Abstract
Red wine intake is associated with a low risk of cardiovascular disease. This effect has been partly attributed to the action of polyphenolic compounds, which decrease the oxidation of plasma low density lipoproteins. Moreover, nitric oxide (•NO) is a vasodilator and polyphenolic compounds induce endothelium-dependent vasorelaxation in vitro.
Here we studied whether a diet rich in dealcoholated red wine (DRW) increases acetylcholine-induced vasorelaxation and whether ingestion of DRW-, quercetin- or catechin-rich diets modifies the •NO-cyclic guanosine-3′,5′-monophosphate (cyclic GMP) pathway and superoxide anion (O2.−) release in aorta in a resting state in rats fed semi-purified diets containing either 35% (v w−1) DRW, 0.3% (w w−1) quercetin or 0.3% (w w−1) catechin for 10 days.
•NO-mediated vasorelaxation induced by acetylcholine was greater in rats fed the DRW-rich diet than in those that received the control diet.
Expression of endothelial •NO synthase (eNOS) was similar in the four dietary groups. The aortic rings of rats fed either the DRW-, quercetin-, or catechin-rich diets showed higher NOS activity, •NO production and cyclic GMP content than those of rats fed the control diet. No changes were observed in O2.− production.
In summary, diets rich in either DRW, quercetin or catechin induced endothelium-dependent vasorelaxation in rat aorta in a resting state through the enhancement of •NO production, without modifying O2.− generation, thus the bioavailability of •NO was increased. The increase in the •NO-cyclic GMP pathway explains the beneficial effect of flavonoids at vascular level.
Keywords: Flavonoids, dealcoholated red wine, rat aorta, nitric oxide, superoxide anion, electron spin resonance spectroscopy, endothelium
Introduction
Ingestion of flavonoids found in wine, tea and various plant foods is inversely correlated with mortality from coronary disease (Renaud & de Lorgeril, 1992; Hertog et al., 1995; Knekt et al., 1996). The benefits of red wine or flavonoid-rich food have been attributed to the antioxidant activity of their polyphenolic compounds (Rice-Evans et al., 1997), and the oxidative modification of low density lipoproteins (LDL) is a key step in the formation of an atherosclerotic lesion (Steinberg et al., 1989). Several studies have shown the protective effect of flavonoids on LDL by measuring their oxidative susceptibility in vitro (Brown et al., 1998), or in ex vivo assays (Fuhrman et al., 1995; Van Het Hof et al., 1999) and the total antioxidant status of lipoproteins (Fuhrman et al., 1995). However, there is still debate about whether red wine and flavonoid-rich food can decrease LDL oxidation ex vivo (Fuhrman et al., 1995; de rijke et al., 1996; Van Het Hof et al., 1999; Caccetta et al., 2000). For many years it was accepted that this was the main mechanism by which flavonoids mediate their beneficial effects.
Some polyphenolic compounds of red wine cause endothelium-dependent vasorelaxation in vitro (Fitzpatrick et al., 1993; Andriambeloson et al., 1997; 1998). The in vivo studies carried out on the protective effects of epicatechin in cerebral ischaemia (Huang et al., 1999), and quercetin in rat brain during global ischaemia and reperfusion (Shutenko et al., 1999) have dealt with the generation of nitric oxide (Van Het Hof et al., 1999NO). This compound, which is continuously synthesized by the vascular endothelium, regulates vascular tone and is also a powerful antioxidant (Rubbo et al., 2000). Van Het Hof et al., 1999NO also inhibits lipid peroxidation in LDL (Hogg et al., 1993; 1998; Rubbo et al., 1995); therefore it is an anti-atherogenic agent (Matthys & Bult, 1997). From results obtained with polyphenols and red wine in vitro, we postulated whether these effects, through •NO, are relevant in vivo in a resting state, because flavonoids ingested as aglycones and glycosides are metabolized. However, the bioavailability of •NO can be reduced by several mechanisms, but most is removed by its reaction with superoxide anion (O2.−), yielding peroxynitrite and peroxynitrous acid, unstable molecules which are potent oxidants.
This study aimed to examine the effect of in vivo administration of a dealcoholated red wine (DRW)-rich diet on acetylcholine-induced vasorelaxation and whether the ingestion of DRW-, quercetin- or catechin-rich diets affects the •NO-cyclic guanosine-3′,5′-monophosphate (cyclic GMP) pathway and O2.− production by aortic tissue in a resting state.
Methods
Animals and diets
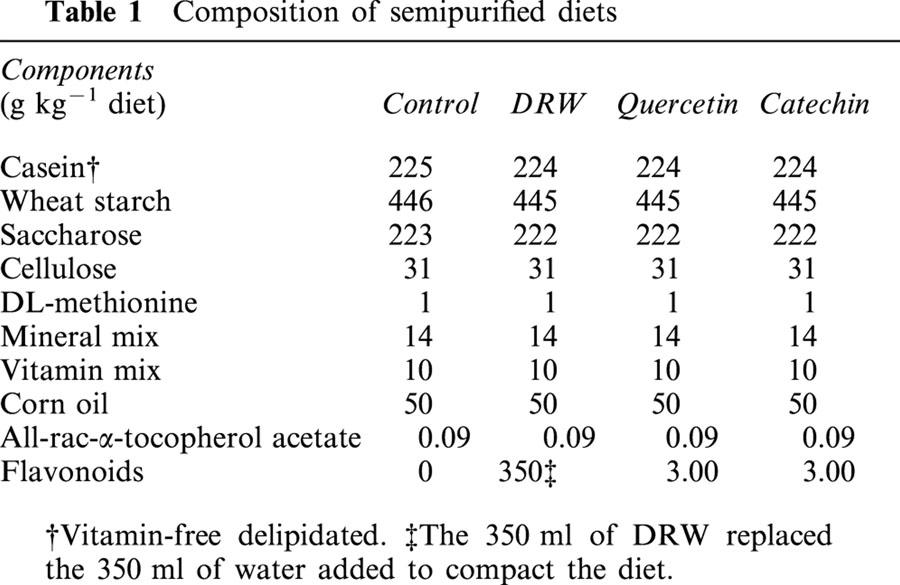
Male Sprague-Dawley rats, which weighed about 175 g, were purchased from Harlan Interfauna Ibérica (Barcelona, Spain). They were housed in temperature-controlled rooms (21 – 23°C), with 40 – 60% humidity, and were subjected to a 12-h light:dark cycle. These rats were divided into four groups and were fed one of the following semi-purified diets for 10 days: (1) a control diet, (2) a 35% (v w−1) DRW diet, (3) a 0.3% (w w−1) quercetin diet or (4) a 0.3% (w w−1) catechin diet (Table 1). To prevent oxidation and loss of antioxidants, these diets were manufactured and stored at −20°C under vacuum until use. Fresh food was provided once a day and rats had free access to food and water. The experimental protocols were reviewed and approved by the Ethical Animal Research Committee of the Faculty of Biology, in accordance with European Community guidelines.
