Abstract
The activation of eosinophils via G-protein-coupled seven transmembran receptors play a necessary role in the recruitment of these cells into tissue. The present study investigates a role for PAF in driving eotaxin production and eosinophil recruitment in an allergic pleurisy model in mice.
The intrapleural injection of increasing doses of PAF (10−11 to 10−9 moles per cavity) induced a dose- and PAF receptor-dependent recruitment of eosinophils 48 h after stimulation.
Intrapleural injection of PAF induced the rapid (within 1 h) release of eotaxin into the pleural cavity of mice and an anti-eotaxin antibody effectively inhibited PAF-induced recruitment of eosinophils.
Eosinophil recruitment in the allergic pleurisy was markedly inhibited by the PAF receptor antagonist UK-74,505 (modipafant, 1 mg kg−1). Moreover, recruitment of eosinophils in sensitized and challenged PAF receptor-deficient animals was lower than that observed in wild-type animals.
Blockade of PAF receptors with UK-74,505 suppressed by 85% the release of eotaxin in the allergic pleurisy.
Finally, the injection of a sub-threshold dose of PAF and eotaxin cooperated to induce eosinophil recruitment in vivo.
In conclusion, the production of PAF in an allergic reaction could function in multiple ways to facilitate the recruitment of eosinophils – by facilitating eotaxin release and by cooperating with eotaxin to induce greater recruitment of eosinophils.
Keywords: Eosinophils, eotaxin, platelet-activating factor, recruitment, chemokines
Introduction
Eosinophils are typically tissue-dwelling cells and appear to play an important role in the pathogenesis of allergic diseases, such as asthma and atopic dermatitis (Cara et al., 1999). There has been much interest in the understanding of the mechanisms underlying eosinophil recruitment in vivo as this knowledge may aid in the development of novel strategies for the treatment of allergic disorders (Teixeira et al., 1995; Giembycz & Lindsay, 1999). The activation of eosinophils via G-protein-coupled seven transmembrane receptors play a necessary role in the recruitment of these cells into tissue and may, thus, be good targets for drug development (Teixeira et al., 1995; 1997a,1997b).
We have recently shown that the cytokine stem cell factor (SCF) played an important role for the migration of eosinophils in an allergic pleurisy model in mice (Klein et al., 2000). Of interest, SCF appeared to drive the local production of LTB4 which cooperated with eotaxin to induce eosinophil recruitment (Klein et al., 2001). Whereas SCF induced significant eotaxin production, the blockade of SCF did not inhibit the release of eotaxin following allergen challenge of sensitized animals (Klein et al., 2001). Thus, it was not clear from our studies the mediator(s) underlying local eotaxin production and release.
Several studies have demonstrated the ability of the lipid mediator platelet-activating factor (PAF) to induce eosinophil recruitment and activation in vivo and in vitro (e.g. Silva et al., 1991; Teixeira et al., 1994; 1997a; Alves et al., 1996). Indeed, PAF induces chemoattraction, activation of several other functions and priming of eosinophils and other cell types (e.g. van der bruggen et al., 1994; Schweizer et al., 1996; Ishii & Shimizu, 2000). Moreover, endogenous production of PAF may be involved in the effector function of eosinophils (e.g.: Tool et al., 1992; Bartemes et al., 1999) and in the modulation of the production of chemokines (e.g.: Maruoka et al., 2000; Au et al., 2001) in response to several stimuli. Here, using a PAF receptor antagonist (UK-74,505) and PAF receptor-deficient animals (PAFR−/−), we investigated whether PAF participated in the cascade of events leading to mediator release and eosinophil recruitment in our allergic pleurisy model.
Methods
Animals
Male BALB/C mice (18 – 22 g) were used throughout these experiments. Animals were housed in a temperature-controlled room with free access to water and food. All experimental procedures have been subjected to evaluation and were approved by the local Animal Ethics Committee. PAF receptor deficient animals were generated as previously described (Ishii et al., 1998) and intercrossed for at least seven generations to establish the BALB/c strain.
Drugs and reagents
Recombinant murine eotaxin was purchased from Peprotech (London, U.K.). Eotaxin was dissolved in water, diluted further in phosphate buffered saline (PBS, pH 7.4) containing 0.01% bovine serum albumin and stored at −20°C until use. Bovine serum albumin, ovalbumin (OVA) and control rabbit serum were purchased from Sigma. PAF was purchased from Calbiochem. The specific and long-acting PAF receptor antagonist UK-74,505 (Modipafant, 4-(chlorophenyl)-1,4-dihydro-3-ethoxycarbonyl-6-methyl-2-[4-(2-methylimidazol[4, 5-c]phenyl-5-[N-(2-pyridyl)carbomoyl]pyridine) (Alabaster et al., 1991; Jezequel et al., 1996) was a gift from Pfizer Global Research and Development, Kent, U.K. UK-74,505 was dissolved in HCl 0.01 N and further diluted in PBS. Control animals received drug vehicle.
