Abstract
Oxide low-density lipoprotein (ox-LDL) is believed to play an important role in early events of atherogenesis, and asymmetric dimethylarginine (ADMA) is associated with the development of endothelial dysfunction. The present study examined the effect of a single injection of native low-density lipoprotein (LDL) on endothelium function and the serum level of ADMA and the effect of probucol on endothelium function and ADMA level in rats.
Endothelial injury was induced by intravenous injection of LDL at the dose of 2, 4, or 6 mg kg−1 for 24, 48, or 72 h, and vasodilator responses to acetylcholine in the aortic rings and serum levels of ADMA, nitrite/nitrate (NO) and malondialdehyde (MDA) were determined.
Pretreatment with LDL markedly reduced endothelium-dependent relaxation in a concentration-dependent manner. Inhibition of vasodilator responses to acetylcholine by LDL was abolished in the presence of L-arginine (3×10−4 M). Serum levels of ADMA and MDA were significantly elevated in the rats pretreated with LDL, while serum level of nitrite/nitrate was markedly decreased.
Pretreatment with probucol significantly improved endothelium-dependent relaxation, decreased concentrations of ADMA and MDA and increased nitrite/nitrate level in the rats treated with LDL. A similar effect was seen in the rats pretreated with an antioxidant vitamin E.
These results suggest that a single injection of native LDL causes endothelial dysfunction by elevation of ADMA levels and that the protective effect of probucol on endothelial cells is related to reduction of ADMA concentration.
Keywords: Asymmetric dimethylarginine, low-density lipoprotein, probucol, endothelium, nitric oxide
Introduction
A great deal of information has been demonstrated that endothelial function is impaired in humans and animals with hypercholesterolaemia. It has been suggested that oxidatively modified low-density lipoprotein (ox-LDL), which inhibits endothelium-dependent relaxation of normal arteries, plays an important role in early events of atherogenesis. Recently, it has been found that methylated arginine compound such as asymmetric dimethylarginine (ADMA), which inhibits nitric oxide synthesis, is significantly raised in hypercholesterotic humans and animals (Böger et al., 1998; Yu et al., 1994; Bode-Böger et al., 1996), and endogenous inhibitors of nitric oxide synthase have been suggested to be related to the development of endothelial dysfunction.
Probucol, a drug in clinical use for treatment of hypercholesterolaemia, is also one of most potent lipophilic antioxidant compounds known. Probucol has been shown to improve the endothelium-dependent relaxation of hypercholesterolaemic animals (Simon et al., 1993). However, the mechanisms responsible for the beneficial effect of probucol on endothelial cells remain unclear. According to facilitation of the elevation of ADMA by lipid peroxide (Xiong et al., 1996) and antioxidant properties of probucol, in the present study we examined whether the protective effect of probucol on endothelial dysfunction by intravenous injection of unmodified human LDL is related to the reduction of ADMA level in rats.
Methods
Animal model
Male Sprague-Dawley rats weighing 160 – 200 g were obtained from Xiang-Ya Medical College. Vascular endothelial injury was induced by a single injection of native LDL. Animals received humane care in compliance with the ‘Guide for the Care and Use of Laboratory Animals' published by the National Institutes of Health (NIH publication 86-23, revised 1986).
Organ chamber experiments
The rats were anaesthetized with sodium pentobarbitone (60 mg kg−1, i.p.). After blood samples were collected from artery, the thoracic aorta was rapidly isolated and cut into rings of 3 mm length. The rings were suspended horizontally between two stainless steel wires and mounted in a 5 ml organ chamber filled with warmed (37°C) and oxygenated (95% O2 and 5% CO2) Krebs' solution. The Krebs' solution had the following composition (mM): NaCl, 119.0; NaHCO3, 25.0; KCl, 4.7; KH2PO4, 1.2; MgSO47H2O, 1.2; CaCl2, 2.5; and glucose, 11.0. One of ring ends was connected to a force transducer. The aortic ring was stretched with 2 g resting force and equilibrated for 60 min, and then precontracted with KCl (60 mM). After a maximal response to KCl was obtained, the rings were washed repeatedly with Krebs' solution and equilibrated again for 30 min. In order to measure vasodilator responses, rings were contracted with phenylephrine to 40∼50% of their maximal contraction. After the contraction stabilized, an accumulative concentration-response curve to acetylcholine (3×10−9-10−6 M) or sodium nitroprusside (3×10−10-10−7 M) was observed.