Table 1.
Composition of semipurified diets
Alcohol was removed from the red wine in a rotary evaporator at 30°C. Vacuum was applied progressively up to −70 bars to avoid mechanical stress. Evaporated ethanol was replaced to the original volume and pH by addition of acidulated distilled water. Gas chromatography was used to determine traces of ethanol in the DRW. The total polyphenol content of the wine and the DRW was measured following the Folin-Ciocalteau method (Singleton & Rossi, 1965) while their phenolic and anthocyanin contents were evaluated by HPLC, following Castellari et al. (1998).
Aortic preparation
At the end of the feeding period, rats were anaesthetized with sodium urethane (1.5 g kg−1 i.p.) and exsanguinated. Thoracic aortae were excised and placed in a phosphate buffer solution (PBS) at pH 7.4. Aortae were carefully cleaned of fat, connective tissue and blood, taking care not to touch the luminal surface, and were then cut into four rings of 4 – 5 mm in length. Segments for vasorelaxation studies were set up in gassed (95% O2 and 5% CO2) PBS. Segments for the determination of cyclic GMP and •NO synthase (NOS) activity were frozen in liquid nitrogen and maintained at −80°C until use.
Effect of DRW on acetylcholine-induced vasorelaxation
Aortic rings with intact endothelium were randomized and fixed between stainless-steel hooks in a bath containing PBS pH 7.4 at 37°C. The hook anchoring the upper end of the ring was connected to the isometric transducer by a silk thread. Rings were equilibrated at an initial tension of 2 g for 60 min and the bath solution was renewed every 15 min and gassed with a mixture of 95% O2 and 5% CO2. After equilibration, these rings were pre-contracted with 2×10−7 M phenylephrine and relaxed with cumulative concentrations of acethylcholine (from 10−8 to 10−4 M) in the presence of 100 U ml−1 superoxide dismutase (SOD). To prevent reaction between O2.− and •NO, SOD was added just before the phenylephrine. Thus, in this condition, the degree of relaxation is an indirect measurement of •NO production (López et al., 2001). The organ bath solution was renewed three times after each assay and rings were allowed to equilibrate for at least 15 min to recover the baseline. Studies were also carried out in the presence of 0.1 mM NG-nitro-L-arginine (L-NNA), an inhibitor of NOS, which was added 20 min before the pre-contraction. The effective molar concentration of acetylcholine which causes 50% of maximal relaxation (EC50) was calculated for each concentration-response curve by fitting data to a linear curve and was expressed as a negative logarithm.
•NO-cyclic GMP pathway parameters
Endothelial NOS (eNOS) protein expression was measured in the supernatant of aortic homogenates subjected to sodium dodecyl sulfate-polyacrilamide gel electrophoresis and subsequently blotted on nitrocellulose membranes at 100 V for 1 h. The blots were developed with a rabbit anti-human eNOS polyclonal antibody (dilution 1 : 5000). Purified bovine eNOS was also loaded (2 μg) on the gels as a positive control and a pre-stained protein standard was used to check transfer efficiency. Bands were quantified by densitometry.
NOS activity in aortic homogenates was measured by the conversion of [3H]-L-arginine to [3H]-L-citrulline using a Cayman kit. [3H]-L-citrulline content was quantified by liquid scintillation counting in a Packard Top-Count counter (Packard Instrument Company, Meriden, CT, U.S.A.). In some experiments, 1 mM NG-nitro-L-arginine methyl ester (L-NAME) HCl, an inhibitor of NOS that generates L-NNA, was added to the incubation medium for 30 min. To address the effect of eNOS on NOS activity, the endothelium of one additional segment was removed by gently rubbing the intima surface with stainless steel wire.
•NO production in a resting state was measured by electron spin resonance (ESR) spectroscopy. Aortic segments were cut into segments, and pre-incubated at 37°C for 20 min in PBS at pH 7.4. They were then exposed to the following amounts of spin trapping agents, 5 mM dietyldithiocarbamic acid (DEDTC) and 50 μM FeSO4.7H2O, and were incubated for 30 min. After incubation, aortic segments were weighed and frozen in liquid nitrogen for posterior ESR analysis. An additional segment from each rat was endothelium denuded or pre-incubated with 1 mM L-NNA. The mononitrosyl iron complex formed with DEDTC (NO-Fe-(DEDTC)2) was measured in a Bruker 300E spectrometer (Bruker Instruments Company, Billerica, MA, U.S.A.) at 77 K (10 mW microwave power, 31985 G amplitude modulation, 9.77 kHz microwave frequency and 100 kHz modulation frequency). The signal from the complex corresponded to the difference in intensity between a maximum at 3440 [G] and a minimum at 3470 [G]. Values were extrapolated to a standard curve made with diethylamine NONOate.
