Abstract
In isolated endothelium-intact or denuded rabbit corpus cavernosum preconstricted with phenylephrine, KMUP-1 (0.001 – 10 μM) caused a concentration-dependent relaxation.
This relaxation of KMUP-1 was attenuated by endothelium removed, high K+ and pretreatments with a soluble guanylyl cyclase (sGC) inhibitor ODQ (1 μM), a NOS inhibitor L-NAME (100 μM), a K+ channel blocker TEA (10 mM), a KATP channel blocker glibenclamide (1 μM), a voltage-dependent K+ channel blocker 4-AP (100 μM) and Ca2+-dependent K+ channel blockers apamin (1 μM) and charybdotoxin (ChTX, 0.1 μM).
The relaxant responses of KMUP-1 (0.01, 0.05, 0.1 μM) together with a PDE inhibitor IBMX (0.5 μM) had additive actions on rabbit corpus cavernosum smooth muscle (CCSM).
KMUP-1 (0.01 – 10 μM) induced increase of intracellular cyclic GMP level in the primary cell culture of rabbit CCSM. This increase in cyclic GMP content was abolished in the presence of ODQ (10 μM).
Both KMUP-1 and sildenafil at 0.2, 0.4, 0.6 mg kg−1 caused increases of intracavernous pressure (ICP) and duration of tumescene (DT) in a dose-dependent manner. These in vivo activities of ICP for sildenafil and KMUP-1 are consistent with those of in vitro effects of cyclic GMP.
KMUP-1 has the following merits: (1) inhibition of PDE or cyclic GMP breakdown, (2) stimulation of NO/sGC/cyclic GMP pathway, and (3) subsequent stimulation of K+ channels, in rabbit CCSM. We suggest that these merits play prominent roles in KMUP-1-induced CCSM relaxation-associated increases of ICP and penile erection.
Keywords: Cyclic GMP, intracavernous pressure, corpus cavernosum, K+ channels
Introduction
Inhibition of type 4 phosphodiesterase (PDE) by xanthine derivatives, including methylxanthine, usually increases intracellular formation of cyclic AMP in various smooth muscle cells. Among them, theophylline is a non-selective PDE inhibitor (Beavo, 1995). KMUP-1, a theophylline-based derivative, has been described to have not only PDE inhibition but also cyclic GMP increasing activities in our previous study (Wu et al., 2001). Type 5 PDE inhibitor, with cyclic GMP increasing activity, has proved to be effective in the treatment of penile dysfunction after oral administration in man (Goldstein et al., 1998). KMUP-1, with PDE inhibition and cyclic GMP increasing activities in rat aortic smooth muscle, was thus supposed similarly to have rabbit cavernosal smooth muscle relaxation and penile erection effects in this study.
Activation of soluble guanylyl cyclase (sGC) induces the formation of cyclic GMP, which is a second messenger of NO action that generally modulates the activity of its effector proteins leading to the vasorelaxation (Schmidt et al., 1993) and also the opening of K+-channel (Murphy & Brayden, 1995). It is reasonable to suggest that activation of sGC and inhibition of phosphodiesterase (PDE) that metabolize the cyclic GMP, may together attribute to the increase of cyclic GMP associated vascular and cavernosal smooth muscle relaxations. YC-1 is a representative of this class of NO-independent sGC activator with PDE inhibition and may lead to a long-lasting cyclic GMP-mediated inhibition of vasoconstriction (Wu et al., 1995; Galle et al., 1999). Recently, BAY 41-2272 and BAY 51-9491 potently stimulate sGC by a mechanism that is also independent of NO (Stasch et al., 2001).
To date, sildenafil has been described to abolish vas deferens contractility initiated by K+ channel blocker (Medina et al., 2000); YC-1 mediate stimulation of Ca2+-IK(Ca) and effectively inhibited the voltage-dependent K+ current IK(V) in GH3 lactotrophs (Wu et al., 2000). In contrast, KMUP-1 displayed not only inhibition of PDE and activation of sGC, but also reversed the K+ channel blockade caused by K+ channel blockers in rat aortic smooth muscle (Wu et al., 2001).
K+ channel openers, acting by liberation of NO, have been shown to relax human isolated CCSMs and produce erection when injected intracorporeally into animals and men (Saito et al., 1998). Many experimental animals have been employed in in vivo studies of penile erection (Rehman et al., 1998; Seyam et al., 1997; Trigo-Rocha et al., 1993; Lue, 1986; Wang et al., 1994; Stief et al., 1998). KMUP-1, a methyl xanthine and piperazine derivative, structurally with six nitrogen atoms as sildenafil and with an ethylpiperazine moiety on the position 7 of xanthine base (Figure 1), combining the PDE inhibition, sGC stimulation, and K+ channels opening activity, has been described to achieve the full relaxation activity in rat aortic smooth muscle (Wu et al., 2001). Taking the above into consideration, we are thus encouraged to use KMUP-1, multiply with above effects in vasculature, to examine its possible effects in rabbit CCSM and penile erection.
Figure 1.
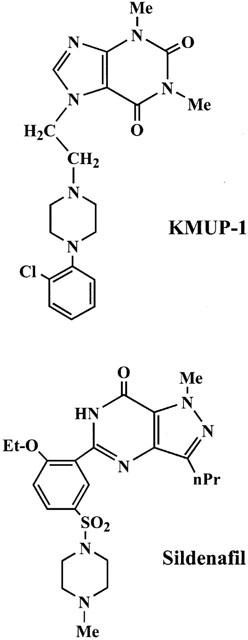
Chemical structures of KMUP-1 and sildenafil.
In this study, we characterized the effects of KMUP-1 on rabbit CCSMs and associated NO/sGC/cyclic GMP activation, retard of induced K+ channels blocking, and PDE inhibiting activities. Notable was found in erectile dysfunction that associated with vascular relaxation, the combination use of PDE inhibitor and sGC stimulator or K+-channel opener was suggested to enhance the results achieved (Martinez-Pineiro et al., 1996). KMUP-1, with those possible activities and intracavernous pressure (ICP) increasing effect, is suggested to be hopeful in the management of erectile dysfunction.
