Abstract
The present study examines the role of migrating leukocytes in the ability of IL-1β to induce the functional up-regulation of B1 receptors, as assessed by kinin B1 agonist-induced oedema in the rat paw.
Pre-treatment with the PAF receptor antagonist WEB 2086 inhibited des-Arg9-BK-induced oedema in IL-1β-treated paws, while the LTB4 receptor antagonist CP105696 had no effect. Des-Arg9-BK-induced paw oedema was also inhibited by pre-treatment with the selectin blocker fucoidin or by an anti-CD-18 monoclonal antibody.
I.d. injection of IL-1β produced a 5 – 10-fold increase of myeloperoxidase (MPO) activity in the rat paw. The increase in MPO activity was significantly inhibited by WEB 2086 (46±9%), fucoidin (68±5%) or the CD-18 antibody (84±3%). In contrast, i.d. injection of TNFα a dose known to upregulate the B1 receptor functionally did not induce any significant increase in MPO activity.
Des-Arg9-BK alone had no effect in MPO activity but enhanced (by about 40%) the response induced by IL-1β, an effect prevented by the B1 receptor antagonist des-Arg9-[Leu8]-BK.
The concentration of TNF-α was increased in the paws after i.d. injection of IL-1β. Pre-treatment with fucoidin, WEB 2086, anti-CD-18 or CP 105695, significantly reversed the local increases in TNF-α concentrations (80±2; 75±4, 73±3 and 40±2%), respectively.
Finally, IL-1β induced an increase of B1 receptor mRNA levels in the rat paw, an effect which was prevented by fucoidin treatment.
Taken together, these results indicate that up-regulation of B1 receptors in the rat paw following IL-1β seems to involve the local recruitment of neutrophils and subsequent local TNF-α production. The cross-talk between kinins, cytokines and leukocytes implicate B1 receptors in chronic inflammatory diseases.
Keywords: Kinins, B1 receptor expression, rat paw oedema, inflammation, neutrophils, cytokines, adhesion molecules
Introduction
Interleukin-1 (IL-1) α and β and related pro-inflammatory cytokines have been depicted as pleiotropic regulators of inflammatory and immune responses, affecting many distinct peripheral and central nervous system functions. The most relevant biological effects of IL-1 include the stimulation of lymphocytes, the activation of leucocytes at the site of inflammation, induction of fever, besides the increase of the production of prostaglandins, nitric oxide and chemokines (Dinarello, 2000a, 2000b). IL-1 downstream pathways are linked to the activation of a wide range of intracellular signalling cascades, such as MAP-kinases and stimulation of transcriptional factors (Saklatvala et al., 1999; Bowie & O'neill, 2000). Therefore, IL-1 has a complex biological role, and its actions are often related to the synthesis of new inflammatory proteins and seems to be dependent on the expression of several inflammatory components (Barnes & Adcock, 1997; Barnes & Karin, 1997).
The kinin system represents one of the inflammatory pathways known to be regulated by cytokines, especially IL-1β. The actions of kinins are mediated by the activation of two seven transmembrane G protein-coupled receptors, namely B1 and B2 receptors (Marceau & Bachvarov, 1998; Calixto et al., 2000). Whereas B2 receptors are constitutive and responsible for the most relevant physiological actions of kinins, B1 receptors are described as atypical, since they are not normally expressed in physiological conditions, but their expression is increased after tissue injury, during chronic inflammatory processes and by the action of certain pro-inflammatory cytokines (for review see: Marceau et al., 1998; Calixto et al., 2000). In fact, recent studies have reported that IL-1β, either in vitro or in vivo, acts as one of the most important modulators of B1 receptor up-regulation (Marceau et al., 1998; Calixto et al., 2000). It has also been demonstrated that up-regulation of B1 receptors by IL-1β requires the stimulation of certain protein kinases, such as protein kinase C, tyrosine kinase and MAP kinases, as well as the activation of the nuclear transcription factor NF-κB (Larrivée et al., 1998; Marceau et al., 1998; Schanstra et al., 1998; Campos et al., 1999). However, recent evidence has suggested that, in spite of the involvement of several signalling mechanisms, the induction of B1 receptors by IL-1β constitutes a very rapid process (Campos et al., 1999; Phagoo et al., 2000).
One of the most important characteristics of IL-1β actions is its well-known ability to induce neutrophil migration and adhesion to the endothelium (Thorlacius et al., 1997; Derevianko et al., 1998; Grutkoski et al., 1999). Despite this evidence, to the best of our knowledge, so far there has been no studies indicating a direct relationship between IL-1β-induced local cell migration and the process of B1 kinin receptors up-regulation in rats. Thus, in the present study we have assessed the role exerted by leukocyte migration in the process of B1 kinin receptors up-regulation in the rat paw, in animals previously treated with IL-1β.
Methods
Measurement of rat paw oedema
Non-fasted male Wistar rats (140 – 180 g) kept in controlled room temperature (22±2°C) under a 12 : 12 h light-dark cycle (lights on 06:00 h) were used. The experiments were conducted according to the procedures described previously (Campos & Calixto, 1995). The animals received a 0.1 ml intradermal (i.d). injection in one hindpaw (right paw) of phosphate buffered saline (PBS, composition mmol L−1): NaCl 137, KCL 2.7 and phosphate buffer 10, containing des-Arg9-BK (100 nmol paw−1). The contralateral paw (left paw) received 0.1 ml of PBS and was used as control. Oedema was measured by use of a plethysmometer (Ugo Basile) at several time-points (10, 20, 30, 60 and 120 min) after injection of des-Arg9-BK. Oedema is expressed in ml as the difference between the right and left paws. In most cases, animals were treated with the cytokine IL-1β (5 ng paw−1; 60 min prior) at the same site as des-Arg9-BK injection, as described beforehand (Campos et al., 1998). In all experiments, the i.d. injections were performed under slight anaesthesia with 2,2,2 tribromoethanol (0.125 g kg−1). The reported experiments were carried out in accordance with current guidelines for the care of laboratory animals and ethical guidelines for investigations of experiments in conscious animals (Zimmerman, 1983).
