatory cells migrated into the plaque.
Nucleotides participates to the formation of a chemotactic gradient both directly (mononuclear phagocytes are known to be attracted towards sites of ATP or UTP release) (Oshimi et al., 1999), and indirectly, by stimulating the release of chemokines such as IL-8 (Warny et al., 2001). A local environment characterized by a high extracellular nucleotide concentration favours proliferation of SMCs and macrophages, and may thus contribute to the expansion of the cellular component of the plaque. ATP-dependent release of cytokines such as IL-1β and TNFα from macrophages may have a major role in the induction of early alterations and in the progression of atheromatosis. In addition, ATP also triggers release of NO and oxygen radicals from macrophages, agents that may have a cytotoxic effect on the arterial wall.
Extracellular ATP itself might have a relevant cytotoxic role within the plaque. It is well known that a crucial step in atheroma progression is lysis of lipid-laden macrophages (foam cells) with the ensuing release of their content into the necrotic core. Many different precipitating factors are involved in this process, one of which could be the sustained stimulation of the P2X7R, a molecule expressed to a high level in macrophages and well known for its potent cytotoxic activity (Murgia et al., 1992; di virgilio et al., 1998). Stimulation with inflammatory cytokines (e.g. IFNγ) enhances expression of some P2R subtypes (e.g. P2X7), and thus is expected to increase cell susceptibility to the cytotoxic effect of ATP (Falzoni et al., 1995). In the cell infiltrate of the atherosclerotic plaque the presence of TH lymphocytes is well documented. These cells are a main source of IFNγ during inflammation and therefore they may affect P2 receptor expression by the macrophages. This may trigger an amplification loop that exacerbates tissue damage. Particular disease conditions may further enhance the contribution of extracellular ATP to the necrotic degeneration of the plaque. Recent experiments have shown that fibroblasts cultured in in vitro conditions that mimick hyperglycaemia are exquisitely sensitive to the cytotoxic effect of ATP (Solini et al., 2000). Furthermore, ATP causes a large release of IL-6 from this cells primed with LPS and phorbol esters. IL-6 is another cytokine that is believed to have a central role in the progression of the plaque and in the acceleration of the arterial lesions observed during diabetes (Morohoshi et al., 1996). Therefore, it is possible that a shift in nucleotide sensitivity is a contributing factor in the pathogenesis of atherosclerotic lesions in diabetes and in other metabolic diseases.
The P2R and extracellular nucleotides might also have a distinct role in the transition from a latent to an overt atherosclerotic disease. It is in fact known that while atherosclerotic lesions are fairly wide spread throughout the population, only a few subjects develop a full blown ischaemic disease. This is thought to be due to the destabilization of the plaque, a phenomenon not necessarily involving rupture. Destabilization causes an alteration in the properties of the endothelial lining that favours thrombosis. Extracellular nucleotides might contribute to this phenomenon in a dual capacity: on the one hand by inducing an increased release of IL-1β and TNFα from inflammatory cells (it is not known whether the endothelium itself might be a source of IL-1β), and on the other by generating a local proaggregant environment at the endothelial surface, or even by causing endothelial cell apoptosis (von albertini et al., 1998; Goepfert et al., 2000; Mallat & Tedgui, 2000). In the complex inflammatory status of the atherosclerotic plaque, MMPs are enjoying increasing interest for their role in the digestion of extracellular matrix and in the weakening of the fibrous cap. These proteases are secreted into the plaque by macrophages, SMCs and lymphocytes, but factors promoting their release and activation are poorly known. Extracellular ATP, acting at the P2X7R, causes a large activation of a MMP expressed on the surface of B lymphocytes (Gu et al., 1998a), therefore it is not unlikely that this nucleotide might also participate in the stimulation of protease activity within the plaque.
Conclusions
Nucleotide receptors are emerging as increasingly important molecules in regulation of the physiology of platelets, leukocytes and cells of the vessel wall. Large clinical trials performed with antagonists of the platelet ADP receptor (ticlodipine and clopidogrel) in patients with atherosclerotic diseases have shown a significant benefit compared to aspirin (Balsano et al., 1990; Jarvis & Simpson, 2000; Savi & Herbert, 2000). New selective and potent antagonists at the various P2 subtypes are actively investigated and have entered experimentation as well as clinical trials, especially as antithrombotic drugs (e.g. ARL 67156, AR-C69931MX and R-99224) (Burnstock & Williams, 2000; Sneddon et al., 2000; Storey, 2001; Sugidachi et al., 2001). The identification of the P2 subtypes expressed by leukocytes, SMCs, endothelial cells and fibroblasts may allow selective inhibition of certain unwanted responses (e.g. cytokine release or proliferation) without affecting others (e.g. release of vasodilatory agents). Furthermore, it is now clear that ectoATP/ADPases and ecto nucleotidases are another promising target for the modulation of platelet aggregation and thrombus formation (Marcus et al., 2000). Thus, from the integration of current awareness on the inflammatory aetiology and pathogenesis of the atheromatous plaque and the increasing knowledge of the complex network of the extracellular nucleotides we can expect the design of innovative approaches to the prevention and treatment of atherosclerosis.
Acknowledgments
The authors wish to thank Dr Anna Morelli for invaluable help. This work was supported by grants by the Italian Ministry of Scientific Research (MURST), the National Research Council of Italy (Target Project on Biotechnology), the Italian Association for Cancer Research (AIRC), the Italian Space Agency (ASI), Telethon of Italy, and by local funds from the University of Ferrara.
