Abstract
5-Hydroxytryptamine (5-HT) is known to produce a number of different effects in the gastrointestinal tract of various species, and has been proposed to play a key role in a number of intestinal disorders in man, including irritable bowel syndrome (IBS), although the receptors involved have yet to be established. The aim of the present study was to investigate the distribution and function of 5-HT2B receptors in human colon, and to establish their possible role in the aetiology of IBS.
The distribution of 5-HT2B receptor mRNA and protein were investigated by quantitative RT – PCR, Western analysis and immunocytochemistry. High levels of both mRNA and protein for 5-HT2B receptors were found throughout the human gastrointestinal tract, and in particular in colon, where 5-HT2B receptors were found predominantly in the longitudinal and circular smooth muscle layers within the muscularis externa, and in the myenteric nerve plexus lying between these two layers.
Electrical field stimulation of longitudinal muscle preparations of human colon mounted in organ baths resulted in neuronally-mediated contractile responses, that were significantly potentiated by application of 5-HT (up to 10−7 M), with a pEC50 of 8.2±0.1 (n=49 donors). The response to 5-HT was inhibited by a number of selective 5-HT2B receptor antagonists.
This study has shown for the first time that, in contrast to animal studies, the excitatory effects of 5-HT in human colon are mediated by 5-HT2B receptors. It is proposed that these receptors contribute to the putative 5-HT-induced colonic smooth muscle hypersensitivity associated with IBS.
Keywords: 5-Hydroxytryptamine, 5-HT2B, human colon, irritable bowel syndrome, motility disorders
Introduction
Physiological and pharmacological studies have indicated that 5-HT may play a pivotal role in mediating sensory and reflex responses in the gastrointestinal tract of various species, including man (Read & Gwee, 1994). Whilst the receptors responsible for these effects in animals have been extensively investigated and subsequently identified, relatively little is known of the 5-HT receptor sub-types responsible for the effects of this amine in the human intestine generally, and in human colon in particular.
Although it is generally accepted that the human small intestine contracts in response to 5-HT, it has been proposed that the predominant response in human colon is relaxation. In human ileum, application of 5-HT induces contraction of both circular and longitudinal muscle layers, via activation of 5-HT1D and 5-HT2B receptors respectively (Borman & Burleigh, 1995; 1997a). In colon circular muscle, however, 5-HT has been shown to induce both smooth muscle relaxation and inhibition of spontaneous contractions, responses attributed to activation of 5-HT4 and/or 5-HT7 receptors (Borman & Burleigh, 1994; Prins et al., 1999; Tam et al., 1994). The response of longitudinal muscle strips to 5-HT is more complex, with some investigators reporting contraction and others reporting relaxation or a combination of contraction and relaxation (Hillier et al., 1994). Although a recent study has indicated that 5-HT-induced excitatory effects in muscle strips of human colon taenia coli may be mediated by a 5-HT4 receptor (Prins et al., 2000), the receptors responsible for the effects in colon inter-taenial longitudinal smooth muscle have not previously been identified.
In addition to effects on smooth muscle activity, application of 5-HT induces profound effects on fluid secretion throughout the human intestine. Exogenous application of 5-HT has been shown to increase chloride ion (and thereby fluid) secretion in human jejunum, ileum and colon, the effect being more pronounced in small than large intestine (Borman & Burleigh, 1997b). In both human jejunum and ileum, and to a lesser extent proximal colon, the 5-HT4 receptor has been shown to mediate the pro-secretory actions of 5-HT, resulting in significant ion and fluid accumulation into the lumen of the intestine (Borman & Burleigh, 1993; 1996; Budhoo et al., 1996). Such activity has been proposed as a possible cause of hypersecretory disorders such as carcinoid syndrome and cholera toxin-induced diarrhoea, which have been associated with increased levels of intestinal 5-HT (Bearcroft et al., 1996).