Involvement of cell migration on des-Arg9-BK-induced oedema in IL-1β pre-treated animals
In separate groups of animals, in order to determine the possible involvement of neutrophil-active chemoattractants in B1 kinin receptors up-regulation, rats were administered the PAF receptor antagonist WEB 2086 (15 μg paw−1) or the LTB4 receptor antagonist CP 105696 (30 μg paw−1) co-injected with the cytokine IL-1β (5 ng paw−1), 60 min before des-Arg9-BK (100 nmol paw−1) injection. In other sets of experiments, in order to examine the participation of adhesion molecules in IL-1β-mediated B1 kinin receptors up-regulation, animals were treated with the non-specific selectin inhibitor fucoidin (10 mg kg−1, i.v.) or with the monoclonal anti-CD18 (integrin β2 chain) antibody (WT3, 1 mg kg−1, i.v.), both administered 15 min before IL-1β treatment. The oedema was induced by i.d. injection of des-Arg9-BK (100 nmol paw−1) and assessed as described before. The doses of all inhibitors have been chosen on the basis of previous studies or through pilot experiments (Dimartino et al., 1989; Williams et al., 1994; Búrigo et al., 1996; Teixeira & Hellewell, 1997).
Measurement of MPO activity
The accumulation of neutrophils in the rat paw was measured by means of tissue MPO activity. Animals received a 0.1 ml i.d. injection of IL-1β (1 and 5 ng paw−1), TNFα (5 ng paw−1) or des-Arg9-BK (100 nmol paw−1) in the right paw and were sacrificed after 60, 30 and 20 min, respectively. In some experiments, animals pretreated with IL-1β (1 and 5 ng paw−1, 60 min) received an i.d. injection of des-Arg9-BK (100 nmol paw−1) in the presence or absence of the selective B1 receptor antagonist des-Arg9-[Leu8]-BK (100 nmol paw−1) and were sacrificed after a further 20 min. PBS-treated paws were used as controls. In other sets of experiments, the MPO activity was assessed in animals that received a co-injection of WEB 2086 (15 μg paw−1) or CP 105696 (30 μg paw−1) or in rats pretreated with fucoidin (10 mg kg−1, i.v.) or with the anti-CD-18 antibody (1 mg kg−1, i.v.), the latter two treatments administered 15 min prior to IL-1β injection.
At the time of sacrifice, the subcutaneous tissue of the paws was removed and assayed for MPO according to a method previously described (Souza et al., 2000b). Briefly, tissues were homogenized at 5% (w v−1) in EDTA/NaCl buffer (pH 4.7) and centrifuged at 3000×g for 15 min, at 4°C. The pellet was re-suspended in hexadecyltrimethyl ammonium bromide 0.5% buffer (pH 5.4) and the samples were frozen and thawed for three times in liquid nitrogen. Upon thawing, the samples were re-centrifuged (3000×g, 15 min, at 4°C) and 25 μl of the supernatant were used for the MPO assay. The enzymatic reaction was assessed with 1.6 mM tetramethylbenzidine, 80 mM NaPO4 and 0.3 mM hydrogen peroxide. The absorbance was measured at 690 nm and the results are expressed in optical density (OD) per mg of dry tissue.
Expression of B1 receptor mRNA in the rat paw
The methodology used was similar to that described by Bélichard et al. (2000) and Schaeffer et al. (2001) with some modifications. Rats received IL-1β (5 ng paw−1) and were sacrificed after various intervals of time (15 – 360 min). In another group of experiments, animals were systemically treated with the non-selective inhibitor of selectins fucoidin (10 mg kg−1, i.v.), 15 min before IL-1β injection (5 ng paw−1, 60 min). Immediately after the sacrifice, the subcutaneous tissue of the paws was removed and frozen in liquid nitrogen. The samples were then homogenized and the total RNA was extracted using the Trizol Reagent (Gibco BRL®). One μg of total RNA were reverse transcribed (RT) using oligo dT as primer (25 μg ml−1) and 200 u of reverse transcriptase (Gibco BRL®), in 20 μl of PCR buffer containing (mM): dNTP 0.5, DTT 10, MgCl2 2.5, KCl 50 and Tris-HCl pH 8.4 20. The samples were incubated for 50 min at 42°C, heated for 15 min at −70° and cooled in ice. After treatment with 2 U of Rnase H (20 min, 37°C), cDNA amplification of a specific sequence of rat B1 receptor and β-actin was performed by PCR using the following primers: sense TGAAGCTGTGAGCTCTTTG and antisense GCCAGTTGAAACGGTTCCC for B1 receptor and sense GTTCCGATGCCCCGAGGATCT and antisense GCATTTGCGGTGCACGATGGA for rat β-actin. β-actin cDNA was used for standardization of the amount of RNA. 5 μl of RT aliquots were mixed in a 20 mM Tris-HCl buffer (pH 8.4) containing: 1.5 mM MgCl2, 300 μM dNTP, 2 μg ml−1 of each primer and 50 u ml−1 of Taq polimerase (Gibco BRL®), in a final volume of 100 μl. The cycling protocol used was the following: 4 min at 94°C, 36 cycles of 35 s at 94°C/45 s at 60°C/45 s at 72°C and finally, 7 min at 72°C. Aliquots of 25 μl were analysed on a 20% TBE (Tris/Borate/EDTA) polyacrylamide gel and stained by ethidium bromide. The size of products are 423 bp for B1 receptor and 361 bp for β-actin. The number of replicates was four.
Measurement of TNFa levels in the rat paw
TNF-α production in the rat paw was assessed using a sandwich ELISA, as previously described (Souza et al., 2000a). Briefly, animals received an i.d. injection of IL-1β (5 ng per paw) and after 60 min, rats were sacrificed and the subcutaneous tissue of the paws removed and placed in a PBS solution containing: Tween 20 0.05%, PMSF 0.1 mM, EDTA 10 mM, Aprotinin A 20 Kl and BSA 0.5%. Tissues were homogenized, centrifuged at 3000×g, for 10 min and the supernatant obtained was stored at −70°C until further analysis. In order to evaluate a role for neutrophils in control of local TNF-α production, neutrophil migration into tissue was prevented by pre-treatment with WEB2086, fucoidin or the anti-CD18 monoclonal antibodies, used in the same doses as described above.