Abbreviations
- ELAM
endothelium leukocyte adhesion molecules
- E-NPP
ecto-nucleotide pyrophosphatase/phosphodiesterase
- E-NTPDase
ecto-nucleoside triphosphate diphosphohydrolase
- GPI
glycosylphosphatidyl inositol
- ICAM-1
intercellular adhesion molecule-1
- IP3
inositol trisphosphate
- LDL
low density lipoprotein
- LPS
lipopolysaccharide
- MCP-1
macrophage chemotactic protein-1
- M-CSF
monocyte-colony stimulating factor
- MMPs
metalloproteinases
- oxLDL
oxidized low density lipoprotein
- PDGF
platelet derived growth factor
- PPi
inorganic pirophosphate
- P2R
P2 receptor
- SMCs
smooth muscle cells
- VCAM-1
vascular cell adhesion molecules-1
- VLA-4
very late antigen-4
References
- AIRAS L., NIEMELA J., SALMI M., PUURUNEN T., SMITH D.J., JALKANEN S. Differential regulation and function of CD73, a glycosyl-phosphatidyl inositol-linked 70-kDa adhesion molecule, on lymphocytes and endothelial cells. J. Cell Biol. 1997;136:421–431. doi: 10.1083/jcb.136.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALAZOVICH K.J., BOXER L.A. Extracellular adenosine nucleotides stimulate protein kinase C activity and human neutrophil activation. J. Immunol. 1990;144:631–637. [PubMed] [Google Scholar]
- BALSANO F., RIZZON P., VIOLI F., SCRUTINIO D., CIMMINIELLO C., AGUGLIA F., PASOTTI C., RUDELLI G. Antiplatelet treatment with ticlodipine in unstable angina. A controlled multicenter clinical trial. The Studio della Ticlodipina nell' Angina Instabile Group. Circulation. 1990;82:296–298. doi: 10.1161/01.cir.82.1.17. [DOI] [PubMed] [Google Scholar]
- BARICORDI O.R., FERRARI D., MELCHIORRI L., CHIOZZI P., HANAU S., CHIARI E., RUBINI M., DI VIRGILIO F. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood. 1996;87:682–690. [PubMed] [Google Scholar]
- BEIGI R.D., DUBYAK G.R. Endotoxin activation of macrophages does not induce ATP release and autocrine stimulation of P2 nucleotide receptors. J. Immunol. 2000;165:7189–7198. doi: 10.4049/jimmunol.165.12.7189. [DOI] [PubMed] [Google Scholar]
- BEIGI R., KOBATAKE E., AIZAWA M., DUBYAK G.R. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am. J. Physiol. 1999;276:C267–C278. doi: 10.1152/ajpcell.1999.276.1.C267. [DOI] [PubMed] [Google Scholar]
- BERCHTOLD S., OGILVIE A.L., BOGDAN C., MUHL-ZURBES P., OGILVIE A., SCHULER G., STEINKASSERER A. Human monocyte-derived dendritic cells express functional P2X and P2Y receptors as well as ecto-nucleotidases. FEBS Lett. 1999;458:424–428. doi: 10.1016/s0014-5793(99)01197-7. [DOI] [PubMed] [Google Scholar]
- BOCHNER B.S., LUSCINSKAS F.W., GIMBRONE M.A., NEWMAN W., STERBINSKY S.A., DERSE-ANTHONY C.P., KLUNK D., SCHLEIMER R.P. Adhesion of human basophils, eosinophils, and neutrophils to interleukin-1-activated human vascular endothelial cells: contribution of endothelial cell adhesion molecules. J. Exp. Med. 1991;173:1553–1557. doi: 10.1084/jem.173.6.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BODIN P., BAILEY D., BURNSTOCK G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br. J. Pharmacol. 1991;103:1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOEYNAEMS J.M., GALAND N. Stimulation of vascular prostacyclin synthesis by extracellular ADP and ATP. Biochem. Biophys. Res. Commun. 1983;112:290–296. doi: 10.1016/0006-291x(83)91829-6. [DOI] [PubMed] [Google Scholar]
- BORN G.V., KRATZER M.A.A. Source and concentration of extracellular adenosine triphosphate during haemostasis in rats, rabbits and man. J. Physiol. 1984;354:419–429. doi: 10.1113/jphysiol.1984.sp015385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYER J.L., DOWNES C.P., HARDEN T.K. Kinetics of activation of phospholipase C by P2 purinergic agonists and guanine nucleotides. J. Biol. Chem. 1989;264:884–890. [PubMed] [Google Scholar]
- BRAMBILLA R., BURNSTOCK G., BONAZZI A., CERUTI S., CATTABENI F., ABBRACCHIO M.P. Cyclo-oxygenase-2-mediates P2Y receptor-induced reactive astrogliosis. Br. J. Pharmacol. 1999;126:563–567. doi: 10.1038/sj.bjp.0702333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUN N., ZHU Y., KRIEGLSTEIN J., CULMSEE C., ZIMMERMANN H. Upregulation of the enzyme chain hydrolyzing extracellular ATP after transient forebrain ischemia in the rat. J. Neurosci. 1998;18:4891–4900. doi: 10.1523/JNEUROSCI.18-13-04891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINER V.A., LUSCHER T.F. Role of vascular endothelial abnormalities in clinical medicine: atherosclerosis, hypertension, diabetes and endotoxemia. Adv. Intern. Med. 1994;39:1–22. [PubMed] [Google Scholar]
- BRYAN R.M., JR, STEENBERG M.L., MARRELLI S.P. Role of endothelium in shear stress-induced constrictions in rat middle cerebral artery. Stroke. 2001;32:1394–1400. doi: 10.1161/01.str.32.6.1394. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechano-sensory transduction. J. Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., WILLIAMS M. P2 purinergic receptors: modulation of cell function and therapeutic potential. J. Pharmacol. Exp. Ther. 2000;295:862–869. [PubMed] [Google Scholar]