Vascular cyclic GMP was evaluated in segments (20 – 40 mg) homogenized in PBS containing 25% trichloroacetic acid and centrifuged at 1500×g for 10 min at 4°C. This parameter was measured in the supernatant using an enzyme immunoassay kit from Cayman. Proteins were determined in the pellet by the Bradford method (Bradford, 1976) using bovine serum albumin as standard.
O2.− production
O2.− production by endothelium functional segments was measured as lucigenin-derived chemiluminescence in the presence of 5 μM lucigenin in a resting state and after stimulation with 100 μM nicotinamine adenine dinucleotide phosphate reduced form (NADPH) (Skatchkov et al., 1999). NG-monomethyl-L-arginine (L-NMMA, 1 mM), an inhibitor of NOS, was used to inhibit the reaction of O2.− with •NO. Other NOS inhibitors interfere with NADPH-dependent reduction (Mayer & Andrews, 1998). To estimate the true O2.− production in a resting state and after stimulation, the values with SOD were subtracted from those obtained in its absence.
Drugs
The red wine used was a common commercial wine made in Spain. L-[2,3,4,5-3H]-Arginine monohydrochloride was purchased from Amersham Pharmacia Biotech (Bucks., U.K.). The diethylamine NONOate, L-NNA, L-NAME, L-NMMA, rabbit antihuman eNOS and bovine eNOS were obtained from Cayman Chemical Company (Miami, FL, U.S.A.). All other compounds were purchased from Sigma Chemical Company (St. Louis, MO, U.S.A.), with the exception of the starch (Panreac Química, Barcelona, Spain) and the mineral and vitamin mix (ICN Biomedicals, Aurora, OH, U.S.A).
Statistics
Results are expressed as the mean±s.e. mean for three rats for acetylcholine-induced vasorelaxation, and for 5 – 6 rats in duplicate for the other assays. Statistical significance was estimated by the Student's t-test for unpaired observations.
Results
Alcohol and polyphenol content in DRW
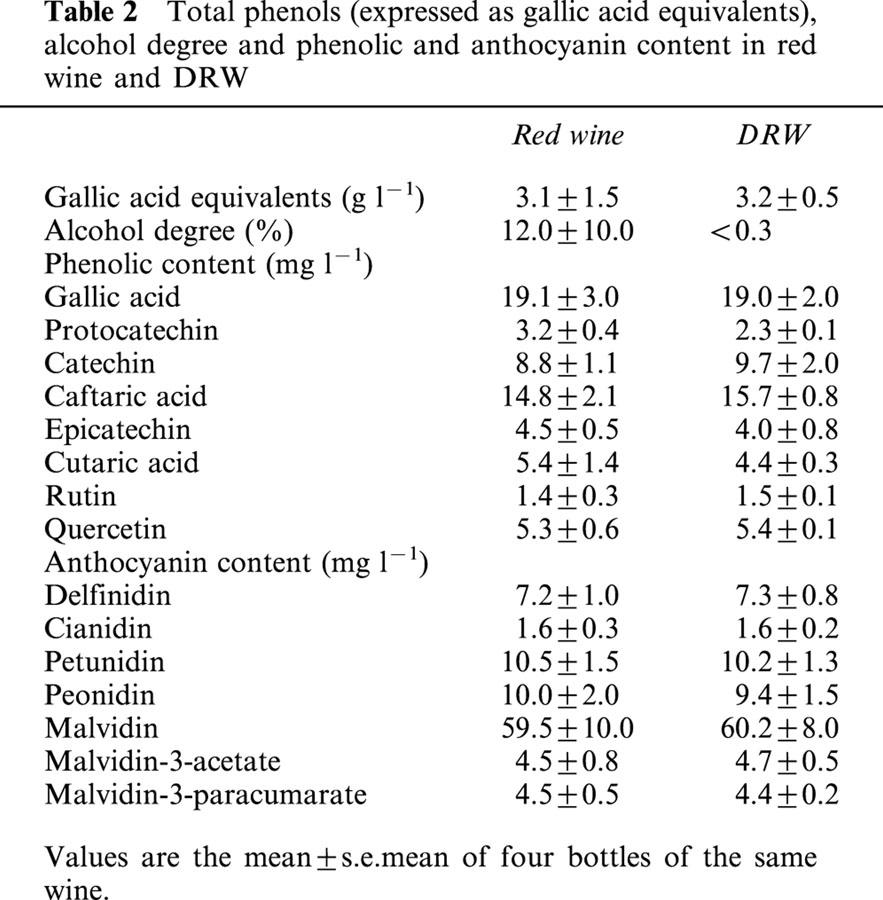
Gas chromatography showed that DRW was non-alcoholic (<3 g l−1 of alcohol). Dealcoholization did not alter the content of total wine polyphenols (3.2 g l−1 and 3.1 g l−1, expressed as gallic acid equivalents in red wine and DRW, respectively). The content of phenolic and anthocyanin compounds in DRW was similar to those of the red wine before the removal of alcohol (Table 2). All groups of rats had a similar daily diet-intake (30 g for the control group) and growth performance (50 g for the control group) during the 10 days of diet.
Table 2.
Total phenols (expressed as gallic acid equivalents), alcohol degree and phenolic and anthocyanin content in red wine and DRW
Acetylcholine-induced vasorelaxation by DRW
Acetylcholine induced cumulative concentration-dependent relaxation in pre-contracted rings. In the DRW group there was a significant difference (P<0.05) in maximal relaxation (75.5±1.8% vs 68.5±1.5% in the control group) in the presence of exogenous SOD (Figure 1), and the EC50 increased (P<0.05) when expressed in −log M (6.94±0.06 vs 6.60±0.08 for the control group), which accounts for a 50% decrease when expressed in mM.
Figure 1.
