Abstract
The identity of the serotonin (5-HT) receptors modulating the transmission of segmental C-fibre mediated signals was studied using an in vitro preparation of the hemisected spinal cord from rat pups.
Responses to trains of stimuli delivered to a lumbar dorsal root were recorded from the corresponding ventral root. The resulting cumulative depolarization (CD) mediated by unmyelinated fibres was quantified in terms of integrated area. The amplitude of the mono-synaptic reflex was also measured. Serotonergic agents were superfused at known concentrations and their effects on the reflexes evaluated.
5-HT had depressant effects on the CD (EC50 34 μM). The rank order of potency of agonists for the depression of the CD was 5-carboxamidotryptamine (5-CT)>α-methylserotonin (α-met-5-HT) ≈5-HT>42-methylserotonin (2-met-5-HT)≈8-OH-DPAT.
All the agonists including 2-met-5-HT and 8-OH-DPAT had strong depressant effects on the mono-synaptic reflex with the following order of potency: 5-CT>48-OH-DPAT>4α-met-5-HT ≈5-HT≈2-met-5-HT.
The inhibitory effects of 5-HT, α-met-5-HT and 5-CT were attenuated by the non-specific 5-HT antagonist methiothepin (1 μM) and by the 5-HT1A/1B antagonist SDZ 21009 (100 nM) but not by the selective 5-HT1A antagonist WAY 100135 (1 μM).
Other antagonists known to block 5-HT2, 5-HT6 and/or 5-HT7 receptors (ketanserin, RO 04-6790, ritanserin and clozapine) did not change the effect of the agonists.
The data suggest an important contribution of 5-HT1B receptors to the inhibition of spinal C-fibre mediated nociceptive reflexes but no experimental support was found for the intervention of 5-HT2, 5-HT6 or 5-HT7 receptors in this in vitro model.
Keywords: Nociceptive reflexes, serotonin receptors, 5-HT1B, 5-HT6, 5-HT7, rat spinal cord
Introduction
The direct administration of serotonin (5-HT) into the spinal cord produces analgesic effects in a number of behavioural tests (see Le Bars, 1988 for a critical review) and there is extensive evidence suggesting a role for 5-HT in the endogenous pain control system (Millan, 1997). At a cellular level, 5-HT induces a range of responses including hyperpolarization, depolarization and changes in excitability of spinal neurones, depolarization of primary afferents and modulation of glutamatergic and peptidergic transmission (Headley et al., 1978; Davies & Roberts, 1981; del mar et al., 1994; Lopez-Garcia & King, 1996; Larkman & Kelly, 1997; Lopez-Garcia, 1998). It is thought that several of these modulating effects at the spinal level can contribute to the depression of synaptic transmission caused by 5-HT (Lopez-Garcia & King, 1996) and therefore to its analgesic effect.
The pharmacological profile of the 5-HT receptors involved in modulating nociceptive transmission has been investigated mostly by means of behavioural studies and to a lesser extent using electrophysiological techniques (see Millan, 1997; Hamon & Bourgoin, 2000 for reviews). Often the results obtained are conflicting and difficult to interpret probably due to the multiplicity of 5-HT receptors with overlapping pharmacological properties. 5-HT3 and several subtypes of 5-HT1 receptors have been proposed to contribute to the modulation of C-fibre mediated spinal transmission (El Yashir et al., 1988; Ali et al., 1994; Peng et al., 1996; Khasabov et al., 1999) but the possible intervention of other receptors has also been suggested (see Hamon et al., 1990 for a review).
Within the last decade four new receptor types for 5-HT have been identified and all of them appear to be present in the spinal cord. Messenger RNAs for 5-HT4, 5-HT5 and 5-HT6 receptors have been detected in cultured sensory neurones or spinal neurones (Chen et al., 1998; Gerard et al., 1996) and the presence of 5-HT7 receptors has been suggested from autoradiographic and electrophysiological studies (Gustafson et al., 1996; Clarke et al., 1997). Interestingly several agonists of 5-HT1 receptors which have been shown to have analgesic-like effects are now known to activate 5-HT6 or 5-HT7 receptors as well. Yet no information is available on the role of these receptors at the spinal level.
The present study was designed to evaluate the relative roles of several 5-HT receptors including 5-HT1,2,3 receptors and some of the newly described 5-HT receptors (5-HT6,7) at inhibiting spinal nociceptive pathways. We analysed the effects of classical 5-HT receptor agonists and antagonists on 5-HT-mediated inhibition of nociceptive dorso-ventral reflexes. Part of this work has been presented in abstract form (Hedo & Lopez-Garcia, 1999a, 1999b).
Methods
All experiments were performed on Wistar rat pups (8 – 12 days old) of either sex weighing between 18 and 29 g. All experimental procedures were performed according to European Union and Spanish Government regulations and were supervised and approved by the University Animal Care Facility.
Preparation of the in vitro hemisected cord
Rats were anaesthetized with urethane (2 g kg−1, i.p.) and their spinal cords extracted following a standard procedure (Lopez-Garcia & Laird, 1998). Briefly, a rostro-caudal laminectomy was performed and the cord was rapidly excised together with attached dorsal and ventral roots. The cord was then hemisected and placed in a recording chamber continuously superfused with oxygenated (95% O2- 5% CO2) artificial cerebrospinal fluid (ACSF) at room temperature. The composition of the ACSF was (in mM): NaCl 128; KCl 1.9; KH2PO4 1.2; MgSO4 1.3; CaCl2 2.4; NaHCO3 26; glucose 10 (pH 7.4). A period of 90 min was allowed for the preparation to stabilize before testing spinal reflexes.
