Abstract
Selective Serotonin Reuptake Inhibitors (SSRIs) are thought to have a delay in therapeutic efficacy because of the need to overcome the inhibitory influence of raphe 5-HT1A autoreceptors. Prolonged SSRI administration has been reported to desensitize these autoreceptors. We have used [35S]-GTPγS autoradiography to determine whether this desensitization occurs at the level of receptor/G protein coupling.
Male mice were injected intraperitoneally once a day with saline or 20 mg kg−1 fluoxetine for either 2 days or 14 days. 5-HT1A receptor binding and coupling to G proteins were assessed using [3H]-8-OH-DPAT and [35S]-GTPγS autoradiography, respectively.
The 5-HT receptor agonist 5-carboxamidotryptamine (5-CT) stimulated [35S]-GTPγS binding in the substantia nigra, as well as in hippocampus and dorsal raphe nucleus. The 5-HT1A receptor antagonist p-MPPF (4-fluoro-N-(2-[4-(2-methoxyphenyl)1-piperazinyl]ethyl)-N-(2-pyridinyl)benzamide) blocked this effect in the latter regions, whereas the 5-HT1B/D antagonist GR-127,935 (2′-methyl-4′-(5-methyl-[1,2,4]oxadiazol-3-yl)-biphenyl-4-carboxylic acid [4-methoxy-3-(4-methyl-piperazin-l-yl)-phenyl]-amide) only decreased labelling in substantia nigra.
Fourteen-day fluoxetine treatment decreased 5-CT-stimulated [35S]-GTPγS binding in dorsal raphe (saline: 112±12% stimulation; fluoxetine: 66±13%), but not in substantia nigra (99±14% vs 103±7%) or hippocampus (157±3% vs 148±18%). Two-day fluoxetine treatment did not alter 5-CT-stimulated [35S]-GTPγS binding in any of the brain areas investigated.
Decreased [35S]-GTPγS binding was not due to receptor down-regulation, since the density of raphe [3H]-8-OH-DPAT binding sites was unaffected by fluoxetine treatment.
These results suggest that the desensitization of presynaptic 5-HT1A receptor function occurs at the level of receptor-G protein interaction on dorsal raphe neurons, and may underlie the therapeutic efficacy of long-term SSRI treatment.
Keywords: Selective Serotonin Reuptake Inhibitors, G protein coupling, [35S]-GTPγS autoradiography, serotonin receptors, serotonin autoreceptors, chronic fluoxetine treatment
Introduction
Several neurotransmitters have been implicated in the pathophysiology of major depression, including the serotonin (5-HT) and noradrenaline systems, but the neurobiological causes of the disease remain unclear (Blier & de Montigny, 1994). More is known on the mechanism of action of antidepressant drugs and several lines of evidence suggest that an enhancement of 5-HT neurotransmission might underlie the therapeutic response to different types of antidepressant treatment. Selective Serotonin Reuptake Inhibitors (SSRIs), including fluoxetine (Prozac®) are among the most effective anti-depressants and SSRIs belonging to different chemical families are thought to exert their effect by increasing extracellular 5-HT concentrations through their blocking of the serotonin reuptake carrier (Perry & Fuller, 1992; Kreiss & Lucki, 1995; Blier et al., 1990).
Microdialysis studies have shown that both acute and repeated fluoxetine administration can increase extracellular 5-HT in striatum, hippocampus and other forebrain areas (Perry & Fuller, 1992; Rutter & Auerbach, 1993; Kreiss & Lucki, 1995). However, the increase in forebrain extracellular 5-HT elicited by acute SSRI administration is limited by a negative feedback involving raphe autoreceptors, with resulting decrease in raphe neuronal activity and 5-HT release (Artigas et al., 1996). Whereas the SSRI-induced increase in extracellular 5-HT is immediate, there is a 2 to 6 week delay in therapeutic efficacy of SSRIs. This delay suggests that 5-HT reuptake inhibition per se is unlikely to account for the therapeutic activity, but that some kind of long term neuro-adaptive change underlies the antidepressant action of SSRIs. The slow onset of therapeutic response to SSRIs is hypothesized to involve the progressive desensitization of somatodendritic 5-HT1A receptors with repeated treatment (Blier & de Montigny, 1994). Such an effect would gradually allow for a return of raphe neuronal activity and increased release of 5-HT in forebrain targets of the dorsal raphe which provides the major serotonergic innervation of the neocortex (O'hearn & Molliver, 1984). This is consistent with the observation that co-administration of a 5-HT1A receptor antagonist decreases the time required for SSRIs to achieve therapeutic efficacy (Artigas et al., 1996).
Of the ∼14 receptors identified to date, the 5-HT1A receptor is one of the best-characterized (for a recent review see Raymond et al., 1999). It shows high affinity for 5-HT, 8-OH-DPAT, as well as for anxiolytic drugs such as buspirone, ipsapirone and gepirone. 5-HT1A receptors are located both presynaptically on the cell bodies and dendrites of serotonergic neurons in the raphe nuclei, and postsynaptically in forebrain areas including hippocampus, entorhinal and prefrontal cortex. Single unit recording (Blier et al., 1988) and microdialysis (Kreiss & Lucki, 1995) studies in rats have shown that 5-HT1A autoreceptors in the dorsal raphe are desensitized following chronic fluoxetine treatment. Chronic fluoxetine therapy lessens the ability of a 5-HT1A agonist, 8-OH-DPAT, to suppress 5-HT release (Kreiss & Lucki, 1995). Most studies have shown that chronic treatment with fluoxetine does not alter 5-HT1A receptor density (Welner et al., 1989; Larsson et al., 1990; Schechter et al., 1990; Hensler et al., 1991, Wieland et al., 1993; Le Poul et al., 1995, Hervás et al., 2001), although not all studies agree (Fanelli & McMonagle-Strucko, 1992). It therefore seems likely that desensitization occurs downstream of the receptor and reflects altered coupling of agonist binding to cellular response.
