Abstract
Proteinase-activated receptor-1 (PAR-1) is activated by thrombin and can be selectively activated by synthetic peptides (PAR-1-activating peptide: PAR-1-AP) corresponding to the receptor's tethered ligand. PAR-1 being expressed by afferent neurons, we investigated the effects of PAR-1 agonists on nociceptive responses to mechanical and thermal noxious stimuli. Intraplantar injection of selective PAR-1-AP increased nociceptive threshold and withdrawal latency, leading to mechanical and thermal analgesia, while control peptide had no effect. Intraplantar injection of thrombin also showed analgesic properties in response to mechanical, but not to thermal stimulus. Co-injection of PAR-1-AP with carrageenan significantly reduced carrageenan-induced mechanical and thermal hyperalgesia, while thrombin reduced carrageenan-induced mechanical but not thermal hyperalgesia. The fact that thrombin is not a selective agonist for PAR-1 may explain the different effects of thrombin and PAR-1-AP. These results identified analgesic properties for selective PAR-1 agonists that can modulate nociceptive response to noxious stimuli in normal and inflammatory conditions.
Keywords: Analgesia, thrombin, proteinase-activated receptors, pain, nociception
Introduction
Proteinases such as thrombin, trypsin, mast cell tryptase, cathepsin G, etc. can act as signalling molecules, through the activation of proteinase-activated receptors (PARs) (Coughlin, 1999; Vergnolle et al., 2001a). The proteolytic cleavage of PARs N-terminal domain reveals a tethered ligand that binds and activates the receptor itself. Synthetic peptides corresponding to this proteolytically revealed new N-terminal domain (PAR-activating peptides) constitute selective agonists for these receptors. Among the PARs family, Proteinase-Activated Receptor-2 (PAR-2) is activated by trypsin and mast cell trypase, while PAR-1, Proteinase-Activated Receptor-3 (PAR-3) and Proteinase-Activated Receptor-4 (PAR-4) are considered thrombin receptors (Coughlin, 1999; Scarborough et al., 1992; Vergnolle et al., 2001a). Thrombin and PAR-1 agonists elicit an inflammatory reaction, partly mediated by a neurogenic mechanism involving the release of substance P and the activation of neurokinin-1 receptors (de garavilla et al., 2001). It has been shown that PAR-1 is expressed on sensory neurons (de garavilla et al., 2001; Steinhoff et al., 2000) but the effects of thrombin or PAR-1 activation on nociceptive response has never been investigated. Recently, we described that PAR-2 agonists signal primary spinal afferent neurons to cause marked and sustained hyperalgesia (Vergnolle et al., 2001b). This study suggested a direct role for PAR-2 and the proteinases that activate this receptor in the transmission of pain. In the present study, we investigated the effects of thrombin and PAR-1 agonists (doses below the threshold to induce an inflammatory reaction) on nociception in response to a noxious mechanical or thermal stimulus. We described that in contrast to PAR-2, PAR-1 agonists did not cause hyperalgesia, but rather induced an increased nociceptive threshold characteristic of analgesia. Moreover, in the inflammatory situation: carrageenan-induced paw inflammation, selective PAR-1 agonists were able to reduce the hyperalgesic response caused by the inflammatory stimulus. These results reveal a possible role for PAR-1 as a modulator of nociceptive signals.
Methods
Animals
Male Wistar (200 – 250 g) rats were obtained from Charles River Breeding Farms Ltd. Montreal, Quebec and were housed in plastic cages in a temperature controlled room. The rats were fed standard pellets and water was provided ad libitum. All procedures were approved by Institutional Animal Care and Ethic Committees.
Chemicals
The PAR-1-AP TFLLR-NH2 was used in this study and was preferred to the peptide corresponding to the tethered ligand of rat PAR-1 (SFLLR-NH2) because of its selectivity for PAR-1: TFLLR-NH2 activates PAR-1 but not PAR-2, PAR-3 or PAR-4, while SFLLR-NH2 activates PAR-1 and PAR-2 (Vergnolle et al., 2001a). PAR-1-activating peptide (TFLLR-NH2) and control peptide (RLLFT-NH2) were obtained from the peptide synthesis facility of the University of Calgary, Faculty of Medicine (director, Dr D. Master). The composition and the purity of peptides were confirmed by HPLC analysis. All the peptides were dissolved in saline. Thrombin and carrageenan were purchased from Sigma St. Louis, U.S.A.