Stimulation and recording
The L5 dorsal root and the corresponding ventral root were placed in tight fitting glass suction electrodes pulled by hand from borosilicate tubing (Clark Electromedical Instruments). Responses to electrical stimulation of the dorsal root were recorded from the ventral root via a DC coupled amplifier (AxoClamp 2B, Axon Instruments Inc.; CA, U.S.A.). The signal was then further amplified with a Neurolog AC-DC amplifier set to DC mode, digitized at 3.3 kHz and stored for off-line computer-aided analysis using a CED 1401 and Spike 2 version 3.14 software (Cambridge Electronic Design, Ltd; Cambridge, U.K.).
Spinal reflexes were elicited with high intensity electrical stimulus (200 μs and 300 μA) previously shown to be about 2 – 3 times threshold for C-fibre stimulation (Hedo et al., 1999). The stimulation test consisted of three single stimulus (at 40 min intervals) and a train of 20 stimulus at 1 Hz.
Drugs
The drugs used were 5-hydroxytryptamine creatinine sulphate (5-HT), 5-carboxamidotryptamine maleate (5-CT), α-methyl-5-hydroxytryptamine (α-met-5-HT), R(+)-8-hydroxy-2-(di-N-propylamino) tetralin hydrobromide (8-OH-DPAT), 2-methyl-5-hydroxytryptamine (2-met-5-HT), methiothepin mesylate, ritanserin, RO 04-6790 (all from RBI-Sigma), (+) WAY 100135 dihydrochloryde (kindly donated by Wyeth Research, U.K.) and SDZ-21009 (kindly donated by Sandoz Pharma, Switzerland).
Drugs were prepared in high concentration aliquots (10 – 50 mM) dissolved in ultrapure water and stored at −20°C or dissolved in DMSO and stored at room temperature in the case of ritanserin and RO 04-6790. All drugs were diluted in ACSF to their final concentrations immediately prior to use.
Experimental protocols
5-HT and all the agonists were applied for a set period of 9 min. For time course experiments electrical stimulation was delivered to the dorsal root at 10 min intervals. After a baseline response was obtained the agonist was superfused so as to deliver a test stimulus during the last minute of superfusion and the stimulation tests were continued until a full recovery was observed. No more than three concentrations of the same agonist or three different agonists were applied to the same preparation.
In a typical experiment with antagonists, electrical stimulation tests were applied before and after 90 min of antagonist superfusion. Thereafter antagonist superfusion was continued until the end of the experiment. The antagonists were tested against 5-HT and the agonists that showed the largest inhibitory effects on the CD. In a single antagonist study 5-HT was first applied and recovery was tested after 20 min of wash, then α-met-5-HT was applied and recovery tested after 45 min of wash followed by a final application of 5-CT and a recovery test after 60 min of wash. The experiments were discarded if the recovery of any of the agonists did not reach 80% of control.
Measurements and data treatment
The responses to trains of stimulus were incremental (Figure 1A) producing a cumulative depolarization (CD). The CD was quantified as the integrated area with a cut-off time of 24 s from first stimulus artefact as in previous reports (see Hedo et al., 1999). The rise rate of the cumulative depolarization was calculated as the final amplitude minus the initial amplitude divided by 18 s. The amplitude of the mono-synaptic reflex to single electrical stimulation and the amplitude of the depolarization induced by superfusion of agonists were also measured.
Figure 1.
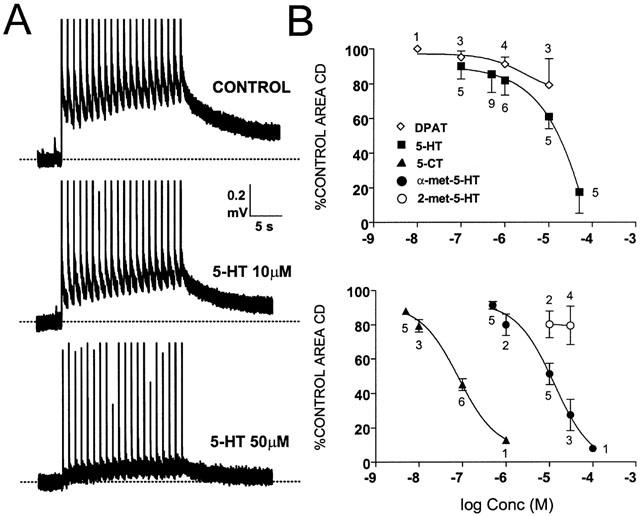
Effects of 5-HT. (A) Shows original recordings obtained from the same experiment. Responses to trains of stimuli produced a cumulative depolarization (CD) which was reduced by 5-HT although a certain slope is still visible during superfusion of 5-HT 50 μM. The large vertical lines correspond to stimulus artefacts in this and subsequent figures. The graphs in (B) show the concentration-response curves for 5-HT and 8-OH-DPAT (upper graph) and for 5-CT, α-met-5-HT and 2-met-5-HT (lower trace) on the integrated area of the CD. The numbers over the data points indicate number of observations.
All data are expressed as mean per cent of control values (±s.e.mean). For statistical analysis paired or unpaired Student t-test were performed on the raw data. EC50 values were obtained from pooled data by four parameter curve fitting using an iterative procedure with commercial software (GraphPad Software Inc., CA, U.S.A.)