Sensitization
Animals were immunized with OVA adsorbed to aluminium hydroxide gel as previously described (Das et al., 1997). Briefly, mice were injected s.c. on days 1 and 8 with 0.2 ml of a solution containing 100 μg of OVA and 70 μg of aluminium hydroxide (Reheiss, Dublin, Ireland).
Leukocyte migration into the pleural cavity induced by PAF or antigen
PAF (10−11 to 10−9 mol per cavity) was injected intrapleurally (i.pl.) in naïve mice, and animals killed at 48 h after the i.pl. injection. In some experiments, low doses of eotaxin and PAF were mixed prior to their i.pl. injection. Sensitized mice were challenged with antigen (OVA) or PBS. The cells present in the pleural cavity were harvested by injecting 2 ml of PBS and total cell counts performed in a modified Neubauer chamber using Turk's stain. Differential cell counts were performed on cytospin preparations (Shandon III) stained with May-Grumwald-Giemsa using standard morphologic criteria to identify cell types. The results are presented as the number of cells per cavity.
PAF receptor antagonist or anti-eotaxin pretreatment
In order to investigate the role of endogenous PAF on the eosinophil recruitment induced by PAF or ovalbumin in immunized animals, the PAF receptor antagonist UK-74,505 (0.1 to 1.0 mg kg−1) was administered i.p. 60 min prior to the stimulus, and the number of infiltrating eosinophils was assessed 48 hours after. This dose range has been shown to be effective at blocking PAF receptors in rodents (Miotla et al., 1998; Borges et al., 2000). A rabbit polyclonal anti-eotaxin antibody was prepared and purified over protein A column as previously described (Ruth et al., 1998). This antibody is specific for eotaxin and not shown to cross-react to other known murine chemokines (Ruth et al., 1998). Anti-eotaxin was administered i.p. at the dose of 100 μg per mouse 60 min prior to the i.pl. administration of PAF. In some experiments, the eosinophil recruitment 48 h after antigen challenge in PAFR−/− mice was compared to that of age- and sex-matched wild-type animals.
Measurement of eotaxin
Frozen supernatants obtained from pleural cavity washes at different times (1 – 24 h) after challenge with PAF (10−9 moles per cavity) were used for eotaxin detection. The effects of the PAF receptor antagonist was assessed by injecting UK-74,505 (1 mg kg−1, i.p.) 60 min prior to antigen challenge. Six hours later, the pleural cavity was washed and the levels of eotaxin assessed on the supernatant.
The concentration of eotaxin protein in pleural effluents was measured by specific ELISA using commercially available antibody pairs and as specified by the supplier (R&D Systems, Minneapolis, MN, USA).
Statistical analysis
All results are presented as the mean±s.e.mean. Normalized data were analysed by one-way ANOVA, and differences between groups were assessed using the Student-Newman-Keuls post-test. A P value <0.05 was considered significant.
Results
PAF induces eosinophil recruitment and eotaxin production in the pleural cavity of mice
The intrapleural injection of increasing doses of PAF (10−11 to 10−9 moles per cavity) induced a dose-dependent recruitment of eosinophils 48 h after stimulation (Figure 1). At this time point, a significant recruitment of mononuclear cells, but not neutrophils, was also observed (data not shown). These effects of PAF were PAF receptor-dependent as demonstrated by the ability of the PAF receptor antagonist UK-74,505 to abrogate PAF-induced eosinophil recruitment (PBS, 0.2±0.1 eosinophils×105 per cavity; PAF 10−9 moles, 1.4±0.3; PAF+UK-74,505 0.1 mg kg−1; 0.4±0.1; PAF+UK-74,505 1.0 mg kg−1, 0.2±0.1; n=5 in each group, P<0.01).
Figure 1.