Drugs and reagents
The following drugs were used: aprotinin, BSA, des-Arg9-BK, fucoidin, 2,2,2 tribromoethanol, PBS tablets, EDTA, hexadecyltrimethyl ammonium, tetramethylbenzidine and PMSF (all from Sigma Chemical Company, St. Louis, MO, U.S.A.). WEB 2086 was a gift of Boehringer-Manhein, Germany, CP 105696 was from Pfizer Global Research and Development, Groton labs, CT, U.S.A. and the anti-CD 18 mAb from Dr Paul Hellewell, University of Sheffield, U.K. NaPO4, NaCl, hydrogen peroxide and Tween 20 were all from Merck, Germany. Recombinant murine cytokine IL-1β (Lot BN091) was obtained from R & D Systems INC., Minneapolis, U.S.A. The stock solutions of des-Arg9-BK and des-Arg9-[Leu8]-BK were prepared in PBS in siliconized plastic tubes maintained at 18°C and diluted to the desired concentration just before use. The other drugs were prepared daily in 0.9% w v−1 NaCl solution.
Statistical analysis
The results are presented as the mean±s.e.mean of 4 – 6 animals. The percentages of inhibition are reported as mean±s.e.mean of inhibitions obtained in each individual experiment at the peak of oedema (20 min after injection of des-Arg9-BK) or at 60 min after IL-1β injection. Statistical comparison of the data was performed by analysis of variance (ANOVA) followed by Dunnett's test or Newman-Keuls test or by use of Student's unpaired t-test. P values less than 0.05 were considered significant.
Results
We have reported previously that the i.d. injection of IL-1β was able to induce the up-regulation of kinin B1 receptor-mediated oedematogenic responses (Campos et al., 1998). Accordingly, the i.d. injection of the selective kinin B1 receptor agonist des-Arg9-BK (100 nmol paw−1) in naive paws induced a very slight increase in rat paw volume (0.08±0.003 ml, n=4), whereas i.d. injection of des-Arg9-BK in animals pre-treated with IL-1β (5 ng paw−1, 60 min before) produced a marked increase in oedema (0.54±0.03 ml, n=4). Histological assessment of sites injected with IL-1β demonstrated that significant accumulation of leukocytes, specially neutrophils, could be seen and correlated with the kinetics of the functional up-regulation of the kinin B1 receptor (Campos et al., 1998 and data not shown).
In order to investigate the role of migrating leukocytes in the functional up-regulation of kinin B1 receptors, animals were pretreated with drugs known to regulate leukocyte recruitment in vivo. As seen in Figure 1A, the oedema induced by des-Arg9-BK in animals pre-treated with IL-1β was significantly inhibited by 37±5% by the co-injection of the PAF receptor antagonist WEB 2086 (15 μg paw−1). In contrast, the co-injection of the LTB4 receptor antagonist CP 105696 (30 μg paw−1), at a dose that significantly inhibited the rat paw oedema caused by arachidonic acid (76±12% inhibition), failed to affect the oedema formation caused by des-Arg9-BK in IL-1β-injected paws (Figure 1B). The treatment of animals with fucoidin (10 mg kg−1, i.v., 15 min) resulted in a significant decrease (43±4%) of des-Arg9-BK-induced paw oedema in rats pre-treated with IL-1β. Similarly, IL-1β-mediated B1 kinin receptor functional up-regulation was also significantly inhibited by the treatment of animals with an anti-CD18 mAb (WT3, 1 mg kg−1, i.v., 15 min) (44+4%) (Figure 2A, B).
Figure 1.
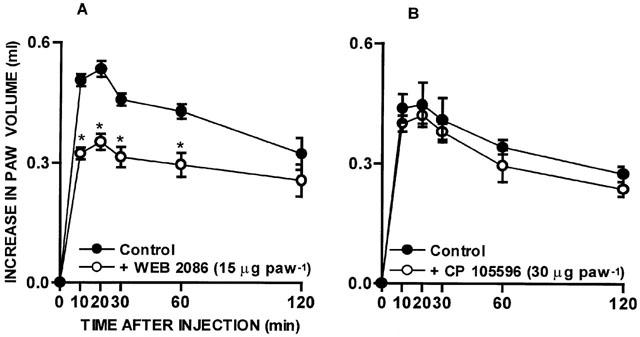
Effect of the treatment with the PAF receptor antagonist WEB 2086 (15 μg paw−1; (A) or with the LTB4 receptor antagonist CP 105696 (30 μg paw−1; (B) on des-Arg9-BK (100 nmol paw−1)-induced rat paw oedema in animals pretreated with IL-1β (5 ng paw−1, 60 min). Each point represents the means±s.e.mean of 4 – 6 experiments. In some cases the error bars are hidden within the symbols. Significantly different from control values *P<0.05 (Student's unpaired t-test).
Figure 2.
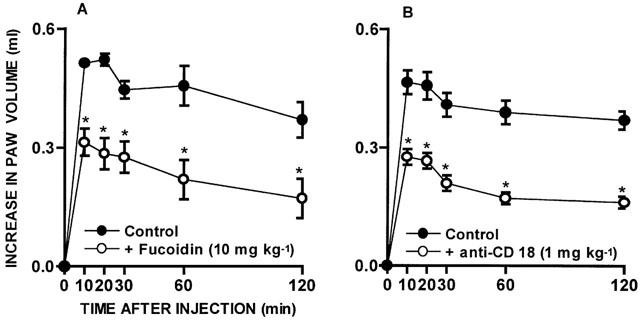
Effect of treatment with the non-selective selectin inhibitor fucoidin (10 mg kg−1, i.v., 15 min; (A) or with an anti-CD18 monoclonal antibody (1 mg kg−1, i.v., 15 min; (B) on des-Arg9-BK (100 nmol paw−1)-induced rat paw oedema in animals pre-treated with IL-1β (5 ng paw−1, 60 min). Each point represents the means±s.e.mean of 4 – 6 experiments. In some cases the error bars are hidden within the symbols. Significantly different from control values *P<0.05 (Student's unpaired t-test).
Intradermal injection of IL-1β (1 and 5 ng paw−1, 60 min) resulted in a marked neutrophil sequestration in the rat paw, as indicated by a 5 – 10-fold increase of MPO activity in IL-1β-treated paws. Intradermal injection of the B1 selective kinin receptor agonist des-Arg9-BK (100 nmol paw−1, 20 min) did not induce any significant neutrophil recruitment when injected alone, but significantly enhanced (38±4%) the neutrophil influx induced by a low dose of IL-1β (1 ng paw−1, 60 min) (Figure 3A). The increase of MPO activity induced by des-Arg9-BK in rats pretreated with IL-1β (1 ng paw−1) was prevented by the co-injection of the B1 receptor antagonist des-Arg9-[Leu8]-BK (100 nmol paw−1) (33±6%) (Figure 3B). On the other hand, des-Arg9-BK injection failed to produce any additional increase in MPO activity, when assessed in rats previously treated with a higher dose (5 ng paw−1, 60 min) of IL-1β (results not shown).