- CAILLAUD D., GALINIER M., ELBAZ M., CARRIE D., PUEL J., FAUVEL J.M., ARNAL J.F. Atherosclerosis: the role of nitric oxide. Presse Med. 2001;30:41–44. [PubMed] [Google Scholar]
- CARIO-TOUMANIANTZ C., LOIRAND G., LADOUX A., PACAUD P. P2X7 receptor activation-induced contraction and lysis in human saphenous vein smooth muscle. Circ. Res. 1998;83:196–203. doi: 10.1161/01.res.83.2.196. [DOI] [PubMed] [Google Scholar]
- CHESSELL I.P., MICHEL A.D., HUMPHREY P.P.A. Properties of the pore-forming P2X7 purinoreceptor in mouse NTW8 microglial cells. Br. J. Pharmacol. 1997;121:1429–1437. doi: 10.1038/sj.bjp.0701278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCKCROFT S., STUTCHFIELD J. ATP stimulates secretion in human neutrophils and HL60 cells via a pertussis toxin-sensitive guanine nucleotide-binding protein coupled to phospholipase C. FEBS Lett. 1989;245:25–29. doi: 10.1016/0014-5793(89)80184-x. [DOI] [PubMed] [Google Scholar]
- COMMUNI D., SUAREZ GONZALES N., DETHEUX M., BREZILLON S., LANNOY V., PARMENTIER M., BOEYNAEMS J-M. Identification of a novel human ADP receptor coupled to G1. J. Biol. Chem. 2001;276:41479–41485. doi: 10.1074/jbc.M105912200. [DOI] [PubMed] [Google Scholar]
- COOPER D.M.F., RODBELL M. ADP is a potent inhibitor of human platelet plasma membrane adenylate cyclase. Nature. 1979;282:517–518. doi: 10.1038/282517a0. [DOI] [PubMed] [Google Scholar]
- CYBULSKY M.I., FRIES J.W.U., WILLIAMS A.J., SULTAN P., EDDY R., BYERS M., SHOWS T., GIMBRONE M.A., COLLINS T. Gene structure, chromosomal location, and basis for alternative mRNA splicing of the human VCAM-1 gene. Proc. Natl. Acad. Sci. USA. 1991;88:7859–7863. doi: 10.1073/pnas.88.17.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CYBULSKY M.I., GIMBRONE M.A. Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:4995, 788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- DENLINGER L.C., FISETTE P.L., GARIS K.A., KWON G., VASQUEZ-TORRES A., SIMON A.D., NGUYEN B., PROCTOR R.A., BERTICS P.J., CORBETT J.A. Regulation of inducible nitric oxide synthase expression by macrophage purinoreceptors and calcium. J. Biol. Chem. 1996;271:337–342. doi: 10.1074/jbc.271.1.337. [DOI] [PubMed] [Google Scholar]
- DIODATI J.G., DAKAK N., GILLIGAN D.M., QUYYUMI A.A. Effect of atherosclerosis on endothelium-dependent inhibition of platelet activation in humans. Circulation. 1998;98:17–24. doi: 10.1161/01.cir.98.1.17. [DOI] [PubMed] [Google Scholar]
- DI VIRGILIO F. The P2Z purinoceptor: an intriguing role in immunity, inflammation, and cell death. Immunol. Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- DI VIRGILIO F., CHIOZZI P., FALZONI S., FERRARI D., SANZ J.M., VENKETARAMAN V., BARICORDI O.R. Cytolytic P2X purinoceptors. Cell Death Diff. 1998;5:191–199. doi: 10.1038/sj.cdd.4400341. [DOI] [PubMed] [Google Scholar]
- DI VIRGILIO F., CHIOZZI P., FERRARI D., FALZONI S., SANZ J.M., MORELLI A., TORBOLI M., BOLOGNESI G., BARICORDI O.R. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97:587–600. doi: 10.1182/blood.v97.3.587. [DOI] [PubMed] [Google Scholar]
- DONG Z.M., CHAPMAN S.M., BROWN A.A., FRENETTE P.S., HYNES R.O., WAGNER D.D. The combined role of P- and E-selectin in atherosclerosis. J. Clin. Invest. 1998;102:145–152. doi: 10.1172/JCI3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBYAK G.R. Purinergic signalling at immunological synapses. J. Auton. Nerv. Syst. 2000;81:64–68. doi: 10.1016/s0165-1838(00)00155-7. [DOI] [PubMed] [Google Scholar]
- DUBYAK G.R., CLIFFORD E.E., HUMPHREYS B.D., KERTESY S.B., MARTIN K.A. Expression of multiple ATP receptor subtypes during the differentiation and inflammatory activation of myeloid leukocytes. Drug Dev. Res. 1996;39:269–278. [Google Scholar]
- ENDERLE M.D., PFOHL M., KELLERMAN N., HAERING H.U., HOFFMEISTER H.M. Endothelial function, variables of fibrinolysis and coagulation in smokers and healthy controls. Haemostasis. 2000;30:149–158. doi: 10.1159/000022537. [DOI] [PubMed] [Google Scholar]
- ENJYOJI K., SEVIGNY J., LIN Y., FRENETTE P., CHRISTIE P.D., SCHULTE AM ESCH J., IMAI M., EDELBERG J.M., RAYBUM H., BEELER D.L., CSIZMADIA E., WAGNER D.D., ROBSON S.C., ROSENBERG R.D. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat. Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- ERB L., LIU J., OCKERHAUSEN J., KONG Q., GARRAD R.C., GRIFFIN K., NEAL C., KRUGH B., SANTIAGO-PEREZ L.I., GONZALES F.A., GRESHAM H.D., TURNER J.T., WEISMAN G.A. An RGD sequence in the P2Y2 receptor interacts with αvβ3 integrins and is required for Go-mediated signal transduction. J. Cell Biol. 2001;153:491–502. doi: 10.1083/jcb.153.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERLINGE D. Extracellular ATP: a growth factor for vascular smooth muscle cells. Gen. Pharmacol. 1998;31:1–8. doi: 10.1016/s0306-3623(97)00420-5. [DOI] [PubMed] [Google Scholar]
- FALK E., FERNÁNDEZ-ORTIZ A. Role of thrombosis in atherosclerosis and its complications. Am. J. Cardiol. 1995;75:3B–11B. doi: 10.1016/0002-9149(95)80003-b. [DOI] [PubMed] [Google Scholar]