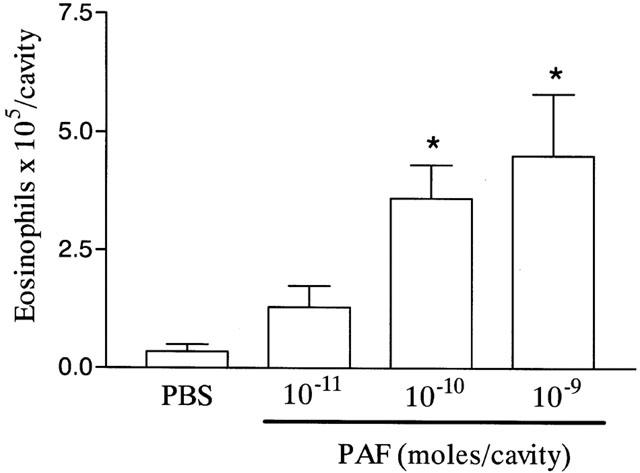
Dose-dependent effects of PAF on the recruitment of eosinophils to the pleural cavity of mice. In dose-response experiments, phosphate-buffered saline (PBS, 100 μl) or PAF (10−11 to 10−9 moles per cavity) were administered into the pleural cavity of naïve mice and the number of infiltrating eosinophils assessed 48 h after injection. The results are expressed as mean±s.e.mean of six mice in each group. *P<0.01 when compared to PBS-injected animals.
Next, we examined whether PAF could induce the release of eotaxin into the pleural cavity of mice. As seen in Figure 2A, significant levels of eotaxin were detected as early as 1 h after the injection of PAF in the pleural cavity of naïve mice. Elevated concentrations of eotaxin were also detected at 3 h and levels had dropped to background 6 h after PAF injection (Figure 2A). Not only was eotaxin induced after injection of PAF, but, more importantly, an anti-eotaxin antibody effectively inhibited the recruitment of eosinophils observed after injection of PAF (Figure 2B).
Figure 2.
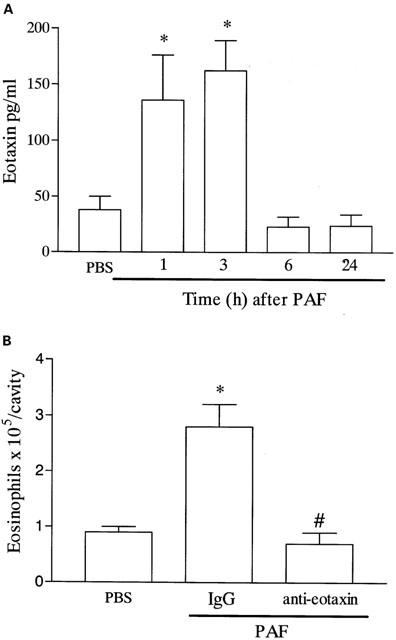
Time-course of the release of eotaxin into the pleural cavity after administration of PAF and effects of an anti-eotaxin polyclonal antibody on PAF-induced eosinophil recruitment. (A) Naïve mice were given an i.pl. injection of PAF (10−9 moles per cavity) and at different times after challenge (1 – 24 h), the pleural cavity of animals were washed, the cells centrifuged, and the supernatants used for the determination of eotaxin using a specific ELISA. (B) Naïve mice were pretreated with non-immune IgG (100 μg, i.p.) or purified anti-eotaxin polyclonal antibody (100 μg, i.p.) 30 min before the i.pl. injection of PAF (10−9 moles per cavity) and the number of infiltrating eosinophils assessed after 48 h. Results are expressed as mean±s.e.mean of six mice in each group. *P<0.01 when compared to PBS-injected mice and #P<0.01 when compared to animals treated with non-immune IgG.
Blockade of PAF receptors regulate the release of eotaxin and eosinophil recruitment in the allergic pleurisy
The possibility that endogenous production of PAF modulated eosinophil recruitment in the allergic pleurisy was investigated by using the PAF receptor antagonist UK-74,505. Pretreatment with UK-74,505 (1 mg kg−1) significantly suppressed by 87% the recruitment of eosinophils observed 48 h after administration of antigen to sensitized mice (Figure 3A). As PAF induced significant local release of eotaxin, we examined whether blockade of PAF receptors would modulate eotaxin production in the allergic pleurisy. Our previous studies have demonstrated that eotaxin release peaks after 6 h in sensitized animals challenged with antigen (Klein et al., 2001). Here, we demonstrate that pretreatment with UK-74,505 inhibited antigen-induced eotaxin production by 85% (Figure 4).
Figure 3.