The range of its biological activities suggests that 5-HT could be involved in a number of pathological events in the gastrointestinal tract, although its physiological role is uncertain. In particular, alterations in the levels of 5-HT are believed to be responsible for the complex symptomology of the irritable bowel syndrome (IBS). IBS is a disorder that is characterized by abdominal pain coupled to constipation, diarrhoea, or both. The underlying cause of IBS has not been elucidated. However, the diversity of actions of 5-HT in the intestine, including the possibility that 5-HT may play a role in visceral hyperalgesia (Sanger, 1996), coupled to the finding that increased levels of 5-HT and its metabolites have been found in the plasma of IBS patients (Bearcroft et al., 1998), have prompted the hypothesis that the amine may play a key role in the aetiology of the disorder (see Gershon, 1999, for example).
Given the potential role of 5-HT in the pathophysiology of IBS, the aim of the present study was to investigate the nature of the 5-HT receptor(s) responsible for the excitatory effects of 5-HT in human colon smooth muscle. In particular, we have investigated the expression of mRNA and protein, as well as function, of 5-HT2B receptors, and have identified a novel role for this receptor in the control of human colonic function.
Methods
Gene expression studies
Primer probe design
The target DNA sequence for the 5-HT2B receptor was retrieved from GenBank, and primer/probe sets were designed using Primer Express (Perkin Elmer ABI software) to amplify a small fragment of the target sequence. The primer/probe sequences were subjected to homology searches against GenBank to confirm that they were specific for the target amplicon. Forward primer: ACGCCTAACATGGTTGACTGTGTC; Reverse primer: TGAGGCTCTCTGTTCGTTGGAA; Probe: AGGTGGCAATGCTGGATGGTTCTCGA.
An assay system has been developed that allows the determination of the abundance of more than one mRNA species in a single tube by using probes for the two genes that have different fluorophores, which are spectrally distinct. This was applied to measure expression of the target gene together with the expression of the ubiquitously expressed glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene, to monitor RNA integrity. The primer/probe set for GAPDH was positioned so that it spans an exon/exon boundary and will therefore only be amplified from cDNA.
RNA isolation
Total RNA was isolated from whole sections of different regions of human intestine, from three different donors, using Trizol™ according to the manufacturer's instructions. The RNA was subjected to a range of quality control checks. Samples were only used for transcription profiling if: (1) The ratio of the optical density (OD) readings for the sample determined at wavelengths of 260 nM and 280 nM was >1.7 indicating that levels of contaminating protein and/or phenol were low; (2) The sample had intact 18 s ribosomal RNA bands as determined by denaturing agarose gel electrophoresis; (3) Actin mRNA transcript levels were above 6000 copies per 100 ng of total RNA as determined by quantitative PCR (QRT – PCR); and (4) QRT – PCR was also used to determine the levels of genomic DNA within the sample. Samples were only used where the level of DNA formed less than 10% of the total nucleic acid.
Quantitative RT – PCR
Copy numbers of mRNA for the 5-HT2B receptor were determined by QRT – PCR using the ABI Taqman sequence detection system. Prior to reverse transcription the total RNA samples were treated with RNase-free DNase to remove any contaminating genomic DNA. In order to ascertain whether any DNA contamination remained following the DNase treatment, all samples were subjected to PCR amplification in the absence of reverse transcriptase. The RNA that had been DNase-treated was then annealed to reverse primers for the target and GAPDH genes. This was carried out in the presence of buffer and the sample was heated to 72°C and then cooled to 55°C. The reverse transcription was carried out by adding MuLV reverse transcriptase and nucleotides to the reaction. This was incubated for 30 min at 37°C to allow synthesis to take place. The sample was then heated to 90°C for 5 min to denature the enzyme. Quantitative sequence detection for both targets was carried out simultaneously on the resulting cDNA that had been made from 100 ng of total RNA. For-ward and reverse primers and probes for target and GAPDH were added along with nucleotides, buffer and AmpliTaq Gold™ Taq polymerase. The PCR reaction took place under the following reactions conditions: 94°C for 12 min, followed by 40 cycles of 94°C for 15 s and 60°C for 30 s.
Determination of mRNA expression levels of 5-HT2B receptors in human intestine
Following extraction, the levels of mRNA for 5-HT2B receptors were determined in full-thickness preparations of each of the following tissues: oesophagus, stomach (antrum, body, fundus and pylorus), liver, pancreas, gallbladder, duodenum, jejunum, ileum, caecum, colon and rectum. The study was repeated in tissues from three separate donors.