- FALZONI S., MUNERATI M., FERRARI D., SPISANI S., MORETTI S., DI VIRGILIO D. The purinergi P2Z receptor of human macrophage cells. Characterization and possible physiological role. J. Clin. Invest. 1995;95:1207–1216. doi: 10.1172/JCI117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRARI D., CHIOZZI P., FALZONI S., DAL SUSINO M., MELCHIORRI L., BARICORDI O.R., DI VIRGILIO F. Extracellular ATP triggers IL-1β release by activating the purinergic P2Z receptor of human macrophages. J. Immunol. 1997a;159:1451–1458. [PubMed] [Google Scholar]
- FERRARI D., CHIOZZI P., FALZONI S., HANAU S., DI VIRGILIO F. Purinergic modulation of interleukin-1β release from microglial cells stimulated with bacterial endotoxin. J. Exp. Med. 1997b;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRARI D., IDZKO M., DICHMANN S., PURLIS D., VIRCHOW C., Jr, NORGAUER J., CHIOZZI P., DI VIRGILIO F., LUTTMANN W. P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett. 2000a;486:217–224. doi: 10.1016/s0014-5793(00)02306-1. [DOI] [PubMed] [Google Scholar]
- FERRARI D., LA SALA A., CHIOZZI P., MORELLI A., FALZONI S., GIROLOMONI G., IDZKO M., DICHMANN S., NORGAUER J., DI VIRGILIO F. The P2 purinergic receptors of human dendritic cells: identification and coupling to cytokine release. FASEB J. 2000b;14:2466–2476. doi: 10.1096/fj.00-0031com. [DOI] [PubMed] [Google Scholar]
- FREYER D.R., BOXER L.A., AXTELL R.A., TODD R.F. Stimulation of human neutrophil adhesive properties by adenine nucleotides. J. Immunol. 1988;141:580–586. [PubMed] [Google Scholar]
- FORGIONE M.A., LEOPOLD J.A., LOSCALZO J. Roles of endothelial dysfunction in coronary artery disease. Curr. Opin. Cardiol. 2000;15:409–415. doi: 10.1097/00001573-200011000-00007. [DOI] [PubMed] [Google Scholar]
- GALIS Z.S., MUSZYNSKI M., SUKHOVA G.K., SIMON-MORRISSEY E., LIBBY P. Enhanced expression of vascular matrix metalloproteinases induced in vitro by cytokines and in regions of human atherosclerotic lesions. Ann. N. Y. Acad. Sci. 1995;748:501–507. doi: 10.1111/j.1749-6632.1994.tb17348.x. [DOI] [PubMed] [Google Scholar]
- GIMBRONE M.A., TOPPER J.N., NAGEL T., ANDERSON K.R., GARCIA-CARDENA G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann. N. Y. Acad. Sci. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. [DOI] [PubMed] [Google Scholar]
- GOEPFERT C., IMAI M., BROOARD S., CSIZMADIA E., KACZMAREK E., ROBSON S.C. CD39 modulates endothelial cell activation and apoptosis. Mol. Med. 2000;6:591–603. [PMC free article] [PubMed] [Google Scholar]
- GOLDBOULT U., NEUFELD H.N. Genetic aspects of arteriosclerosis. Arteriosclerosis. 1988;6:357–377. doi: 10.1161/01.atv.6.4.357. [DOI] [PubMed] [Google Scholar]
- GORSKI D.H., WALSH K. Mitogen-responsive nuclear factors that mediate growth control signals in vascular myocytes. Cardiovasc. Res. 1995;30:585–592. [PubMed] [Google Scholar]
- GU B., BENDALL L.J., WILEY J.S. Adenosine triphosphate-induced shedding of CD23 and L-selectin (CD62L) from lymphocytes is mediated by the same receptor but different metalloproteases. Blood. 1998a;92:946–951. [PubMed] [Google Scholar]
- GU L., OKADA Y., CLINTON S.K., SUKHOVA G.K., LIBBY P., ROLLINS B.J. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low-density lipoprotein receptor-deficient mice. Mol. Cell. 1998b;2:275–281. doi: 10.1016/s1097-2765(00)80139-2. [DOI] [PubMed] [Google Scholar]
- GU B.J., ZHANG W.Y., BENDALL L.J., CHESSELL I.P., BUELL G.N., WILEY J.S. Expression of P2X7 purinoceptors on human lymphocytes and monocytes: evidence for non-functional P2X7 receptors. Am. J. Physiol. Cell Physiol. 2000;279:C1189–C1197. doi: 10.1152/ajpcell.2000.279.4.C1189. [DOI] [PubMed] [Google Scholar]
- HARPER S., WEBB T.E., CHARLTON S.J., NG L.L., BOARDER M.R. Evidence that P2Y4 nucleotide receptors are involved in the regulation of rat aortic smooth muscle cells by UTP and ATP. Br. J. Pharmacol. 1998;124:703–710. doi: 10.1038/sj.bjp.0701895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASENSTAB D., LEA H., HART C.E., LOK S., CLOWES A.W. Tissue factor overexpression in rat arterial neointima models thrombosis and progression of advanced atherosclerosis. Circulation. 2000;101:2651–2657. doi: 10.1161/01.cir.101.22.2651. [DOI] [PubMed] [Google Scholar]
- HEITZER T., YLA-HERTTUALA S., LUOMA J., KURZ S., MUNZEL T., JUST H., OLSCHEWSKI M., DREXLER H. Cigarette smoking potentiates endothelial dysfunction of forearm resistance vessels in patients with hypercholesterolemia. Role of oxidized LDL. Circulation. 1996;93:1346–1353. doi: 10.1161/01.cir.93.7.1346. [DOI] [PubMed] [Google Scholar]