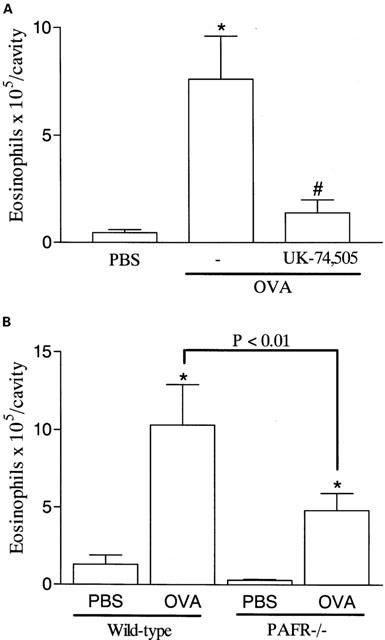
Effects of the PAF receptor antagonist, UK-74,505, and of PAF receptor deficiency on the eosinophil recruitment induced by allergen challenge in sensitized mice. (A) Mice were pretreated with UK-74,505 (1 mg kg−1, i.p.) 60 min before the i.pl. injection of antigen (OVA, 1 μg per cavity) in sensitized mice and the number of infiltrating eosinophils assessed after 48 h. (B) Immunized wild-type or PAFR−/− received an i.pl. injection of antigen (OVA, 1 μg per cavity) and the number of infiltrating eosinophils assessed after 48 h. Results are expressed as means±s.e.mean of six mice in each group. *P<0.01 when compared to PBS-injected mice and #P<0.01 when compared to animals treated with drug vehicle.
Figure 4.
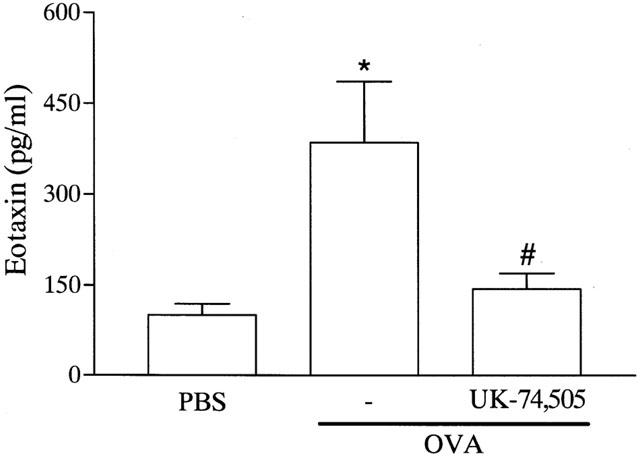
Effects of the PAF receptor antagonist, UK-74,505, on the release of eotaxin induced by allergen challenge in sensitized mice. Mice were pretreated with UK-74,505 (1 mg kg−1, i.p.) 60 min before the i.pl. injection of antigen (OVA, 1 μg per cavity) in sensitized mice and the levels of eotaxin on the pleural wash assessed after 6 h. Results are expressed as means±s.e.mean of six mice in each group. *P<0.01 when compared to PBS-injected mice and #P<0.01 when compared to animals treated with drug vehicle.
To confirm an important role of PAF receptors for the migration of eosinophils in vivo, PAFR−/− and wild-type mice were sensitized and challenged with antigen. As demonstrated in Figure 3B, eosinophil recruitment in PAFR−/− mice was inhibited by 50% as compared to wild-type mice. In PAFR−/− mice, the injection of PAF failed to induce a significant recruitment of eosinophils as compared to PBS-injected animals (wild-type: PBS, 0.2±0.1×105 eosinophils per cavity; PAF 10−9 moles per cavity, 2.7±0.8; PAFR−/−, PAF 10−9 moles per cavity, 0.4±0.1, P<0.05, n=4).
Cooperation between PAF and eotaxin
Next, we investigated whether sub-threshold doses of PAF and eotaxin could cooperate to induce eosinophil recruitment in vivo. As seen in Figure 5, a sub-threshold dose of eotaxin (10 ng per cavity) and PAF (10−11 moles per cavity) failed to induce eosinophil recruitment in the pleural cavity of naïve mice when injected alone. Nevertheless, concomitant injection of PAF and eotaxin induced significant eosinophil recruitment that was greater than the somation of either mediator when injected alone (Figure 5). The injection of the mediators alone or in combination failed to affect significantly the levels of circulating eosinophils at 1, 4, 24 and 48 h after challenge, as compared to PBS-injected mice (data not shown).
Figure 5.
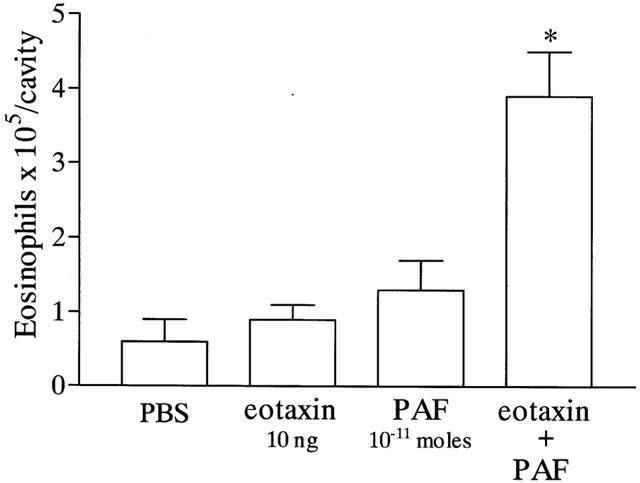
Cooperative effects of the injection of sub-threshold doses of eotaxin and PAF on eosinophil recruitment into the pleural cavity of mice. Mice were injected with phosphate-buffered saline (PBS, 100 μl), eotaxin (10 ng), PAF (10−11 moles) or with PAF and eotaxin and the number of infiltrating eosinophils assessed after 48 h. The results are expressed as means±s.e.mean of six mice in each group. *P<0.01 when compared to mice injected with PBS or the mediators alone.