Protein expression studies
Preparation of human tissue extracts
Snap frozen samples of human tissue (approximately 1 g each piece) were added to 10 volumes of ice-cold 50 mM HEPES (pH 7.4), 1 mM EDTA, 1 mM EGTA, 250 mM sucrose, 0.2 mM PMSF. The tissue was homogenized using an Ultra-Turrax homogenizer on full speed for 10 s. Sodium dodecyl sulphate (SDS) was added at a final concentration of 1%, for 10 min at room temperature, to solubilize proteins. Insoluble material was removed by centrifugation at 1000×g for 10 min at room temperature. The supernatant was decanted and re-centrifuged at 40,000×g for 15 min at room temperature. Protein was determined by the BCA method using BSA as a standard. The final protein extracts were stored at −20°C until use.
Dot-blotting of 5-HT2B receptor protein
The level of 5-HT2B receptor protein expression was determined in protein extracts from human ileum or colon. In brief, protein extracts were diluted to 0.4 mg protein.ml in 62.5 mM Tris-HCl (pH 6.8), 1% (w v−1) SDS. Immunoblotting was performed using a 96-well perspex-blotting manifold (Life Technologies). Before use, the manifold was washed in detergent, and rinsed in distilled water. A nitrocellulose sheet, pre-soaked with distilled water and a buffer containing 25 mM Tris (pH 8.3), 192 mM glycine, 20% methanol, was loaded into the manifold and clamped in place by vacuum pressure. Protein samples (100 μl well−1) were added to wells and incubated for 1 h at room temperature.
The resultant protein blot was removed from the manifold and rinsed in phosphate-buffered saline (PBS) for 5 min. The blot was stained with Ponceau S for 15 min to visualize the protein bound and to inactivate endogenous alkaline phosphatase enzyme. Destaining was achieved using PBS before blocking in PBS containing 1% (w v−1) Marvel, 1% (w v−1) BSA, 1% (v v−1) sheep serum for 1 h at room temperature. Anti-5-HT2B antibody (Pharmingen) diluted to 0.1 μg ml−1 in PBS, 1% Marvel, 0.25% BSA (reagent dilutent) was added to the blot and incubated for 90 min at 37°C. After thorough washing, bound antibody was localized using an anti-mouse fluorescein-linked secondary antibody (1 : 600; Amersham) and an anti-fluorescein alkaline phosphatase conjugate (diluted in 1 : 2500 in Tris-buffered saline, 1% Marvel, 0.25% BSA). Finally, bound conjugate was localized using enhanced chemifluorescence and imaged using a fluorimager (STORM; Molecular Dynamics). The resultant image was quantified using ImageQuant software. Control values obtained using non-immune mouse IgG as the primary antibody were subtracted from the results for the anti-5-HT2B antibody to correct for endogenous fluorescence or residual alkaline phosphatase activity in protein extracts. Western analysis confirmed the specificity and affinity of the antibody for the human 5-HT2B receptor.
Immunocytochemistry studies
Tissue preparation
Fresh frozen (10 μm) or formalin-fixed paraffin-embedded (5 – 7 μm) sections of human colon were mounted onto silane-coated slides. Frozen sections were stored at −80°C until use, while paraffin-embedded sections were stored at room temperature (RT). On the day of the study, frozen sections were brought to RT and air-dried for 1 h. These sections were fixed in acetone for 30 min and air-dried for 1 h. Paraffin-embedded sections were rehydrated in graded alcohols.