5-HT1A receptors are coupled to G proteins of the Gi family, which include Gi1, Gi2, Gi3, Go and Gz proteins (Raymond et al., 1993; Barr et al., 1997). We have previously shown that activation of this type of receptors can be visualized on frozen brain sections of normal rats and guinea-pigs using [35S]-GTPγS labelling (Waeber & Moskowitz, 1997), a technique developed by Sim et al. (1995). Chronic treatment with the full 5-HT1A receptor agonist 8-OH-DPAT has been shown to decrease [35S]-GTPγS labelling induced by 5-HT1A receptor activation in rat raphe nucleus (Hensler & Durgam, 2001). A related study using the azapirone anxiolytic buspirone, a partial 5-HT1A receptor agonist, reported decreased 8-OH-DPAT induced [35S]-GTPγS labelling in lateral septum as well as raphe nucleus (Sim-Selley et al., 2000). Interestingly, electrophysiological experiments have shown that chronic treatment with other azapirone drugs, gepirone and ipsapirone, did not affect 5-HT1A receptor activity in hippocampus but led to desensitization of 5-HT1A autoreceptors in dorsal raphe nucleus, in a way very similar to the effect of chronic treatment with SSRIs (Blier & de Montigny, 1987; Schechter et al., 1990). We therefore hypothesized that 5-HT1A autoreceptor desensitization in the raphe following chronic fluoxetine treatment was likely to occur at the level of receptor-G protein interaction.
In the present study, we used agonist-stimulated [35S]-GTPγS receptor autoradiography to demonstrate that chronic treatment with the SSRI fluoxetine affects receptor coupling, but not density, in the same way as chronic treatment with 5-HT1A receptor agonists (Sim-Selley et al., 2000; Hensler & Durgam, 2001).
Methods
Materials
[35S]-GTPγS was obtained from New England Nuclear (Boston, MA, U.S.A.) (specific activity 1000 – 1500 Ci mmol−1). [3H]-8-OH-DPAT was obtained from Amersham (Arlington Heights, IL, U.S.A.) (specific activity, 205 Ci mmol−1). GDP was purchased from Sigma Chemical (St. Louis, MO, U.S.A.). 5-carboxamidotryptamine, p-MPPF dihydrochloride (4-fluoro-N-(2-[4-(2-methoxyphenyl)1-piperazinyl]ethyl)-N-(2-pyridinyl)benzamide) and DPCPX (8-cyclopentyl-1,3-dipropylxanthine) were from Sigma-RBI (Natick, MA, U.S.A.). GR-127,935 (2′-methyl-4′-(5-methyl-[1,2,4]oxadiazol-3-yl)-biphenyl-4-carboxylic acid [4-methoxy-3-(4-methyl-piperazin-1-yl)-phenyl]-amide) was provided by Glaxo.
Tissues
Adult male Swiss Webster mice (25 – 30 g, Taconic, Germantown, NY, U.S.A.) received either 2-day or 14-day treatment consisting of intraperitoneal injections once a day (1000 – 1100 h) with saline or 20 mg kg−1 fluoxetine (dissolved at a concentration of 2 mg ml−1). Twenty-four hours after the last injection, they were sedated with inhaled CO2 and decapitated. The whole brain and upper cervical spinal cord were dissected and frozen in isopentane cooled at −40°C. Hindbrains (posterior to bregma-2.5 mm) were cut into 14 μm sections using a cryostat-microtome (Microm HM505E). Sections were thaw-mounted onto Superfrost Plus glass slides (Fisher Scientific, Pittsburgh, PA, U.S.A.) and stored at −80°C for less than 3 weeks.
Autoradiography
[35S]-GTPγS binding was visualized as previously described (Sim et al., 1995; Waeber & Moskowitz, 1997; Happe et al., 2001) with minor modifications. Sections were thawed to room temperature 15 min before the experiment and incubated for 15 min at room temperature in 50 mM glycylglycine buffer (pH 7.4) containing 150 mM NaCl and 1 mM EGTA, and for a further 30 min in the same buffer supplemented with 3 mM MgCl2, 0.2 mM dithiothreitol, and 2 mM GDP. In order to observe agonist-induced binding, sections were incubated in the appropriate concentration of agonist for 60 min at 30°C in buffer containing 2 mM GDP, 100 nM DPCPX (to block adenosine A1 receptors stimulated by endogenously formed adenosine; Laitinen, 1999), 10 μM pargyline, and 0.1 nM [35S]-GTPγS. Slides were then washed for 5 min twice in ice-cold buffer (without GDP), dipped briefly in ice-cold deionized water, dried under a stream of cold air, and exposed to Kodak Bio-Max film along with 14C-labelled polymer standards (Amersham) for 24 h.
5-HT1A receptor binding sites were visualized in sections consecutive to those used for [35S]-GTPγS autoradiography as follows. Sections were thawed to room temperature 15 min before the experiment and incubated for 15 min at room temperature in 0.17 M Tris·HCl (pH 7.4) containing 4 mM CaCl2. Sections were then incubated for 60 min in buffer supplemented with 2 nM [3H]-8-OH-DPAT 10 μM pargylin and 0.01% ascorbic acid, washed twice for 5 min each in ice-cold buffer, briefly dipped in ice-cold deionized water, dried under a stream of cold air and exposed to Amersham 3H-Hyperfilm for 2 weeks along with 3H-labelled polymer standards (Amersham).