Figure 3.
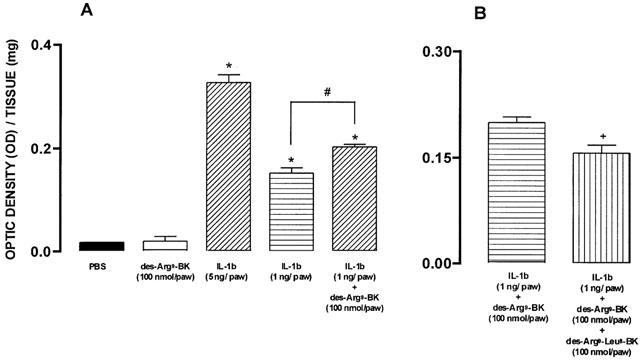
(A) Effect of IL-1β (1 and 5 ng paw−1, 60 min) or des-Arg9-BK (100 nmol paw−1, 20 min), given alone or after IL-1β (1 ng paw−1) i.d. injection, on MPO activity in the rat paw. (B) Effect of the co-injection of the B1 receptor selective antagonist des-Arg9-[Leu8]-BK (100 nmol paw−1) on IL-1β (1 ng paw−1, 60 min) plus des-Arg9-BK (100 nmol paw−1, 20 min)-induced increase in MPO activity in the rat paw. Each column represents the means±s.e.mean of four experiments. Significantly different from values obtained in PBS- (*), IL-1β- (#) or IL-β plus des-Arg9-BK (+) injected paws, P<0.05 (one-way ANOVA followed by Newman-Keuls test).
The i.d. injection of TNFα (5 nmol paw−1, 30 min) previously shown to induce the functional upregulation of B1 receptor (Campos et al., 1998) had no significant effects on the recruitment of neutrophils when compared with saline treated animals, as assessed by the MPO activity in the paws (0.0323±0.0026 optic density per tissue mg in TNFα treated animals versus 0.0368±0.0018 optic density per tissue mg in saline-treated rats, n=3).
The effects of strategies which interfere with leukocyte migration on the functional up-regulation of kinin B1 receptors induced by IL-1β correlated with their ability to prevent the influx of neutrophils, as assessed by the tissue levels of MPO. As such, IL-1β (5 ng paw−1, 60 min)-induced increase in MPO activity was significantly prevented by the co-injection of the PAF receptor antagonist WEB 2086 (15 μg paw−1) (46±9%), by the systemic treatment with fucoidin (10 mg kg−1, i.v.) (68±5%) or by the anti-CD18 antibody (1 mg kg−1, i.v.) (84±3%) (Figure 4).
Figure 4.
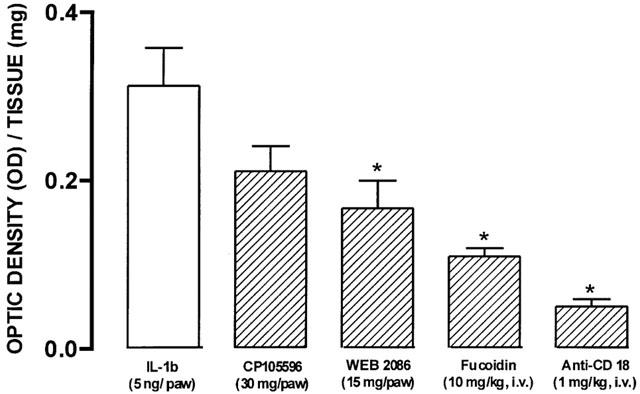
Effect of treatment with CP 105696 (30 μg paw−1), WEB 2086 (15 μg paw−1), fucoidin (10 mg kg−1, i.v., 15 min) or with the β2 integrin-CD18 antibody (1 mg kg−1, i.v., 15 min) on IL-1β (5 ng paw−1, 60 min)-induced increase in MPO activity in the rat paw. Each column represents the means±s.e.mean of four experiments. Significantly different from values obtained in IL-1β-injected paws (*) P<0.05 (one-way ANOVA followed by Dunnett's test).
Next, we investigated whether strategies which blocked neutrophil influx could also interfere with the IL-1β-induced production of TNF-α concentrations. The injection of IL-1β resulted in a marked increase of TNFα levels (Figure 5). Pretreatment with fucoidin, WEB 2086, the anti-CD18 antibody or CP-105695, significantly reversed the local increases in TNF-α concentrations (80±2; 75±4, 73±3 and 40±2%), respectively (Figure 5).
Figure 5.
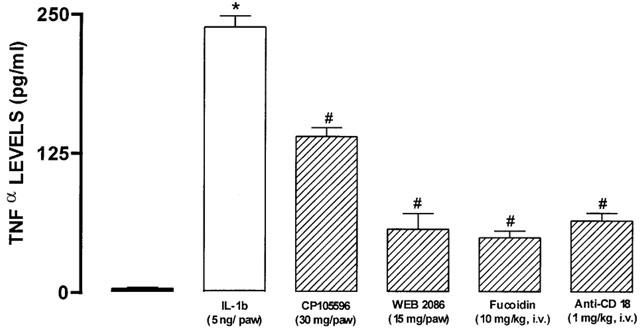
Effect of treatment with CP 105696 (30 μg/paw), WEB 2086 (15 μg paw−1), fucoidin (10 mg kg−1, i.v., 15 min) or with the β2 integrin-CD18 antibody (1 mg kg−1, i.v., 15 min) on IL-1β (5 ng paw−1, 60 min)-induced increase in TNFα levels in the rat paw. Each column represents the means±s.e.mean of 4 – 5 experiments. Significantly different from values obtained in PBS- (*) or IL-1β-injected paws (#) P<0.05. (one-way ANOVA followed by Newman-Keuls test).