Control experiments
Several control experiments were run at various points throughout the main experiments described above. In order to test the stability of the signal during prolonged recordings, trains of stimulus were delivered to the dorsal root at the scheduled times but in the absence of agonists or antagonists (n=3). In order to discard an effect of the solvent DMSO this was superfused in isolation (0.1% in ACSF) over prolonged periods of time (up to 2 h) and trains of stimuli delivered every 10 or 20 min (n=4). Since the effects of all serotonergic agonists used were inhibitory we superfused acetylcholine (acetylcholine chloride from RBI) at a range of concentrations (1 – 100 μM) for 9 min previous to delivery of electrical stimuli (n=5). None of these treatments produced changes in the area of the CD exceeding 9% of initial values.
Results
A total of 84 rat pups were used for the present study. The ventral root recordings showed spontaneous activity in the form of fast deflexions of the recording trace. Under stable conditions and prior to drug application the cumulative depolarization (CD) evoked by trains of electrical stimulus had a mean integrated area of 11.7±0.8 mV.s (range 5 – 18 mV.s) and rise rate was 11±1 μV/s (range 4 – 20 μV/s). Under these conditions the mean amplitude of the mono-synaptic reflex was 3.4±1.1 mV (range 0.6 – 7.5 mV).
Effects of 5-HT receptor agonists
All the agonists used in the present experiments including 5-HT depressed the integrated area of the CD. These effects were concentration-dependent and reversible. Figure 1A shows an example of the inhibitory effect of 5-HT at two different concentrations and Figure 1B shows the concentration-response curves for the agonists tested. Table 1 summarizes the EC50 values for the inhibition of the CD. The most potent agonist was 5-CT followed by α-met-5-HT and 5-HT itself. These agonists produced an immediate depression of the CD which outlasted the drug application time (see Table 1). In contrast 8-OH-DPAT and 2-met-5-HT were weak agonists reducing the area of the CD to 79±18% and to 80±8% of control values respectively at 10 μM. At this high concentration 8-OH-DPAT depressed the responses to the first 2 – 3 stimuli of the train to a greater extent than the responses to the following stimuli (see Figure 2). As a consequence the rise rate of the CD increased to 132% of control during superfusion with 8-OH-DPAT 10 μM (P⩽0.05). None of the other agonists including 5-HT modified the rise rate at concentrations close to their EC50.
Table 1.
EC50 values for the inhibition of the cumulative depolarization (CD) and the mono-synaptic reflex (MSR).
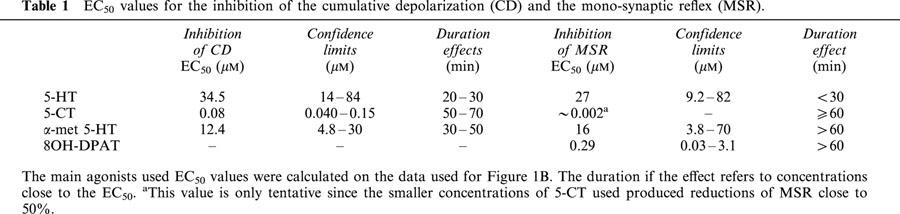
Figure 2.
Effects of 8-OH-DPAT. (A) Shows responses to repetitive dorsal root stimulation in control ACSF (left) and during superfusion with 8-OH-DPAT 10 μM. Note the greater effect of 8-OH-DPAT on the first second of the response (horizontal arrow) than on the development of the response upon further stimulation. (B) Shows responses to single stimuli in control ACSF and during superfusion of 8-OH-DPAT 1 μM obtained from a different experiment. Note the strong reduction of the mono-synaptic reflex (vertical arrow).
All the 5-HT receptor agonists tested showed a concentration-dependent inhibitory effect on the mono-synaptic reflex. The order of potency of the agonists was 5-CT>48-OH-DPAT>4α-met-5-HT≈5-HT (see Table 1). 2-met-5-HT applied at 10 and 30 μM reduced the MSR to 82 and 44% of control values respectively, and therefore its EC50 value is likely to be close to that for 5-HT. The strong effect of 8-OH-DPAT on the MSR contrasted with its modest effect on the CD (see Figure 2).
Recovery of the MSR after drug application was very slow for 5-CT, 8-OH-DPAT and α-met-5-HT and outlasted the effects of these compounds on the CD (see Table 1). For this reason, the effects of the antagonists on the agonist-induced depression of the MSR were not assessed.
Both 5-HT and α-met-5-HT produced a similar depolarization of motoneurones which was visualized as a slow baseline deflection (0.2 – 0.7 mV at 10 μM) but none of the other agonists did. 5-HT, 5-CT and α-met-5-HT produced a clear inhibition of the ongoing activity. These effects appeared to be faster and of greater magnitude the higher the concentration used but were not quantified or analysed further.
Effects of 5-HT receptor antagonists
Several of the antagonists used had a direct effect on the CD. Methiothepin (1 – 5 μM), ritanserin (5 μM) and RO 04-6790 (5 μM) produced a significant increment of the integrated area of the CD (between 115% and 130% of control) and clozapine (5 μM) produced a very marked increase (to 180% of control). In contrast SDZ-21009 (>0.5 μM) and WAY 100135 (>1 μM) showed a tendency to decrease the CD.