Image analysis
[3H]-8-OH-DPAT and [35S]-GTPγS binding in selected brain regions were assessed by measuring the optical density of the autoradiograms using a computerized image analysis system (M4; Imaging Research, St. Catharines, Ontario, Canada). Agonist-induced [35S]-GTPγS binding is expressed as percentage of basal binding.
Data analysis
Concentration-effect curves were obtained from fitting autoradiographic measurement data points by non-linear regression using Grafit (Erithacus Software, Staines, U.K.). The equation used was Stim=Emax/(1+EC50/Ago), where Stim is the stimulated binding (in per cent over basal), Emax is the maximal binding, EC50 is the concentration of agonist resulting in half-maximal [35S]-GTPγS binding, and Ago is the agonist concentration.
To compare concentration/effect curves in saline and treated animals, the improvement of fit using two different curves (to fit data from saline- and fluoxetine-treated mice separately) over the simpler model using a single curve (to fit the pooled values from both saline- and fluoxetine-treated mice) was assessed by calculating the F statistics:
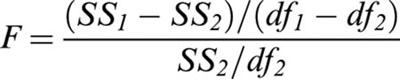 |
where the subscript 1 refers to the simplest model (single concentration/effect curves fitted to pooled data), SSi is the sum of squares of the respective residuals and dfi the respective degrees of freedom.
Basal [35S]-GTPγS binding in different regions were compared within the 2-day and 14 day treatment groups with a t-test and a Bonferroni correction (since sections from the short- and long-term treatment groups were labelled in separate experiments, no attempt was made to compare basal [35S]-GTPγS labelling between these groups).
Results
As previously described in the rat and guinea-pig (Waeber & Moskowitz, 1997), basal [35S]-GTPγS binding was heterogeneous, with relatively higher levels in interpeduncular nucleus, central grey and dorsal raphe (Figure 1). At variance with these two species however, little basal binding was found in mouse superficial grey layer of the superior colliculus. This difference is unlikely to be related to the different buffer used in the present study (Happe et al., 2001), since rat sections incubated together with mouse section did shown relatively intense basal [35S]-GTPγS labelling in the superior colliculus (data not shown).
Figure 1.
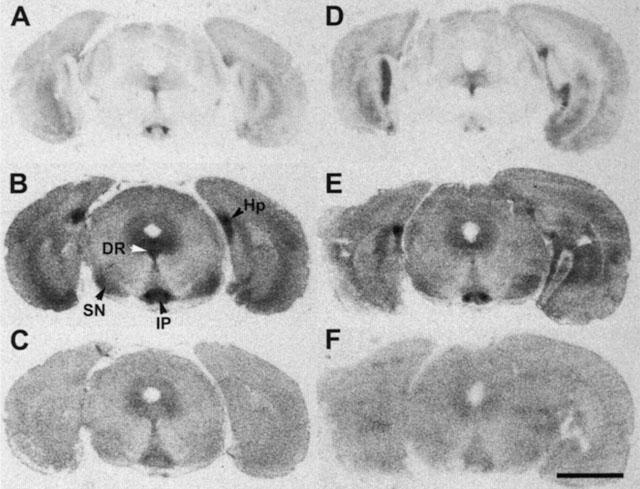
Distribution of binding sites for the selective 5-HT1A radioligand [3H]-8-OH-DPAT in representative coronal sections of a mouse treated for 14 days with saline (A) or 20 mg kg−1 fluoxetine (D). Note the similar density of binding site over the dorsal raphe (DR). (B and E) Show 5-carboxamidotryptamine (5-CT) induced [35S]-GTPγS labelling in sections consecutive to those shown in (A and D). Note the marked decrease in labelling in the dorsal raphe, but the similar density of labelling in the substantia nigra (SN), interpeduncular nucleus (IP) and hippocampus (Hp). (C and F) Show basal [35S]-GTPγS binding, relatively homogeneous at this brain level, with slightly higher intensity in the central grey and interpeduncular nucleus. Quantification revealed significantly decreased basal [35S]-GTPγS binding in the substantia nigra, but not dorsal raphe of mice treated with fluoxetine for 14 days (see Table 1). Scale bar is 2 mm.
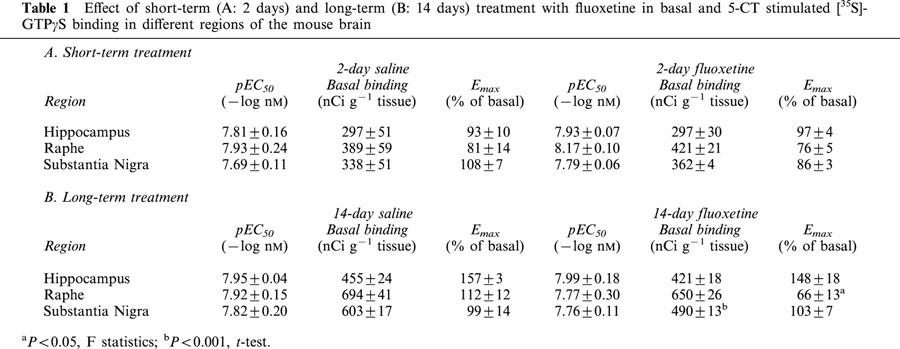
No significant differences in the level of basal [35S]-GTPγS binding were observed in the different treatment groups in the dorsal raphe and hippocampus (Table 1). Long-term (14-day), but not short-term (2-day) fluoxetine treatment significantly decreased basal [35S]-GTPγS labelling in substantia nigra.