Methods
Animals
Male New Zealand white rabbits (2.5 – 3 kg) were provided from National Laboratory Animal Breeding and Research Center (Taipei, Taiwan) and housed under conditions of constant temperature and controlled illumination. Food and water were available ad libitum. The study was approved by the Animal Care and Use Committee of the Kaohsiung Medical University.
Drugs and chemicals
Tetraethylammonium (TEA), 4-aminopyridine (4-AP), glibenclamide, phenylephrine, 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), apamin, charybdotoxin, Nw-nitro-L-arginine methyl ester (L-NAME) and 3-isobutyl-1-methylxanthine (IBMX) were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Sildenafil citrate was supplied by the Cadila Healthcare Ltd. (Maninagar, India). All other reagents used were from E. Merck (Darmstadt, Germany). KMUP-1, synthesized in this laboratory, was dissolved in 10% absolute alcohol, 10% propylene glycol and 2% 1N HCl at 10 mM. Serial dilutions were made in distilled water.
Disruption of endothelium
The endothelium lining the lacunar spaces of rabbit corpus cavernosum was disrupted and/or removed by detergent treatment using a modification of a protocol for blood vessels, described elsewhere (Kim et al., 1991). The intact, isolated penis was placed in a tray containing chilled physiological salt solution (PSS). A 21-gauge minicatheter was inserted into each corporal body at the proximal end of the penis. A third minicatheter was inserted into the distal end, below the glans penis, where the right and left corpora communicate. While the distal and one proximal minicatheter were clamped, 3 ml of CHAPS (wt vol−1) in saline was infused into the remaining proximal catheter. After a short interval (∼20 s), the clamped minicatheters were opened and the preparation was washed by infusion of saline. The corpora cavernosa were then removed and tested for endothelial integrity. Of the tissues treated with CHAPS, 75% did not relax or relaxed poorly (<10% of maximal relaxation) to acetylcholine (Ach, 1 μM) were considered to be functionally denuded of endothelium.
Tissue procurement and organ bath experiments
Male rabbits were killed with pentobarbitone and their penises excised rapidly and cut longitudinally into equal strips to give 3 – 6 segments. These segments were incubated in Kerbs-bicarbontate solution (in mM: NaCl 118; NaHCO3, 25; KCl 4.7; KH2PO4 1.2; MgSO4 1.2; glucose 11; CaCl2, 2.5; pH 7.3 – 7.4) maintained at 37°C and aerated with 95% O2 and 5% CO2. Isometric tension was recorded with a force displacement transducer (UGO BASILE, Model 7004, Italy). Rabbit CCSMs were stretched to a resting tension of 2 g and then contracted with phenylephrine (PE, 10 μM). Tissues were also treated with guanethidine (10 μM) to block contractions caused by noradrenaline released from adrenergic neuron and also partially deplete tissue stores of catecholamines by inhibition of the vesicular transport system, and treated with atropine (1 μM) to prevent muscarinic effects caused by acetylcholine. When the stable constriction to PE was reached, concentration-response curves to KMUP-1 and sildenafil (0.001 – 10 μM) were constructed. Data were expressed as a percentage of the maximum contractile response to PE. To examine the possible action mechanisms of KMUP-1, the rabbit CCSMs were pretreated with a sGC inhibitor ODQ (1 μM), a NOS inhibitor L-NAME (100 μM), a K+ channel blocker TEA (10 mM), a KATP channel blocker glibenclamide (1 μM), a voltage-dependent K+ channel blocker 4-AP (100 μM) and Ca2+-dependent K+ channel blockers apamin (1 μM) and charybdotoxin (0.1 μM) for 30 min prior to the addition of KMUP-1. In addition, the rabbit CCSMs pretreated with ODQ (1 μM), L-NAME (100 μM) and above mentioned K+ channel blockers for 30 min prior to the addition of sildenafil were also examined. To observe whether the rabbits CCSM relaxation of KMUP-1 are affected by a nonselective PDE inhibitor, we investigated the action of KMUP-1 in the presence of IBMX (0.5 μM). In another experiment, rabbit CCSMs were preconstricted with 60 mM KCl. The KCl solution was prepared by substituting NaCl with KCl (60 mM) in an equimolar amount.
Culture of rabbit corpus cavernosum smooth muscle cells
Rabbit CCSMs were obtained as sterile surgical specimens, the tissue was washed and cut into 1 to 2 mm pieces and placed into culture dishes with Dulbecco's modified Eagle's medium (DMEM) containing 20% foetal bovine serum (FBS), penicillin (100 u ml−1), streptomycin (100 u ml−1) and 2 mM Glutamine. After explants attached to the culture dish, usually 1 to 2 days, DMEM supplement with 10% FBS, penicillin, streptomycin, and glutamine were added. Smooth muscle cells migrated out from the explants in 3 – 5 days. At this time, the explants were removed, and cells were allowed to achieve confluence. Cells were detached using 0.05% trypsin, 0.02% EDTA at 37°C for 5 min to establish secondary cultures. Cultures were maintained for no more than four passages. Cellular homogeneity was further confirmed by the presence of smooth muscle specific α-myosin and α-actin immunoreactivity. Indirect immunofluorescence staining for a variety of antigens was carried out by first plating the cells on chamber slides fixing the cells in 3.7% formaldehyde-phosphate buffered saline for 10 min and permeabilizing the cells with phosphate buffered saline 0.1% Triton X-100. Cells were then stained with either a mouse monoclonal antibody directed against the amino terminal 10 amino acids of α-smooth muscle actin (Boehringer Mannheim, Indianapolis, IN, U.S.A.) and α-myosin (Moreland et al., 1995). All were stained with fluorescein-labelled goat anti-mouse IgG antibody (Boehringer Mannheim).