Measure of nociception
The mechanical nociceptive flexion reflex and the paw withdrawal latency in response to a radiant heat stimulus were evaluated respectively with a Ugo Basile Analgesymeter and a plantar test apparatus (Stoelting, Chicago, IL, U.S.A.) as previously described (Vergnolle et al., 2001b). After a baseline measure (time 0), PAR-1-AP (TFLLR-NH2), control peptide (RLLFT-NH2), thrombin or boiled thrombin were injected (in a total volume of 100 μl) into the rat paw under light halothane anaesthesia. Nociceptive thresholds and paw withdrawal latency were then measured at different times after this intraplantar injection. For the dose-response curves, nociceptive threshold and withdrawal latency were measured 1 h after the intraplantar injection. The same procedure was used in rats that received an intraplantar injection of carrageenan (2% in 50 μl), but the peptides and thrombin were diluted in a final volume of 50 μl and were injected separately from carrageenan. Mechanical and thermal hyperalgesia were defined as a significant decrease in respectively nociceptive threshold and withdrawal latency, while analgesia was defined as a significant increase in nociceptive threshold and withdrawal latency.
Assessment of inflammation
As an index of oedema formation, paw volume was measured with a hydroplethismometer in conscious rats, before and after the intraplantar injections. Myeloperoxidase (MPO) activity was measured in rat paw tissues, 6 h after the intraplantar injection and according to previously described methods (de garavilla et al., 2001; Steinhoff et al., 2000; Vergnolle et al., 2001b).
Assessment of neurotoxicity of PAR-1-AP and thrombin
To address the possibility of a direct neurotoxic action of PAR-1 agonists on the physiological properties of axons, we stimulated and recorded from rat sciatic nerve axons in vivo exposed to local application of the agents. Multifibre motor conduction studies of sciatic-tibial fibres were carried out under pentobarbitone anaesthesia (65 mg kg−1) in Sprague-Dawley rats (weight 275 – 500 g) by stimulating at the sciatic notch and knee and recording the M (compound muscle action) potential with subcutaneous platinum electrodes dorsally over the tibial innervated interosseous muscles of the hind paw. Fibre conduction recordings in motor axons exposed to near nerve thrombin (5 u) or TFLLR-NH2 (10 μg) were carried out before, and every 15 min for 2 h, after injection of the peptide. Conduction velocities were calculated in the notch to knee segment and the amplitude of the M potential measured from baseline to peak. The M potential amplitude reflects numbers of conduction motor units, but does decline by approximately 25% with prolonged recordings from local hindpaw.
Statistical analysis
Data are presented as mean±s.e.m. Withdrawal latency and nociceptive threshold were analysed with a one-way analysis of variance followed by Dunnett's test. Data from fibre conduction studies were analysed by one-way analysis of variance (ANOVA) with appropriate Bonferroni. With all statistical analysis, P<0.05 was considered significant.
Results
PAR-1 agonists induce analgesia
Intraplantar administration of 10 μg of the selective PAR-1 agonist TFLLR-NH2 significantly increased nociceptive threshold and withdrawal latency from 30 to 90 min after its injection (Figure 1a – d). Nociceptive threshold and withdrawal latency returned to basal by 3 h after TFLLR-NH2 injection. In contrast, administration of the control peptide RLLFT-NH2 had no effect on nociceptive threshold or withdrawal latency. A dose response study indicated that a lower dose of TFLLR-NH2 (1 μg paw−1) was still effective in reducing nociceptive threshold (Figure 1b) and withdrawal latency (Figure 1d). Intraplantar administration of TFLLR-NH2 can induce inflammation of the paw. As a result, we determined the dose of TFLLR-NH2 that produced analgesia but did not cause inflammation. While the intraplantar injection of 500 μg of TFLLR-NH2 caused granulocyte infiltration as observed by increased MPO activity (Figure 1e), and caused marked oedema (Figure 1f), doses up to 10 μg of TFLLR-NH2 did not induce granulocyte infiltration or oedema, but still induced analgesia (Figure 1a – f).