The antagonists were tested against the agonists that showed larger inhibitory effects on the CD at concentrations close to their respective EC50 values – i.e. 5-HT (10 μM), α-met-5-HT (10 μM) and 5-CT (0.1 μM) – in a fixed temporal sequence (see Methods section). The results obtained were compared to the effects of the agonists applied in the same manner but in the absence of antagonists (n=4). The inhibitory effect of each agonist when applied in sequence was equal to that found when the agonist was applied to a fresh cord (i.e. 5-CT 0.1 μM reduced the area of the CD to 45±4% of control when applied at the end of a sequence and to 41±4% when applied to a fresh cord; difference not significant). The effects of the antagonists on the agonist-induced depression of the CD are summarized in Figure 3.
Figure 3.
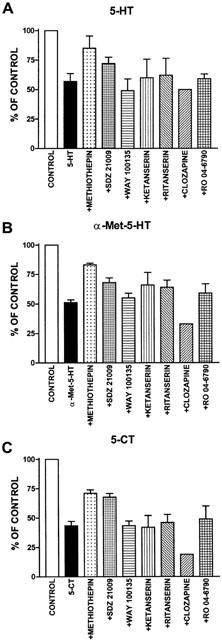
Graphs show the effects of (A) 5-HT, (B) α-met-5-HT and (C) 5-CT on the integrated area of the cumulative depolarization applied in the absence (black bars) and the presence of a series of antagonists (as specified). Agonists and antagonists were applied at the following concentrations: 5-HT and α-met-5-HT, 10 μM; 5-CT, 100 nM; methiothepin, 1 μM; SDZ-21009, 100 nM; WAY 100135, 1 μM; Ketanserin, ritanserin, clozapine and RO 04-6790, 5 μM. Numbers of observations 2 – 4 (see Results for details).
Methiothepin
This non-specific 5-HT receptor antagonist, applied at 1 μM (n=3), clearly attenuated the depressant effects of 5-HT, α-met-5-HT and 5-CT on the integrated area of the CD (see Figure 3A – C). During superfusion with methiothepin 5 μM (n=2) none of the agonists caused reductions of the integrated area of the CD larger than 9% (not shown). Furthermore this antagonist blocked the depolarization caused by 5-HT and α-met-5-HT.
SDZ-21009
This 5-HT1A/1B receptor antagonist applied at 100 nM (n=4) reduced the inhibitory effects of 5-HT, α-met-5-HT and 5-CT (see Figure 3A – C). In order to test further the effects of SDZ-21009, more detailed time course experiments were run for each agonist in the presence (n=6 – 8) and absence (n=6 – 8) of this compound. These experiments are summarized in Figure 4A. In the presence of SDZ-21009 all three agonists had a significantly smaller peak effect and a faster recovery than in control ACSF. An example of the effects of SDZ-21009 on 5-CT-induced depression of the CD is shown in Figure 4B. Higher concentrations of this antagonist (1 – 5 μM; n=3) produced mixed agonist-antagonist actions on the CD compromising the interpretation of the data.
Figure 4.
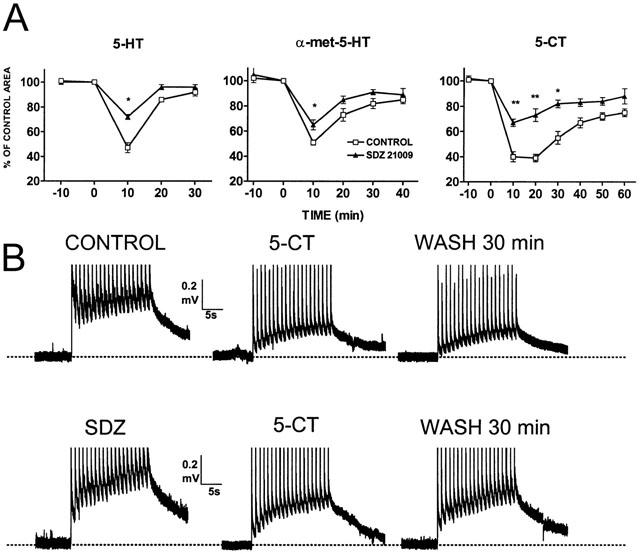
Effects of SDZ 21009. Each graph in (A) shows a comparison between control responses to 5-HT (10 μM), α-met-5-HT (10 μM) and 5-CT (0.1 μM) (hollow squares, n=6 – 8) and responses in the presence of SDZ-21009 100 nM (filled triangles; n=6 – 8). Test stimulus were given at 10 min intervals. The peak effect of the agonist was significantly smaller (* for P⩽0.5; ** for P⩽0.01). (B) shows original recordings obtained from two different preparations (upper and lower traces). The upper traces show the effect of 5-CT during application of the drug and after 30 min of wash in control ACSF. The lower traces show the same sequence in SDZ-21009 containing ACSF.
WAY 100135
Superfusion of this specific 5-HT1A receptor antagonist at 1 μM (n=2) or at 5 μM (n=1) did not produce any signs of antagonism on any of the three agonists (see Figure 3A – C).
Ketanserin and ritanserin
Neither of the classical 5-HT2 receptor antagonists ketanserin and ritanserin (5 μM, n=3 both drugs) blocked the depression of the CD induced by the agonists. An attenuation of the effect of 5-HT and α-met-5-HT but not of 5-CT was observed (see Figure 3A – C). Both antagonists blocked the motoneuronal depolarizations to 5-HT and α-met-5-HT.