Immunocytochemistry
Anti-5-HT2B receptor antibody (IgG1 isotype) was purchased from Pharmingen Inc., U.S.A. (Catalogue number 60531A, Lot number MO17437). All incubations and washes were carried out on an orbital shaker at RT and all washes were in phosphate-buffered saline (PBS) unless otherwise stated. Endogenous peroxidase activity was quenched by incubation in 1% H2O2 : 0.1% NaN3 in PBS for frozen sections, and 3% H2O2 in distilled water for paraffin-embedded sections (30 min each). At this stage, an antigen retrieval step was performed on paraffin-embedded sections. These sections were microwaved in 0.01 M citrate buffer for 20 min followed by cooling slowly in tap water. All sections were then incubated for 30 min with 10% blocking serum (Vector Universal Elite Kit). Subsequently, sections were incubated with the 5-HT2B receptor antibody (1 – 2 μg ml−1 in PBS) for 16 to 72 h at 4°C. Control sections were incubated with mouse IgG at 1 – 2 μg ml−1. Sections were then washed (2×5 min) and incubated with a biotinylated universal secondary antibody (Vector Universal Elite Kit) for 30 min, followed by washing (2×5 min) and incubation with Vectastain Elite ABC reagent (Vector Universal Elite Kit) for 30 min. Subsequently, sections were incubated with 3′,3-diaminobenzidine tetrachloride (0.025% w v−1) :H2O2 (0.02% v v−1) in 0.05 M Tris buffer (pH 7.6) for 5 min, followed by washing in distilled water. Sections were then counterstained in Mayer's haematoxylin (1 min), dehydrated to xylene and cover-slipped with DPX mountant (BDH Laboratories). Immunostained sections were viewed with a Zeiss Axioplan2 microscope.
Pharmacology
Sections of human colon were cut open along their longitudinal axis. The sections were pinned out flat and the mucosa carefully removed using sharp dissecting scissors. Once the mucosa was removed, the section was turned over to reveal the three taenia coli (taenia mesencolica, taenia omentalis and taenia libera) and the muscle bands that lie between them. Longitudinal muscle strips (2 mm wide by 20 mm long) were then cut from the tissue between the taenia coli and suspended between stainless steel hooks in organ chambers containing oxygenated (95% O2 : 5% CO2) Krebs solution at 37°C. The composition of the Krebs solution was as follows in mM: NaCl 118.2, KCl 4.69, MgSO4.7H2O 1.18, KH2PO4 1.19, glucose 11.1, NaHCO3 25.0, CaCl2.6H2O 2.5.
Tissues were placed under a tension equivalent to 10 mN and left to equilibrate for a period of at least 60 min. Responses were recorded using isometric transducers coupled to an Apple Macintosh computer via a MacLab interface. After 60 min, the longitudinal muscle sections of human colon were stimulated electrically (at sub-maximal voltage with 60 s between successive stimulations) using parallel platinum wire electrodes and a Multistim D330 pulse stimulator. Upon electrical stimulation, the strips of human colon longitudinal smooth muscle responded with a rapid contraction. Tissues were stimulated for 10 s every 60 s, at 15 V and with a 1 ms pulse width, at a range of frequencies from 0.1 to 40 Hz (with at least 3 min at each frequency). After a maximum response, stimulation was halted and the tissues washed three times, with 5 min between, and left to equilibrate for 30 min. After this time, electrical stimulation was recommenced, using the same stimulation parameters as previously and a frequency that produced sub-maximal contractions, and was allowed to stabilize. Once the response to electrical stimulation had stabilized (stimulated responses differed by no more than 10%), the strips were exposed to increasing concentrations of 5-HT (or 5-HT receptor agonists, cumulative concentration-effect curves with a minimum of 3 min contact time at each concentration), in the absence or presence of selective receptor antagonists (incubated for 30 min prior to exposure to 5-HT). In this way, a single concentration-effect curve to 5-HT was generated in each preparation, either in the absence or presence of antagonist. A concentration ratio (CR) was therefore generated between the EC50 values for 5-HT in the absence and presence of antagonist, in tissue from the same donor.
Data analysis
To calculate antagonist pKB values, the mean CR was plotted as log10 (CR-1) against log antagonist molar concentration according to the method of Arunlakshana & Schild (1959). If the slope of the plot did not differ significantly from one, it was constrained to unity to calculate an apparent pKB value. Where only one concentration of antagonist had been tested, apparent pA2 values were calculated for individual data points according to the method of Mackay (1978). Where appropriate, statistical comparisons were carried out by ANOVA or Student's t-test, with P<0.05 being taken to indicate statistical significance.