Measurement of intracellular cyclic GMP content
Intracellular cyclic GMP concentrations in rabbit CCSM cells were assayed as was previously described (Wu et al., 2001). In brief, cells were finally grown in 24-well plates (105 cells well−1). At confluence, monolayer cells were washed with PBS and then incubated with KMUP-1 and sildenafil (0.01 – 10 μM) in the presence of 100 μM IBMX for 10 min as described by Park et al. (1998). Incubation was terminated by the addition of 6% trichloroacetic acid (TCA). Cell suspensions were sonicated and then centrifuged at 2500×g for 15 min at 4°C. Then, the supernatants were lyophilized and cyclic GMP of each sample was determined using a commercially available radioimmunoassay kit (Amersham Pharmacia Biotech, Buckinghamshire, U.K.).
Measurement of intracavernous pressure
Male rabbits were used for the investigation. After sedation with an intramuscular injection of ketamine (10 mg kg−1), the rabbits were anaesthetized with intraperitoneal pentobarbitone (30 mg kg−1) and maintained with 10 mg kg−1 if needed. The animals breathed spontaneously. The rabbits were then placed in the supine position, and the body temperature was maintained at 37°C. The femoral artery was cannulated for continuous monitoring of systemic arterial pressure (SAP) and heart rate (HR) via a pressure transducer (Spectramed, Model P10EZ, U.S.A.). Under sterile conditions, the skin overlying the penis was incised and the corpora cavernosum was exposed at the root of the penis. A 25-gauge needle was inserted into the CCSMs for pressure recording. The needle was connected to a three-way stopcock, thus permitting the intracavernous injection of the drugs. The tube was filled with heparinized saline (50 iu 2 h−1) to prevent clotting.
Intracavernous injection
In 48 rabbits, divided into two groups, eight animals in each group for one dose, KMUP-1 and sildenafil (0.2, 0.4, 0.6 mg kg−1) were, respectively, injected into CCSMs in a identical volume of less than 0.2 ml. Normal saline in increasing volumes (0.05, 0.1, 0.2 ml) was injected in four rabbits as a control group. The effects of KMUP-1 and normal saline on the intracavernous pressure (ICP) and on the duration of action were evaluated. In order to minimize the effect of the previous drug, the cavernous body was flushed with 0.2 ml normal saline before each injection and the time interval between each injection was at least 1.5 h.
Statistical evaluation of data
The results are expressed as mean±s.e.mean. Statistical differences were determined by independent and paired Student's t-test in unpaired and paired samples, respectively. Whenever a control group was compared with more than one treated group, the one way ANOVA or two way repeated measures ANOVA was used. When the ANOVA manifested a statistical difference, the Dunnett's or Student-Newman-Keuls test was applied. The P value less than 0.05 was considered to be significant in all experiments. Analysis of the data and plotting of the figures were done with the aid of software (SigmaStat and SigmaPlot, Version 5.0, San Rafael, CA, U.S.A.) run on an IBM compatible computer.
Results
Corpus cavernosum smooth muscle relaxant activity
As shown in Figure 2, cumulative concentrations of KMUP-1 (0.001 – 10 μM) produced concentration-dependent relaxations both in endothelium-denuded (E−) and endothelium-intact (E+) CCSMs, indicating that KMUP-1-induced endothelium-independent relaxation. However, KMUP-1 did have a significant rightward shift in the response curve after endothelium denudation, suggesting that at least part of the observed effect is endothelium-dependent. The estimated EC50 value for KMUP-1 in E+ CCSMs was −log EC50=7.19±0.09. Additionally, KMUP-1 completely relaxed the CCSM strips at 10 μM even to 120% relaxations. In contrast, sildenafil (−log EC50=6.96±0.11) also induced similar relaxations (see Figure 5).
Figure 2.
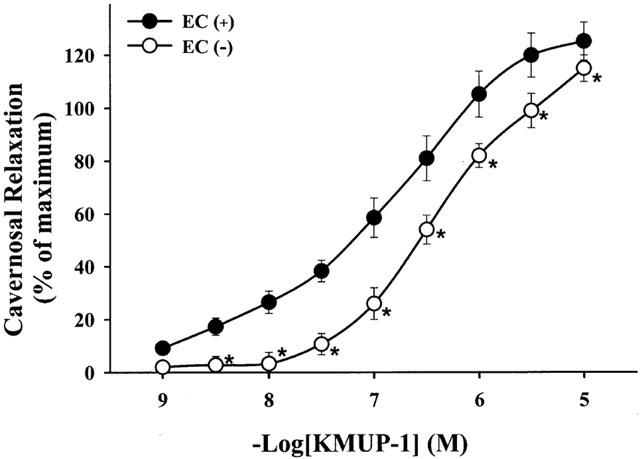
Effects of KMUP-1 on phenylephrine (10 μM)-precontracted rabbit corpus cavernosum in the endothelium-denuded EC (−) and endothelium-intact EC (+) corporeal smooth muscle strips. *P<0.05, n=12 as compared with the KMUP-1 (two way repeated measures ANOVA followed by Student-Newman-Keuls test).
Figure 5.
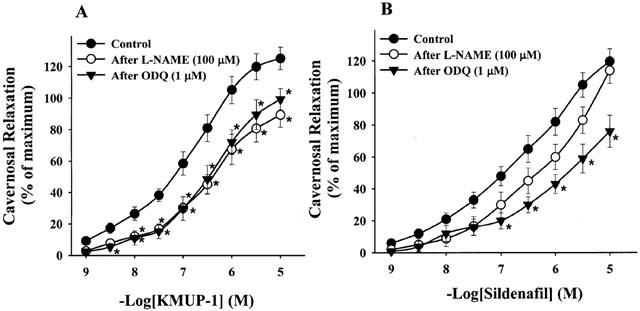
Effects of KMUP-1 (A) and Sildenafil (B) on phenylephrine (10 μM)-precontracted rabbit corpus cavernosum in the absence and presence of L-NAME (100 μM), ODQ (1 μM). *P<0.05, n=12 as compared with the control, respectively (two way repeated measures ANOVA followed by Student-Newman-Keuls test).