Combined superfusion of ketanserin and SDZ-21009
In order to check whether the effects of 5-HT2 and 5-HT1B receptor antagonists were additive SDZ-21009 (100 nM) and ketanserin (5 μM) were superfused together (n=3) and their combined effect on α-met-5-HT analysed. During superfusion of both antagonists, α-met-5-HT reduced the CD to only 83±4% of control whereas during separate superfusion of SDZ-21009 and ketanserin, α-met-5-HT reduced the CD to 68±4% and to 66±10% of control respectively. This is consistent with an additive effect of the combined antagonists.
RO 04-6790 and Clozapine
Superfusion of the 5-HT6 receptor antagonist RO 04-6790 (5 μM; n=3) produced only a small attenuation of the effects of the agonists. In order to further test the effects of this antagonist a new series of six experiments were run with an identical structure to those described for SDZ-21009. These experiments confirmed a small attenuation of the agonist-induced effect which was not statistically significant. Superfusion of clozapine (5 μM; n=2) active at 5-HT6/7 receptors did not show antagonism of 5-HT or the other agonists. In fact, larger depressant effects of the agonists were observed in the presence of clozapine. However these effects may be due to the large increase of the signal produced by clozapine applied in isolation.
Discussion
As a starting point for the discussion of the present results it is important to note that the inhibitory actions of 5-HT on nociceptive reflexes were blocked by the generic 5-HT antagonist methiothepin indicating that the effects studied were due to the activation of 5-HT receptors. Other factors that might have explained a decrease of the signals recorded such as fatigue of the preparation due to prolonged recordings or the use of solvents or non-specific drug actions were ruled out by control experiments. We therefore believe that the inhibitory effects of 5-HT on the cumulative depolarization represent an electrophysiological correlate of the analgesic actions reported for 5-HT and related compounds.
The order of potency of agonists that mimicked the inhibition of the CD does not coincide with any subtype of 5-HT receptor defined so far. The most potent agonist at inhibiting the CD, 5-CT, has affinity for 5-HT1 and for 5-HT5,6&7 receptors and α-met-5HT, the second most potent agonist, is regarded as a selective 5-HT2 receptor agonist (Hoyer et al., 1994). Yet, our results suggest that 5-HT1B receptors mediate, at least in part, the inhibition of nociceptive reflexes caused by all these agents. SDZ-21009 is a well established 5-HT1A/1B antagonist which has, in addition, a well documented β-blocker action (Schoeffter & Hoyer, 1989; Hoyer et al., 1990). We have recently showed that β-adrenoceptor agonists fail to mimic noradrenaline effects on nociceptive reflexes under identical experimental conditions (Hedo & Lopez-Garcia, 2001) and these results are consistent with the low expression of β-adrenoceptors in the spinal cord (Nicholas et al., 1998). Therefore the blocking action of SDZ-21009 observed in the present study may be attributed to the blockade of 5-HT1A or 5-HT1B receptors. In addition, a specific 5-HT1A receptor antagonist (WAY 100135) failed to modify the response to 5-HT. Surprisingly SDZ-21009 attenuated not only the inhibitory effects of 5-HT and 5-CT but also those of α-met 5-HT.
The effects of α-met-5-HT are interesting for several reasons. This compound, like 5-HT itself, depolarized motoneurones therefore reducing the drive for further depolarization upon arrival of synaptic input. The present results show that ketanserin and ritanserin blocked the agonist-induced depolarization producing only a small attenuation of 5-HT- and α-met-5-HT-induced inhibition of nociceptive reflexes. This observation is consistent with previous data indicating that depolarization of motoneurones is mediated by 5-HT2 receptors (Wallis & Elliot, 1991) and shows that motoneurone depolarization has only a marginal impact on the integrated area of the CD that we have studied. Under the present experimental conditions, 5-HT and α-met-5-HT may activate 5-HT2 and 5-HT1B receptors although the truly inhibitory effect of these compounds are mediated by 5-HT1B receptors. This view is supported by the additive effects of 5-HT1B and 5-HT2 antagonists when applied together. It is also interesting to note that neither ketanserin nor ritanserin modified the effects of 5-CT which in turn did not produce motoneuronal depolarization at the low concentrations used.
This interpretation is consistent with results presented by other authors showing that activation of 5-HT1B receptors has antinociceptive actions in behavioural tests, reduces the receptive fields of nociceptive neurones and depresses responses to nociceptive stimulation in spinal neurones (El Yashir et al., 1988; Eide et al., 1990; Murphy & Zemlan, 1990; Ali et al., 1994). Since the 5-HT1B receptor is located on terminals of afferent fibres and descending serotonergic fibres, a presynaptic mechanism has been proposed (see Hamon et al., 1990 for a review). Despite all these considerations, in our hands, the blocking action of SDZ-21009 was only partial. This leaves the possibility of the involvement of other 5-HT receptors opened to discussion.