Determination of ADMA and SDMA concentrations
Blood samples were centrifugated at 1300×g for 15 min (4°C) and the serum was deprotein with 5-sulfosalicylic acid (5-SSA) (St Louis, MO, U.S.A.). The supernatant was used for measurement of ADMA with high-performance liquid chromatography (HPLC) as described previously with some modification (Chen et al., 1997). HPLC was carried out using a Shimadzu LC-6A liquid chromatograph with Shmadzu SCL-6A system controller and Shimadzu SIC-6A autosampler. O-Phthaldiadehyde adducts of methylated amino acids and internal standard ADMA produced by precolumn mixing were monitored using a model RF 530 fluorescence detector set at λex=338 and λem=425 nm on a Resolve C18 column. Samples were eluted from the column using a linear gradient containing mobile phase A composed of 0.05 mol l−1 (pH 6.8) sodium acetate-methanol-tetrahydrofuran (81 : 18 : 1 v v v−1) and mobile phase B composed of 0.05 mol l−1 sodium acetate-methanol-tetrahydrofuran (22 : 77 : 1 v v v−1) at a flow-rate of 1 ml min−1.
Determination of MDA and nitrite/nitrate concentrations
The content of thiobarbituric acid reactive substance reflecting level of lipid peroxide was measured spectrophotometrically, by previously described methods (Bessman & Gardner, 1983) and expressed as the amount of MDA.
The serum level of nitric oxide was determined indirectly as the content of nitrite and nitrate. Plasma level of nitrite/nitrate (NO) was measured as previously described (Feng et al., 2001). Briefly, nitrate was converted to nitrite with aspergillus nitrite reductase, and the total nitrite was measured with the Griess reagent. The absorbance was determined at 540 nm with a spectrophotometer.
Determination of creatinine concentration
The serum level of creatinine was determined by routine methods.
Experimental protocols
The first series of experiments was designed to examine the dose response of LDL-induced endothelial dysfunction. LDL at the dose of 2, 4 or 6 mg kg−1 was injected intravenously.
The second series of experiments was designed to test the time-course of LDL-induced endothelial dysfunction. The rats were pretreated with LDL (4 mg kg−1) for 24, 48, or 72 h before the experiment.
The third series of experiments was designed to evaluate the effect of probucol and vitamin E on LDL-induced endothelial injury. The rats were pretreated with probucol (75 or 150 mg kg−1, i.g.) or vitamin E (100 mg kg−1, i.g.) once a day for 5 days and then treated with LDL (4 mg kg−1, 48 h). Probucol was dissolved in a vehicle containing 10% Gum acacia and 0.5% saline carboxymethyl cellulose. For the studies on the effect of L-arginine on endothelial injury by LDL, rings were exposed to L-arginine (3×10−4 M) for 15 min before addition of phenylephrine to increase vascular tone.
Reagents
Native low-density lipoprotein, phenylephrine, acetylcholine, L-arginine and probucol were purchased from Sigma. Vitamin E and sodium nitroprusside were obtained from Xiamen Fish Liver Oil Factory (Xiamen, China) and Shuanghe Pharmaceutical Co. Ltd (Beijing, China), respectively. Nitric oxide and malondialdehyde assay kits were from Ju-Li Biological Medical Engineering Institute (Nanjing, P.R. China).
Statistic analysis
Results are expressed as means±s.e.mean. The data were analysed by ANOVA followed by the unpaired Student's t-test for multiple comparisons. The significance level was chosen as P<0.05.
Results
Vasodilator responses
Phenylephrine was added to increase smooth muscle tone in the rat aortic rings. Vasoconstrictor responses to phenylephrine (10−6 M) were significantly increased in the rats treated with LDL. The tension was 0.98±0.02 and 0.85±0.04 g for control and LDL, respectively (n=8, P<0.05). In presence of phenylephrine, acetylcholine (3×10−9-10−6 M) caused a concentration-dependent relaxation in the isolated rat aorta. After pretreatment with LDL (2, 4 or 6 mg kg−1) for 48 h, vasodilator responses to acetylcholine were significantly decreased (Figure 1A). Vasodilator responses to sodium nitroprusside (3×10−10 – 10−7 M) were not affected by pretreatment with LDL. EC50 value for sodium nitroprusside was 8.98±0.05 and 9.02±0.08 from control and LDL, respectively (n=8, P>0.05).
Figure 1.