Effects on K+ channels
KMUP-1 (0.001 – 10 μM) caused a concentration-dependent relaxation in phenylephrine-contracted CCSMs. However, KMUP-1 had a great reduction of relaxation in the presence of high K+ (60 mM) (Figure 3). CCSMs relaxation of KMUP-1 were inhibited by a K+ channel blocker TEA (−log EC50=5.37±0.05), a KATP channel blocker glibenclamide (−log EC50=6.57±0.15), a voltage-dependent K+ channel blocker 4-AP (−log EC50=5.83±0.17) and Ca2+-dependent K+ channel blockers apamin (−log EC50= 5.85±0.11) and charybdotoxin (−log EC50=5.63±0.09) (Figure 4A). At high concentrations (1 – 10 μM), CCSMs relaxation of sildenafil (−log EC50=6.96±0.11) were also attenuated by a K+ channel blocker TEA (−log EC50= 6.22±0.15), a voltage-dependent K+ channel blocker 4-AP (−log EC50=6.47±0.14) and Ca2+-dependent K+ channel blockers apamin (−log EC50=6.58±0.16) and charybdotoxin (−log EC50=6.37±0.19), but not by a KATP channel blocker glibenclamide (−log EC50=7.13±0.12) (Figure 4B).
Figure 3.
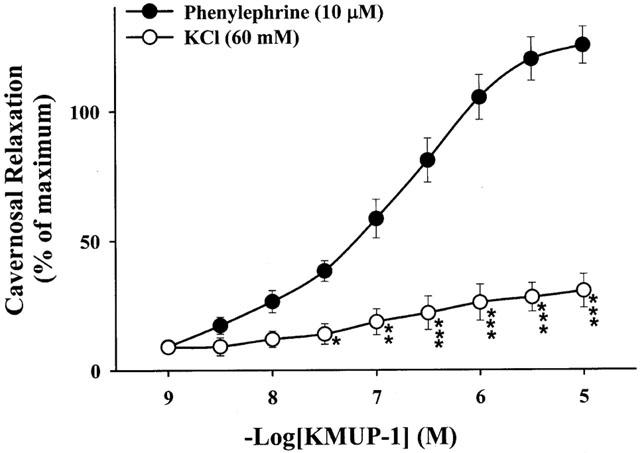
Effects of KMUP-1 on the rabbit corpus cavernosum, precontracted with phenylephrine (10 μM) and 60 mM KCl, respectively. *P<0.05, **P<0.01, ***P<0.001, n=12 as compared with the KMUP-1 (two way repeated measures ANOVA followed by Student-Newman-Keuls test).
Figure 4.
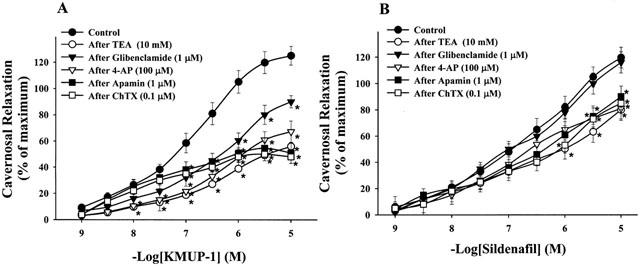
Effects of KMUP-1 (A) and Sildenafil (B) on phenylephrine (10 μM)-precontracted rabbit corpus cavernosum in the absence and presence of potassium channel blockers. *P<0.05, n=12 as compared with the KMUP-1 (two way repeated measures ANOVA followed by Student-Newman-Keuls test).
Effects on NO synthase and soluble guanylyl cyclase
The relaxations of CCSMs elicited by KMUP-1 (−log EC50=7.19±0.09) were significantly inhibited by the pretreatment with a NOS inhibitor L-NAME (−log EC50=6.51±0.08) and a sGC inhibitor ODQ (−log EC50=6.79±0.12), respectively (Figure 5A). On the other hand, the relaxations produced by sildenafil (−log EC50=6.96±0.11) were not inhibited by L-NAME (−log EC50=6.40±0.05), but not inhibited by ODQ (−log EC50=5.88±0.15) significantly (Figure 5B).
Inhibition of phosphodiesterase activity
Effects of KMUP-1 on CCSMs were investigated after inhibition of PDE activity by IBMX. KMUP-1 (−log EC50=7.21±0.12) and IBMX (0.5 μM) were additively (−log EC50=8.65±0.14) to induce relaxation (Figure 6).
Cyclic GMP enhancing activity in corpus cavernosum smooth muscle cells
Effects of KMUP-1 on cyclic GMP levels were examined in primary rabbit CCSMs cells in the presence of IBMX (100 μM). The amounts of basal release of cyclic GMP with and without IBMX were 1.5±0.11 and 0.75±0.13 pmol mg−1 well−1, respectively (n=3). It meant that IBMX alone displayed a significant effect on cyclic GMP levels in CCSM. Both KMUP-1 and sildenafil at 0.01 – 10 μM increased the cyclic GMP levels (Figure 7), which were inhibited by the pretreatment with ODQ (10 μM) (Figure 8).
Figure 7.
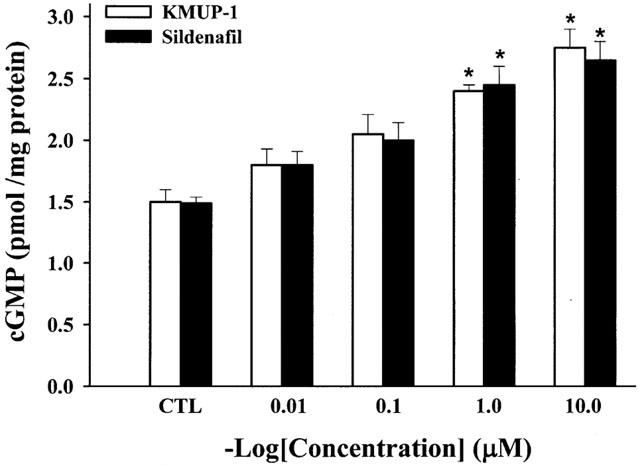
Effects of KMUP-1 (0.01, 0.1, 1, 10 μM) and sildenafil (0.01, 0.1, 1, 10 μM) on cyclic GMP levels in rabbit corpus cavernosum smooth muscle cells. Each value represents the mean±s.e. from three independent experiments. *P<0.05 as compared with the control (ANOVA followed by Dunnett's test). CTL: solvent control.