Human tissues
All samples of human tissue were obtained through medically qualified intermediaries with the informed consent of the donor or donor's next of kin, and with approval of the local research ethics committee. The tissues were transported to Pharmagene in phosphate-buffered saline solution on ice. For mRNA expression studies, sections were obtained from the region of intestine indicated from multi-organ donors (n=23 donors), or from sections of intestine removed at operation for carcinoma (n=6 donors). All tissues from multi-organ donors were obtained via intermediaries who had obtained from the donor's next of kin the express permission for the use of those tissues for research purposes. The age range for these donors was 17 – 79 years, with mean age (±s.e.mean) of 44.8±2.4 years. For immunohistochemistry studies, sections of distal (descending) colon were obtained from specimens removed at operation for carcinoma in situ of the rectum. For organ bath studies, sections of colon (10 ascending and 39 descending or sigmoid) were obtained from 49 donors, 26 male and 23 female, age range 40 – 96 (mean age±s.e.mean of 66.9±1.8 years), undergoing operations for carcinoma (n=39), diverticular disease (n=4), Crohns disease (n=1), ulcerative colitis (n=3) or polyps (n=2). For these studies, tissues were kept at 4°C until the experiment, which was carried out within 24 h of removal of the tissue from the patient. In all cases, the section of colon was judged to be macroscopically normal by a consultant histopathologist.
Materials
In addition to the above, the following chemicals were used for this study: alpha-methyl-5-hydroxytryptamine (α-Me-5-HT; Tocris Cookson), 5-hydroxytryptamine creatinine sulphate (RBI), 2-methyl-5-hydroxytryptamine maleate (2-Me-5-HT; RBI), 5-methoxytryptamine hydrochloride (5-MeOT; RBI), cisapride (synthesized in-house), RS-127445 (2-amino-4-(4-fluoronaphth-1-yl)-6-isopropylpyrimidine; synthesized in-house), SB-206553 (5-methyl-1-(3-pyridylcarbamoyl)-1,2,3,5-tetrahydropyrrolo[2,3-f]indole; synthesized in-house), rauwolscine hydrochloride (RBI), yohimbine hydrochloride (RBI), methiothepin maleate (Tocris Cookson), SB-204741 (N-(1-methyl-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea; synthesized in-house), SB-242084 (6-chloro-5-methyl-1-[6-(2-methylpyridin-3-yloxy)pyridin-3-yl-carbamoyl]indoline; synthesized in-house), ketanserin tartrate (RBI) and methysergide maleate (Tocris Cookson).
Results
Gene expression
In sections of human gastrointestinal tract, each obtained from three independent donors, 5-HT2B receptor mRNA was expressed in all tissues tested (Figure 1). There were no significant differences in mRNA expression levels between different regions of the GI tract.
Figure 1.
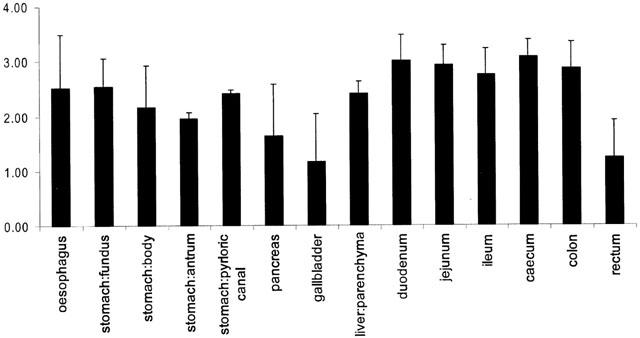
Expression of mRNA for 5-HT2B receptors in human gastrointestinal tract. Data are expressed as mean log copy numbers (per 100 ng of total RNA) from three donors, with standard errors indicated by bars.
Protein expression
5-HT2B receptors were detected in both human ileum and colon. In colon, highest expression was seen in smooth muscle, with little or no protein expression detected in the mucosa or taenia coli (Figure 2, data from a single donor). A similar pattern of expression was seen in ileum.
Figure 2.