- HIBELL A.D., KIDD E.J., CHESSEL I.P., HUMPHREY P.P.A., MICHEL A.D. Apparent species differences in the kinetic properties of P2X7 receptors. Br. J. Pharmacol. 2000;130:167–173. doi: 10.1038/sj.bjp.0703302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLOPETER G., JANTZEN H.-M., VINCENT D., LI G., ENGLAND L., RAMAKRISHNAN V., YANG R-B., NURDEN P., NURDEN A., JULIUS D., CONLEY P.B. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- HOU M., MOLLER S., EDVINSSON L., ERLINGE D. Cytokines induce upregulation of vascular P2Y2 receptors and increased mitogenic responses to UTP and ATP. Arterioscler. Thromb. Vasc. Biol. 2000;20:2064–2069. doi: 10.1161/01.atv.20.9.2064. [DOI] [PubMed] [Google Scholar]
- HOURANI S.M., HALL D.A. Receptors for ADP on human blood. Trends Pharmacol. Sci. 1994;15:103–108. doi: 10.1016/0165-6147(94)90045-0. [DOI] [PubMed] [Google Scholar]
- HRAFKNELSDOTTIR T., ERLINGE D., JERN S. Extracellular nucleotides ATP and UTP induce a marked acute release of tissue-type plasminogen activator in vivo in man. Thromb. Haemost. 2001;85:875–881. [PubMed] [Google Scholar]
- HU Y., FISETTE P.L., DENLINGER L.C., GUADARRAMA A.G., SOMMER J.A., PROCTOR R.A., BERTICS P.J. Purinergic receptor modulation of lipopolysaccharide signaling and inducible nitric-oxide synthase expression in Raw 264.7 macrophages. J. Biol. Chem. 1998;273:27170–27175. doi: 10.1074/jbc.273.42.27170. [DOI] [PubMed] [Google Scholar]
- HUMPHREYS B.D., DUBYAK G.R. Induction of the P2Z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-γ in the human THP-1 monocytic cell line. J. Immunol. 1996;157:5627–5637. [PubMed] [Google Scholar]
- IMAI M., GOEPFERT C., KACZMAREK E., ROBSON S.C. CD39 modulates IL-1 release from activated endothelial cells. Biochem. Biophys. Res. Commun. 2000;270:272–278. doi: 10.1006/bbrc.2000.2410. [DOI] [PubMed] [Google Scholar]
- JARVIS B., SIMPSON K. Clopidogrel: a review of its use in the prevention of atherothrombosis. Drugs. 2000;60:347–377. doi: 10.2165/00003495-200060020-00012. [DOI] [PubMed] [Google Scholar]
- JIN J., DASARI V.R., SISTARE F.D., KUNAPULI S.P. Distribution of P2Y receptor subtypes on hematopoietic cells. Br. J. Pharmacol. 1998;123:789–794. doi: 10.1038/sj.bjp.0701665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHN G.R., SIMPSON J.E., WOODROOFE M.N., LEE S.C., BROSNAN C.F. Extracellular nucleotides differentially regulate interleukin-1β signalling in primary astrocytes: inplication for inflammatory gene expression. J. Neurosci. 2001;21:4134–4142. doi: 10.1523/JNEUROSCI.21-12-04134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KACZMAREK E., KOZIAK K., SEVIGNY J., SIEGEL J.B., ANRATHER J., BEAUDOIN A.R., BACH F.H., ROBSON S.C. Identification and characterization of CD39 vascular ATP diphosphohydrolase. J. Biol. Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- KHAKH B.S., BURNSTOCK G., KENNEDY C., KING B.F., NORTH A., SEGUELA P., VOIGT M., HUMPHREY P.P.A. International Union of Pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- KIECHL S., EGGER G., MAYR M., WIEDERMANN C.J., BONORA E., OBERHOLLENZER F., MUGGEO M., XU Q., WICK G., POEWE W., WILLEIT J. Chronic infections and the risk of carotid atherosclerosis: prospective results from a large population stud. Circulation. 2001;103:1064–1070. doi: 10.1161/01.cir.103.8.1064. [DOI] [PubMed] [Google Scholar]
- KIM M., JIANG L.-H., WILSON H.L., NORTH R.A., SURPRENANT A. Proteomic and functional evidence for a P2X7 receptor signalling complex. EMBO J. 2001;20:6347–6358. doi: 10.1093/emboj/20.22.6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORENAGA R., YAMAMOTO K., OHURA N., SOKABE T., KAMIYA A., ANDO J. Sp1-mediated downregulation of P2X4 receptor gene transcription in endothelial cells exposed to shear stress. Am. J. Physyiol. Heart Circ. Physiol. 2001;280:H2214–H2221. doi: 10.1152/ajpheart.2001.280.5.H2214. [DOI] [PubMed] [Google Scholar]
- KUNAPULI S., DANIEL J.L. P2 receptor subtypes in the cardiovascular system. Biochem. J. 1998;336:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNAPULI S.P. Interplay of P2 receptor subtypes in platelet function. Haematologica. 2000;85:27–31. [Google Scholar]
- KULLO J.J., GAU G.T., TAJIK A.J. Novel risk factors for atherosclerosis. Mayo Clin. Proc. 2000;75:369–380. doi: 10.4065/75.4.369. [DOI] [PubMed] [Google Scholar]
- LAGES B., WEISS H.J. Secreted dense granule adenine nucleotides promote calcium influx and the maintenance of elevated cytosolic calcium levels in stimulated human platelets. Thromb. Haemost. 1999;81:286–292. [PubMed] [Google Scholar]
- LAZAROWSKI E.R., HARDEN T.K. Quantitation of extracellular UTP using a sensitive enzymatic assay. Br. J. Pharmacol. 1999;127:1272–1278. doi: 10.1038/sj.bjp.0702654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEON C., HECHLER B., VIAL C., LERAY C., CAZENAVE J.P., GACHET C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakarioblastic cells. FEBS Lett. 1997;403:26–30. doi: 10.1016/s0014-5793(97)00022-7. [DOI] [PubMed] [Google Scholar]
- LEWIS C.J., EVANS R.J. P2X receptor immunoreactivity in different arteries from the femoral, pulmonary, cerebral, coronary and renal circulations. J. Vasc. Res. 2001;38:332–340. doi: 10.1159/000051064. [DOI] [PubMed] [Google Scholar]