Discussion
There are many experimental and clinical studies supporting a role for eosinophil recruitment and function in the pathophysiology of allergic diseases (Cara et al., 1999). Thus, strategies which limit eosinophil migration and/or function in vivo may be relevant as novel therapy for the treatment of allergic diseases (Teixeira et al., 1995; Giembycz & Lindsay, 1999). Chemokines are among the mediators which are thought to play an important role for the migration of eosinophils (and other leukocytes) in vivo (Murphy et al., 2000). We have been particularly interested in understanding the mediators of the inflammatory process which regulate chemokine production and how chemokines interact with other mediators to induce the recruitment of eosinophils in vivo. We have previously demonstrated a role for the cooperation between SCF-driven LTB4 release and eotaxin in mediating eosinophil recruitment in vivo (Klein et al., 2000; 2001). Here, we investigated a role for PAF in driving eotaxin production and eosinophil recruitment in the allergic pleurisy model.
In agreement with in vivo studies in experimental animals and in humans (Henocq & Vargaftig, 1986; Silva et al., 1991; Teixeira et al., 1994; 1997a), injection of PAF induced a significant recruitment of eosinophils into the pleural cavity of mice. Interestingly, PAF-induced eosinophil recruitment was preceded by a rapid increase in the release of eotaxin into the pleural cavity. The kinetics of eotaxin release following PAF is slightly faster than that observed after antigen challenge in the allergic pleurisy and other models (Gonzalo et al., 1996; Humbles et al., 1997; Klein et al., 2001). Moreover, PAF-induced eosinophil recruitment was suppressed by pretreatment with an anti-eotaxin antibody. Overall these results clearly demonstrate the ability of the exogenous addition of PAF to induce eosinophil recruitment in vivo in an eotaxin-dependent manner.
We have previously shown that eotaxin was released in the allergic pleurisy model and was greatly responsible for the eosinophil recruitment in response to antigen challenge (Klein et al., 2001). Thus, it was of interest to examine whether endogenous production of PAF and action on PAF receptors could modulate the production of eotaxin and subsequent eosinophil recruitment in the allergic pleurisy in mice. Our results demonstrate that blockade of PAF receptors with UK-74,505 inhibited the recruitment of eosinophils in the allergic pleurisy model. Moreover, eosinophil recruitment in PAFR−/− animals was clearly lower than that observed in wild-type animals, albeit the inhibition observed was of lesser intensity than after UK-74,505 treatment. One distinct possibility that arises from these results is that chronic PAF receptor deficiency may trigger compensatory mechanisms not observed after acute treatment with the PAF receptor antagonist. These issues are being investigated in our laboratory at present.
Not only was eosinophil recruitment suppressed, but also the PAF receptor antagonist inhibited to a great extent the release of eotaxin after antigen challenge. Altogether, our results suggest that PAF is a major modulator of eotaxin production in the allergic pleurisy model and that blockade of eotaxin release may underlie the inhibitory effects of PAF receptor antagonists on the recruitment of eosinophils. Other studies have previously demonstrated the ability of PAF to modulate chemokine production by different cell types in response to a range of different stimuli (e.g. Maruoka et al., 2000; Au et al., 2001). However, this is, to the best of our knowledge, the first demonstration of the important role of PAF in modulating eotaxin release in vivo.
One final interesting observation was the synergistic effect of sub-threshold doses of PAF and eotaxin to induce the recruitment of eosinophils in vivo. These results are in agreement with our previous study demonstrating the ability of another lipid mediator, LTB4, to cooperate with eotaxin to induce eosinophil recruitment in vivo (Klein et al., 2001). In addition, one other study has also shown the synergistic effects of the administration of PAF and eotaxin on eosinophil recruitment (assessed as tissue content of eosinophil peroxidase) and airway hyperresponsiveness in the guinea-pig lung (Fukuyama et al., 2000). One important suggestion that derives from these studies is that in an allergic reaction, smaller quantities of different mediators (e.g. PAF/LTB4 and eotaxin) may be necessary and sufficient to mediate a full recruitment of inflammatory cells. Thus, although mediator redundancy does occur in vivo, a range of different mediators must cooperate to obtain a final adequate response, ie. eosinophil migration. The corollary of the latter affirmative is that blockade of one or other mediator may be sufficient to suppress the functional response observed. Thus and in addition to the coordinated (temporal) effects of mediator release (Lukacs et al., 1999; Gonzalo et al., 1998), mediator cooperation may explain the ability of distinct strategies to suppress completely eosinophil migration in several models of allergic inflammation.