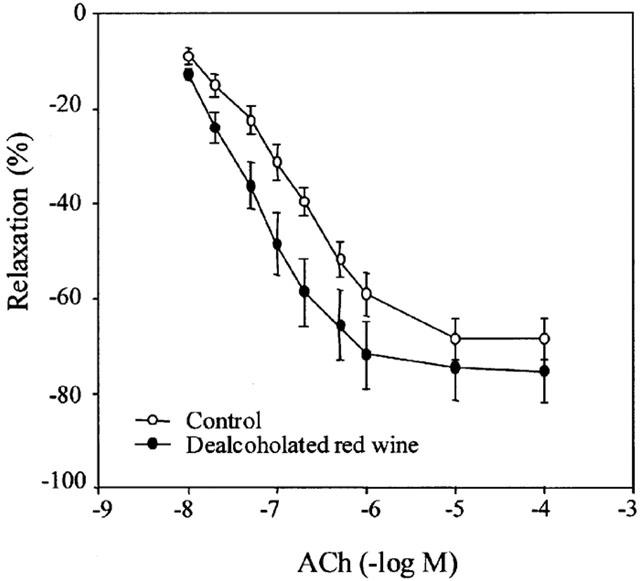
Concentration-response curves to acetylcholine in the presence of 60 U ml−1 SOD in intact aortic rings from the control and dealcoholated red wine groups. Values are the mean±s.e.mean of three rats, the s.e.mean is shown by vertical lines.
Effect of flavonoid-rich diets on the •NO-cyclic GMP pathway
The mean expression of eNOS (relative densitometric units) was similar in the four dietary groups (Figure 2).
Figure 2.
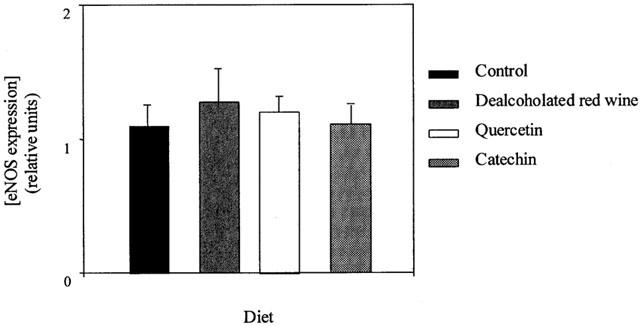
eNOS expression in aortic homogenates from rats fed a control, dealcoholated red wine-, quercetin- or catechin-rich diet. Values are expressed as relative units of densitometry (one unit corresponds to 2 μg of bovine eNOS) and are the mean±s.e.mean of five rats, the s.e.mean is shown by vertical lines.
NOS activity was significantly higher in aortic homogenates from rats fed the DRW-, quercetin-, and catechin-rich diets (180±13, 140±13 and 120±14 pmol mg of tissue protein−1 h−1, respectively) than in the control group (74±6 pmol mg of tissue protein−1 h−1) (Figure 3). In all groups, 98% of the citrulline formation was blocked by the addition of 100 μM L-NAME. NOS activity in endothelium-denuded rings was undetectable, indicating that the increased activity of NOS in segments with functional endothelium was due to eNOS.
Figure 3.
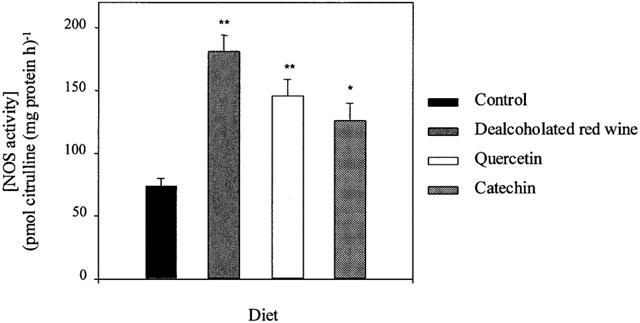
NOS activity measurements as conversion of [3H]-arginine to [3H]-citrulline in aortic homogenates from rats fed a control, dealcoholated red wine-, quercetin- or catechin-rich diet. Values are the mean±s.e. mean of five rats .*P<0.05, **P<0.01 significantly different compared with control, by the Student's unpaired t-test, the s.e.mean is shown by vertical lines.
DRW-, quercetin- and catechin-rich diets significantly increased the resting •NO production by aortae (Figure 4), as shown by the ESR detection of the •NO-Fe-(DEDTC)2 complex in aortic rings with functional endothelium (Figure 5). After incubation with 1 mM L-NNA, the signal was reduced by 90 – 95% in all groups. The endothelium-denuded segments gave a very weak signal in all groups (data not shown), indicating that the endothelium is the main source of •NO.
Figure 4.
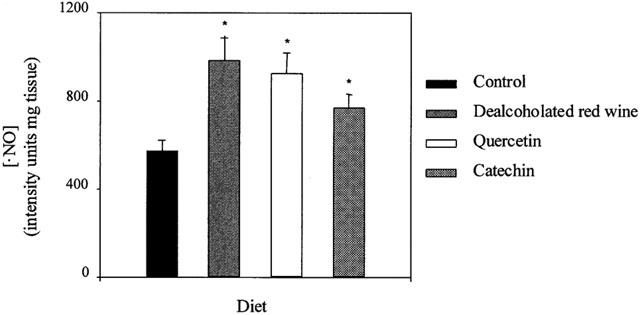
•NO production in intact aortic segments from rats fed a control, dealcoholated red wine-, quercetin- or catechin-rich diets. Values are the mean±s.e.mean of five rats.*P<0.01 significantly different compared with control, by the Student's unpaired t-test, the s.e.mean is shown by vertical lines.
Figure 5.
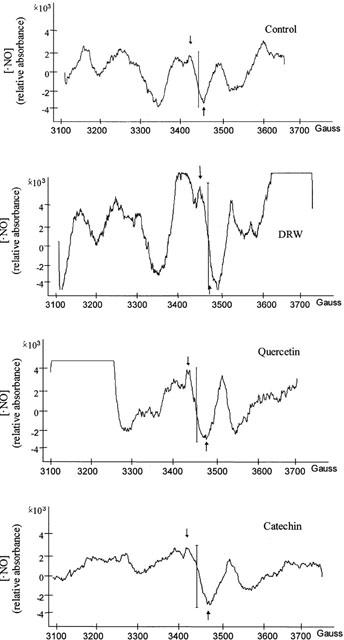
Representative ESR spectra of rat aorta from the control and dealcoholated red wine-, quercetin- or catechin-rich diets. The signal from the NO-Fe-(DEDTC)2 complex is indicated by two arrows.