- LI H., CYBULSKY M.I., GIMBRONE M.A., LIBBY P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecole, in rabbit aortic endothelium. Arterioscl. Thromb. 1993;13:197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- LIBBY P., GENG Y.J., AIKAWA M., SCHOENBECK U., MACH F., CLINTON S.K., SUKHOVA G.K., LEE R.T. Macrophages and atherosclerotic plaque stability. Curr. Op. Lipidol. 1996;7:330–335. doi: 10.1097/00041433-199610000-00012. [DOI] [PubMed] [Google Scholar]
- LIU Q.H., BOHLEN H., TITZER S., CHRISTENSEN O., DIEHL V., HESCHLER J., FLEISCHMANN B.K. Expression and a role of functionally coupled P2Y receptors in human dendritic cells. FEBS Lett. 1999;445:402–408. doi: 10.1016/s0014-5793(99)00161-1. [DOI] [PubMed] [Google Scholar]
- MALLAT Z., TEDGUI A. Apoptosis in the vasculature: mechanisms and functional importance. Br. J. Pharmacol. 2000;130:947–962. doi: 10.1038/sj.bjp.0703407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS A.J., BROEKMAN M.J., DROSOPOULOS J.H.F., ISLAM N., ALYONYCHEVA T.N., SAFIER L.B., HAJJAR K.A., POSNETT D.N., SCHOENBORN M.A., SCHOOLEY K.A., GAYLE R.B., MALISZEWSKI C.R. The endothelial cell ecto-ADPase responsible for inhibition of platelet function is CD39. J. Clin. Invest. 1997;99:1351–1360. doi: 10.1172/JCI119294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS A.J., BROEKMAN M.J., DROSOPULOS J.H.F., ISLAM N., GAYLE R.B. , III, PINSKY D.J., MALISZEWSKI C.R. Human ecto-ADPase/CD39: thromboregulation via a novel pathway. Haematologica. 2000;85:53–57. [Google Scholar]
- MATHIE R.T., RALEVIC V., ALEXANDER B., BURNSTOCK G. Nitric oxide is the mediator of ATP-induced dilatation of the rabbit hepatic arterial vascular bed. Br. J. Pharmacol. 1991;103:1602–1606. doi: 10.1111/j.1476-5381.1991.tb09834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYERS K.M., HOLMSEN H., SEACHORD C.L. Comparative study of platelet dense granule constituents. Am. J. Physiol. 1982;243:R454–R461. doi: 10.1152/ajpregu.1982.243.3.R454. [DOI] [PubMed] [Google Scholar]
- MIRAS-PORTUGAL M.T., GUALIX J., MATEO J., DIAZ-HERNANDEZ M., GOMEZ-VILLAFUERTE R., CASTRO E., PINTOR J. Diadenosine polyphosphates, extracellular function and catabolism. Prog. Brain Res. 1999;120:397–409. doi: 10.1016/s0079-6123(08)63572-4. [DOI] [PubMed] [Google Scholar]
- MOROHOSHI M., FUJISAWA K., UCHIMURA I., NUMANO F. Glucose-dependent interleukin-6 and tumor necrosis factor production by human peripheral blood monocytes in vitro. Diabetes. 1996;45:954–959. doi: 10.2337/diab.45.7.954. [DOI] [PubMed] [Google Scholar]
- MURGIA M., PIZZO P., STEINBERG T.H., DI VIRGILIO F. Characterization of the cytotoxic effect of extracellular ATP in J774 macrophages. Biochem. J. 1992;288:897–901. doi: 10.1042/bj2880897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURATA J., LEE H.J., CLAIR T., KRUTZSCH H.C., ARESTAD A.A., SOBEL M.E., LIOTTA L.A., STRACKE M.L. cDNA cloning of the human motility-stimulating protein, autotaxin, reveals a homology with phosphodiesterase. J. Biol. Chem. 1994;269:30479–30484. [PubMed] [Google Scholar]
- NORTH R.A., SURPRENANT A. Pharmacology of cloned P2X receptors. Ann. Rev. Pharmacol. Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- NYGARD O., NORDREHAUG J.E., REFSUM H., UELAND P.M., FARSTAD M., VOLLSET S.E. Plasma homocysteine levels and mortality in patients with coronary artery disease. N. Engl. J. Med. 1997;337:230–236. doi: 10.1056/NEJM199707243370403. [DOI] [PubMed] [Google Scholar]
- OIKE M., KIMURA C., KOYAMA T., YOSHIKAWA M., ITO Y. Hypotonic stress-induced dual Ca2+ responses in bovine aortic endothelial cells. Ann. J. Physiol. Heart Circ. Physiol. 2000;279:H630–H638. doi: 10.1152/ajpheart.2000.279.2.H630. [DOI] [PubMed] [Google Scholar]
- OKAWA A., NAKAMURA I., GOTO S., MORIYA H., NAKAMURA Y., IKEGAWA S. Mutation in Npps in a mouse model for ossification of the posterior longitudinal ligament of the spine. Nat. Genet. 1998;19:271–273. doi: 10.1038/956. [DOI] [PubMed] [Google Scholar]
- ORYU M., SAKAMOTO H., OGAWA Y., TANAKA S., SAKAMOTO N. Effects of released products from platelets on neutrophilic adhesion to endothelial cells and nylon fibers. (1996) J. Leukoc. Biol. 1996;60:77–80. doi: 10.1002/jlb.60.1.77. [DOI] [PubMed] [Google Scholar]
- OSHIMI Y., MIYAZAKI S., ODA S. ATP-induced Ca2+ response mediated by P2U and P2Y purinoceptors in human macrophages: signalling from dying cells to macrophages. Immunology. 1999;98:220–227. doi: 10.1046/j.1365-2567.1999.00858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARKER J.C., SNOW R.L. Influence of external ATP on permeability and metabolism of dog red blood cells. Am. J. Physiol. 1972;223:888–893. doi: 10.1152/ajplegacy.1972.223.4.888. [DOI] [PubMed] [Google Scholar]
- PERREGAUX D., GABEL C.A. Interleukin-1β maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J. Biol. Chem. 1994;269:15195–15203. [PubMed] [Google Scholar]
- PERREGAUX D., GABEL C.A. Post-translational processing of murine IL-1: evidence that ATP-induced release of IL-1α and IL-1β occurs via a similar mechanism. J. Immunol. 1998;160:2469–2477. [PubMed] [Google Scholar]