Finally, the results show that the time-dependent functional increase of B1 receptors following IL-1β injection (5 ng paw−1, 15 – 360 min, number of replicates=4) (Campos et al., 1998) was accompanied by a marked increase in kinin B1 receptor mRNA expression in the rat paw, as assessed by RT – PCR (Figure 6A). Furthermore, IL-1β (5 ng paw−1, 60 min)-induced increase in B1 receptor mRNA was significantly inhibited by the systemic treatment with fucoidin (49±5%) (Figure 6B).
Figure 6.
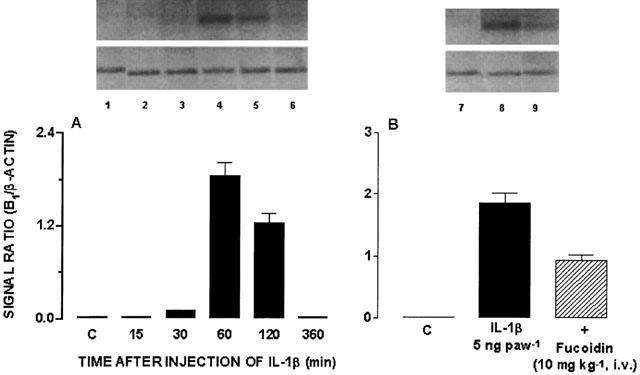
(A) Time-dependent effect of IL-1β (5 ng paw−1, 15 – 360 min) injection on kinin B1 receptor expression in the rat paw. (B) Effect of systemic treatment with fucoidin (10 mg kg−1, i.v., 15 min) on IL-1β (5 ng paw−1, 60 min)-induced increase in B1 receptor expression. Bottom, graphic representation of B1/β-actin signals ratio. 1. PBS; 2. IL-1β 15 min; 3. IL-1β 30 min; 4. IL-1β 60 min; 5. IL-1β 120 min; 6. IL-1β 360 min; 7. PBS; 8. IL-1β 60 min; 9. Fucoidin 10 mg kg−1, i.v. Number of replicates=4.
Discussion
The crosstalk between cytokines and kinin receptors has been extensively investigated in the last years. Several lines of evidence have demonstrated that pro-inflammatory cytokines are able to regulate B1 receptors expression through complex signalling mechanisms involving the activation of downstream kinases and transcription factors, especially NF-κB (Larrivée et al., 1998; Campos et al., 1999; Phagoo et al., 2000). In addition, the induction of B1 kinin receptors by other stimuli, including lipopolysaccharide of E. coli or tissue trauma, likely involves the secondary production of cytokines, such as IL-1β and TNF-α (Marceau, 1995; Marceau et al., 1998). In this way, pro-inflammatory cytokines seem to exert a pivotal role in the mechanisms mediating kinin responses, as they are capable of regulating B1 kinin receptor expression during certain inflammatory conditions. Despite the experimental evidence demonstrating the possible mechanisms responsible for B1 receptor up-regulation, the process is not entirely understood. The results of the present study demonstrated that IL-1β-induced up-regulation of B1 kinin receptor in the rat paw was largely dependent on the ability of the cytokine to induce local accumulation of neutrophils with consequent local TNF-α production. Neutrophil influx was PAF receptor- and cell adhesion molecule-dependent.
The release in tissue of chemoattractant molecules which act on seven-transmembrane G protein-associated receptors is an essential step for the recruitment of leukocytes (Mansson et al., 2000; Rossi & Zlotnik, 2000; von andrian & Mackay, 2000). Thus, we reasoned that IL-1β was also inducing the local release of chemoattractant molecules that would drive the local influx of neutrophils. Here, we assessed the role of two of these mediators, PAF and LTB4, known to be potent and effective inducers of neutrophil recruitment and activation (Liu et al., 1996; Crooks & Stockley, 1998). In addition, PAF and LTB4 can be induced by IL-1β in different conditions (Nourshargh et al., 1995; Showell et al., 1998; Wen et al., 1998; Serou et al., 1999; Wang & Sun, 2000) and there are good pharmacological antagonists to evaluate their role in vivo. The data of the present study show that B1 kinin receptors-mediated oedema in rats pretreated with IL-1β was significantly inhibited by the co-injection of a selective PAF receptor antagonist. It has been reported that IL-1β mediated leukocyte extravasation in mesenteric microvessels involves the formation of PAF (Nourshargh et al., 1995). In addition, the pre-treatment with PAF receptor antagonists prevents IL-1β-mediated cyclooxygenase-2 induction in primary hippocampal neuronal culture cells (Serou et al., 1999). In contrast to the effects of the PAF receptor antagonist, the LTB4 receptor antagonist had no significant effect on des-Arg9-BK-induced paw oedema in IL-1β-treated rats. Together, the data above suggest that neutrophil recruitment in response to IL-1β administration in the rat paw might be in part mediated by the endogenous release of PAF.
Our data also suggest that B1 receptors up-regulation after i.d. injection of IL-1β involves a process dependent on the activation of adhesion molecules. This conclusion derives from the observation that des-Arg9-BK-induced paw oedema was significantly inhibited by a non-selective selectin inhibitor, fucoidin, or by an anti-CD18 antibody. These results are in agreement with the ability of IL-1β to induce the expression of adhesion molecules in the endothelium and, hence, to induce the influx of leukocytes to sites of inflammation (Meager, 1999; Mansson et al., 2000). Indeed, IL-1β and other pro-inflammatory cytokines are capable of triggering the activation of protein kinase cascades and NF-κB which, in turn, promote the expression of adhesion molecules, including selectins and integrins (Barnes & Karin, 1997; Stylianou & Saklatvala, 1998).
The injection of IL-1β in the rat paw induced a significant influx of neutrophils, as assessed by a 5 – 10-fold increase in MPO activity. MPO is stored in cytoplasmatic granules of neutrophils and represents an indirect, but relevant measurement of neutrophil sequestration. Blockade of PAF-receptors with WEB 2086 or of selectin- and CD18-dependent interactions with fucoidin and anti-CD mAb, respectively, markedly reduced the local neutrophil influx. As discussed above, these same inhibitors blocked not only IL-1β-induced increase in MPO activity, but also reduced in a significant manner the functional up-regulation of B1 receptors in rats pre-treated with IL-1β. Overall, these results argue that the recruitment of neutrophils in response to IL-1β administration is an essential prerequisite for the expression of a functional B1 receptor. Our results evaluating the expression of B1 receptor mRNA expression in the rat paw reinforce the latter concept. Indeed, the present work demonstrates for the first time a rapid up-regulation of B1 receptor mRNA after injection of IL-1β. Interestingly, this increase of B1 mRNA expression following IL-1β pre-treatment correlated well with the functional up-regulation of the receptor (Campos et al., 1998; 1999). Furthermore, the ability of fucoidin to inhibit B1 receptor mRNA reinforces the dependence of B1 receptor induction on neutrophil recruitment.