Figure 8.
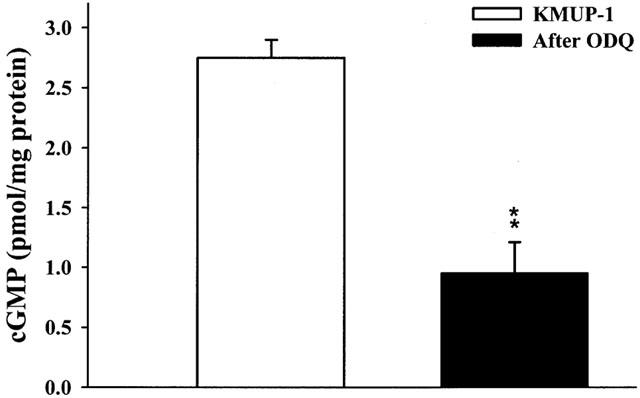
Effects of KMUP-1 (10 μM) on cyclic GMP levels in rabbit corpus cavernosal smooth muscle cells and in the absence or presence of ODQ (10 μM) and methylene blue (100 μM). Each value represents the mean±s.e. from three independent experiments. **P<0.01 as compared with the KMUP-1 (ANOVA followed by Dunnett's test).
Increase of ICP
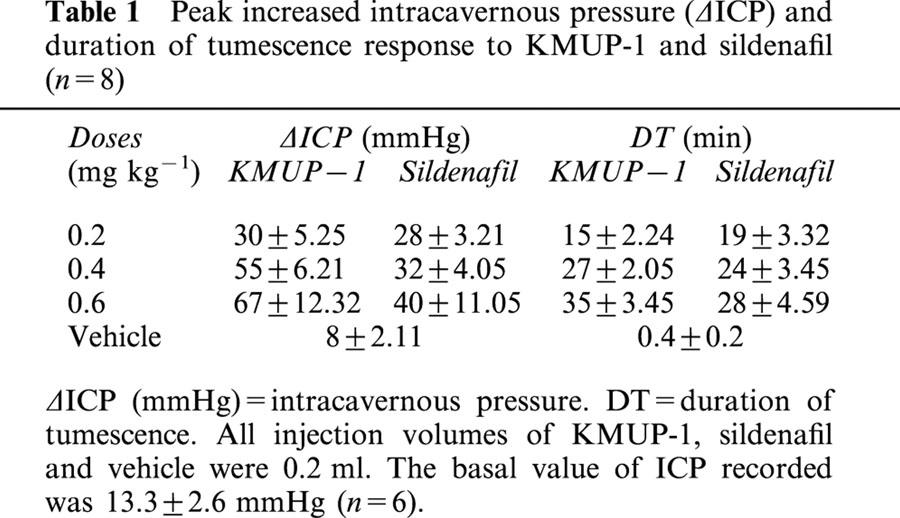
To examine whether KMUP-1 was with corporeal relaxation-associated penile erection activity, we measured the ICP of rabbits. As shown in Table 1, the basal value of ICP recorded was 13.3±2.6 mmHg (n=6). Intracavernous injection of KMUP-1 induced tumescence as documented by a sustained increase in ICP. During the injection periods, the SAP and HR were unchanged (data not shown). Injection of saline induced a transient rise in ICP in a volume-dependent manner. Nevertheless, the pressure rises often returned to the resting level within 1 min and the spike-like pressure curves were different from those of KMUP-1 and sildenafil. We believed that the transient rise in ICP was due to the volume effect of saline. Administration of KMUP-1 and sildenafil (0.2, 0.4, 0.6 mg kg−1) induced dose-dependent elevations in ICP. We also found tumescence of the penile shaft did not show full erection in some cases. There are no significant differences between two compounds in their responses to ICP (Table 1).
Table 1.
Peak increased intracavernous pressure (δICP) and duration of tumescence response to KMUP-1 and sildenafil (n=8)
Discussion
The nonadrenergic/noncholinergic neurotransmitter NO plays a crucial role in attenuating smooth muscle contraction, inducing smooth muscle relaxation, and penile erection. Several vasoactive agents, including NO and PDE inhibitors, initiate and/or enhance CCSM relaxation (Soderling & Beavo, 2000). Soluble guanylyl cyclase, when activated by NO, catalyzes the formation of cyclic GMP from GTP, whereas cyclic GMP-specific phosphodiesterases (PDEs) catalyze the hydrolysis of cyclic GMP to GMP. Termination of signal transduction by hydrolysis of cyclic GMP depends on the specificity and expression of PDE isozymes in the target tissues (Juilfs et al., 1999). One such class of drugs is sildenafil, an inhibitor of cyclic GMP-specific PDE, for use in male erectile dysfunction (Wallis et al., 1999).
The physiologic regulation of penile tumescence involves a balance between relaxant and contractile events. Relaxation is mainly promoted by endothelium-dependent mechanisms and stimulation of nitrergic nerves. In contrast, the adrenergic neuro-transmission has been reported as a promoter of penile flaccidity through the activation of alpha-adrenergic receptors (Angulo et al., 2001). Here, we observed that KMUP-1 possesses concentration-dependent relaxant activities in rabbit CCSMs. KMUP-1 produced rabbit CCSM relaxations in both endothelium-intact and deprived muscles. This relaxation of KMUP-1 was reduced by removing endothelium from CCSMs. Guanethidine and atropine treatment had no significant effects on KMUP-1-induced relaxations. These results suggest that KMUP-1-caused relaxation is un-associated with adrenergic and cholinergic neuronal function. The relaxant effect of KMUP-1 on endothelium deprived and NOS inhibitor-pretreated CCSMs still exist. These facts indicated that KMUP-1 might have NO-independent relaxant effects on CCSMs.