Figure 1.
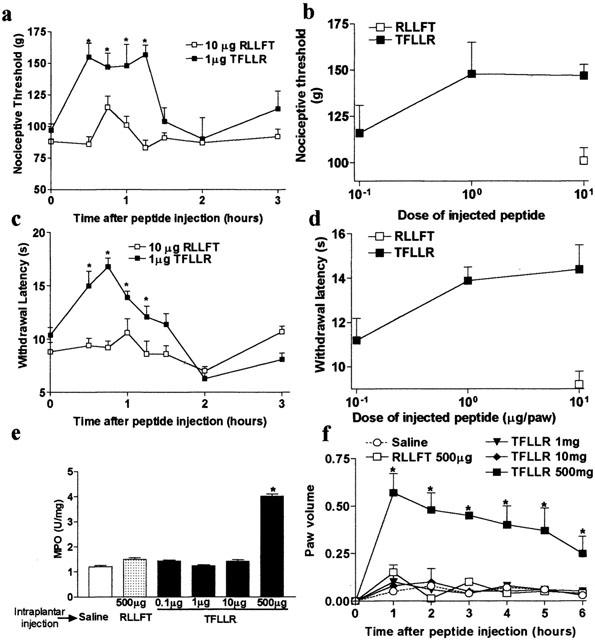
PAR-1-induced mechanical (a,b) (nociceptive threshold) and thermal (c,d) (withdrawal latency) analgesia, granulocyte infiltration (e) and paw oedema (f) in rats. n=6 – 9 rats per group. For (a), (c) and (f), *P<0.05 compared to basal measurement (time 0) and for (e), *P<0.01 compared to control peptide (RLLFT-NH2) injected group.
Thrombin induces mechanical analgesia and thermal hyperalgesia
Thrombin (5 u paw−1), which can activate PAR-1, also caused a significant increase in nociceptive threshold characteristic of analgesia, when injected into the rat hindpaw (Figure 2a,b). A maximum effect of thrombin on nociceptive threshold was observed at 30 min after its injection, while nociceptive threshold returned to baseline levels by 90 min. The dose-response curve of thrombin analgesia response to a mechanical stimulus showed that 5 u thrombin paw−1 produced a maximal response (Figure 2b). A higher dose (10 u thrombin paw−1) did not cause analgesia. In contrast to TFLLR-NH2, thrombin intraplantar injection reduced withdrawal latency and caused thermal hyperalgesia from 30 min to 2 h after the injection. This effect was dose-dependent (Figure 2d). The doses of 1 and 5 u paw−1 of thrombin did not cause granulocyte infiltration as observed by MPO activity levels (Figure 2e), but the intraplantar injection of 5 u paw−1 of thrombin caused small but significant oedema (Figure 2f). The dose of 10 u paw−1 of thrombin caused both granulocyte infiltration and marked oedema (Figure 2e,f). Injection of boiled thrombin did not affect nociceptive threshold withdrawal latency and did not cause changes in paw volume or MPO activity (Figure 2a – f).
Figure 2.
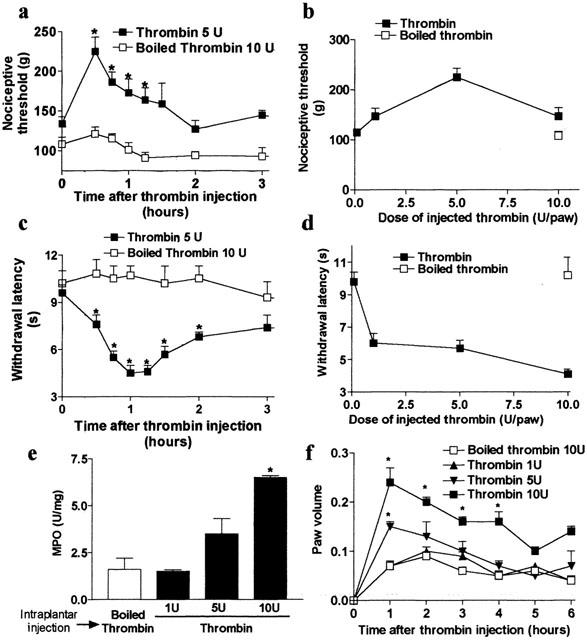
Thrombin-induced mechanical analgesia (a,b) and thermal hyperalgesia (c,d), granulocyte infiltration (e) and paw oedema (f) in rats. n=6 – 9 rats per group. For (a), (c) and (f), *P<0.05 compared to basal measurement (time 0) and for (e), *P<0.01 compared to boiled thrombin injected group.