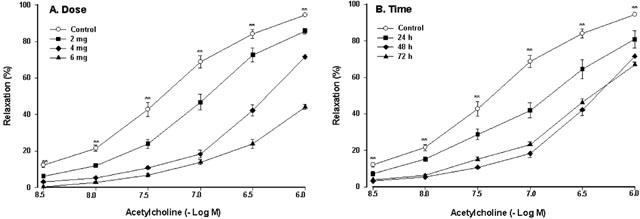
The dose-effect curve (A) and the time-effect curve (B) of vasodilator responses to acetylcholine in the isolated rat thoracic aorta in the rats pretreated with native LDL. Native LDL was given intravenously. The aorta rings were precontracted by phenylephrine (10−6 M). Values are means±s.e.mean, n=6∼8. **P<0.01 compared with control.
As shown in Figure 1B, pretreatment with LDL (4 mg kg−1) for 24, 48 or 72 h markedly decreased vasodilator responses to acetylcholine, and the maximal inhibitory effect of LDL was at 48 h.
Probucol (75 or 150 mg kg−1) significantly attenuated inhibition of vasodilator responses to acetylcholine by LDL. Vitamin E (100 mg kg−1), an antioxidant, also significantly attenuated inhibition of vasodilator responses to acetylcholine by LDL (Figure 2A). However, probucol or vitamin E itself had no effect on vasodilator responses to acetylcholine.
Figure 2.
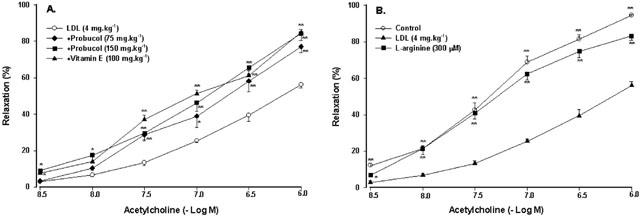
Effect of probucol, vitamin E or L-arginine on endothelium-dependent relaxation to acetylcholine. Probucol and vitamin E were given orally (A). Rings was exposed to L-arginine (B). The rats were treated with LDL at the dose of 4 mg kg−1 for 48 h. Values are means±s.e.mean, n=6∼8. *P<0.05, **P<0.01 compared with LDL.
As shown in Figure 2B, exposure of rings to L-arginine (3×10−4 M), a substrate of NO synthase, reversed the inhibition of vasodilator responses to acetylcholine by LDL.
Serum concentrations of ADMA and SDMA
Pretreatment with LDL at the dose of 2 mg kg−1 for 48 h only caused a slight increase in concentrations of ADMA (P>0.05), while at higher doses (4 or 6 mg kg−1) LDL caused a significant increase in concentrations of ADMA (Figure 3A). Pretreatment with LDL (4 mg kg−1) for 24 h only caused a slight increase in concentration of ADMA (P>0.05), while pretreatment with LDL (4 mg kg−1) for 48 or 72 h significantly increased the serum level of ADMA (Figure 3B). LDL had no effect on the serum concentrations of symmetric dimethylarginine (SDMA) (SDMA was 0.349±0.125, 0.356±0.14, 0.452±0.18 and 0.418±0.11 mol l−1 for control, 2, 4 or 6 mg kg−1 LDL, respectively, n=8, P>0.05).
Figure 3.
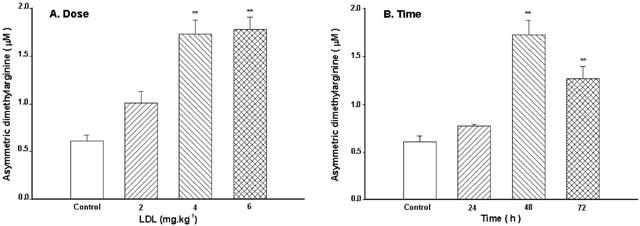
Effect of LDL on serum levels of ADMA. The dose-effect (A) and the time-effect (B) relationship of LDL. Native LDL was given intravenously. Values are means±s.e.mean, n=6∼8. **P<0.01 compared with control.
Probucol (75 or 150 mg kg−1) significantly inhibited the elevated concentration of ADMA by LDL. Vitamin E (100 mg kg−1) also markedly inhibited the elevated concentration of ADMA by LDL (Figure 4). Probucol or vitamin E itself had no effect on concentrations of ADMA.
Figure 4.