NO has been shown to be the major EDRF in the penile CCSM (Kim et al., 1991). Relaxation of CCSMs appears to occur via NO-elicited activation of guanylate cyclase and cyclic GMP formation (Christ et al., 1993). The CCSM relaxant effects of KMUP-1 was significantly blunted but not inhibited by pretreatment with the sGC inhibitor and the NOS inhibitor. We suggest that other mechanisms of relaxation are activated in addition to the stimulation of NO/sGC/cyclic GMP pathway. On the other hand, we observed that sildenafil-induced relaxations did not show any significant inhibition by L-NAME in rabbit CCSMs, in contrast to our KMUP-1. Therefore, we suggested that sildenafil-induced relaxation appears to be endothelium-independent in CCSMs and is consistent with a previous report (Mcauley et al., 2001). Here, we further confirmed that the partial inhibition of sildenafil-induced relaxation by ODQ is most likely due to the inhibition of basal guanylyl cyclase activity (Mcauley et al., 2001), which is also shown in our KMUP-1.
In this study, we observed that the combination of KMUP-1 and IBMX have an additive effect on CCSMs relaxations. Recently, we have demonstrated that KMUP-1 affected cyclic GMP breakdown at 100 μM, due to its inhibition on the enzyme activity of PDE in human platelets (Wu et al., 2001). Furthermore, KMUP-1 significantly raised the intracellular cyclic GMP levels in a concentration-dependent manner in primary rabbit CCSM cells. These results further confirm that KMUP-1 activate the NO/sGC/cyclic GMP pathway and inhibit the PDE or cyclic GMP breakdown, and therefore elevate the intracellular cyclic GMP levels leading to the CCSM relaxation as previously described in rat aortic smooth muscle (Wu et al., 2001). In this regard, we suggest that the order for the activation of sGC is KMUP-1>sildenafil. In contrast, the order for PDE inhibition is sildenafil>KMUP-1.
K+ channel opener reduces the tissue tension or contractile force in response to relaxation of the CCSM (Anderson, 1993). Vasodilators depend on the K+ channel mechanism reducing their relaxant effects when exposed to high K+ solutions, because an increase in extracellular K+ attenuates the K+ gradient across the plasma membrane, thus rendering the K+ channel-activating mechanism ineffective (Khan et al., 1998). K+ channels can regulate corporeal smooth muscle tone, and also play a significant role in corporeal smooth muscle tone (Christ et al., 1993). These authors further suggested that impairment in K+ channels activity may contribute to erectile dysfunction. Thus, the possibility of K+ channels activation by KMUP-1 was further investigated. The importance of K+ channel-mediated hyperpolarization of KMUP-1 was provided by the differential potency of KMUP-1 in relaxing PE-induced versus KCl-induced contractions. KMUP-1 produced relaxation in this way, since its effect was almost completely blunted in high K+ (60 mM) condition. In these situations, KMUP-1-induced increase in K+ efflux would not hyperpolarize CCSMs sufficiently to inhibit transmembrane Ca2+ influx as in aortic smooth muscle (Wu et al., 2001). Relaxant effects of KMUP-1, reduced by a K+ channel blocker TEA, a KATP channel blocker glibenclamide (Lee et al., 1999), a voltage-dependent K+ channel blocker 4-AP (Sobey & Faraci, 1999) and Ca2+-dependent K+ channel blockers apamin (Nakagawa et al., 1989) and ChTX (Garcia et al., 1995) further suggest the relaxant effect of KMUP-1 might be partly associated with K+ channel activities. On the contrary, sildenafil-induced relaxation is not altered by the pretreatment with glibenclamide and is decreased by other K+ channel blockers only at concentrations between 1 and 10 μM. Obviously, our results from IC50 value of KMUP-1 and sildenafil in the experiments indicate the order for the opening activity of K+ channel is KMUP-1>sildenafil. Therefore, to what extent the relaxant effects of KMUP-1 results from its opening activity of K+ channels will be worthy of further investigation using conventional ruptured patch recording techniques. Indeed, in this field of research, selective inhibition of sGC by ODQ on the KCa channel activity has been described in coronary artery smooth muscle performed by patch clamp method (Li et al., 1998). The sequence of possible actions of KMUP-1 is also illustrated in Figure 9.
Figure 9.
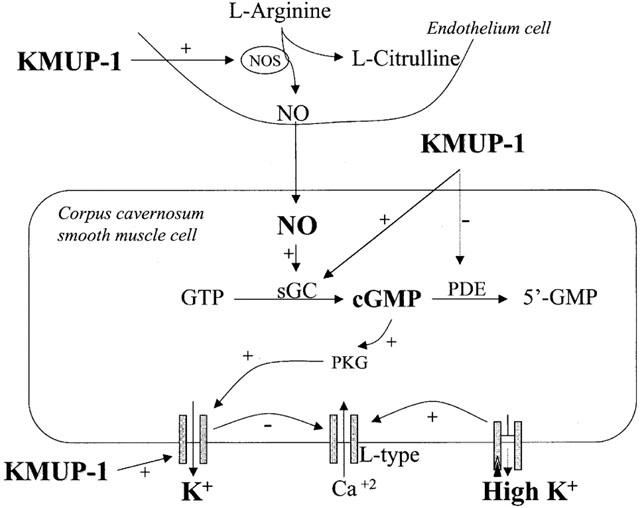
Hypothetical action mechanisms of KMUP-1 in corpus cavernosum smooth muscle.
In the present study, intracavernous injection of KMUP-1 dose-dependently resulted in rises of ICP, without significant change in blood pressure. Our KMUP-1 displayed similar duration of tumescence as sildenafil. The results presented here provide the evidence, as previously (Wu et al., 2001), that the CCSM relaxant activities of KMUP-1 are mediated via inhibition of PDE and associated cyclic GMP metabolism, K+ channels activity, and activation of NO/sGC/cyclic GMP pathway. As shown in Figure 9, obtained accumulation of cyclic GMP may further enhance the K+ efflux, leading to blunt of Ca2+ influx-associated contractility in CCSMs. Combination of these multiple pathways may thus attribute to significant relaxation of CCSMs and associated penile erection. Here, it is suggested that PDE inhibition, cyclic GMP increasing, and K+ channel activities of KMUP-1 are the crucial determinants for its CCSM relaxation effects in rabbit. Therefore, we suggest that the in vivo findings of ICP for KMUP-1 and sildenafil are consistent with the proposed mode of action derived from the in vitro experiments. These potent CCSMs relaxant and penile erection activities of KMUP-1 might be useful to treat erectile dysfunction.