We have previously shown that pre-treatment of animals with anti-TNF-α abrogates the ability of IL-1β to upregulate the kinin B1 receptor (Campos et al., 1999). Moreover, we have also demonstrated that local TNF-α production was dependent on the influx of neutrophils following ischaemia and reperfusion injury of the superior mesenteric artery in rats (Souza et al., 2000a). It was of interest then to investigate whether IL-1β-induced neutrophil influx could drive the local production of TNF-α, explaining the ability of neutrophils to induce the functional up-regulation of kinin B1 receptors. This was particularly relevant as TNF-α did not induce significant neutrophil recruitment, as assessed by the local increase in MPO activity, at doses known to induce the functional upregulation of B1 receptors (Campos et al., 1998). Here, we show that the injection of IL-1β into the rat paw induced a significant local production of TNF-α immunoreactivity. More importantly and in agreement with our previous studies in another model of acute neutrophil-dependent inflammation (Souza et al., 2000a), blockade of neutrophil influx with fucoidin, the anti-CD18 mAb or the PAF receptor antagonist effectively blocked local TNF-α production. Unexpectedly, the LTB4 receptor antagonist partially inhibited the local production of TNF-α without interfering with neutrophil recruitment or B1 receptor functional upregulation. We do not have an explanation for these partial inhibitory effects of the LTB4 receptor antagonist. One distinct possibility which arises from our previous work (Souza et al., 2000b) is that LTB4 receptors may be necessary for neutrophil activation and, thus, partially responsible for the local production of TNF-α, but not to an extent that would not interfere with B1 receptor functional upregulation.
It is not clear from our studies how infiltrating neutrophils drive the local production of TNF-α. Nevertheless, the distinct ability of neutrophils to produce different oxygen species and inflammatory mediators and to interact with other cell types may underlie the observation above. For example, in some conditions, such as after i.d. IL-1β injection, neutrophil-derived oxidants can act as second messengers in IL-1β signalling pathways (Derevianko et al., 1998). Whatever the underlying explanation, the present finding may bear relevance to many inflammatory conditions where infiltrating neutrophils are a major pathological feature. Thus, the results presented above demonstrate that the influx of neutrophils is necessary for the local production of TNF-α which may in turn drive the functional up-regulation of kinin B1 receptors.
In addition to inducing local TNF-α release, neutrophils have been shown to express kinin B1 receptors (Araújo et al., 2001) and could, thus, be a direct target of the B1 receptor agonist-induced paw oedema. Nevertheless, the i.d. injection of the selective kinin B1 receptor agonist des-Arg9-BK did not induce any alteration in MPO activity when injected alone in the rat paw. In fact, when compared with other chemotatic factors, kinins, including BK, are described as weak inducers of cell migration. So, it has been suggested that the involvement of kinins during cell recruitment, might be dependent on the interaction with other mediators of inflammation (Böckmann & Paegelow, 2000). Here, we show that des-Arg9-BK was able to induce a further increase of MPO activity (about 40%) in rats pre-treated with a low dose of IL-1β (1 ng paw−1). These results are in accordance with previous observations (Ahluwalia & Perretti, 1996; McLean et al., 2000) which demonstrate the ability of des-Arg9-BK in stimulating neutrophil influx after IL-1β priming. In addition, our results also suggest the involvement of B1 receptors in des-Arg9-BK-induced responses, as the increase in MPO activity was significantly prevented by the co-injection of the B1 receptor antagonist des-Arg9−[Leu8]-BK. Thus, cytokines and kinins may participate cooperatively in the fine regulation of neutrophil migration during the inflammatory process. As such, IL-1β-induced neutrophil migration results in the TNFα-dependent up-regulation of B1 receptors, that in turn might amplify the cell influx in order to perpetuate the inflammatory response.
Interestingly and in contrast to the fundamental role of neutrophils shown here, it has been previously reported that depletion of circulating neutrophils had no effect on B1 receptor-induced fall in blood pressure in LPS-treated rabbits (Bouthillier et al., 1987). The reasons for the discrepancy is not known but there are several reasons which could account for the different need of neutrophils for local versus systemic B1 receptor expression. For example, the cell types responding to the inflammatory stimuli and releasing B1 receptor-inducing pro-inflammatory cytokines (such as TNF-α) could be different in the two different experiments. In this regard, we have previously demonstrated that strategies which block neutrophil influx were capable of abrogating the local, but not systemic, production of TNF-α following mesenteric ischemia and reperfusion injury in rats (Souza et al., 2000a).
In conclusion, the results of the present study demonstrate that the injection of IL-1β induces neutrophil recruitment into the paw via a mechanism that involves the participation of PAF receptors and adhesion molecules. The recruited neutrophils induce the local production of TNF-α which appears to drive the local up-regulation of B1 receptors. The important cross-talk between the kinin system and cytokines suggest that studies evaluating the blockade of kinin receptors, specially the B1 receptor, may be warranted in chronic inflammatory diseases where pro-inflammatory cytokines are expressed in great levels.