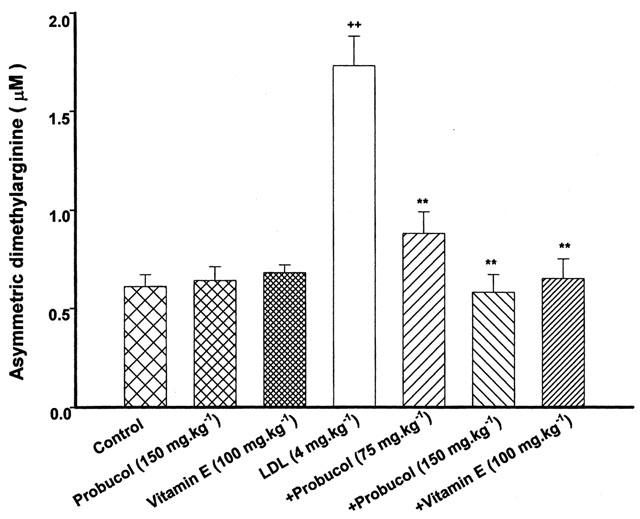
Effect of probucol or vitamin E on serum levels of ADMA. Probucol and vitamin E were given orally. The rats were treated with LDL at the dose of 4 mg kg−1 for 48 h. Values are means±s.e.mean, n=6∼8. ++P<0.01 compared with control, **P<0.01 compared with LDL.
Serum concentrations of nitrite/nitrate
Pretreatment with LDL at the dose of 2 mg kg−1 for 48 h caused a slight decrease in concentrations of nitrite/nitrate (P>0.05), while at higher doses (4 or 6 mg kg−1) LDL caused a significant decrease in concentrations of nitrite/nitrate (Figure 5A). After pretreatment with LDL (4 mg kg−1) for 24, 48 or 72 h, serum concentrations of nitrite/nitrate were significantly decreased (Figure 5B).
Figure 5.
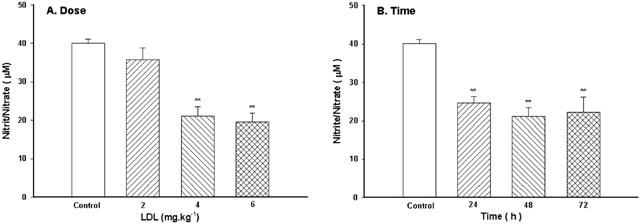
Effect of LDL on serum levels of NO. The dose-effect (A) and the time-effect (B) relationship of LDL. Native LDL was given intravenously. Values are means±s.e.mean, n=6∼8. **P<0.01 compared with control.
Probucol (75 or 150 mg kg−1) attenuated the decreased level of nitrite/nitrate by LDL. Vitamin E also attenuated significantly the decreased level of nitrite/nitrate by LDL (Figure 6). Probucol or vitamin E itself had no effect on concentrations of nitrite/nitrate.
Figure 6.
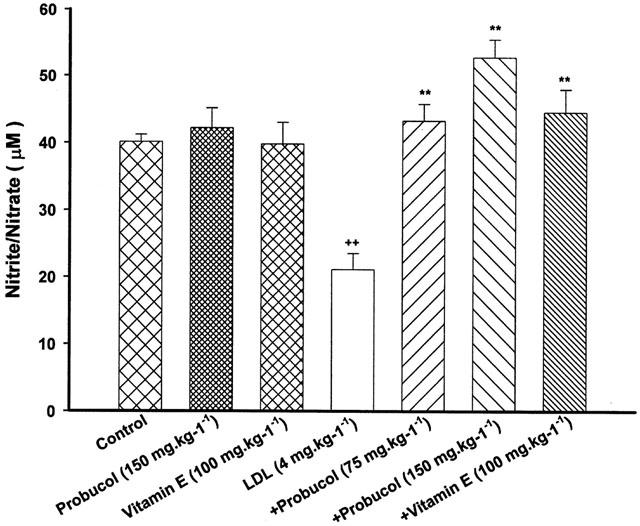
Effect of probucol or vitamin E on serum levels of NO. Probucol and vitamin E were given orally. The rats were treated with LDL at the dose of 4 mg kg−1 for 48 h. Values are means±s.e.mean, n=6∼8. ++P<0.01 compared with control, **P<0.01 compared with LDL.
Serum concentrations of MDA
Pretreatment with LDL at the dose of 2 mg kg−1 for 48 h only caused a slight increase in concentrations of MDA (P>0.05), while at higher doses (4 or 6 mg kg−1) LDL caused a significant increase in concentrations of MDA (Figure 7A). After pretreatment with LDL (4 mg kg−1) for 24, 48 or 72 h, serum concentrations of MDA were significantly increased (Figure 7B).
Figure 7.
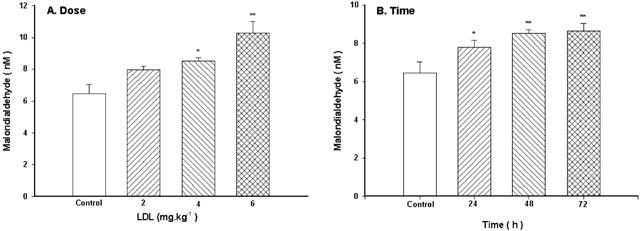
Effect of LDL on serum concentrations of MDA. The dose-effect (A) and the time-effect (B) relationship of LDL. Native LDL was given intravenously. Values are means±s.e.mean, n=6∼8. *P<0.05, **P<0.01 compared with control.