Aortic rings with functional endothelium from rats fed either the DRW-, quercetin- or catechin-rich diet showed an approximate 2 fold increase in cyclic GMP content compared with controls (Figure 6).
Figure 6.
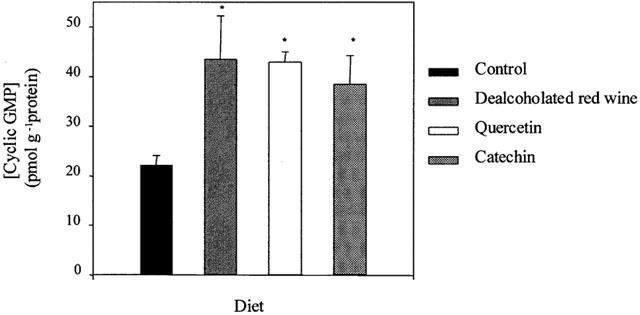
Cyclic GMP content in aortic homogenates from rats fed a control, dealcoholated red wine-, quercetin- or catechin-rich diets. Values are the mean±s.e.mean of five rats. *P<0.001 significantly different compared with control, by the Student's unpaired t-test, the s.e.mean is shown by vertical lines.
Effect of flavonoid-rich diets on O2.− production
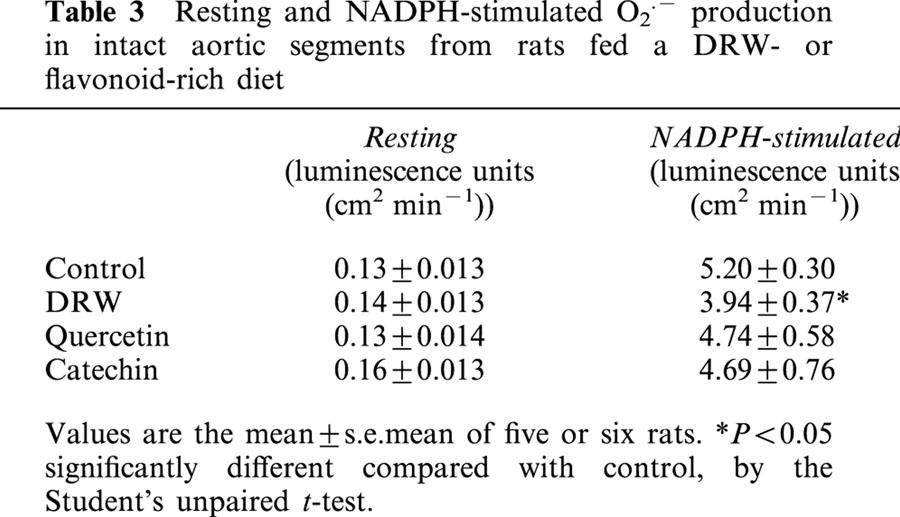
O2.− production by aortic segments in a resting state was similar in the four groups (Table 3). NADPH-stimulated O2.− production was reduced by 30% (P<0.05) in segments from rats fed DRW, and rats that received the quercetin- or catechin-rich diets showed a O2.− production similar to controls (Table 3).
Table 3.
Resting and NADPH-stimulated O2·.− production in intact aortic segments from rats fed a DRW- or flavonoid-rich diet
Discussion
This study provides the first direct evidence that DRW-, quercetin-, and catechin-rich diets induce an endothelium-dependent increase in •NO without modifying the O2.− release in a resting condition in rat aorta. The •NO released contributes to the relaxation of the vascular smooth muscle. Our results indicate that several compounds found in red wine, other than alcohol, have a vasorelaxant effect in vivo.
Many studies have focused on the beneficial cardiovascular effect of polyphenolic compounds in humans. There is growing interest in identifying the mechanisms of action of dietary polyphenolic compounds of red wine: catechin (present in considerable concentration), quercetin, resveratrol, oligomeric tannins and anthocyanins, present in low concentrations. The antioxidant activity of flavonoids protects against atherosclerosis, on the one hand, by reducing the susceptibility of LDL to oxidation (Esterbauer et al., 1992), and on the other, because of their vasodilator properties observed in vitro (Duarte et al., 1993; Herrera et al., 1996; Andriambeloson et al., 1997; 1998; Flesch et al., 1998; Chen & Pace-Asciak, 1996).
The relaxation of blood vessels is subjected to a complex control mechanism. Increased relaxation of vascular smooth muscle can result from increased eNOS expression and/or NOS activity or from activation of guanylyl cyclase, all of which lead to the accumulation of cyclic GMP (Moncada et al., 1991). In this sense, there is interest in developing compounds which stimulate the •NO-cyclic GMP pathway in order to treat cardiovascular disease (Lemos et al., 1999). Our results show that ingestion of a diet rich in DRW, quercetin or catechin increases NOS activity, •NO production and cyclic GMP content in rat aorta in a resting state and that these changes are endothelium-dependent. The absence of an increase in eNOS expression indicates that the mechanism of action of flavonoids is not transcriptional.