- PHIPPS R.P. Atherosclerosis: the emerging role of inflammation and the CD40-CD40L system. Proc. Natl. Acad. Sci. USA. 2000;97:6930–6932. doi: 10.1073/pnas.97.13.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULVIRENTI T.J., YIN J.L., CHAUFOUR X., MCLACHLAN C., HAMBLY B.D., BENNET M.R., BARDEN J.A. P2X (purinergic) receptor redistribution in rabbit aorta following injury to endothelial cells and cholesterol feeding. J. Neurocytol. 2000;29:623–631. doi: 10.1023/a:1010828302936. [DOI] [PubMed] [Google Scholar]
- RAINGER G.E., NASH G.B. Cellular pathology of atherosclerosis: smooth muscle cells prime cocultured endothelial cells for enhanced leukocyte adhesion. Circ. Res. 2001;88:615–622. doi: 10.1161/01.res.88.6.615. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidine. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RASSENDREN F., BUELL G., VIRGINIO C., COLLO G., NORTH R.A., SURPRENANT A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J. Biol. Chem. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- RATHBONE M.P., MIDDLEMISS P.J., GYSBERS J.W., DE FORGE S., COSTELLO P., DEL MAESTRO R.F. Purine nucleosides and nucleotides stimulate proliferation of a wide range of cell types. In Vitro Cell. Dev. Biol. 1992;28A:529–536. doi: 10.1007/BF02634137. [DOI] [PubMed] [Google Scholar]
- ROBSON S.C., KACZMAREK E., SIEGEL J.B., CANDINAS D., KOZIAK K., MILLAN M., HANCOCK W.W., BACH F.H. Loss of ATP diphosphohydrolase activity with endothelial cell activation. J. Exp. Med. 1997;185:153–163. doi: 10.1084/jem.185.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ DEL CASTILLO A., TORRES M., DELICADO E.G., MIRAS-PORTUGAL M.T. Subcellular distribution studies of diadenosine polyphosphates-Ap4A and Ap5A-in bovine adrenal medulla: presence in chromaffine granules. J. Neurochem. 1988;51:1696–1703. doi: 10.1111/j.1471-4159.1988.tb01147.x. [DOI] [PubMed] [Google Scholar]
- SAVI P., HERBERT J.M. Pharmacology of ticlodipine and clopidogrel. Haematologica. 2000;85:73–77. [Google Scholar]
- SHAH P.K. Link between infection and atherosclerosis. Who are the culprits: viruses, bacteria, both, or neither. Circulation. 2001;103:5–6. doi: 10.1161/01.cir.103.1.5. [DOI] [PubMed] [Google Scholar]
- SIKORA A., LIU J., BROSNAN C., BUELL G., CHESSELL I., BLOOM B.R. Cutting edge: purinergic signaling regulates radical-mediated bacterial killing mechanisms in macrophages through a P2X7-independent mechanism. J. Immunol. 1999;163:558–561. [PubMed] [Google Scholar]
- SLUYTER R., BARDEN J.A., WILEY J.S. Detection of P2X purinergic receptors on human B lymphocytes. Cell Tissue Res. 2001;304:231–236. doi: 10.1007/s004410100372. [DOI] [PubMed] [Google Scholar]
- SNEDDON P., WESTFALL T.D., TODOROV L.D., TODOROVA S.M., WESTFALL D.P., NICKEL P., KENNEDY C. The effect of P2 receptor antagonists and ATPase inhibition on sympathetic purinergic neurotransmission in the guinea pig isolated vas deferens. Br. J. Pharmacol. 2000;129:1089–1094. doi: 10.1038/sj.bjp.0703163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLINI A., CHIOZZI P., FALZONI S., MORELLI A., FELLIN R., DI VIRGILIO F. High glucose modulates P2X7 receptor-mediated function in human primary fibroblasts. Diabetologia. 2000;43:1248–1256. doi: 10.1007/s001250051520. [DOI] [PubMed] [Google Scholar]
- SOLINI A., CHIOZZI P., MORELLI A., FELLIN R., DI VIRGILIO F. Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes, microvesicle formation and IL-6 release. J. Cell Sci. 1999;112:297–305. doi: 10.1242/jcs.112.3.297. [DOI] [PubMed] [Google Scholar]
- SOLLE M., LABASI J., PERREGAUX D.G., STAM E., PETRUSHOVA N., KOLLER B.H., GRIFFITHS R.J., GABEL C.A. Altered cytokine production in mice lacking P2X7 receptors. J. Biol. Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- SOUSLOVA V., CESARE P., DING Y., AKOPIAN A.N., STANFA L., SUZUKI R., CARPENTER K., DICKENSON A., BOYCE S., HILL R., NEBENIUS-OOSTHUIZEN D., SMITH A.J., KIDD E.J., WOOD J.N. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- SPERLAGH B., HASKO G., NEMETH Z., VIZI E.S. ATP released by LPS increases nitric oxide production in Raw 264.7 macrophage cell line via P2Z/P2X7 receptors. Neurochem. Int. 1998;33:209–215. doi: 10.1016/s0197-0186(98)00025-4. [DOI] [PubMed] [Google Scholar]
- STARY H.C. Evolution and progression of atherosclerotic lesions in coronary arteries of children and young adults. Arteriosclerosis. 1989;9:I19–I32. [PubMed] [Google Scholar]
- STOREY F. The P2Y12 receptor as a therapeutic target in cardiovascular disease. Platelets. 2001;12:197–209. doi: 10.1080/09537100120058739. [DOI] [PubMed] [Google Scholar]
- SUH B.C., KIM J.S., NAMGUNG U., HA H., KIM K.T. P2X7 nucleotide receptor mediation of membrane pore formation and superoxide generation in human promyelocytes and neutrophils. J. Immunol. 2001;166:6754–6763. doi: 10.4049/jimmunol.166.11.6754. [DOI] [PubMed] [Google Scholar]