Probucol (75 or 150 mg kg−1) significantly inhibited the elevated concentration of MDA by LDL. An increased in the level of MDA by LDL was also inhibited by pretreatment with vitamin E (Figure 8). Probucol or vitamin E itself had no effect on concentrations of MDA.
Figure 8.
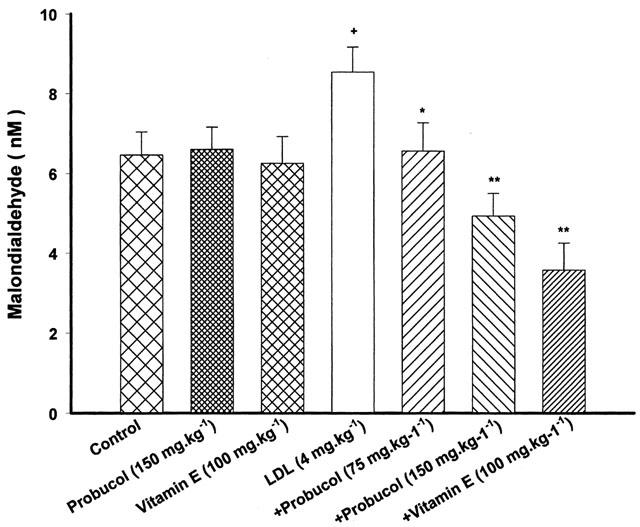
Effect of probucol or vitamin E on serum levels of MDA. Probucol and vitamin E were given orally. The rats were treated with LDL at the dose of 4 mg kg−1 for 48 h. Values are means±s.e.mean, n=6∼8. +P<0.05 compared with control, *P<0.05, **P<0.01 compared with LDL.
Serum concentrations of creatinine
There were no differences in serum concentrations of creatinine between the control and LDL group. The level of creatinine was 33.4±3.3 and 35.5±4.2 μmol l−1 for control and LDL, respectively (n=8, P>0.05).
Discussion
There is growing evidence that ox-LDL plays a key role in the development of atherosclerotic lesions. Ox-LDL exhibits numerous biological effects, including endothelial dysfunction, activation of endothelial adhesiveness, monocyte differentiation and adhesion, and smooth muscle cell proliferation (Kugiyama et al., 1990; Frostegard et al., 1990; 1991; Koba et al., 2000). However, most of these studies were observed in isolated vascular tissues or cultured endothelial cells. Recently, it has been reported that intravenous injection of cholesterol oxidation products causes endothelial dysfunction and increases macromolecular permeability in rabbits (Rong et al., 1998). Others have shown that a single injection of native LDL (4 mg kg−1) causes a rapid accumulation of LDL in the arterial wall, where it becomes oxidatively modified within 6 to 12 h, and induces an inflammatory reaction such as activation of nuclear factor-κB and the expression of intercellular adhesion molecule-1 in the arterial walls in rats (Calara et al., 1998). In the present study, we tested the effect of a single injection of native LDL on endothelium-dependent relaxation and the level of NO. The results showed that a single injection of native LDL markedly decreased in endothelium-dependent relaxation to acetylcholine and in serum concentrations of NO and increased vasoconstrictor responses to phenylephrine in this animal model.