The bioavailability of compounds depends on their intestinal absorption and metabolism. However, few studies on the bioavailability of flavonoids in red wine have been carried out. Donovan et al. (1999) detected only catechin metabolites in human plasma after red wine intake and quercetin and catechin aglycones were not recovered in rat plasma after ingestion because they were metabolized by the intestine and liver (Manach et al., 1996). Thus, it is difficult to extrapolate the endothelium-•NO-derived vasorelaxation observed in vitro to an in vivo situation because flavonoids are metabolized and interact with other dietary components and thus their clinical relevance was limited. Metabolites are more hydrophilic than their initial polyphenolic forms, and therefore are more likely to circulate in plasma. Fitzpatrick et al. (1993) assumed that quercetin does not contribute to the endothelium-dependent relaxation in vivo since its solubility in water is limited and relaxation was not reversed by NOS inhibitors. However, some indirect evidences indicate a possible role in vivo. Rutinosids accumulate between the endothelial layer and the vascular smooth muscle cells (Neumann et al., 1992) and DRW intake increases the flow-mediated dilatation of the brachial artery in humans (Agewall et al., 2000). Moreover, Shutenko et al. (1999), by perfusing rats with quercetin, showed an increase in •NO in brain during global ischaemia and reperfusion. Our in vivo results demonstrate that some of the polyphenolic compounds of red wine, or their metabolites reach blood vessels and induce •NO-mediated relaxation. The administration of catechin at the same concentration as quercetin had a less pronounced effect on the •NO-cyclic GMP pathway. These observations can be explained in two ways. Firstly, quercetin or quercetin metabolites undergo enterohepatic circulation, while catechin is quickly eliminated in urine (Manach et al., 1999) or secondly, because catechin metabolites are less strongly bound to albumin than quercetin, their elimination is accelerated (Manach et al., 1999). It is likely that after ingestion, the presence of flavonoids or their metabolites in blood vessels may be smaller than the concentration used in studies in vitro.
Oxidative stress is involved in the endothelial dysfunction associated with cardiovascular pathologies (Durante et al., 1988; Cai & Harrison, 2000). This dysfunction can be caused by a decrease in the release of •NO and/or •NO breakdown by the reaction of •NO with O2.−, yielding peroxynitrite and peroxynitrous acid or with other biochemical sinks that accelerate •NO removal. Sensitive techniques for the analysis of O2.− have been described (Tarpey & Fridovich, 2001) and chemiluminescence allows access to intracellular sites of O2.− generation. Lucigenin has been widely used as a chemiluminescent substrate but has also been questioned (Tarpey et al., 1999) because O2.− is generated by lucigenin itself. However, we have used 5 μM lucigenin rather than 250 μM to measure O2.− in vascular tissues and lucigenin at 5 μM elicits marked chemiluminescence signal without stimulating additional O2.− production (Skatchkov et al., 1999). Our results showed a significant decrease in O2.− release at vascular level after ingesting DRW. This fact, together with the absence of effect of quercetin- and catechin-rich diets reinforce the significance of increased bioavailability of •NO and the vasorelaxant effect of DRW or the flavonoids studied. Furthermore, red wine contains a wide range of polyphenols. Additive effects in vitro between quercetin and resveratrol had been described and moderate consumption of red wine may play a significant role in preventing coronary heart disease (Chen & Pace-Asciak, 1996). However, resveratrol, a natural phytoalexin, is usually present in low concentrations in young wine because of short fermentation with grape skins, which leaves little time for extraction and thus, resveratrol was not detected in our wine by the method used. The quercetin- and catechin-rich diets administered to the rats contained 1000 times more of these constituents than the DRW-rich diet. It was likely that the mixture of flavonoids and other DRW phenolic compounds may strengthen their specific activity and may explain the marked effect of DRW with respect to the ingestion of isolated flavonoids. Furthermore, we chose a young wine, rich in total phenols. According to Flesch et al. (1998), endothelium-dependent vasodilator effects appear to be specific for wines with a high phenolic content. In our study it was difficult to compare the effects of quercetin and catechin with those of DRW because of the range of concentrations used. However, the decrease in O2.− production by aortic rings from rats fed a DRW-rich diet should be noted. Further work is needed to clarify the underlaying mechanism of action of flavonoids in the •NO-cyclic GMP pathway.
The increased bioavailability of •NO by dietary flavonoids at the blood vessel level in a resting state is important for two reasons with respect to hypertension and atherosclerosis. On the one hand, •NO is a vasodilator and on the other, it is a potent antioxidant of LDL in vitro (Hogg et al., 1993; Hogg & Kalyanaraman, 1998; Rubbo et al., 1995), by inhibiting radical chain propagation reactions and thus acting as an anti-atherogenic agent (Hogg et al., 1993; Matthys & Bult, 1997). Some components of red wine such as flavonoids contribute to the prevention and treatment of cardiovascular diseases by increasing •NO levels in the vascular system without increasing O2.− production as we demonstrate in the present study and thus, without increasing the formation of peroxynitrite-derived pro-oxidants.
In conclusion, DRW intake induced an •NO-mediated relaxation of rat aorta. Flavonoids, such as quercetin and catechin, and also the broad range of polyphenols found in red wine are involved in this in vivo vascular response because they increase NOS activity and the subsequent •NO production by blood vessels, and cyclic GMP content.
Acknowledgments
This work was supported by CICYT PM 98-0182. We thank Tania Yates for help in the preparation of the English manuscript and N. Clos from the Serveis Científico-Tècnics of the Universitat de Barcelona for technical assistance.