Acknowledgments
This work was supported by grants from CNPq, FINEP, PRONEX and FAPESP (Brazil). M.M. Campos is a PhD student receiving a grant from CNPq
Abbreviations
- BK
bradykinin
- BSA
bovine serum albumine
- des-Arg9-BK
des-Arg9-bradykinin
- i.d.
intradermal
- IL
interleukin
- LTB4
leukotriene B4
- MAP-kinase
mitogen-activated protein kinase
- MPO
myeloperoxidase
- NF-κB
nuclear factor κB
- PAF
platelet activating factor
- PBS
phosphate buffered saline, PMSF, phenylmethylsulphonylfluoride
- TNF-α
tumour necrosis factor-α
References
- AHLUWALIA A., PERRETTI M. Involvement of bradykinin B1 receptors in the polymorphonuclear leucocyte accumulation induced by IL-1β in vivo in the mouse. J. Immunol. 1996;156:269–274. [PubMed] [Google Scholar]
- ARAÚJO R.C., KETTRITZ R., FICHTNER I., PAIVA A.C.M., PESQUERO J.B., BADER M. Altered neutrophil homeostasis in kinin B1 receptor-deficient mice. Biol. Chem. 2001;382:91–95. doi: 10.1515/BC.2001.014. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., ADCOCK I.M. NF-κB: a pivotal role in asthma and a new target for therapy. Trends Pharmacol. Sci. 1997;18:47–50. doi: 10.1016/s0165-6147(97)89796-9. [DOI] [PubMed] [Google Scholar]
- BARNES P.J., KARIN M. Nuclear factor-κB - a pivotal transcription factor in chronic inflammatory diseases. N. Eng. J. Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- BÉLICHARD P., LANDRY M., FAYE P., BACHVAROV D.R., BOUTHILLIER J., PRUNEAUS D., MARCEAU F. Inflammatory hyperalgesia induced by zymosan in the plantar tissue of the rat: effect of kinin receptor antagonists. Immunopharmacology. 2000;46:139–147. doi: 10.1016/s0162-3109(99)00165-4. [DOI] [PubMed] [Google Scholar]
- BÖCKMANN S., PAEGELOW I. Kinins and kinin receptors: importance for the activation of leukocytes. J. Leukoc. Biol. 2000;68:587–592. [PubMed] [Google Scholar]
- BOUTHILLIER J., DEBLOIS D., MARCEAU F. Studies on the induction of pharmacological responses to des-Arg9-bradykinin in vitro and in vivo. Br. J. Pharmacol. 1987;92:257–264. doi: 10.1111/j.1476-5381.1987.tb11319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOWIE A., O'NEILL L.A. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J. Leukoc. Biol. 2000;67:508–514. doi: 10.1002/jlb.67.4.508. [DOI] [PubMed] [Google Scholar]
- BÚRIGO A.C., CALIXTO J.B., MEDEIROS Y.S. Pharmacological profile of rat pleurisy induced by Bothrops jararaca venom. J. Pharm. Pharmacol. 1996;48:106–111. doi: 10.1111/j.2042-7158.1996.tb05887.x. [DOI] [PubMed] [Google Scholar]
- CALIXTO J.B., CABRINI D.A., FERREIRA J., CAMPOS M.M. Kinins in pain and inflammation. Pain. 2000;87:1–5. doi: 10.1016/S0304-3959(00)00335-3. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., CALIXTO J.B. Involvement of B1 and B2 receptors in bradykinin-induced rat paw oedema. Br. J. Pharmacol. 1995;114:1005–1013. doi: 10.1111/j.1476-5381.1995.tb13305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E.P., CALIXTO J.B. Modulation of kinin B1 receptor-mediated rat paw oedema by IL-1β and TNFα. Peptides. 1998;19:1269–1276. doi: 10.1016/s0196-9781(98)00087-4. [DOI] [PubMed] [Google Scholar]
- CAMPOS M.M., SOUZA G.E.P., CALIXTO J.B. In vivo B1 kinin-receptor up-regulation. Evidence for involvement of protein kinases and nuclear factor kappaB pathways. Br. J. Pharmacol. 1999;127:1851–1859. doi: 10.1038/sj.bjp.0702715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROOKS S.W., STOCKLEY R.A. Leukotriene B4. Int J. Biochem. Cell. Biol. 1998;30:173–178. doi: 10.1016/s1357-2725(97)00123-4. [DOI] [PubMed] [Google Scholar]
- DEREVIANKO A., GRAEBER T., D'AMICO R., SIMMS H.H. The role of neutrophil-derived oxidants as second messengers in interleukin-1β-stimulated cells. Shock. 1998;10:54–61. doi: 10.1097/00024382-199807000-00010. [DOI] [PubMed] [Google Scholar]
- DIMARTINO M.J., WOLFF C.E., CAMPBELL G.K., HANNA N. The pharmacology of arachidonic acid-induced rat PMN leukocyte infiltration. Agents and Actions. 1989;27:325. doi: 10.1007/BF01972812. [DOI] [PubMed] [Google Scholar]
- DINARELLO C.A. Proinflammatory cytokines. Chest. 2000a;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- DINARELLO C.A. The role of the interleukin-1 receptor antagonist in blocking inflammation mediated by interleukin-1. N. Engl. J. Med. 2000b;343:732–734. doi: 10.1056/NEJM200009073431011. [DOI] [PubMed] [Google Scholar]
- GRUTKOSKI P.S., D'AMICO R., AYALA A., SIMMAS H.H. IL-1β stimulation induces paracrine regulation of PMN function and apoptosis. Shock. 1999;12:373–381. doi: 10.1097/00024382-199911000-00006. [DOI] [PubMed] [Google Scholar]
- LARRIVÉE J.-F., BACHVAROV D.R., HOULE F., LANDRY J., HUOT J., MARCEAU F. Role of mitogen-activated protein kinases in the expression of the B1 kinin receptors induced by tissue injury. J. Immunol. 1998;160:1419–1426. [PubMed] [Google Scholar]
- LIU L., MUL F.P., KUIJPERS T.W., LUTTER R., ROOS D., KNOL E.F. Neutrophil transmigration across monolayers of endothelial cells and airway epithelial cells is regulated by different mechanisms. Ann. NY Acad. Sci. 1996;796:21–29. doi: 10.1111/j.1749-6632.1996.tb32563.x. [DOI] [PubMed] [Google Scholar]
- MANSSON P., ZHANG X.W., JEPPSSON B., JOHNELL O., THORLACIUS H. Critical role of P-selectin-dependent rolling in tumor necrosis factroα-induced leukocyte adhesion and extravascular recruitment in vivo. Naunyn-Shmiedrberg's Arch. Pharmacol. 2000;362:190–196. doi: 10.1007/s002100000268. [DOI] [PubMed] [Google Scholar]