In conclusion, the production of PAF in an allergic reaction could function in multiple ways to facilitate the recruitment and activation of eosinophils – by facilitating eotaxin release, by cooperating with eotaxin to induce greater recruitment of eosinophils (the present study), and by priming and activating the eosinophils which reached the tissues (van der bruggen et al., 1994; Schweizer et al., 1996; Liu et al., 1998; Ishii & Shimizu, 2000). As eosinophils are thought to play a major role in allergic diseases and PAF appears to be a major regulator of eosinophil recruitment/function in experimental animals, it would be reasonable to suggest that PAF receptor antagonists would be an ideal therapeutic target for the treatment of these diseases. However, at least in the case of asthma, several clinical studies have failed to demonstrate a beneficial effect of PAF receptor antagonists (Kuitert et al., 1995; Evans et al., 1997; reviewed in Ishii & Shimizu, 2000). Having the latter trials in mind, it will be important to examine whether PAF receptor activation also plays a major role in the production of eotaxin (and other chemokines) following allergen challenge in other experimental models and in humans.
Acknowledgments
We are grateful to CNPq, PADCT, FAPEMIG and CAPES for financial support. A Klein is on study leave from Universidade Federal do Mato Grosso do Sul.
Abbreviations
- LTB4
Leukotriene B4
- OVA
ovalbumin
- PAF
platelet-activating factor
- PAFR−/−
PAF receptor-deficient
- PBS
phosphate-buffered saline
- SCF
stem cell factor
References
- ALABASTER V.A., KEIR R.F., PARRY M.J., DE SOUZA R.N. UK-74505, a novel and selective PAF antagonist, exhibits potent and long lasting activity in vivo. Agents Actions Suppl. 1991;34:221–227. [PubMed] [Google Scholar]
- ALVES A.C., PIRES A.L., CRUZ H.N., SERRA M.F., DIAZ B.L., CORDEIRO R.S., LAGENTE V., MARTINS M.A. Selective inhibition of phosphodiesterase type IV suppresses the chemotactic responsiveness of rat eosinophils in vitro. Eur. J. Pharmacol. 1996;312:89–96. doi: 10.1016/0014-2999(96)00357-3. [DOI] [PubMed] [Google Scholar]
- AU B.T., TEIXEIRA M.M., COLLINS P.D., WILLIAMS T.J. Blockade of PAF receptors controls IL-8 production by regulating the activation of neutrophil CD11/CD18. Eur. J. Pharmacol. 2001;425:65–71. doi: 10.1016/s0014-2999(01)01141-4. [DOI] [PubMed] [Google Scholar]
- BARTEMES K.R., MCKINNEY S., GLEICH G.J., KITA H. Endogenous platelet-activating factor is critically involved in effector functions of eosinophils stimulated with IL-5 or IgG. J. Immunol. 1999;162:2982–2989. [PubMed] [Google Scholar]
- BORGES C.M., SILVEIRA M.R., BEKER M.A., FREIRE-MAIA L., TEIXEIRA M.M. Scorpion venom-induced neutrophilia is inhibited by a PAF receptor antagonist in the rat. J. Leukoc. Biol. 2000;67:515–519. doi: 10.1002/jlb.67.4.515. [DOI] [PubMed] [Google Scholar]
- CARA D.C., NEGRAO-CORREA D., TEIXEIRA M.M. Mechanisms underlying eosinophil trafficking and their relevance in vivo. Histol. Histopathol. 1999;15:899–920. doi: 10.14670/HH-15.899. [DOI] [PubMed] [Google Scholar]
- DAS A.M., FLOWER R.J., HELLEWELL P.G., TEIXERA M.M., PERRETTI M. A novel murine model of allergic inflammation to study the effect of dexamethasone on eosinophil recruitment. Br. J. Pharmacol. 1997;121:97–104. doi: 10.1038/sj.bjp.0701122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS D.J., BARNES P.J., CLUZEL M., O'CONNOR B.J. Effects of a potent platelet-activating factor antagonist, SR27417A, on allergen-induced asthmatic responses. Am. J. Respir. Crit. Care Med. 1997;156:11–16. doi: 10.1164/ajrccm.156.1.9611112. [DOI] [PubMed] [Google Scholar]