The role of 5-HT1A receptors in the modulation of nociceptive reflexes has been intensively discussed in the literature and conflicting pro-and anti-nociceptive effects have been reported. (El Yashir et al., 1988; Gjerstad & Hole, 1996; Eide et al., 1990; Ali et al., 1994; Murphy & Zemlan, 1989; Hololean et al., 1992). In our model, 8-OH-DPAT had a depressant effect on nociceptive reflexes which was observed in the first seconds of the response to repetitive stimulation. In contrast, 8-OH-DPAT was unable to prevent a fast development of the CD. It is important to note that this effect on nociceptive reflexes appeared at high concentrations (10 μM) whereas profound and long-lasting effects of 8-OH-DPAT were observed on the non-nociceptive mono-synaptic reflex at much lower concentrations (0.1 – 1 μM). Clarke et al. (1997) reported an excitatory action of 8-OH-DPAT on spinal reflexes which was more consistent with a non-specific activation of 5-HT7 receptors than with the activation of 5-HT1A receptors. It is possible that in the present experiments excitatory and inhibitory actions of this compound may have cancelled each other out whereas in other studies, a more spatially restricted application of the drug led to net inhibitory or excitatory effects. Nevertheless, the failure of WAY 100135 to prevent the effects of 5-HT suggests that 5-HT1A receptors do not play a mayor role in the overall modulation of nociceptive reflexes in this isolated preparation. The role of 5-HT1D receptors in the modulation of nociceptive reflexes has only recently been addressed and few observations exists in this regard. It has been shown that blockade of 5-HT1D receptors depress responses of trigeminal dorsal horn neurones to noxious mechanical stimulation but does not affect responses of spinal dorsal horn neurones (Cumberbatch et al., 1998). In the present study the superfusion of ketanserin and ritanserin which have antagonist activity at 5-HT1D receptors (see Boess & Martin, 1994) fail to block the effects of 5-CT.
The possible intervention of 5-HT6 and 5-HT7 receptors was assessed by superfusion of antagonists reported to block selectively (RO 04-6790; Sleight et al., 1998) or non-selectively these receptors (ritanserin and clozapine; see Monsma et al., 1993; Roth et al., 1994). At the high concentrations used, RO 04-6790 produced only a small and non-significant attenuation of the agonist-induced depression which was not confirmed by clozapine. Therefore the main conclusion that can be drawn is that neither 5-HT6 nor 5-HT7 receptors play a major role in the inhibition of nociceptive reflexes induced by exogenous 5-HT. It is noteworthy that all the antagonists with an action on 5-HT6 receptors produced an increase in baseline responses including RO 04-6790, methiothepin, ritanserin and clozapine. It is therefore possible that blockade of this receptor reveals a tonic inhibitory role for endogenous 5-HT which has been previously reported in the isolated spinal cord (Wallis & Elliot, 1991). This may also explain the increased responses to noxious stimuli after administration of methiothepin found in behaving animals (Eide et al., 1987). The very few reports in which the role of spinal 5-HT7 receptors has been addressed are more consistent with potentiation than with inhibition of afferent input (Cardenas et al., 1999; Clarke et al., 1997).
Finally we observed that 2-met-5-HT, a 5-HT3 receptor agonist, produced significant but small depression of nociceptive reflexes. The role of 5-HT3 receptors has been investigated in the context of spinal nociceptive processing in several studies and the general view is that they play a role in the inhibition of nociceptive input probably via a presynaptic mechanism (see Glaum et al., 1988; Peng et al., 1996; Khasabov et al., 1999; but see also Xu et al., 1994). The present results support this view.
In summary, our results lend strong experimental support to the hypothesis of the involvement of 5-HT1B receptors in the inhibition of spinal nociceptive reflexes. A coadjuvant role for 5-HT3 receptors is also suggested by the present results. In contrast, no evidence has been found to infer the intervention of 5-HT2, 5-HT6 or 5-HT7 receptors.
Acknowledgments
The authors want to thank Dr J.M.A. Laird for the discussion of the results and revision of the English version of this manuscript. Financial support was obtained from Spanish Ministry of Science and Technology (SAF-2000-0199) and a Contrato Programa from the Madrid Regional Government. (Principal investigator: Dr F. Cervero).
Abbreviations
- ACSF
Artificial cerebrospinal fluid
- CD
Cumulative depolarization
- 5-CT
5-Carboxamidotryptamine
- DMSO
Dimethyl sulphoxide
- 8-OH-DPAT
R(+)-8-hydroxy-2-(di-N-propylamino) tetralin hydrobromide
- α-met-5-HT
α-methyl-5-hydroxytryptamine
- 2-met-5-HT
2-methyl-5-hydroxytryptamine
References