PAR-1-induced analgesia is not related to axonal neurotoxicity
M potential amplitudes and sciatic-tibial conduction velocity did not significantly change during exposure to either agent. The findings identified no evidence of direct or nonspecific toxicity on axons exposed to thrombin or TFLLR-NH2.
PAR-1 agonists attenuate inflammatory hyperalgesia
Intraplantar injection of carrageenan caused a significant reduction of nociceptive threshold and withdrawal latency, characteristic of mechanical and thermal hyperalgesia, respectively. This is denoted by the decreased area under the curve compared to saline intraplantar injection (Figure 3a,b). Intraplantar administration of the selective PAR-1 agonist TFLLR-NH2 markedly reduced mechanical (Figure 3a) and thermal (Figure 3b) hyperalgesia caused by carrageenan. The control peptide RLLFT-NH2 had no effect. The dose of TFLLR-NH2 that inhibited carrageenan-induced hyperalgesia did not affect the inflammatory reaction as shown by the lack of change in MPO activity or paw oedema between carrageenan+TFLLR-NH2− or carrageenan+RLLFT-NH2− treated groups (Figure 3c,d).
Figure 3.
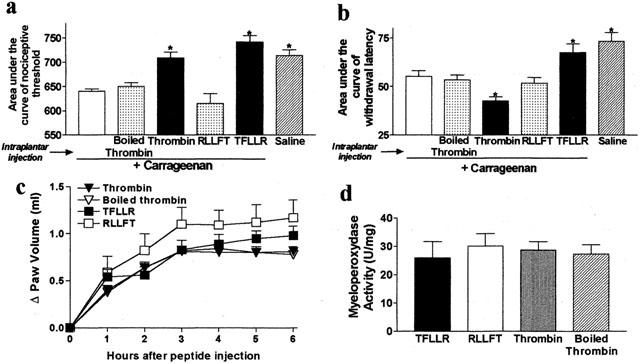
Effects of intraplantar co-injection of carrageenan and selective PAR-1 agonists and thrombin on carrageenan-induced inflammatory hyperalgesia (a,b), granulocyte infiltration (c) and oedema (d). The changes in nociceptive threshold and withdrawal latency were calculated for each rat as the area under the curve versus time (over a 6 h period) and results are presented as mean±s.e.mean. For all, n=8 rats per group and for (a and b), *P<0.05 compared to carrageenan alone. No significant differences were observed in all groups for (c) and (d).
Thrombin attenuates inflammatory mechanical hyperalgesia but augments inflammatory thermal hyperalgesia
When injected into the paw of rats that have concomitantly received carrageenan, thrombin showed a mechanical anti-hyperalgesic effect as shown by a significant increase of the area under the curve of nociceptive threshold when compared to rats that were treated with carrageenan alone (Figure 3a). In contrast, thrombin increased the carrageenan-induced hyperalgesic response to a thermal stimulus, as the area under the curve of withdrawal latency was significantly reduced in rats that received thrombin+carrageenan compared to rats treated with carrageenan alone (Figure 3b). Inactivated thrombin (boiled) had no effect on carrageenan-induced mechanical or thermal hyperalgesia. Thrombin had no effect on the magnitude of the inflammatory reaction induced by carrageenan as observed by the lack of change in MPO activity or paw volume when compared to rats injected with the boiled enzyme (Figure 3c,d).