- MARCEAU F. Kinin B1 receptors: a review. Immunopharmacology. 1995;30:1–26. doi: 10.1016/0162-3109(95)00011-h. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., BACHVAROV D.R. Kinin receptors. Clin. Rev. Allergy Inflamm. 1998;16:385–401. doi: 10.1007/BF02737658. [DOI] [PubMed] [Google Scholar]
- MARCEAU F., HESS J.F., BACHVAROV D.R. The B1 receptors for kinins. Pharmacol Rev. 1998;50:357–386. [PubMed] [Google Scholar]
- MCLEAN P.G., AHLUWALIA A., PERRETTI M. Association between kinin B1 receptor expression and leukocyte trafficking across mouse mesenteric poscapillary venules. J. Exp. Med. 2000;192:367–380. doi: 10.1084/jem.192.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEAGER A. Cytokine regulation of cellular adhesion molecule expression in inflammation. Cytokine Growth Factor Rev. 1999;10:27–39. doi: 10.1016/s1359-6101(98)00024-0. [DOI] [PubMed] [Google Scholar]
- NOURSHARGH S., LARKIN S.W., DAS A., WILLIAMS T.J. Interleukin-1-induced leukocyte extravasation across rat mesenteric microvessels is mediated by platelet-activating factor. Blood. 1995;85:2553–2558. [PubMed] [Google Scholar]
- PHAGOO S.B., YAQOOB M., HERRERA-MARTINEZ E., MCINTYRE P., JONES C., BURGESS G.M. Regulation of bradykinin receptor gene expression in human lung fibroblasts. Eur. J. Pharmacol. 2000;397:237–246. doi: 10.1016/s0014-2999(00)00323-x. [DOI] [PubMed] [Google Scholar]
- ROSSI D., ZLOTNIK A. The biology of chemokines and their receptors. Ann. Rev. Immunol. 2000;18:217–243. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- SAKLATVALA J., DEAN J., FINCH A. Protein kinase cascades in intracellular signalling by interleukin-1 and tumor necrosis factor. Biochem. Soc. Symp. 1999;64:63–67. [PubMed] [Google Scholar]
- SCHAEFFER P., LAPLACE M.-C., SAVI P., PRABONNAUD V., SALEL V., HERBERT J.-M. Detection of bradykinin B1 receptors in rat aortic smooth muscle cells. Biochem. Pharmacol. 2001;61:291–298. doi: 10.1016/s0006-2952(00)00554-2. [DOI] [PubMed] [Google Scholar]
- SCHANSTRA J.P., BATAILLÉ E., CASTAÑO M.E.M., BARASCUD Y., HIRTZ C., PESQUERO J.B., PECHER C., GAUTHIER F., GIROLAMI J.-P., BASCANDS J.-L. The B1-agonist [des-Arg10]-kallidin activates transcription factors NF-κB and induces homologous up-regulation of the bradykinin B1-receptor in cultured human lung fibroblasts. J. Clin. Invest. 1998;101:2080–2091. doi: 10.1172/JCI1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEROU M.J., DECOSTER M.A., BAZAN N.G. Interleukin-1β activates expression of cyclooxygenase-2 and inducible nitric oxide synthase in primary hippocampal neuronal culture: platelet activating factor as a preferential mediator of cyclooxygenase-2 expression. J. Neurosci. Res. 1999;58:593–598. [PubMed] [Google Scholar]
- SHOWELL H.J., CONKLYN M.J., ALPERT R., HINGORANI G.P., WRIGHT K.F., SMITH M.A., STAM E., SALTER E.D., SCAMPOLI D.N., MELTZER S., REITER L.A., KOCH K., PISCOPIO A.D., CORTINA S.R., LOPEZ-ANAYA A., PETTIPHER E.R., MILICI A.J., GRIFFITHS R.J. The preclinical pharmacological profile of the potent and selective leukotriene B4 antagonist CP 195543. J. Pharmacol. Exp. Ther. 1998;285:946–954. [PubMed] [Google Scholar]
- SOUZA D.G., CARA D.C., CASSALI G.D., COUTINHO S.F., SILVEIRA M.R., ANDRADE S.P., POOLE S.P., TEIXEIRA M.M. Effects of the PAF receptor antagonist UK74505 on local and remote reperfusion injuries folloxing ischaemia mesenteric artery in the rat. Br. J. Pharmacol. 2000a;131:1800–1808. doi: 10.1038/sj.bjp.0703756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUZA D.G., COUTINHO S.F., SILVEIRA M.R., CARA D.C., TEIXEIRA M.M. Effects of a BLT receptor antagonist on local and remote reperfusion injuries after transient ischemia of the superior mesenteric artery in rats. Eur. J. Pharmacol. 2000b;403:121–128. doi: 10.1016/s0014-2999(00)00574-4. [DOI] [PubMed] [Google Scholar]
- STYLIANOU E., SAKLATVALA J. Interleukin-1. Int. J. Biochem. Cell. Biol. 1998;30:1075–1079. doi: 10.1016/s1357-2725(98)00081-8. [DOI] [PubMed] [Google Scholar]
- TEIXEIRA M.M., HELLEWELL P.G. The effect of the selectin binding polysaccharide fucoidin on eosinophil recruitment in vivo. Br. J. Pharmacol. 1997;120:1059–1066. doi: 10.1038/sj.bjp.0701024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORLACIUS H., LINDBOM L., RAUD J. Cytokine-induced leukocyte rolling in mouse cremaster muscle arterioles is P-selectin dependent. Am. J. Physiol. 1997;272:H1725–H1729. doi: 10.1152/ajpheart.1997.272.4.H1725. [DOI] [PubMed] [Google Scholar]
- VON ANDRIAN U.H., MACKAY C.R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- WANG J.H., SUN G.Y. Platelet activating factor (PAF) antagonists on cytokine induction of iNOS and sPLA2 in immortalized astrocytes. Neurochem. Res. 2000;25:613–619. doi: 10.1023/a:1007550801444. [DOI] [PubMed] [Google Scholar]
- WEN F.Q., WATANABE K., YOSHIDA M. Eicosanoid profile in culture human pulmonary artery smooth muscle cells treated with IL-1β and TNFα. Prostaglandins Leukot. Essent. Fatty Acids. 1998;59:71–75. doi: 10.1016/s0952-3278(98)90054-0. [DOI] [PubMed] [Google Scholar]
- WILLIAMS F.M., KUS M., TANDA K., WILLIAMS T.J. Effect of duration of ischaemia on reduction of myocardial infarct size by inhibition of neutrophil accumulation using an anti-CD18 monoclonal antibody. Br. J. Pharmacol. 1994;111:1123–19987. doi: 10.1111/j.1476-5381.1994.tb14861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