Figure 6.
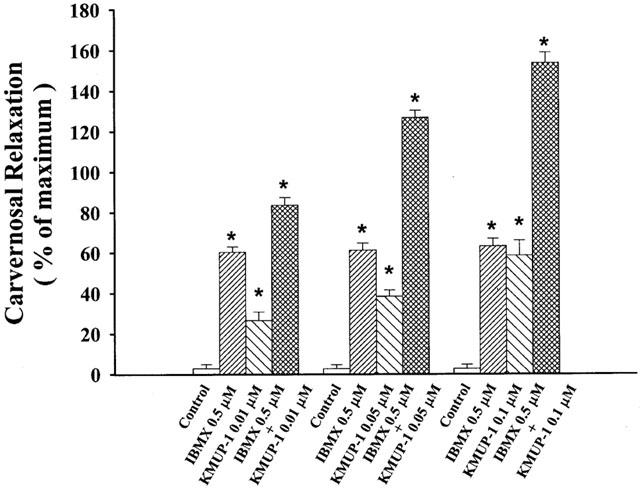
Additive effects of KMUP-1 (0.01, 0.05, 0.1 μM) and IBMX (0.5 μM) on phenylephrine (10 μM)-precontracted rabbit carvernosal strips. Each value represents the mean±s.e., *P<0.05, n=8 as compared with the control value. (ANOVA followed by Dunnett's test). Control: solvent control.
Acknowledgments
This work was supported by grants from the National Science Council: NSC-90-2320-B-037-009-M59 to Dr Ing-Jun Chen and NSC-90-2315-B-037-002 to Dr Bin-Nan Wu and Vital Pharm Co., Ltd. (Kaohsiung, Taiwan).
Abbreviations
- CCSM
corpus cavernosum smooth muscle
- cyclic GMP
guanosine 3′,5′-cyclic monophosphate
- ICP
intracavernous pressure
- KATP channels
ATP-sensitive potassium channels
- NO
nitric oxide
- NOS
nitric oxide synthase
- PDE
phosphodiesterase
- PE
phenylephrine
- sGC
soluble guanylyl cyclase
References
- ANDERSON K.E. Pharmacology of lower urinary tract smooth muscles and penile erectile tissues. Pharmacol. Rev. 1993;45:253–308. [PubMed] [Google Scholar]
- ANGULO J., CUEVAS P., FERNANDEZ A., GABANCHO S., SAENZ DE TEJADA I. Combination of phentolamine and L-arginine or sildenafil synergistically improves neurogenic relaxation of rabbit corpus cavernosum smooth muscle. Urology. 2001;57:585–589. doi: 10.1016/s0090-4295(00)01032-3. [DOI] [PubMed] [Google Scholar]
- BEAVO J.A. Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol. Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- CHRIST G.J., SPRAY D.C., BRINK P.R. Characterization of K currents in cultured human corporal smooth muscle cells. J. Androl. 1993;14:319–328. [PubMed] [Google Scholar]
- GALLE J., ZABEL U., HUBNER U., HATZELMANN A., WAGNER B., WANNER C., SCHMIDT H.H. Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity. Br. J. Pharmacol. 1999;127:195–203. doi: 10.1038/sj.bjp.0702495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARCIA M.L., KNAUS H.G., MUNUJOS P., SLAUGHTER R.S., KACZOROWSKI G.J. Charybdotoxin and its effects on potassium channels. Am. J. Physiol. 1995;269:C1–C10. doi: 10.1152/ajpcell.1995.269.1.C1. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I., LUE T.F., PADMA-NATHAN H., ROSEN R.C., STEERS W.D., WICKER P.A. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N. Engl. J. Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- JUILFS D.M., SODERLING S., BURNS F., BEAVO J.A. Cyclic GMP as substrate and regulator of cyclic nucleotide phosphodiesterases (PDEs) Rev. Physiol. Biochem. Pharmacol. 1999;135:67–104. doi: 10.1007/BFb0033670. [DOI] [PubMed] [Google Scholar]
- KHAN S.A., HIGDON N.R., MEISHERI K.D. Coronary vasorelaxation by nitroglycerin: involvement of plasmalemmal calcium-activated K+ channels and intracellular Ca+2 stores. J. Pharmacol. Exp. Ther. 1998;284:838–846. [PubMed] [Google Scholar]
- KIM N., AZADZOI K.M., GOLDSTEIN I., SAENZ DE TEJADA I. A nitric oxide-like factor mediates nonadrenergic-noncholinergic neurogenic relaxation of penile corpus cavernosum smooth muscle. J. Clin. Invest. 1991;88:112–118. doi: 10.1172/JCI115266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE S.W., WANG H.Z., CHRIST G.J. Characterization of ATP-sensitive potassium channels in human corporal smooth muscle cells. Int. J. Impot. Res. 1999;11:179–188. doi: 10.1038/sj.ijir.3900398. [DOI] [PubMed] [Google Scholar]
- LI P.L., JIN M.W., CAMPBELL W.B. Effect of selective inhibition of soluble guanylyl cyclase on the K(Ca) channel activity in coronary artery smooth muscle. Hypertension. 1998;31:303–308. doi: 10.1161/01.hyp.31.1.303. [DOI] [PubMed] [Google Scholar]
- LUE T.F. The mechanism of penile erection in the monkey. Semin. Urol. 1986;4:217–224. [PubMed] [Google Scholar]
- MARTINEZ-PINEIRO L., GALICIA DE. PEDRO. I., CISNEROS J., CUERVO E., FRIAS INIESTA J., MARTINEZ-PINEIRO J.A. New features of pharmacologic treatment of erectile dysfunction. Arch. Esp. Urol. 1996;49:270–276. [PubMed] [Google Scholar]
- MCAULEY I.W., KIM N.N., MIN K., GOLDSTEIN I., TRAISH A.M. Intracavernosal sildenafil facilitates penile erection independent of the nitric oxide pathway. J. Androl. 2001;22:623–628. [PubMed] [Google Scholar]
- MEDINA P., SEGARRA G., TORONDEL B., CHUAN P., DOMENECH C., VILA J.M., LLUCH S. Inhibition of neuroeffector transmission in human vas deferens by sildenafil. Br. J. Pharmacol. 2000;131:871–874. doi: 10.1038/sj.bjp.0703657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORELAND R.B., TRAISH A., MCMILLIN M.A., SMITH B., GOLDSTEIN I., SAENZ DE TEJADA I. PGE1 suppresses the induction of collagen synthesis by transforming growth factor-beta 1 in human corpus cavernosum smooth muscle. J. Urol. 1995;153:826–834. [PubMed] [Google Scholar]