NO, an important local regulator factor in cardiovascular tissues, is synthesized from L-arginine by NO synthase in endothelial cells. It possesses complex cardiovascular actions such as regulating vascular smooth muscle tone, protecting endothelial cells, and inhibiting vascular smooth muscle cell proliferation (Hegarty et al., 2001; Zanetti et al., 2000; Ignarro et al., 2001). L-arginine analogues such as ADMA, which is present in blood of both humans and animals, can inhibit NO synthesis in vivo (Vallance et al., 1992) and in vitro (Kurose et al., 1995). It has been documented that endogenous ADMA is formed by protein arginine methyltransferases (PRMTs), which utilizes S-adenosylmethionine as methyl group donor, and metabolized by a enzyme dimethylarginine dimethylaminohydrolase (DDAH), which hydrolyzes ADMA to L-citrulline and dimethylamine asymmetric dimethylarginine (Leiper & Vallance, 1999; Böger et al., 2000b; Ito et al., 1999). There is growing evidence that endothelial dysfunction in some cardiovascular diseases such as hypercholesterolaemia, heart failure and hypertension is associated with elevation of ADMA, and endogenous inhibitors of nitric oxide synthase have been thought as a novel predictor of endothelial dysfunction (Böger et al., 1998; Usui et al., 1998; Surdacki et al., 1999). And exogenous supplement with L-arginine decreased endothelium injury by hypercholesterolemia (Duan et al., 2000). In the present study, a single injection of native LDL caused a marked decrease in vasodilator responses to acetylcholine concomitantly with an increase in the level of ADMA, and exogenous supplement with L-arginine, a substrate of NO synthase, attenuated significantly the inhibition of endothelium-dependent relaxation by LDL. Previous investigations have shown that the level of endogenous ADMA is raised in rabbits and monkeys with chronic hypercholesterolaemia (Xiong et al., 1996; Böger et al., 2000a). In culture endothelial cells, native LDL or ox-LDL induces an increase in the level of ADMA, which is related to the increased activity of PRMTs and the decreased activity of DDAH (Böger et al., 2000b; Ito et al., 1999). Studies in clinic have also shown that serum concentrations of ADMA are increased in patients with hypercholesterolemia (Böger et al., 1998). Our results and findings of other authors suggest that ox-LDL caused the elevated level of ADMA, with a subsequent inhibition of nitric oxide synthesis, resulting in endothelial dysfunction.
Probucol, which has a potent antioxidant activity, inhibits peroxidant generation (Simon et al., 1993) and blocks the oxidative modification of LDL (Parthasarathy et al., 1986; Daugherty et al., 1989). Probucol can preserve endothelial function in hypercholesterolemic animals (Kita et al., 1987), and the beneficial effect of probucol on endothelial cells is ascribed to its antioxidant activity (Kaneko et al., 1996). As mentioned above, incubation of endothelial cells with ox-LDL causes a significant increase in the level of ADMA (Böger et al., 2000b). Our previous work has also showed that chronic hypercholesterolemia caused a marked increase in the serum level of ADMA concomitantly with the elevated concentration of lipid peroxidant in rabbits (Xiong et al., 1996). These findings suggest that an increase in the level of ADMA may be due to elevation of lipid peroxidant. Based on antioxidant properties of probucol, it is probable that the protective effect of probucol on endothelial function is related to the reduction of ADMA level. In the present study, LDL caused an increase in serum concentrations of ADMA with the elevated level of lipid peroxidant, and probucol treatment significantly reduced the elevated level of both ADMA and lipid peroxidant by LDL. To further confirm whether the effect of probucol is related to its antioxidant property, the effect of vitamin E, which is a potent chain-breaking antioxidant but dose not affect lipid metabolism, on ADMA level was observed. The results revealed that supplement with vitamin E also decreased concentrations of MDA and ADMA and improved the endothelium-dependent vasodilation. A similar effect has been seen in hypercholesterolemic rabbits (Xiong et al., 1996). These findings support the hypothesis that protective effect of probucol on endothelium is related to inhibition of the elevated level of ADMA via reduction of lipid peroxides.
In summary, this study suggests that a single injection of native LDL produces endothelial dysfunction via elevation of ADMA levels in rats. The present results also suggest that the beneficial effect of probucol on endothelial cells is related to reduction of ADMA concentration.
Acknowledgments
This work was supported by a grant from State Education Commission, P.R. China.