Abbreviations
- Cyclic GMP
cyclic guanosine-3′,5′-monophosphate
- DEDTC
diethyldithiocarbamic acid
- DRW
dealcoholated red wine
- eNOS
endothelial nitric oxide synthase
- ESR
electron spin resonance
- LDL
low density lipoproteins
- L-NAME
NG-nitro-L-arginine methyl ester
- L-NMMA
NG-monomethyl-L-arginine
- L-NNA
NG-nitro-L-arginine
- NADPH
nicotinamine adenine dinucleotide phosphate reduced form
- •NO
nitric oxide
- NOS
nitric oxide synthase
- O2.−
superoxide anion
- PAGE
polyacrilamide gel electrophoresis
- SDS
sodium dodecyl sulfate
- SOD
superoxide dismutase
References
- AGEWALL S., WRIGHT S., DOUGHTY R.N., WHALLEY G.A., DUXBURY M., SHARPE N. Does a glass of wine improve endothelial function. Eur. Heart J. 2000;21:74–78. doi: 10.1053/euhj.1999.1759. [DOI] [PubMed] [Google Scholar]
- ANDRIAMBELOSON E., KLESCHYOV A.L., MULLER B., BERETZ A., STOCLET J.C., ANDRIANTSITOHAINA R. Nitric oxide production and endothelium-dependent vasorelaxation induced by wine polyphenols in rat aorta. Br. J. Pharmacol. 1997;120:1053–1058. doi: 10.1038/sj.bjp.0701011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANDRIAMBELOSON E., MAGNIER C., HAAN-ARCHIPOFF G., LOBSTEIN A., ANTON R., BERETZ A., STOCLET J.C., ANDRIANTSITOHAINA R. Natural dietary polyphenolic compounds cause endothelium-dependent vasorelaxation in rat thoracic aorta. J. Nutr. 1998;128:2324–2333. doi: 10.1093/jn/128.12.2324. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BROWN J.E., KHODR H., HIDER R.C., RICE-EVANS C. Structural dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties. Biochem. J. 1998;330:1173–1178. doi: 10.1042/bj3301173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CACCETTA R.A., CROFT K.D., BEILIN L.J., PUDDEY I.B. Ingestion of red wine significantly increases plasma phenolic acid concentrations but does not acutely affect ex vivo lipoprotein oxidizability. Am. J. Clin. Nutr. 2000;71:67–74. doi: 10.1093/ajcn/71.1.67. [DOI] [PubMed] [Google Scholar]
- CAI H., HARRISON D.G. Endothelial dysfunction in cardiovascular diseases. The role of oxidant stress. Circ. Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- CASTELLARI M., ARFELLI G., RIPONI C., AMATI A. Evolution of phenolic compounds in red winemaking as affected by must oxygenation. Am. J. Enol. Viticult. 1998;49:91–94. [Google Scholar]
- CHEN C.K., PACE-ASCIAK C.R. Vasorelaxing activity of resveratrol and quercetin in isolated rat aorta. Gen. Pharmacol. 1996;27:363–366. doi: 10.1016/0306-3623(95)02001-2. [DOI] [PubMed] [Google Scholar]
- DE RIJKE Y.B., DEMACKER P.N., ASSEN N.A., SLOOTS L.M., KATAN M.B., STALENHOEF A.F. Red wine consumption does not affect oxidizability of low-density lipoproteins in volunteers. Am. J. Clin. Nutr. 1996;63:329–334. doi: 10.1093/ajcn/63.3.329. [DOI] [PubMed] [Google Scholar]
- DONOVAN J.L., BELL J.R., KASIM-KARAKAS S., GERMAN J.B., WALZEM R.L., HANSEN R.J., WATERHOUSE A.L. Catechin is present as metabolites in human plasma after consumption of red wine. J. Nutr. 1999;129:1662–1668. doi: 10.1093/jn/129.9.1662. [DOI] [PubMed] [Google Scholar]
- DUARTE J., VIZCAÍNO F.P., UTRILLA P., JIMENEZ J., TAMARGO J., ZARZUELO A. Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen. Pharmacol. 1993;24:857–862. doi: 10.1016/0306-3623(93)90159-u. [DOI] [PubMed] [Google Scholar]
- DURANTE W., SEN A.K., SUNAHARA F.A. Impairment of endothelium-dependent relaxation in aortae from spontaneously diabetic rats. Br. J. Pharmacol. 1988;94:463–468. doi: 10.1111/j.1476-5381.1988.tb11548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTERBAUER H., GEBICKI J., PUHL H., JÜRGENS G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Rad. Biol. Med. 1992;13:341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- FITZPATRICK D.F., HIRSCHFIELD S.L., COFFEY R.G. Endothelium-dependent vasorelaxing activity of wine and other grape products. Am. J. Physiol. 1993;265:H774–H778. doi: 10.1152/ajpheart.1993.265.2.H774. [DOI] [PubMed] [Google Scholar]
- FLESCH M., SCHWARZ A., BÖHM M. Effects of red and white wine on endothelium-dependent vasorelaxation of rat aorta and human coronary arteries. Am. J. Physiol. 1998;275:H1183–H1190. doi: 10.1152/ajpheart.1998.275.4.H1183. [DOI] [PubMed] [Google Scholar]
- FUHRMAN B., LAVY A., AVIRAM M. Consumption of red wine with meals reduces the susceptibility of human plasma and low-density lipoprotein to lipid peroxidation. Am. J. Clin. Nutr. 1995;61:549–554. doi: 10.1093/ajcn/61.3.549. [DOI] [PubMed] [Google Scholar]
- HERRERA M.D., ZARZUELO A., JIMENEZ J., MARHUENDA E., DUARTE J. Effects of flavonoids on rat aortic smooth muscle contractility: structure-activity relationships. Gen. Pharmacol. 1996;27:273–277. doi: 10.1016/0306-3623(95)02010-1. [DOI] [PubMed] [Google Scholar]
- HERTOG M.G.L., KROMHOUT D., ARAVANIS C., BLACKBURN H., BUZINA R., FIDANZA F., GIAMPAOLI S., JANSEN A., MENOTTI A., NEDELJKOVIC S. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study Arch. Intern. Med. 1995155381–386.et al [PubMed] [Google Scholar]
- HOGG N., KALYANARAMAN B. Nitric oxide and low-density lipoprotein oxidation. Free Rad. Res. 1998;28:593–600. doi: 10.3109/10715769809065815. [DOI] [PubMed] [Google Scholar]