Table 1.
Effect of short-term (A: 2 days) and long-term (B: 14 days) treatment with fluoxetine in basal and 5-CT stimulated [35S]-GTPγS binding in different regions of the mouse brain
The potent non-selective 5-HT receptor agonist 5-carboxamidotryptamine (5-CT) stimulated [35S]-GTPγS binding in hippocampus (molecular layer of dentate gyrus and strata oriens and radiatum of CA3), dorsal raphe, interpeduncular nucleus, and substantia nigra (Figure 1). In the presence of the specific 5-HT1A receptor antagonist p-MPPF (1 μM), 5-CT-stimulated [35S]-GTPγS binding virtually disappeared in hippocampus and dorsal raphe (Figure 2). This finding indicates that 5-CT-enhanced [35S]-GTPγS labelling is accounted for by 5-HT1A receptors in dorsal raphe and hippocampus, confirming previous reports in rats (Waeber & Moskowitz, 1997; Sim et al., 1997; Alper & Nelson, 1998; Dupuis et al., 1998), and in agreement with the distribution of binding sites for the specific 5-HT1A ligand [3H]-8-OH-DPAT in consecutive sections of the same mice (Figure 1A,D). In contrast, 5-CT-induced [35S]-GTPγS binding in substantia nigra was markedly decreased by the 5-HT1B/1D receptor antagonist GR-127,935 (1 μM), but was unaffected by the 5-HT1A receptor antagonist p-MPPF, suggesting that the relevant receptors in this region belong to the 5-HT1B/1D subtype.
Figure 2.
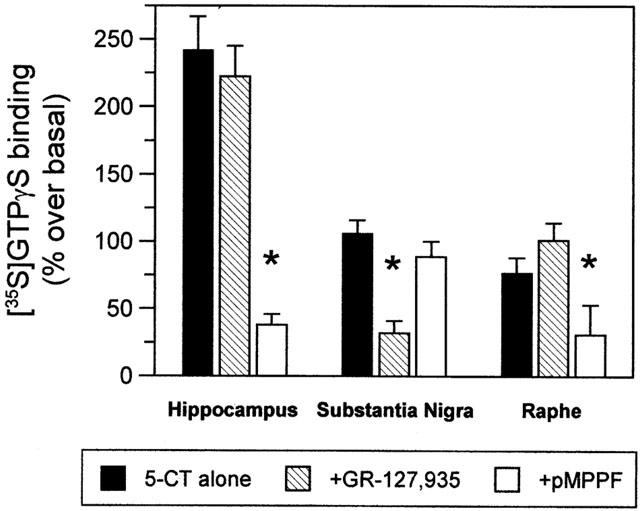
Pharmacological profile of [35S]-GTPγS binding induced by 10 μM 5-CT in mouse hippocampus, substantia nigra and dorsal raphe (bars represent mean±s.e.mean, n=5. Representative of two independent experiments). The 5-HT1A receptor antagonist p-MPPF (1 μM) virtually abolished 5-CT-induced [35S]-GTPγS binding in hippocampus and dorsal raphe, regions where the 5-HT1B/1D receptor antagonist GR-127,935 (1 μM) showed no effect. In contrast, labelling in substantia nigra was markedly attenuated by GR-127,935, but unaffected by p-MPPF. *P<0.05 (one-way analysis of variance, followed by Dunnett's post hoc test).
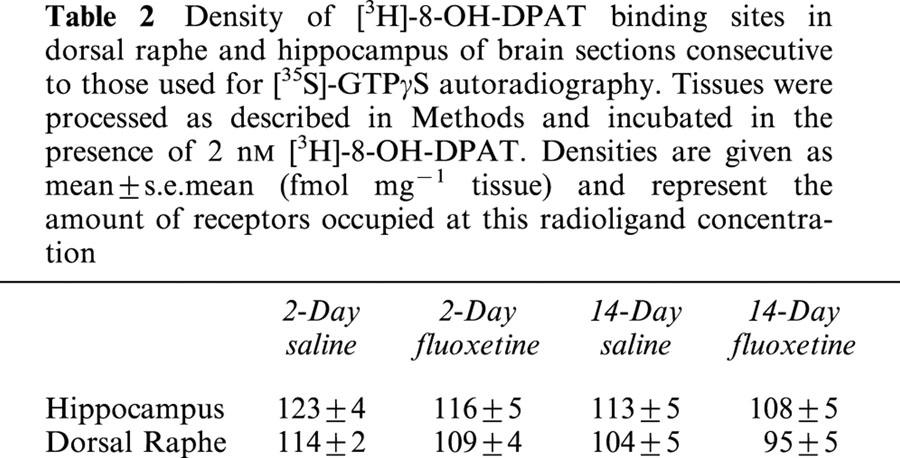
There was no significant difference in the EC50 value for 5-CT in the different regions or treatment groups (Table 1). In contrast, 14-day fluoxetine treatment significantly decreased the maximal [35S]-GTPγS binding in dorsal raphe nucleus, but not in the other brain regions investigated (Figure 3). Short-term fluoxetine treatment (2 days) did not affect EC50 values or maximal [35S]-GTPγS binding in any brain area (Table 1). None of the treatments altered the density of [3H]-8-OH-DPAT binding sites in the regions studied (Table 2).
Figure 3.