Abbreviations
- ADMA
asymmetric dimethylarginine
- LDL
low-density lipoprotein
- MDA
malondialdehyde
- NO
nitric oxide
- ox-LDL
oxide low-density lipoprotein
- SDMA
symmetric dimethylarginine
- SNP
sodium nitroprusside
References
- BESSMAN J.D., GARDNER F.H. Platelet size in thrombocytopenia due to sepsis. Surg. Gynecol. Obstet. 1983;156:177–180. [PubMed] [Google Scholar]
- BODE-BÖGER S.M., BÖGER R.H., KIENKE S., JUNKER W., FRÖLICH J.C. Elevated L-arginine/dimethylarginie ratio contributes to enhanced systemic NO production by dietary L-arginine in hypercholesterolemic rabbits. Biochem. Biophys. Res. Commun. 1996;219:598–603. doi: 10.1006/bbrc.1996.0279. [DOI] [PubMed] [Google Scholar]
- BÖGER R.H., BODE-BÖGER S.M., SYDOW K., HEISTAD D.D., LENTZ S.R. Plasma concentration of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, is elevated in monkeys with hyperhomocyst(e)inemia or hypercholesterolemia. Arterioscler. Thromb. Vasc. Biol. 2000a;20:1557–1564. doi: 10.1161/01.atv.20.6.1557. [DOI] [PubMed] [Google Scholar]
- BÖGER R.H., BODE-BÖGER S.M., SZUBA A., TSAO P.S., CHAN J.R., TANGPHAO O., BLASCHKE T.F., COOKE J.P. Asymmetric dimethylarginine: a novel risk factor for endothelial dysfunction: its role in hypercholesterolemia. Circulation. 1998;98:1842–1847. doi: 10.1161/01.cir.98.18.1842. [DOI] [PubMed] [Google Scholar]
- BÖGER R.H., SYDOW K., BORLAK J., THUM T., LENZEN H., SCHUBERT B., TSIKAS D., BODE-BÖGER S.M. LDL cholesterol upregulates synthesis of asymmetrical dimethylarginine in human endothelial cells: involvement of S-adenosylmethionine-dependent methyltransferases. Circ. Res. 2000b;87:99–105. doi: 10.1161/01.res.87.2.99. [DOI] [PubMed] [Google Scholar]
- CALARA F., DIMAYUGA P., NIEMANN A., THYBERG J., DICZFALUSY U., WITZTUM J.L., PALINSKI W., SHAH P.K., CERCEK B., NILSSON J., REGNSTROM J. An animal model to study local oxidation of LDL and its biological effects in the arterial wall. Arterioscler. Thromb. Vasc. Biol. 1998;18:884–893. doi: 10.1161/01.atv.18.6.884. [DOI] [PubMed] [Google Scholar]
- CHEN B.M., XIA L.W., ZHAO R.Q. Determination of NG,NG-dimethylarginine in human plasma by high-performance liquid Chromatography. J. Chromatogr. B. Biomed. Sci. Appl. 1997;692:467–471. doi: 10.1016/s0378-4347(96)00531-2. [DOI] [PubMed] [Google Scholar]
- DAUGHERTY A., ZWEIFEL B.S., SCHONFELD G. Probucol attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Br. J. Pharmacol. 1989;98:612–618. doi: 10.1111/j.1476-5381.1989.tb12635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUAN J., MUROHARA T., IKEDA H., KATOH A., SHINTANI S., SASAKI K., KAWATA H., YAMAMOTO N., IMAIZUMI T. Hypercholesterolemia inhibits angiogenesis in response to hindlimb ischemia: nitric oxide-dependent mechanism. Circulation. 2000;102:III370–iii376. doi: 10.1161/01.cir.102.suppl_3.iii-370. [DOI] [PubMed] [Google Scholar]
- FENG Q., LU X., JONES D.L., SHEN J., ARNOLD J.M. Increased inducible nitric oxide synthase expression contributes to myocardial dysfunction and higher mortality after myocardial infarction in mice. Circulation. 2001;104:700–704. doi: 10.1161/hc3201.092284. [DOI] [PubMed] [Google Scholar]
- FROSTEGARD J., HAEGERSTRAND A., GIDLUND M., NILSSON J. Biologically modified LDL increases the adhesive properties of endothelial cells. Atherosclerosis. 1991;90:119–126. doi: 10.1016/0021-9150(91)90106-d. [DOI] [PubMed] [Google Scholar]
- FROSTEGARD J., NILSSON J., HAEGERSTRAND A., HAMSTEN A., WIGZELL H., GIDLUND M. Oxidized low density lipoprotein induces differentiation and adhesion of human monocytes and the monocytic cell line U937. Proc. Natl. Acad. Sci. U.S.A. 1990;87:904–908. doi: 10.1073/pnas.87.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEGARTY N.J., YOUNG L.S., KIRWAN C.N., O'NEILL A.J., BOUCHIER-HAYES D.M., SWEENEY P., WATSON R.W., FITZPATRICK J.M. Nitric oxide in unilateral ureteral obstruction: Effect on regional renal blood flow. Kid. Int. 2001;59:1059–1065. doi: 10.1046/j.1523-1755.2001.0590031059.x. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., BUGA G.M., WEI L.H., BAUER P.M., WU G., DEL SOLDATO P. Role of the arginine-nitric oxide pathway in the regulation of vascular smooth muscle cell proliferation. Proc. Natl. Acad. Sci. U.S.A. 2001;98:4202–4208. doi: 10.1073/pnas.071054698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITO A., TSAO P.S., ADIMOOLAM S., KIMOTO M., OGAWA T., COOKE J.P. Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation. 1999;99:3092–3095. doi: 10.1161/01.cir.99.24.3092. [DOI] [PubMed] [Google Scholar]