- ALI Z., WU G., KOZLOV A., BARASI S. The actions of 5-HT1 agonists and antagonists on nociceptive processing in the rat spinal cord: results from behavioural and electrophysiological studies. Brain Res. 1994;661:83–90. doi: 10.1016/0006-8993(94)91184-3. [DOI] [PubMed] [Google Scholar]
- BOESS F.G., MARTIN I.L. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- CARDENAS C.G., MAR L.P., VYSOKANOV A.V., ARNOLD P.B., CARDENAS L.M., SURMEIER D.J., SCROGGS R.S. Serotonergic modulation of hyperpolarization-activated current in acutely isolated rat dorsal root ganglion neurons. J. Physiol. 1999;518:507–523. doi: 10.1111/j.1469-7793.1999.0507p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN J.J., VASKO M.R., WU X., STAEVA T.P., BAEZ M., ZGOMBICK J.M., NELSON D.L. Multiple subtypes of serotonin receptors are expressed in rat sensory neurons in culture. J. Pharmacol. Exp. Ther. 1998;287:1119–1127. [PubMed] [Google Scholar]
- CLARKE R.W., OGILVIE J., HOUGHTON A.K. Enhancement and depression of spinal reflexes by 8-hydroxy-2-(di-n-propylamino)tetralin in the decerebrated and spinalized rabbit: involvement of 5-HT1A- and non-5-HT1A-receptors. Br. J. Pharmacol. 1997;122:631–638. doi: 10.1038/sj.bjp.0701430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUMBERBATCH M.J., HILL R.G., HARGREAVES R.J. Differential effects of the 5-HT1B/1D receptor agonist naratriptan on trigeminal versus spinal nociceptive responses. Cephalagia. 1998;18:659–664. doi: 10.1046/j.1468-2982.1998.1810659.x. [DOI] [PubMed] [Google Scholar]
- DAVIES J.E., ROBERTS M.H. 5-Hydroxytryptamine reduces substance P responses on dorsal horn interneurones: a possible interaction of neurotransmitters. Brain Res. 1981;217:399–404. doi: 10.1016/0006-8993(81)90018-4. [DOI] [PubMed] [Google Scholar]
- DEL MAR L.P., CARDENAS C.G., SCROGGS R.S. Serotonin inhibits high-threshold Ca2+ channel currents in capsaicin-sensitive acutely isolated adult rat DRG neurons. J. Neurophysiol. 1994;72:2551–2554. doi: 10.1152/jn.1994.72.5.2551. [DOI] [PubMed] [Google Scholar]
- EIDE P.K., BERGE O.G., HUNSKAAR S. Test-dependent changes in nociception after administration of the putative serotonin antagonist metitepin in mice. Neuropharmacology. 1987;26:1121–1126. doi: 10.1016/0028-3908(87)90257-7. [DOI] [PubMed] [Google Scholar]
- EIDE P.K., JOLY N.M., HOLE K. The role of spinal cord 5-HT1A and 5-HT1B receptors in the modulation of a spinal nociceptive reflex. Brain Res. 1990;536:195–200. doi: 10.1016/0006-8993(90)90025-7. [DOI] [PubMed] [Google Scholar]
- EL-YASSIR N., FLEETWOOD-WALKER S.M., MITCHELL R. Heterogeneous effects of serotonin in the dorsal horn of rat: the involvement of 5-HT1 receptor subtypes. Brain Res. 1988;456:147–158. doi: 10.1016/0006-8993(88)90356-3. [DOI] [PubMed] [Google Scholar]
- GERARD C., EL MESTIKAWY S., LEBRAND C., ADRIEN J., RUAT M., TRAIFFORT E., HAMON M., MARTRES M.P. Quantitative RT-PCR distribution of serotonin 5-HT6 receptor mRNA in the central nervous system of control or 5,7-dihydroxytryptamine-treated rats. Synapse. 1996;23:164–173. doi: 10.1002/(SICI)1098-2396(199607)23:3<164::AID-SYN5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- GJERSTAD J.T.J., HOLE K. The effect of 5-HT1A receptor stimulation on nociceptive dorsal horn neurones in rats. Eur. J. Pharmacol. 1996;318:315–321. doi: 10.1016/s0014-2999(96)00819-9. [DOI] [PubMed] [Google Scholar]
- GLAUM S.R., PROUDFIT H.K., ANDERSON E.G. Reversal of the antinociceptive effects of intrathecally administered serotonin in the rat by a selective 5-HT3 receptor antagonist. Neurosci. Lett. 1988;95:313–317. doi: 10.1016/0304-3940(88)90677-5. [DOI] [PubMed] [Google Scholar]
- GUSTAFSON E.L., DURKIN M.M., BARD J.A., ZGOMBICK J., BRANCHEK T.A. A receptor autoradiographic and in situ hybridization analysis of the distribution of the 5-HT7 receptor in rat brain. Br. J. Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMON M., BOURGOIN S.Role of serotonin and other neuroactive molecules in the physiopathogenesis of migraine. Current hypotheses Pathol. Biol. (Paris) 200048619(Abstract) [PubMed] [Google Scholar]
- HAMON M., COLLIN E., CHANTREL D., DAVAL G., VERGE D., BOURGOIN S., CESSELIN F.Serotonin receptor and the modulation of pain Serotonin and Pain 199053ed. Besson, J.M., p
- HEADLEY P.M., DUGGAN A.W., GRIERSMITH B.T.Selective reduction by noradrenalin and serotonin of nociceptive responses of cat dorsal horn neurones Brain Res. 1978145185(Abstract) [DOI] [PubMed] [Google Scholar]
- HEDO G., LAIRD J.M., LOPEZ-GARCIA J.A. Time-course of spinal sensitization following carrageenan-induced inflammation in the young rat: a comparative electrophysiological and behavioural study in vitro and in vivo. Neuroscience. 1999;92:309–318. doi: 10.1016/s0306-4522(98)00734-9. [DOI] [PubMed] [Google Scholar]
- HEDO G., LOPEZ-GARCIA J.A.Serotonergic effects on the wind-up and long latency components of rat spinal reflexes in vitro J. Physiol. (Lond) 1999a51597(Abstract) [Google Scholar]