- HOGG N., KALYANARAMAN B., JOSEPH J., STRUCK A., PARTHASARATHY S. Inhibition of low-density lipoprotein oxidation by nitric oxide. Potential role in atherogenesis. FEBS Lett. 1993;334:170–174. doi: 10.1016/0014-5793(93)81706-6. [DOI] [PubMed] [Google Scholar]
- HUANG Y., CHAN N., LAU C., YAO X., CHAN F., CHEN Z. Involvement of endothelium/nitric oxide in vasorelaxation induced by purified green tea (-) epicatechin. Biochim. Biophys. Acta. 1999;1427:322–328. doi: 10.1016/s0304-4165(99)00034-3. [DOI] [PubMed] [Google Scholar]
- KNEKT P., JARVINEN R., REUNANEN A., MAATELA J. Flavonoid intake and coronary mortality in Finland: a cohort study. Br. J. Med. 1996;312:478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMOS V.S., FREITAS M.R., MULLER B., LINO Y.D., QUEIROGA C.E., CORTES S.F. Dioclein, a new nitric-oxide- and endothelium-dependent vasodilator flavonoid. Eur. J. Pharmacol. 1999;386:41–46. doi: 10.1016/s0014-2999(99)00747-5. [DOI] [PubMed] [Google Scholar]
- LÓPEZ D., SÁNCHEZ J., CABALLERO C., PUIG-PARELLADA P., MITJAVILA M.T. Free radicals production in aortic rings from rats fed a fish oil-rich diet. Am. J. Physiol. 2001;280:H2929–H2935. doi: 10.1152/ajpheart.2001.280.6.H2929. [DOI] [PubMed] [Google Scholar]
- MANACH C., TEXIER O., MORAND C., CRESPY V., RÉGÉRAT F., DEMIGNÉ C., RÉMÉSY C. Comparison of the bioavailability of quercetin and catechin in rats. Free Rad. Biol. Med. 1999;27:1259–1266. doi: 10.1016/s0891-5849(99)00159-8. [DOI] [PubMed] [Google Scholar]
- MANACH C., TEXIER O., RÉGÉRAT F., AGULLO G., DEMIGNÉ C., RÉMÉSY C. Dietary quercetin is recovered in rat plasma as conjugated derivatives of isorhamnetin and quercetin. J. Nutr. Biochem. 1996;7:375–380. [Google Scholar]
- MATTHYS K.E., BULT H. Nitric oxide function in atherosclerosis. Mediators Inflamm. 1997;6:3–21. doi: 10.1080/09629359791875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYER B., ANDREWS P. Nitric oxide synthases: catalytic function and progress towards selective inhibition. Arch. Pharmacol. 1998;358:127–133. doi: 10.1007/pl00005233. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M., HIGGS E.A. Nitric oxide: physiology, pathophysiology and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- NEUMANN H., CARLSSON K., BROM G. Uptake and localization of O-(betahydroxyethyl)-rutinosides in the venous wall, measured by laser scanning microscopy. Eur. J. Clin. Pharmacol. 1992;43:423–426. doi: 10.1007/BF02220620. [DOI] [PubMed] [Google Scholar]
- RENAUD S., DE LORGERIL M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- RICE-EVANS C., MILLER N.J., PAGANGA G. Antioxidant properties of phenolic compounds. Trends in Plant Science. 1997;2:152–159. [Google Scholar]
- RUBBO H., PARTHASARATHY S., BARNES S., KIRK M., KALAYANARAMAN B., FREEMAN B.A. Nitric oxide inhibition of lipoxygenase-dependent liposome and low-density lipoprotein oxidation: termination of radical chain propagation reactions and formation of nitrogen-containing oxidized lipid derivatives. Arch. Biochem. Biophys. 1995;324:15–25. doi: 10.1006/abbi.1995.9935. [DOI] [PubMed] [Google Scholar]
- RUBBO H., RADI R., ANSELMI D., KIRK M., BARNES S., BUTLER J., EISERICH J.P., FREEMAN B.A. Nitric oxide reaction with lipid peroxyl radicals spares α-tocopherol during lipid peroxidation. J. Biol. Chem. 2000;275:10812–10818. doi: 10.1074/jbc.275.15.10812. [DOI] [PubMed] [Google Scholar]
- SHUTENKO Z., HENRY Y., PINARD E., SEYLAZ J., POTIER P., BERTHET F., GIRARD P., SERCOMBE R. Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem. Pharmacol. 1999;57:199–208. doi: 10.1016/s0006-2952(98)00296-2. [DOI] [PubMed] [Google Scholar]
- SINGLETON S.L., ROSSI J.A. Colorimetric of total phenols with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965;16:144–158. [Google Scholar]
- SKATCHKOV M.P., SPERLING D., HINK U., MULSCH A., HARRISON D.G., SINDERMANN I., MEINERTZ T., MUNZEL T. Validation of lucigenin as a chemiluminescent probe to monitor vascular superoxide as well as basal vascular nitric oxide production. Biochem. Biophys. Res. Commun. 1999;254:319–324. doi: 10.1006/bbrc.1998.9942. [DOI] [PubMed] [Google Scholar]
- STEINBERG D., PARTHASARATHY S., CAREW T.E., KHOO J.C., WITZTUM J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- TARPEY M.M., FRIDOVICH I. Methods of detection of vascular reactive species nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ. Res. 2001;89:224–236. doi: 10.1161/hh1501.094365. [DOI] [PubMed] [Google Scholar]
- TARPEY M.M., WHITE C.R., SUAREZ E., RICHARDSON G., RADI R., FREEMAN B.A. Chemiluminescent detection of oxidants in vascular tissue. Lucigenin but not coelenterazine enhances superoxide formation. Circ. Res. 1999;84:1203–1211. doi: 10.1161/01.res.84.10.1203. [DOI] [PubMed] [Google Scholar]
- VAN HET HOF K.H., WISEMAN S.A., YANG C.S., TIJBURG L.B. Plasma and lipoprotein levels of tea catechins following repeated tea consumption. Proc. Soc. Exp. Biol. Med. 1999;220:203–209. doi: 10.1046/j.1525-1373.1999.d01-34.x. [DOI] [PubMed] [Google Scholar]