- KANEKO M., HAYASHI J., SAITO I., MIYASAKA N. Probucol downregulates E-selectin expression on cultured human vascular endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1996;16:1047–1051. doi: 10.1161/01.atv.16.8.1047. [DOI] [PubMed] [Google Scholar]
- KITA T., NAGANO Y., YOKODE M., ISHII K., KUME N., OOSHIMA A., YOSHIDA H., KAWAI C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc. Natl. Acad. Sci. U.S.A. 1987;84:5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOBA S., PAKALA R., WATANABE T., KATAGIRI T., BENEDICT C.R. Synergistic interaction between thromboxane A2 and mildly oxidized low density lipoproteins on vascular smooth muscle cell proliferation. Prostagland. Leukot. Essent. Fatty Acids. 2000;63:329–335. doi: 10.1054/plef.2000.0223. [DOI] [PubMed] [Google Scholar]
- KUGIYAMA K., KERNS S.A., MORRISETT J.D., ROBERTS R., HENRY P.D. Impairment of endothelium-dependent arterial relaxation by lysolecithin in modified low-density lipoproteins. Nature. 1990;344:160–162. doi: 10.1038/344160a0. [DOI] [PubMed] [Google Scholar]
- KUROSE I., WOLF R., GRISHAM M.B., GRANGER D.N. Effects of an endogenous inhibitor of nitric oxide synthesis on postcapillary venules. Am. J. Physiol. 1995;268:H2224–H2231. doi: 10.1152/ajpheart.1995.268.6.H2224. [DOI] [PubMed] [Google Scholar]
- LEIPER J., VALLANCE P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc. Res. 1999;43:542–548. doi: 10.1016/s0008-6363(99)00162-5. [DOI] [PubMed] [Google Scholar]
- PARTHASARATHY S., YOUNG S.G., WITZUM J.L., PITTMAN R.C., STEINBERG D. Probucol inhibits oxidative modification of low density lipoprotein. J. Clin. Invest. 1986;77:641–644. doi: 10.1172/JCI112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RONG J.X., RANGASWAMY S., SHEN L., DAVE R., CHANG Y.H., PETERSON H., HODIS H.N., CHISOLM G.M., SEVANIAN A. Arterial injury by cholesterol oxidation products causes endothelial dysfunction and arterial wall cholesterol accumulation. Arterioscler. Thromb. Vasc. Biol. 1998;18:1885–1894. doi: 10.1161/01.atv.18.12.1885. [DOI] [PubMed] [Google Scholar]
- SIMON B.C., HANDENSCHILD C.C., COHEN R.A. Preservation of endothelium-dependent relaxation in atherosclerotic rabbit aorta by probucol. J. Cardiovasc. Pharmacol. 1993;21:893–901. doi: 10.1097/00005344-199306000-00007. [DOI] [PubMed] [Google Scholar]
- SURDACKI A., NOWICKI M., SANDMANN J., TSIKAS D., BÖGER R.H., BODE-BÖGER S.M., KRUSZELNICKA-KWIATKOWSKA O., KOKOT F., DUBIEL J.S., FROELICH J.C. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J. Cardiovasc. Pharmacol. 1999;33:652–658. doi: 10.1097/00005344-199904000-00020. [DOI] [PubMed] [Google Scholar]
- USUI M., MATSUOKA H., MIYAZAKI H., UEDA S., OKUDA S., IMAIZUMI T. Increased endogenous nitric oxide synthase inhibitor in patients with congestive heart failure. Life Sci. 1998;62:2425–2430. doi: 10.1016/s0024-3205(98)00225-2. [DOI] [PubMed] [Google Scholar]
- VALLANCE P., LEONE A., CALVER A., COLLIER J., MONCADA S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339:572–575. doi: 10.1016/0140-6736(92)90865-z. [DOI] [PubMed] [Google Scholar]
- XIONG Y., LI Y.J., YU X.J., LIU G.Z., LI N.S. Endogenous inhibitor of nitric oxide synthesis and lipid peroxidation in hyperlipidemic rabbits. Zhongguo Yao Li Xue Bao. 1996;17:149–152. [PubMed] [Google Scholar]
- YU X.J., LI Y.J., XIONG Y. Increase of an endogenous inhibitor of nitric oxide synthesis in serum of high cholesterol fed rabbits. Life Sci. 1994;54:753–758. doi: 10.1016/0024-3205(94)00443-9. [DOI] [PubMed] [Google Scholar]
- ZANETTI M., KATUSIC Z.S., O'BRIEN T. Expression and function of recombinant endothelial nitric oxide synthase in human endothelial cells. J. Vasc. Res. 2000;37:449–456. doi: 10.1159/000054077. [DOI] [PubMed] [Google Scholar]