- SUGIDACHI A., ASAI F., YONEDA K., IWAMURA R., OGAWA T., OTSUGURO K., KOIKE H. Antiplatelet action of R-99224, an active metabolite of a novel thienopyridine-type Gi-linked P2T antagonist, CS-747. Br. J. Pharmacol. 2001;132:47–54. doi: 10.1038/sj.bjp.0703761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUN B., LI J., OKAHARA K., KAMBAYASHI J. P2X1 purinoceptor in human platelets. J. Biol. Chem. 1998;273:11544–11547. doi: 10.1074/jbc.273.19.11544. [DOI] [PubMed] [Google Scholar]
- SURPRENANT A., RASSENDREN F., KAWASHIMA E., NORTH R.A., BUELL G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- TONETTI M., STURLA L., BISTOLFI T., BENATTI U., DE FLORA A. Extracellular ATP potentiates nitric oxide synthase expression induced by lipopolysaccharide in RAW 264.7 murine macrophages. Biochim. Biophys. Res. Commun. 1994;203:430–435. doi: 10.1006/bbrc.1994.2200. [DOI] [PubMed] [Google Scholar]
- VAN DAELE P., VAN COEVORDEN A., ROGER P.P., BOEYNAEMS J.M. Effects of adenine nucleotides on the proliferation of aortic endothelial cells. Circ. Res. 1992;70:82–90. doi: 10.1161/01.res.70.1.82. [DOI] [PubMed] [Google Scholar]
- VASSORT G. Adenosine 5′-triphosphate: a P2 purinergic agonist in the myocardium. Physiol. Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- VISCHER U.M., WOLLHEIM C.B. Purine nucleotides induce regulated secretion of von Willebrand factor: involvement of cytosolic Ca2+ and cyclic adenosine monophosphate-dependent signalling in endothelial exocytosis. Blood. 1998;91:118–127. [PubMed] [Google Scholar]
- VON ALBERTINI M., PALMETSHOFER A., KACZMAREK E., KOZIAK K., STROKA D., GREY S.T., STUHLMEIER K.M., ROBSON S.C. Extracellular ATP and ADP activate transcription factor NF-kappa B and induce endothelial cell apoptosis. Biochem. Biophys. Res. Commun. 1998;248:822–829. doi: 10.1006/bbrc.1998.9055. [DOI] [PubMed] [Google Scholar]
- VON KUGELGEN I., WETTER A. Molecular pharmacology of P2Y receptors. Naunyn Schmiedeberg's Arch. Pharmacol. 2000;362:310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- WANG D.J., HUANG N.N., GONZALES F.A., HEPPEL L.A. Multiple signal transduction pathways lead to extracellular ATP-stimulated mitogenesis in mammalian cells: I. Involvement of protein kinase C-dependent and -independent pathways. J. Cell Physiol. 1991;146:473–482. doi: 10.1002/jcp.1041460319. [DOI] [PubMed] [Google Scholar]
- WARNY M., ABOUDOLA S., ROBSON S.C., SEVIGNY J., COMMUNI D., SOLTOFF S.P., KELLY C.P. P2Y6 nucleotide receptor mediates monocyte IL-8 production in response to UDP or lipopolysaccharide. J. Biol. Chem. 2001;276:26051–26056. doi: 10.1074/jbc.M102568200. [DOI] [PubMed] [Google Scholar]
- WATSON M.L., GRIX S.P., JORDAN N.J., PLACE G.A., DODD S., LEITHEAD J., POLL C.T., YOSHIMURA T., WESTWICK J. Interleukin 8 and monocyte chemoattractant protein 1 production by cultured human airway smooth muscle cells. Cytokine. 1998;10:346–352. doi: 10.1006/cyto.1997.0350. [DOI] [PubMed] [Google Scholar]
- WEBB T.E., BOLUYT M.O., BARNARD E.A. Molecular biology of P2Y purinoceptors: expression in rat heart. J. Auton. Pharmacol. 1996;16:303–307. doi: 10.1111/j.1474-8673.1996.tb00040.x. [DOI] [PubMed] [Google Scholar]
- WHITE P.J., KUMARI R., PORTER K.E., LONDON N.J., NG L.L., BOARDER M.R. Antiproliferative effect of UTP on human arterial and venous smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H2735–H2742. doi: 10.1152/ajpheart.2000.279.6.H2735. [DOI] [PubMed] [Google Scholar]
- YAGI K., NISHINO I., EGUCHI M., KITAGAWA M., MIURA Y., MIZOGUCHI T. Involvement of ecto-ATPase as an ATP receptor in the stimulatory effect of extracellular ATP on NO release in bovine aorta endothelial cells. Biochem. Biophys. Res. Commun. 1994;203:1237–1243. doi: 10.1006/bbrc.1994.2315. [DOI] [PubMed] [Google Scholar]
- YAMAMOTO K., KORENAGA R., KAMIYA A., QI Z., SOKABE M., ANDO J. P2X4 receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H285–H292. doi: 10.1152/ajpheart.2000.279.1.H285. [DOI] [PubMed] [Google Scholar]
- YANG S., CHEEK D.J., WESTFALL D.P., BUXTON I.L. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ. Res. 1994;74:401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- YEGUTKIN G.G., HENTTINEN T., JALKANEN S. Extracellular ATP formation on vascular endothelial cells is mediated by ecto-nucleotide kinase activities via phosphotransfer reactions. FASEB J. 2001;15:251–260. doi: 10.1096/fj.00-0268com. [DOI] [PubMed] [Google Scholar]
- ZHENG J.S., O'NEILL L., LONG X., WEBB T.E., BARNARD E.A., LAKATTA E.G., BOLUYT M.O. Stimulation of P2Y receptors activates c-fos gene expression and inhibits DNA synthesis in cultured cardiac fibroblasts. Cardiovasc. Res. 1998;37:718–728. doi: 10.1016/s0008-6363(97)00245-9. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg's Arch. Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN H., BRAUN N. Ecto-nucleotidases-molecular structures, catalytic properties, and functional roles in the nervous system. Prog. Brain Res. 1999;120:371–385. [PubMed] [Google Scholar]