- FUKUYAMA S., INOUE H., AIZAWA H., OIKE M., KITAURA M., YOSHIE O., HARA N. Effect of eotaxin and platelet-activating factor on airway inflammation and hyperresponsiveness in guinea pigs in vivo. Am. J. Respir. Crit. Care Med. 2000;161:1844–1849. doi: 10.1164/ajrccm.161.6.9905039. [DOI] [PubMed] [Google Scholar]
- GIEMBYCZ M.A., LINDSAY M.A. Pharmacology of the eosinophil. Pharmacol. Rev. 1999;51:213–340. [PubMed] [Google Scholar]
- GONZALO J.A., LLOYD C.M., KREMER L., FINGER E., MARTINEZ C., SIEGELMAN M.H., CYBULSKY M., GUTIERREZ-RAMOS J.C. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines, and adhesion receptors. J. Clin. Invest. 1996;98:2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONZALO J.A., LLOYD C.M., WEN D., ALBAR J.P., WELLS T.N.C., PROUDFOOT A.E., MARTINEZ-A C., DORF M., BJERKE T., COYLE A.J., GUTIERREZ-RAMOS J.C. The coordinated action of CC chemokines in the lung orchestrates allergic inflammation and airway hyperresponsiveness. J. Exp. Med. 1998;188:157–167. doi: 10.1084/jem.188.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENOCQ E., VARGAFTIG B.B. Accumulation of eosinophils in response to intracutaneous PAF-acether and allergens in man. Lancet. 1986;14:1378–1379. doi: 10.1016/s0140-6736(86)91683-1. [DOI] [PubMed] [Google Scholar]
- HUMBLES A.A., CONROY D.M., MARLEAU S., RANKIN S.M., PALFRAMAN R.T., PROUDFOOT A.E., WELLS T.N., LI D., JEFFERY P.K., GRIFFITHS-JOHNSON D.A., WILLIAMS T.J., JOSE P.J. Kinetics of eotaxin generation and its relationship to eosinophil accumulation in allergic airways disease: analysis in a guinea pig model in vivo. J. Exp. Med. 1997;186:601–612. doi: 10.1084/jem.186.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHII S., KUWAKI T., NAGASE T., MAKI K., TASHIRO F., SUNAGA S., CAO W.H., KUME K., FUKUCHI Y., IKUTA K., MIYAZAKI J., KUMADA M., SHIMIZU T. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating receptor. J. Exp. Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHII S., SHIMIZU T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog. Lipid Res. 2000;39:41–82. doi: 10.1016/s0163-7827(99)00016-8. [DOI] [PubMed] [Google Scholar]
- JEZEQUEL S.G., UDEN S., WASTALL P. Modipant, a new PAF antagonist: pharmacokinetics and disposition in rat, dog and man. Xenobiotica. 1996;26:963–975. doi: 10.3109/00498259609052498. [DOI] [PubMed] [Google Scholar]
- KLEIN A., TALVANI A., CARA D.C., GOMES K.L., LUKACS N.W., TEIXEIRA M.M. SCF plays a major role in the recruitment of eosinophils in allergic pleurisy in mice via the production of LTB4. J. Immunol. 2000;164:4271–4276. doi: 10.4049/jimmunol.164.8.4271. [DOI] [PubMed] [Google Scholar]
- KLEIN A., TALVANI A., SILVA P.M., MARTINS M.A., WELLS T.N., PROUDFOOT A., LUCKACS N.W., TEIXEIRA M.M. Stem cell factor-induced leukotriene B(4) production cooperates with eotaxin to mediate the recruitment of eosinophils during allergic pleurisy in mice. J. Immunol. 2001;167:524–531. doi: 10.4049/jimmunol.167.1.524. [DOI] [PubMed] [Google Scholar]
- KUITERT L.M., ANGUS R.M., BARNES N.C., BARNES P.J., BONE M.F., CHUNG K.F., FAIRFAX A.J., HIGENBOTHAM T.W., O'CONNOR B.J., PIOTROWSKA B. Effect of a novel potent platelet-activating factor antagonist, modipafant, in clinical asthma. Am. J. Respir. Crit. Care Med. 1995;151:1331–1335. doi: 10.1164/ajrccm.151.5.7735582. [DOI] [PubMed] [Google Scholar]
- LIU L., ZUURBIER A.E., MUL F.P., VERHOEVEN A.J., LUTTER R., KNOL E.F., ROOS D. Triple role of platelet-activating factor in eosinophil migration across monolayers of lung epithelial cells: eosinophil chemoattractant and priming agent and epithelial cell activator. J. Immunol. 1998;161:3064–3070. [PubMed] [Google Scholar]