- HEDO G., LOPEZ-GARCIA J.A.A methiothepin-sensitive receptor mediates the serotonergic modulation of spinal nociceptive reflexes in vitro Society for Neuroscience Abstracts 1999b25923(Abstract) [Google Scholar]
- HEDO G., LOPEZ-GARCIA J.A. Alpha-1A adrenoceptors modulate potentiation of spinal nociceptive pathways in the rat spinal cord in vitro. Neuropharmacology. 2001;41:862–869. doi: 10.1016/s0028-3908(01)00117-4. [DOI] [PubMed] [Google Scholar]
- HOLOHEAN A.M., HACKMAN J.C., SHOPE S.B., DAVIDOFF R.A. Serotonin1A facilitation of frog motoneuron responses to afferent stimuli and to N-methyl-D-aspartate. Neuroscience. 1992;48:469–477. doi: 10.1016/0306-4522(92)90506-w. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- HOYER D., SCHOEFFTER P., WAEBER C., PALACIOS J.M. Serotonin 5-HT1D receptors. Ann. N. Y. Acad. Sci. 1990;600:168–181. doi: 10.1111/j.1749-6632.1990.tb16880.x. [DOI] [PubMed] [Google Scholar]
- KHASABOV S.G., LOPEZ-GARCIA J.A., ASGHAR A.U., KING A.E. Modulation of afferent-evoked neurotransmission by 5-HT3 receptors in young rat dorsal horn neurones in vitro: a putative mechanism of 5-HT3 induced anti-nociception. Br. J. Pharmacol. 1999;127:843–852. doi: 10.1038/sj.bjp.0702592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARKMAN P.M., KELLY J.S. Modulation of IH by 5-HT in neonatal rat motoneurones in vitro: mediation through a phosphorylation independent action of cAMP. Neuropharmacology. 1997;36:721–733. doi: 10.1016/s0028-3908(97)00021-x. [DOI] [PubMed] [Google Scholar]
- LE BARS D.Serotonin and Pain Neuronal Serotonin 1988171eds. Osborne, N.N. & Hamon, N., p
- LOPEZ-GARCIA J.A. Serotonergic modulation of the responses to excitatory amino acids of rat dorsal horn neurons in vitro: implications for somatosensory transmission. Eur. J. Neurosci. 1998;10:1341–1349. doi: 10.1046/j.1460-9568.1998.00153.x. [DOI] [PubMed] [Google Scholar]
- LOPEZ-GARCIA J.A., KING A.E. Pre- and post-synaptic actions of 5-hydroxytryptamine in the rat lumbar dorsal horn in vitro: implications for somatosensory transmission. Eur. J. Neurosci. 1996;8:2188–2197. doi: 10.1111/j.1460-9568.1996.tb00740.x. [DOI] [PubMed] [Google Scholar]
- LOPEZ-GARCIA J.A., LAIRD J.M. Central antinociceptive effects of meloxicam on rat spinal cord in vitro. Neuroreport. 1998;9:647–651. doi: 10.1097/00001756-199803090-00016. [DOI] [PubMed] [Google Scholar]
- MILLAN M.J.The role of Descending Noradrenergic and Serotonergic Pathways in the Modulation of Nociception: Focus on Receptor Multiplicity The Pharmacology of Pain 1997385–446.eds. Dickenson, A. & Besson, J.M., pp
- MONSMA F.J.J., SHEN Y., WARD R.P., HAMBLIN M.W., SIBLEY D.R. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol. Pharmacol. 1993;43:320–327. [PubMed] [Google Scholar]
- MURPHY R.M., ZEMLAN F.P. Functional change in the 5-HT presynaptic receptor in spinal cord of aged rats. Neurobiol. Aging. 1989;10:95–97. doi: 10.1016/s0197-4580(89)80016-8. [DOI] [PubMed] [Google Scholar]
- MURPHY R.M., ZEMLAN F.P. Selective serotonin1A/1B agonists differentially affect spinal nociceptive reflexes. Neuropharmacology. 1990;29:463–468. doi: 10.1016/0028-3908(90)90168-q. [DOI] [PubMed] [Google Scholar]
- NICHOLAS A.P., PIERIBONE V.A., HOKFELT T. Cellular localisation of messenger RNA for beta-1 and beta-2 adrenergic receptors in rat brain. Neuroscience. 1993;56:1023–1039. doi: 10.1016/0306-4522(93)90148-9. [DOI] [PubMed] [Google Scholar]
- PENG Y.B., LIN Q., WILLIS W.D. The role of 5-HT3 receptors in periaqueductal gray-induced inhibition of nociceptive dorsal horn neurons in rats. J. Pharmacol. Exp. Ther. 1996;276:116–124. [PubMed] [Google Scholar]
- ROTH B.L., CRAIGO S.C., CHOUDHARY M.S., ULUER A., MONSMA F.J.J., SHEN Y., MELTZER H.Y., SIBLEY D.R. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J. Pharmacol. Exp. Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- SCHOEFFTER P., HOYER D. 5-Hydroxytryptamine 5-HT1B and 5-HT1D receptors mediating inhibition of adnylate cyclase activity. Pharmacological comparison with special reference to the effects of yohimbine, rauwolscine and some beta-adrenoceptor antagonists. Naunyn Schmiedebergs Arch. Pharmacol. 1989;340:285–292. doi: 10.1007/BF00168512. [DOI] [PubMed] [Google Scholar]
- SLEIGHT A.J., BOESS F.G., BOS M., BOURSON A. The putative 5-ht6 receptor: localization and function. Ann. N. Y. Acad. Sci. 1998;861:91–96. doi: 10.1111/j.1749-6632.1998.tb10178.x. [DOI] [PubMed] [Google Scholar]
- WALLIS D.I., ELLIOTT P.The electrophysiology of 5-HT Serotonin: Molecular biology, receptors and functional effects 1991Basel: Birkhauser Verlag; 203–219.eds. Fozard, J.R. & Saxena, P.R., pp [Google Scholar]
- XU W., QIU X.C., HAN J.S. Serotonin receptor subtypes in spinal antinociception in the rat. J. Pharmacol. Exp. Ther. 1994;269:1182–1189. [PubMed] [Google Scholar]