Discussion
The discovery of the proteinase-activated receptor system has pointed out new potential roles for proteinases that can act not only as degradative enzymes but also as signalling molecules through the activation of G protein-coupled receptors (Coughlin, 1999; Scarborough et al., 1992; Vergnolle et al., 2001a). Proteinases are largely released during inflammatory processes, and recent findings have suggested a role for proteinases like thrombin, trypsin and tryptase in inducing and maintaining inflammatory reactions through the activation of PAR-1 and PAR-2 (Vergnolle et al., 1999a, 1999b; 2001a; Vergnolle, 1999). Two recent studies have shown that PAR-1 and PAR-2 are expressed by primary spinal afferent neurons and that their selective agonists elicit acute inflammation partly via a neurogenic mechanism (de garavilla et al., 2001; Steinhoff et al., 2000). The presence of PARs on afferent C-fibres suggests that PARs might be implicated in nociceptive pathways. Indeed, in a previous study, we showed that PAR-2 activation signals nociceptive neurons to cause a marked and sustained hyperalgesia. This suggested a role for proteinases and PAR-2 in pain transmission (Vergnolle et al., 2001b). In the present study, we have identified an opposite effect for PAR-1. Activation of PAR-1 by selective agonists attenuated nociception, caused analgesia in non-inflammatory conditions and reduced inflammatory hyperalgesia. These effects of PAR-1 agonists on the pain response were independent of modulation of the inflammatory response.
Our study showed that local application of selective PAR-1 agonists attenuated the nociceptive response to noxious mechanical or thermal stimuli and significantly decreased inflammatory mechanical and thermal hyperalgesia induced by intraplantar injection of carrageenan. Thrombin, which activates PAR-1 by cleaving its N-terminal domain reproduced the effects of the selective PAR-1 agonist TFLLR-NH2 on mechanical analgesia and also inhibited carrageenan-induced mechanical hyperalgesia. However, thrombin had opposite effects on nociception in response to a thermal stimulus. Intraplantar injection of thrombin caused thermal hyperalgesia (Figure 2c) and amplified the thermal carrageenan-induced hyperalgesia (Figure 3b). The hyperalgesic effects of thrombin cannot be explained by a pro-inflammatory effect of thrombin since doses of thrombin that caused hyperalgesia (1 and 5 u paw−1, Figure 2c,d) did not increase MPO activity or cause oedema (Figure 2e,f). Co-injection of thrombin with carrageenan did not amplify the inflammatory response caused by carrageenan (Figure 3c,d). Moreover, if inflammation was responsible for thrombin hyperalgesic effect in response to a thermal noxious stimulus, hyperalgesia instead of analgesia would also have been observed in response to a mechanical stimulus. The hyperalgesic effect of thrombin may be explained by the fact that thrombin is not a selective agonist for PAR-1. Thrombin's actions are mediated by activation of PAR-1, but also by activation of PAR-3 and PAR-4 (although PAR-3 is considered as a co-factor for PAR-4 activation (Nakanishi-Matsui et al., 2000). Thrombin's actions can also be mediated by its non-catalytic site (Bar-Shavit et al., 1983; 1984; Herbert et al., 1994). Thus, it is possible that thrombin causes thermal hyperalgesia by activating a receptor different from PAR-1 and located on nociceptive fibres. Thrombin is also known to have anti-inflammatory properties, but the analgesic properties of thrombin on nociceptive response to a mechanical stimulus can also not be explained by a decreased inflammatory response. Doses of thrombin that caused mechanical analgesia (1 and 5 u paw−1, Figure 2a,b) did not modify MPO activity of paw volume (Figure 2e,f). Furthermore, co-injection of thrombin with carrageenan did not reduce the inflammatory response caused by carrageenan (Figure 3c,d). The lack of analgesia induced by the intraplantar injection of 10 u thrombin paw−1 (Figure 2b) might be explained by the inflammatory reaction caused by this dose, which significantly increased MPO and paw volume (Figure 2e,f).
Thrombin and selective PAR-1-APs have been shown to induce neuronal damage (Weinstein et al., 1998; Turgeon et al., 1999; Festoff et al., 2000). We wanted to address the possibility that the analgesic effect we observed after the intraplantar injection of PAR-1 agonists might be due to a toxic effect of these agonists on axons. Multifibre motor conduction studies of sciatic-tibial fibres directly exposed to either thrombin or the selective PAR-1 agonist TFLLR-NH2 showed that M potential amplitudes and conduction velocity did not significantly change with exposure to either agent. These results confirmed that the analgesic effects of PAR-1 agonists are not related to non-specific neurotoxicity.