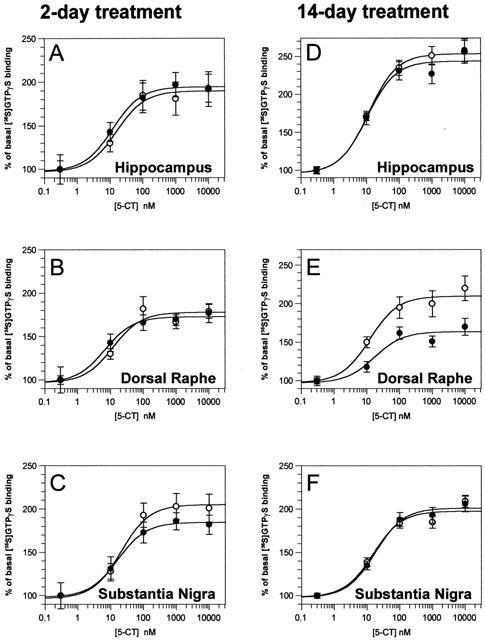
Concentration/response curves for the 5-carboxamidotryptamine (5-CT) induced [35S]-GTPγS binding in different brain regions of mice treated with saline (empty circle) or 20 mg kg−1 fluoxetine (full circles) for 2 days (left column) or 14 days (right column). Data points are the means±s.e.mean of 8 – 10 different animals per group. No attempt was made to compare data from the 2-day treatment group with data from the 14-day treatment group, because tissue sections were 35S-GTPγS-labelled in different experiments. The only significant treatment-related difference was found in the dorsal raphe of mice treated with fluoxetine for 14 consecutive days, in which [35S]-GTPγS was significantly decreased (see Table 1).
Table 2.
Density of [3H]-8-OH-DPAT binding sites in dorsal raphe and hippocampus of brain sections consecutive to those used for [35S]-GTPγS autoradiography. Tissues were processed as described in Methods and incubated in the presence of 2 nM [3H]-8-OH-DPAT. Densities are given as mean±s.e.mean (fmol mg−1 tissue) and represent the amount of receptors occupied at this radioligand concentration
Discussion
The main finding of the present study is that long-term (14-day) treatment with the SSRI fluoxetine selectively uncouples 5-HT1A receptors from G proteins in the dorsal raphe nucleus, but not in hippocampus. This effect is not observed after short-term (2-day) treatment. In addition, 5-HT1B receptor coupling in the substantia nigra is not altered after either short-term or long-term treatment.
The decrease in 5-CT induced [35S]-GTPγS labelling in dorsal raphe nucleus is unlikely to be due to a decrease in 5-HT1A receptor density, since the density of [3H]-8-OH-DPAT labelling in the raphe of fluoxetine-treated mice was not significantly different from that found in the raphe of vehicle-treated mice. Numerous previous studies have found that chronic fluoxetine administration does not alter 5-HT1A receptor binding (see Introduction for references). In addition, Hensler & Durgam (2001) have recently reported decreased 5-HT1A receptor coupling in raphe nucleus without alteration in receptor density after 14-day treatment with the 5-HT1A receptor agonist 8-OH-DPAT.
Interestingly, in a study very similar to ours, Li et al. (1997) failed to observe any effect of chronic fluoxetine treatment on the G protein coupling of raphe 5-HT1A receptors. In their study, 5-HT1A receptors were labelled in frozen brain sections with [3H]-8-OH-DPAT in the absence or presence of the GTP analogue guanylylimidodiphosphate (Gpp(NH)p). Differences in species and treatment regimen could account for the contrasting outcomes, but it is more likely that most 5-HT1A receptors in dorsal raphe exist in an uncoupled, low-affinity state, insensitive to Gpp(NH)p. Indeed, Li et al. (1997) found that less than 25% of [3H]-8-OH-DPAT binding in dorsal raphe and hippocampus was inhibited by this GTP analogue. Direct activation of [35S]-GTPγS binding therefore seems to be a more powerful technique to detect alteration in 5-HT1A receptor coupling after chronic treatment with serotonergic agents.
The fluoxetine dose that we chose has been shown to increase 5-HT content in microdialysate from mouse striatum and hippocampus for well over 200 min (Knobelman et al., 2001). We therefore hypothesize that dorsal raphe 5-HT1A receptor uncoupling results from the long-term receptor stimulation by 5-HT released from the serotonergic neurons. However, it is unlikely that the differential effect of fluoxetine treatment on raphe 5-HT1A receptors vs hippocampal 5-HT1A receptors is due to a smaller elevation in extracellular 5-HT in the latter area, since the same region-specific pattern has been observed after chronic systemic treatment with a full (Hensler & Durgam, 2001) and a partial (Sim-Selley et al., 2000) 5-HT1A receptor agonist.
The mechanism mediating the uncoupling of raphe 5-HT1A receptors following treatment with fluoxetine or 5-HT1A receptor agonists and the reason for its regional selectivity are unclear. Raphe 5-HT1A receptors are localized presynaptically to both dendritic processes and somata, whereas hippocampal 5-HT1A receptors are postsynaptic and associated exclusively with dendritic spines (Kia et al., 1996). It is possible that sub-cellular localization influences the protein environment of the receptors (e.g. G protein subtypes, kinases), which could modify the development of desensitization. [35S]-GTPγS autoradiography can be best applied to receptors coupled to Pertussis-sensitive G proteins, but there is no indication that [35S]-GTPγS binds differentially to Gi1, Gi2, Gi3, or Go (Waeber & Moskowitz, 1997). Although 5-HT1A receptors preferentially couple to Gi3 proteins, they can activate all inhibitory G proteins (Raymond et al., 1999). It is conceivable that 5-HT1A receptors in dorsal raphe are coupled to a particular G protein subtype more prone to down-regulation. Indeed, using immunoaffinity chromatography followed by immunoblotting with subtype-specific anti-Gα antibodies, Mannoury-La-cour et al. (2001) found that 5-HT1A receptors are physically coupled to Go in hippocampus, and to Gi3 in raphe nuclei. Although we did not observe any treatment-related difference in basal [35S]-GTPγS labelling in the latter region, this observation was made at a single [35S]-GTPγS concentration. Further studies will be useful to quantify the level of specific G proteins in dorsal raphe after chronic fluoxetine treatment. Li et al. (1996) have shown that chronic fluoxetine treatment reduces the concentration of Gi1 and Gi3 in hypothalamus, and Go and Gi2 in midbrain, while the latter two protein were unaltered in frontal cortex despite 22 days of treatment. The decrease that they observed in the midbrain could reflect the reduction in basal [35S]-GTPγS binding that we found in the substantia nigra of fluoxetine-treated mice.