- LUKACS N.W., OLIVEIRA S.H., HOGABOAM C.M. Chemokines and asthma: redundancy of function or a coordinated effort. J. Clin. Invest. 1999;104:995–999. doi: 10.1172/JCI8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARUOKA S., HASHIMOTO S., GON Y., TAKESHITA I., HORIE T. PAF-induced RANTES production by human airway smooth muscle cells requires both p38 MAP kinase and Erk. Am. J. Respir. Crit. Care Med. 2000;161:922–929. doi: 10.1164/ajrccm.161.3.9906059. [DOI] [PubMed] [Google Scholar]
- MIOTLA J.M., JEFFERY P.K., HELLEWELL P.G. Platelet-activating factor plays a pivotal role in the induction of experimental lung injury. Am. J. Respir. Cell. Mol. Biol. 1998;18:197–204. doi: 10.1165/ajrcmb.18.2.2846. [DOI] [PubMed] [Google Scholar]
- MURPHY P.M., BAGGIOLINI M., CHARA I.F., HERBERT C.A., HORUK R., MATSUSHIMA K., MILLER L.M., OPPENHEIM J.J., POWER C.A. International union of Pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- RUTH J.H., LUKACS N.W., WARMINGTON K.S., POLAK T.J., BURDICK M., KUNKEL S.L., STREITER R.M., CHENSUE S.W. Expression and participation of eotaxin during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granuloma formation. J. Immunol. 1998;161:4276–4282. [PubMed] [Google Scholar]
- SCHWEIZER R.C., VAN KESSEL-WELMERS B.A., WARRINGA R.A., MAIKOE T., RAAIJMAKERS J.A., LAMMERS J.W., KOENDERMAN L. Mechanisms involved in eosinophil migration. Platelet-activating factor-induced chemotaxis and interleukin-5-induced chemokines are mediated by different signals. J. Leukoc. Biol. 1996;59:347–356. doi: 10.1002/jlb.59.3.347. [DOI] [PubMed] [Google Scholar]
- SILVA P.M., MARTINS M.A., FARIA NETO H.C., CORDEIRO R.S., VARGAFTIG B.B. Generation of an eosinophilotactic activity in the pleural cavity of platelet-activating factor-injected rats. J. Pharmacol. Exp. Ther. 1991;257:1039–1044. [PubMed] [Google Scholar]
- TEIXEIRA M.M., GIEMBYCZ M.A., LINDSAY M.A., HELLEWELL P.G. Pertussis toxin reveals distinct early signalling events in platelet-activating factor, leukotriene B4- and C5a-induced eosinophil homotypic aggregation in vitro and recruitment in vivo. Blood. 1997a;89:4566–4573. [PubMed] [Google Scholar]
- TEIXEIRA M.M., REYNIA S., ROBINSON M., SHOCK A., WILLIAMS T.J., WILLIAMS F.M., ROSSI A.G., HELLEWELL P.G. Role of CD11/CD18 in mediating cutaneous inflammation in the guinea pig. Br. J. Pharmacol. 1994;111:811–818. doi: 10.1111/j.1476-5381.1994.tb14810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA M.M., WELLS T.N.C., LUKACS N.W., PROUDFOOT A.E.E., KUNKEL S.L., WILLIAMS T.J., HELLEWELL P.G. Chemokine-induced eosinophil recruitment: Evidence of a role for endogenous eotaxin in an in vivo allergy model in mouse skin. J. Clin. Invest. 1997b;100:1656–1666. doi: 10.1172/JCI119690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEIXEIRA M.M., WILLIAMS T.J., HELLEWELL P.G. Mechanisms and pharmacological manipulation of eosinophil accumulation in vivo. Trends Pharmacol. Sci. 1995;16:418–423. doi: 10.1016/s0165-6147(00)89092-6. [DOI] [PubMed] [Google Scholar]
- TOOL A.T., KOENDERMAN L., KOK P.T., BLOM M., ROOS D., VERHOEVEN A.J. Release of platelet-activating factor is important for the respiratory burst induced in human eosinophils by opsonized particles. Blood. 1992;79:2729–2732. [PubMed] [Google Scholar]
- VAN DER BRUGGEN T., KOK P.T., RAAIJMAKERS J.A., LAMMERS J.W., KOENDERMAN L. Cooperation between Fc gamma receptor II and complement receptor type 3 during activation of platelet-activating factor release by cytokine-primed human eosinophils. J. Immunol. 1994;153:2729–2735. [PubMed] [Google Scholar]