- MURPHY M.E., BRAYDEN J.E. Apamin-sensitive K+ channels mediate an endothelium-dependent hyperpolarization in rabbit mesenteric arteries. J. Physiol. 1995;489:723–734. doi: 10.1113/jphysiol.1995.sp021086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAGAWA A., NAKAMURA S., ARITA M. Possible increases in potassium conductance by apamin in mammalian ventricular papillary muscles: a comparison with the effects on enzymatically isolated ventricular cells. J. Cardiovasc. Pharmacol. 1989;14:38–45. doi: 10.1097/00005344-198907000-00008. [DOI] [PubMed] [Google Scholar]
- PARK K., MORELAND R.B., GOLDSTEIN I., ATALA A., TRAISH A. Sildenafil inhibits phosphodiesterase type 5 in human clitoral corpus cavernosum smooth muscle. Biochem. Biophys. Res. Commun. 1998;249:612–617. doi: 10.1006/bbrc.1998.9206. [DOI] [PubMed] [Google Scholar]
- REHMAN J., CHRIST G., MELMAN A., FLEISCHMANN J. Intracavernous pressure responses to physical and electrical stimulation of the cavernous nerve in rats. Urology. 1998;51:640–644. doi: 10.1016/s0090-4295(97)00693-6. [DOI] [PubMed] [Google Scholar]
- SAITO M., OHMURA M., KONDO A. Does potassium induce the release of nitric oxide in the rabbit corpus cavernosum. Urol. Res. 1998;26:137–141. doi: 10.1007/s002400050036. [DOI] [PubMed] [Google Scholar]
- SCHMIDT H.H.H.W., LOHMANN S.M., WALTER U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim. Biophys. Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- SEYAM R.M., BEGIN L.R., TU L.M., DION S.B., MERLIN S.L., BROCK G.B. Evaluation of a no-needle penile injector: a preliminary study evaluating tissue penetration and its hemodynamic consequences in the rat. Urology. 1997;50:994–998. doi: 10.1016/S0090-4295(97)00541-4. [DOI] [PubMed] [Google Scholar]
- SOBEY C.G., FARACI F.M. Inhibitory effect of 4-aminopyridine on responses of the basilar artery to nitric oxide. Br. J. Pharmacol. 1999;126:1437–1443. doi: 10.1038/sj.bjp.0702439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SODERLING S.H., BEAVO J.A. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr. Opin. Cell Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- STASCH J.P., BECKER E.M., ALONSO-ALIJA C., APELER H., DEMBOWSKY K., FEURER A., GERZER R., MINUTH T., PERZBORN E., PLEISS U., SCHRODER H., SCHROEDER W., STAHL E., STEINKE W., STRAUB A., SCHRAMM M. NO-independent regulatory site on soluble guanylate cyclase. Nature. 2001;410:212–215. doi: 10.1038/35065611. [DOI] [PubMed] [Google Scholar]
- STIEF C.G., UCKERT S., BECKER A.J., TRUSS M.C., JONAS U. The effect of the specific phosphodiesterase (PDE) inhibitors on human and rabbit cavernous tissue in vitro and in vivo. J. Urol. 1998;159:1390–1393. [PubMed] [Google Scholar]
- TRIGO-ROCHA F., HSU G.L., DONATUCCI C.F., LUE T.F. The role of cyclic adenosine monophosphate, cyclic guanosine monophosphate, endothelium and nonadrenergic, noncholinergic neurotransmission in canine penile erection. J. Urol. 1993;149:872–877. doi: 10.1016/s0022-5347(17)36250-x. [DOI] [PubMed] [Google Scholar]
- WALLIS R.M., CORBIN J.D., FRANCIS S.H., ELLIS P. Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am. J. Cardiol. 1999;83:3C–12C. doi: 10.1016/s0002-9149(99)00042-9. [DOI] [PubMed] [Google Scholar]
- WANG R., DOMER F.R., SIKKA S.C., KADOWITZ P.J., HELLSTROM W.J. Nitric oxide mediates penile erection in cats. J. Urol. 1994;151:234–237. doi: 10.1016/s0022-5347(17)34923-6. [DOI] [PubMed] [Google Scholar]
- WU B.N., LIN R.J., LIN C.Y., SHEN K.P., CHIANG L.C., CHEN I.J. A xanthine-based KMUP-1 with cyclic GMP enhancing and K+ channels opening activities in rat aortic smooth muscle. Br. J. Pharmacol. 2001;134:265–274. doi: 10.1038/sj.bjp.0704231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU C.C., KO F.N., KUO S.C., LEE F.Y., TENG C.M. YC-1 inhibited human platelet aggregation through NO-independent activation of soluble guanylate cyclase. Br. J. Pharmacol. 1995;116:1973–1978. doi: 10.1111/j.1476-5381.1995.tb16400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU S.N., HWANG T., TENG C.M., LI H.F., JAN C.R. The mechanism of actions of 3-(5′-(hydroxymethyl-2′-furyl)-1-benzyl indazole (YC-1) on Ca(2+)-activated K(+) currents in GH(3) lactotrophs. Neuropharmacology. 2000;39:1788–1799. doi: 10.1016/s0028-3908(00)00025-3. [DOI] [PubMed] [Google Scholar]