A study of the time course of 5-HT1A receptor desensitization using electrophysiological recordings showed that the potency of 8-OH-DPAT to depress the firing of raphe neurons was reduced as early as after a 3-day fluoxetine treatment (Le Poul et al., 1995). Although we did not study the time course of 5-HT1A receptor uncoupling, our results are compatible with this study, since we do not observe any decrease in 5-CT induced [35S]-GTPγS binding after 2 days of fluoxetine treatment. Chronic treatment with the full 5-HT1A receptor agonist 8-OH-DPAT significantly attenuated 5-HT1A receptor-stimulated [35S]-GTPγS binding in dorsal raphe after both 7 and 14 days (Hensler & Durgam, 2001). It is therefore quite possible that changes in [35S]-GTPγS binding could have been observed after less than the full 14-day fluoxetine treatment that we employed.
It is interesting to note that the effects of long-term blockade of 5-HT re-uptake sites with fluoxetine is similar to those of chronic treatment with 5-HT1A receptor agonists (Sim-Selley et al., 2000; Hensler & Durgam, 2001), but not to those of genetic deletion of the 5-HT transporter. Studies in 5-HT transporter knockout mice showed that the decreased agonist induced [35S]-GTPγS binding in dorsal raphe was due to a reduced of 5-HT1A receptor density, but not to altered G-protein coupling or G-protein levels (Fabre et al., 2000; Li et al., 2000). Similar changes in 5-HT1B receptors were observed in the substantia nigra of these mice (Fabre et al., 2000). It is possible that the absence of the transporter during development produces more complex changes than transient pharmacological blockade in adult brain.
Similar results have recently been obtained in Substance P (Neurokinin-1) receptor knockout mice (Froger et al., 2001). These mice show decreased 5-HT1A receptor density and agonist-induced [35S]-GTPγS binding in dorsal raphe, but not in hippocampus (see above). This observation, taken together with the fact that the substance P receptor antagonist MK-869 shows therapeutic efficacy in depressed patients (Kramer et al., 1998), substantiates the hypothesis that enhancement of 5-HT neurotransmission underlies the therapeutic response to different types of antidepressant treatments (Blier & de Montigny, 1994).
In conclusion, the time course and regional selectivity of the changes as well as the lack of effect on the receptor density observed in the present study are similar to previous observations following chronic administration of antidepressant drugs (Blier & de Montigny, 1994). Although the present study does not rule out the occurrence of changes downstream of the G protein level or in regions other than the ones investigated, the present findings suggest that uncoupling between raphe 5-HT1A receptors and their signalling G proteins plays an important role in the delayed therapeutic effects of SSRIs. Further studies are needed to determine whether these alterations are brought about by post-translational modification of the receptors (e.g. phosphorylation), changes in the G protein population, or in another interacting molecule. Understanding specific mechanisms contributing to long-term fluoxetine-induced 5-HT1A receptor desensitization on dorsal raphe neurons could lead to the development of more effective antidepressants with a reduced delay in therapeutic activity.
Abbreviations
- Gpp(NH)p
guanylylimidodiphosphate
- GTPγS
guanosine-5′-O-(3-thio)triphosphate
- SSRI
Selective Serotonin Reuptake Inhibitors
References
- ALPER R.H., NELSON D.L. Characterization of 5-HT1A receptor-mediated [35S]GTPγS binding in rat hippocampal membranes. Eur. J. Pharmacol. 1998;343:303–312. doi: 10.1016/s0014-2999(97)01547-1. [DOI] [PubMed] [Google Scholar]
- ARTIGAS F., ROMERO L., DE MONTIGNY C., BLIER P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- BARR A.J., BRASS L.F., MANNING D.R. Reconstitution of receptors and GTP-binding regulatory proteins (G proteins) in Sf9 cells. A direct evaluation of selectivity in receptor G protein coupling. J. Biol. Chem. 1997;272:2223–2229. doi: 10.1074/jbc.272.4.2223. [DOI] [PubMed] [Google Scholar]