It has been established that cyclic AMP and adenyl cyclase have been implicated in nociceptive pathways and contribute to inflammatory pain (Taiwo & Levine, 1991; Sluka, 1997). Several studies have also shown that the thrombin receptor PAR-1 is negatively coupled to adenyl cyclase (Manolopoulos et al., 1997; Seiler et al., 1996). Thus, the PAR-1 induced analgesia might be explained by inhibition of adenyl cyclase. We are presently investigating this hypothesis. Thrombin is believed to be the endogenous agonist for PAR-1, but pharmacological studies have shown that a high concentration of trypsin is also able to activate PAR-1. We cannot rule out the possibility that PAR-1 action on neurons might be activated by a proteinase other than thrombin. However, if thrombin is the endogenous PAR-1 agonist, our results show that in inflammatory situations thrombin release might exert an anti-hyperalgesic (or analgesic) effect. The PAR-1-mediated analgesic effect of thrombin would be effective only in response to a mechanical stimulus since we have shown in the present study that thrombin caused hyperalgesia in response to a thermal stimulus. In vivo, the PAR-1-mediated analgesic effect of thrombin may be reduced due to other effects of thrombin on different fibres and on receptors other than PAR-1. On the other hand, selective PAR-1 agonists might constitute effective anti-nociceptive drugs.
We showed that peripheral activation of PAR-1 by selective PAR-1 agonists induced mechanical and thermal analgesia. Local application of PAR-1 agonists is also able to markedly reduce inflammatory hyperalgesia. These findings reveal a previously unknown mechanism of modulation of pain transmission through the activation of PAR-1. Although their efficacy in a clinical setting has yet to be demonstrated, the present study highlights PAR-1 selective agonists as possible analgesic agents.
Acknowledgments
This work was supported by grants from the Canadian Institute of Health Research and NicOx, S.A. to N. Vergnolle.
Abbreviations
- M
Compound Muscle Action
- MPO
Myeloperoxydase
- PAR-1-AP
PAR-1-activating peptide
- PAR-1
Proteinase-Activated Receptor-1
- PAR-2
Proteinase-Activated Receptor-2
- PAR-3
Proteinase-Activated Receptor-3
- PAR-4
Proteinase-Activated Receptor-4
- PARs
Proteinase-Activated Receptors
References
- BAR-SHAVIT R., KAHN A., MUDD M., WILNER G., MANN K., FENTON J. Localization of a chemotactic domain in human thrombin. Biochemistry. 1984;23:397–400. doi: 10.1021/bi00298a001. [DOI] [PubMed] [Google Scholar]
- BAR-SHAVIT R., KAHN A., WILNER G., FENTON J. Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science. 1983;220:728–731. doi: 10.1126/science.6836310. [DOI] [PubMed] [Google Scholar]
- COUGHLIN S.R. How the protease thrombin talks to cells. Proc. Natl. Acad. Sci. U.S.A. 1999;96:11023–11027. doi: 10.1073/pnas.96.20.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE GARAVILLA L., VERGNOLLE N., YOUNG S.H., ENNES H., STEINHOFF M., OSSOVSKAYA V.S., D'ANDREA M.R., MAYER E.A., WALLACE J.L., HOLLENBERG M.D., ANDRADE-GORDON P., BUNNETT N.W. Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism. Br. J. Pharmacol. 2001;133:975–987. doi: 10.1038/sj.bjp.0704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FESTOFF B.W., D'ANDREA M.R., CITRON B.A., SALCEDO R.M., SMIRNOVA I.V., ANDRADE-GORDON P. Motor neuron cell death in wobbler mutant mice follows overexpression of the G-protein-coupled protease-activated receptor for thrombin. Mol. Med. 2000;6:410–429. [PMC free article] [PubMed] [Google Scholar]