- BLIER P., CHAPUT Y., DE MONTIGNY C. Longterm 5-HT reuptake blockade, but not monoamine oxidase inhibition, decreases the function of terminal 5-HT autoreceptors: an electrophysiological study in the rat brain. Naunyn-Schmiedeberg's Arch. Pharmacol. 1988;337:246–254. doi: 10.1007/BF00168834. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C. Modification of 5-HT neuron properties by sustained administration of the 5-HT1A agonist gepirone: electrophysiological studies in the rat brain. Synapse. 1987;1:470–480. doi: 10.1002/syn.890010511. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C. Current advances and trends in the treatment of depression. Trends Pharmacol. Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- BLIER P., DE MONTIGNY C., CHAPUT Y. A role for the serotonin system in the mechanism of action of antidepressant treatments: preclinical evidence. J. Clin. Psychiatry. 1990;51 Suppl.:14–20. [PubMed] [Google Scholar]
- DUPUIS D.S., PALMIER C., COLPAERT F.C., PAUWELS P.J. Autoradiography of serotonin 5-HT1A receptor-activated G proteins in guinea pig brain sections by agonist-stimulated [35S]GTPγS binding. J. Neurochem. 1998;70:1258–1268. doi: 10.1046/j.1471-4159.1998.70031258.x. [DOI] [PubMed] [Google Scholar]
- FANELLI R.J., MCMONAGLE-STRUCKO K. Alteration of 5-HT1A receptor binding sites following chronic treatment with ipsapirone measured by quantitative autoradiography. Synapse. 1992;12:75–81. doi: 10.1002/syn.890120109. [DOI] [PubMed] [Google Scholar]
- FABRE V., BEAUFOUR C., EVRARD A., RIOUX A., HANOUN N., LESCH K.P., MURPHY D.L., LANFUMEY L., HAMON M., MARTRES M.P. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur. J. Neurosci. 2000;12:2299–2310. doi: 10.1046/j.1460-9568.2000.00126.x. [DOI] [PubMed] [Google Scholar]
- FROGER N., GARDIER A.M., MORATALLA R., ALBERTI I., LENA I., BONI C., DE FELIPE C., RUPNIAK N.M., HUNT S.P., JACQUOT C., HAMON M., LANFUMEY L. 5-Hydroxytryptamine (5-HT)1A Autoreceptor Adaptive Changes in Substance P (Neurokinin 1) Receptor Knock-Out Mice Mimic Antidepressant-Induced Desensitization. J. Neurosci. 2001;21:8188–8197. doi: 10.1523/JNEUROSCI.21-20-08188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAPPE H.K., BYLUND D.B., MURRIN L.C. Agonist-stimulated [35S]GTPγS autoradiography: optimization for high sensitivity. Eur. J. Pharmacol. 2001;422:1–13. doi: 10.1016/s0014-2999(01)01043-3. [DOI] [PubMed] [Google Scholar]
- HENSLER J.G., COVACHICH A., FRAZER A. A quantitative autoradiographic study of 5-HT1A receptor regulation. Effect of 5,7-dihydroxytryptamine and antidepressant treatments. Neuropsychopharmacology. 1991;4:131–144. [PubMed] [Google Scholar]
- HENSLER J.G., DURGAM H. Regulation of 5-HT1A receptor-stimulated [35S]GTPγS binding as measured by quantitative autoradiography following chronic agonist administration. Br. J. Pharmacol. 2001;132:605–611. doi: 10.1038/sj.bjp.0703855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERVAS I., VILARO T., ROMERO L., SCORZA C., MENGOD G., ARTIGAS F. Desensitization of 5-HT1A autoreceptors by a low chronic fluoxetine dose. Effect of the concurrent administration of WAY-100635. Neuropsychopharmacology. 2001;24:11–20. doi: 10.1016/S0893-133X(00)00175-5. [DOI] [PubMed] [Google Scholar]
- KIA H.K., BRISORGUEIL M.J., HAMON M., CALAS A., VERGÉ D. Ultrastructural localization of 5-hydroxytryptamine1A receptors in the rat brain. J. Neurosci. Res. 1996;46:697–708. doi: 10.1002/(SICI)1097-4547(19961215)46:6<697::AID-JNR7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- KNOBELMAN D.A., HEN R., LUCKI I. Genetic regulation of extracellular serotonin by 5-hydroxytryptamine1A and 5-hydroxytryptamine1B autoreceptors in different brain regions of the mouse. J. Pharmacol. Exp. Ther. 2001;298:1083–1091. [PubMed] [Google Scholar]
- KRAMER M.S., CUTLER N., FEIGHNER J., SHRIVASTAVA R., CARMAN J., SRAMEK J.J., REINES S.A., LIU G., SNAVELY D., WYATT-KNOWLES E., HALE J.J., MILLS S.G., MACCOSS M., SWAIN C.J., HARRISON T., HILL R.G., HEFTI F., SCOLNICK E.M., CASCIERI M.A., CHICCHI G.G., SADOWSKI S., WILLIAMS A.R., HEWSON L., SMITH D., RUPNIAK N.M., et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- KREISS D.S., LUCKI I. Effects of acute and repeated administration of antidepressant drugs on extracellular levels of 5-hydroxytryptamine measured in vivo. J. Pharmacol. Exp. Ther. 1995;274:866–876. [PubMed] [Google Scholar]
- LAITINEN J.T. Selective detection of adenosine A1 receptor-dependent G-protein activity in basal and stimulated conditions of rat brain [35S]guanosine 5′-(gamma-thio)triphosphate autoradiography. Neuroscience. 1999;90:1265–1279. doi: 10.1016/s0306-4522(98)00571-5. [DOI] [PubMed] [Google Scholar]
- LARSSON L.G., RENYI L., ROSS S.B., SVENSSON B., ANGEBY-MOLLER K. Different effects on the responses of functional pre- and postsynaptic 5-HT1A receptors by repeated treatment of rats with the 5-HT1A receptor agonist 8-OH-DPAT. Neuropharmacology. 1990;29:86–91. doi: 10.1016/0028-3908(90)90047-u. [DOI] [PubMed] [Google Scholar]
- LE POUL E., LAARIS N., DOUCET E., LAPORTE A.M., HAMON M., LANFUMEY L. Early desensitization of somato-dendritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn-Schmiedeberg's Arch. Pharmacol. 1995;352:141–148. doi: 10.1007/BF00176767. [DOI] [PubMed] [Google Scholar]
- LI Q., BATTAGLIA G., VAN DE KAR L.D. Autoradiographic evidence for differential G-protein coupling of 5-HT1A receptors in rat brain: lack of effect of repeated injections of fluoxetine. Brain Res. 1997;769:141–151. doi: 10.1016/s0006-8993(97)00693-8. [DOI] [PubMed] [Google Scholar]
- LI Q., MUMA N.A., VAN DE KAR L.D. Chronic fluoxetine induces a gradual desensitization of 5-HT1A receptors: Reductions in hypothalamic and midbrain Gi and Go proteins and in neuroendocrine responses to a 5-HT1A agonist. J. Pharmacol. Exp. Ther. 1996;279:1035–1042. [PubMed] [Google Scholar]
- LI Q., WICHEMS C., HEILS A., LESCH K.P., MURPHY D.L. Reduction in the density and expression, but not G-protein coupling, of serotonin receptors (5-HT1A) in 5-HT transporter knock-out mice: gender and brain region differences. J. Neurosci. 2000;20:7888–7895. doi: 10.1523/JNEUROSCI.20-21-07888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANNOURY-LA-COUR C., EL MESTIKAWY S., RUMAJOGEE P., BERNARD R., MIQUEL M.-C., HAMON M., LANFUMEY L.Regional differences in G proteins coupled to 5-HT1A receptors in the rat brain Soc. Neurosci. 200127380.7(abstract) [Google Scholar]
- O'HEARN E., MOLLIVER M.E. Organization of raphe-cortical projections in rat: a quantitative retrograde study. Brain Res. Bull. 1984;13:709–726. doi: 10.1016/0361-9230(84)90232-6. [DOI] [PubMed] [Google Scholar]
- PERRY K.W., FULLER R.W. Effect of fluoxetine on serotonin and dopamine concentration in microdialysis fluid from rat striatum. Life Sci. 1992;50:1683–1690. doi: 10.1016/0024-3205(92)90423-m. [DOI] [PubMed] [Google Scholar]
- RAYMOND J.R., MUKHIN Y.V., GETTYS T.W., GARNOVSKAYA M.N. The recombinant 5-HT1A receptor: G protein coupling and signalling pathways. Br. J. Pharmacol. 1999;127:1751–1764. doi: 10.1038/sj.bjp.0702723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAYMOND J.R., OLSEN C.L., GETTYS T.W. Cell-specific physical and functional coupling of human 5-HT1A receptors to inhibitory G protein alpha-subunits and lack of coupling to Gs alpha. Biochemistry. 1993;32:11064–11073. doi: 10.1021/bi00092a016. [DOI] [PubMed] [Google Scholar]
- RUTTER J.J., AUERBACH S.B. Acute uptake inhibition increases extracellular serotonin in the rat forebrain. J. Pharmacol. Exp. Ther. 1993;265:1319–1324. [PubMed] [Google Scholar]
- SCHECHTER L.E., BOLANOS F.J., GOZLAN H., LANFUMEY L., HAJ-DAHMANE S., LAPORTE A.M., FATTACCINI C.M., HAMON M. Alterations of central serotonergic and dopaminergic neurotransmission in rats chronically treated with ipsapirone: biochemical and electrophysiological studies. J. Pharmacol. Exp. Ther. 1990;255:1335–1347. [PubMed] [Google Scholar]
- SIM L.J., SELLEY D.E., CHILDERS S.R. In vitro autoradiography of receptor-activated G proteins in rat brain by agonist-stimulated guanylyl 5′-[gamma-[35S]thio]-triphosphate binding. Proc. Natl. Acad. Sci. U.S.A. 1995;92:7242–7246. doi: 10.1073/pnas.92.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIM L.J., XIAO R., CHILDERS S.R. In vitro autoradiographic localization of 5-HT1A receptor-activated G-proteins in the rat brain. Brain Res. Bull. 1997;44:39–45. doi: 10.1016/s0361-9230(97)00061-0. [DOI] [PubMed] [Google Scholar]
- SIM-SELLEY L.J., VOGT L.J., XIAO R., CHILDERS S.R., SELLEY D.E. Region-specific changes in 5-HT1A receptor-activated G-proteins in rat brain following chronic buspirone. Eur. J. Pharmacol. 2000;389:147–153. doi: 10.1016/s0014-2999(99)00875-4. [DOI] [PubMed] [Google Scholar]
- WAEBER C., MOSKOWITZ M.A. 5-Hydroxytryptamine1A and 5-Hydroxytryptamine1B receptors stimulate [35S] Guanosine-5′-O-(3-thio)triphosphate binding to rodent brain sections as visualized by in vitro autoradiography. Mol. Pharmacol. 1997;52:623–631. doi: 10.1124/mol.52.4.623. [DOI] [PubMed] [Google Scholar]
- WELNER S.A., DE MONTIGNY C., DESROCHES J., DESJARDINS P., SURANYICADOTTE B.E. Autoradiographic quantification of serotonin1A receptors in rat brain following antidepressant drug treatment. Synapse. 1989;4:347–352. doi: 10.1002/syn.890040410. [DOI] [PubMed] [Google Scholar]
- WIELAND S., FISCHETTE C.T., LUCKI I. Effect of chronic treatments with tandospirone and imipramine on serotonin-mediated behavioral responses and monoamine receptors. Neuropharmacol. 1993;32:561–573. doi: 10.1016/0028-3908(93)90052-5. [DOI] [PubMed] [Google Scholar]