- HERBERT J., DUPUY E., LAPLACE M., ZINI J., BAR-SHAVIT R., TOBELEM G. Thrombin induces endothelial cell growth via both a proteolytic and non-proteolytic pathway. Biochem. J. 1994;303:227–231. doi: 10.1042/bj3030227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANOLOPOULOS V., FENTON J.W., LELKES P. The thrombin receptor in adrenal medullary microvascular endothelial cells is negatively coupled to adenylyl cyclase through a Gi protein. Biochim. Biophys. Acta. 1997;1356:321–332. doi: 10.1016/s0167-4889(97)00002-5. [DOI] [PubMed] [Google Scholar]
- NAKANISHI-MATSUI M., ZHENG Y.W., SULCINER D.J., WEISS E.J., LUDEMAN M.J., COUGHLIN S.R. PAR3 is a cofactor for PAR4 activation by thrombin. Nature. 2000;404:609–613. doi: 10.1038/35007085. [DOI] [PubMed] [Google Scholar]
- SCARBOROUGH R.M., NAUGHTON M.A., TENG W., HUNG D.T., ROSE J., VU T.K., WHEATON V.I., TURCK C.W., COUGHLIN S.R. Tethered ligand agonist peptides. Structural requirements for thrombin receptor activation reveal mechanism of proteolytic unmasking of agonist function. J. Biol. Chem. 1992;267:13146–13149. [PubMed] [Google Scholar]
- SEILER S., MICHEL I.M., FENTON J.W. Involvement of the ‘tethered-ligand' receptor in thrombin inhibition of platelet adenylate cyclase. Biochem. Biophys. Res. Com. 1996;182:1296–1302. doi: 10.1016/0006-291x(92)91873-o. [DOI] [PubMed] [Google Scholar]
- SLUKA K. Activation of the cAMP transduction cascade contributes to the mechanical hyperalgesia and allodynia induced by intradermal injection of capsaicin. Br. J. Pharmacol. 1997;122:1165–1173. doi: 10.1038/sj.bjp.0701486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINHOFF M., VERGNOLLE N., YOUNG S., TOGNETTO M., AMADESI S., ENNES H., TREVISANI M., HOLLENBERG M.D., WALLACE J.L., CAUGHEY G., MITCHELL S., WILLIAMS L., GEPPETTI P., MAYER E., BUNNETT N. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- TAIWO Y., LEVINE J.D. Further confirmation of the role of adenyl cyclase and of cAMP-dependent protein kinase in primary afferent hyperalgesia. Neuroscience. 1991;44:131–135. doi: 10.1016/0306-4522(91)90255-m. [DOI] [PubMed] [Google Scholar]
- TURGEON V., MILLIGAN C., HOUENOU L. Activation of the protease-activated receptor (PAR-1) induces motoneuron degeneration in the developing avian embryo. J. Neuropathol. Exp. Neurol. 1999;58:499–504. doi: 10.1097/00005072-199905000-00009. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J. Immunol. 1999;163:5064–5069. [PubMed] [Google Scholar]
- VERGNOLLE N., BUNNETT N.W., SHARKEY K.A., BRUSSEE V., COMPTON S., GRADY E.F., CIRINO G., GERARD N., BASBAUM A., ANDRADE-GORDON P., HOLLENBERG M.D., WALLACE J.L. Proteinase-activated receptor-2 and hyperalgesia: a novel pain pathway. Nat. Med. 2001b;7:821–826. doi: 10.1038/89945. [DOI] [PubMed] [Google Scholar]
- VERGNOLLE N., HOLLENBERG M.D., SHARKEY K., WALLACE J.L. Characterization of the inflammatory response to proteinase-activated receptor-2 (PAR-2)-activating peptides in the rat paw. Br. J. Pharmacol. 1999b;127:1083–1090. doi: 10.1038/sj.bjp.0702634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., HOLLENBERG M.D., WALLACE J.L. Pro- and anti-inflammatory actions of thrombin: a distinct role for proteinase-activated receptor-1 (PAR1) Br. J. Pharmacol. 1999a;126:1262–1268. doi: 10.1038/sj.bjp.0702408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N., WALLACE J.L., BUNNETT N.W., HOLLENBERG M.D. Protease-activated receptors in inflammation, neuronal signaling and pain. Trends Pharmacol. Sci. 2001a;22:146–152. doi: 10.1016/s0165-6147(00)01634-5. [DOI] [PubMed] [Google Scholar]
- WEINSTEIN J., LAU A., BRASS L.F., CUNNINGHAM D. Injury-related factors and conditions down-regulate the thrombin receptor (PAR-1) in human neuronal cell line. J. Neurochem. 1998;71:1034–1050. doi: 10.1046/j.1471-4159.1998.71031034.x. [DOI] [PubMed] [Google Scholar]