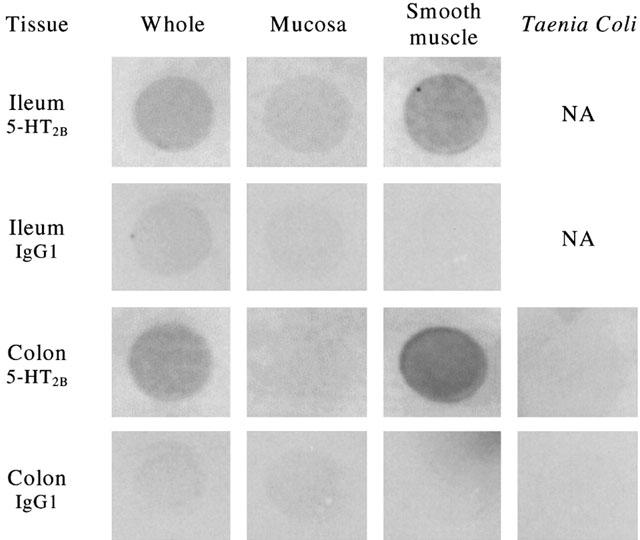
Dot-blotting of protein extracts from human ileum and colon with anti-5-HT2B antibody or control (mouse IgG1) antibody. Tissues are full thickness (whole), or sub-divided into mucosa and smooth muscle, and isolated taenia coli for colon. NA indicates not applicable to ileum. Data were obtained in tissues from a single donor.
Immunocytochemistry
In sections of human colon (from a single donor), 5-HT2B receptor-like immunoreactivity (5-HT2BR-ir) was observed in several cell types. In the muscularis externa, moderate 5-HT2BR-ir was seen in the circular and longitudinal muscle layers, while moderate to strong staining was observed in putative myenteric nerve plexuses lying between the two muscle layers (Figure 3). Confirmation that 5-HT2BR-ir was localized in the nerve plexus was obtained by labelling a neighbouring section of colon with a neuronal marker antibody, neurofilament 68 (Figure 4, upper and middle panel). The possibility that glial cells are also labelled can not be excluded. The control section, incubated with mouse IgG, showed no discernible staining (Figure 4 lower panel).
Figure 3.
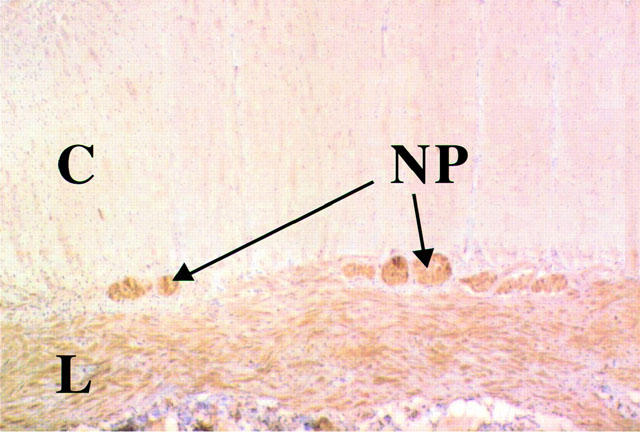
5-HT2B receptor-like immunoreactivity in a paraffin-embedded section of human colon. In the muscularis externa, immunoreactivity was highest in the longitudinal muscle layer (L) and in the nerves of the myenteric nerve plexus (N), with lower expression in the circular muscle layer (C).
Figure 4.
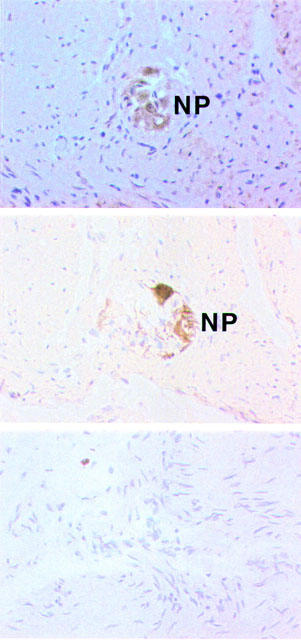
5-HT2B receptor-immunoreactivity (5-HT2BR-ir) and neurofilament-immunoreactivity (ir) in frozen sections of colon. 5-HT2BR-ir in muscle and nerve plexus (NP, upper panel), Neurofilament-ir in a neighbouring section showing localization of the nerve plexus (middle panel), and control IgG1 antibody (lower panel).
Pharmacology
Electrical stimulation (15 V, 1 ms pulse width, at sub-maximal frequency, for 10 s every 60 s) caused highly reproducible, transient, contractile responses of isolated preparations of human colon smooth muscle. The responses were inhibited by tetrodotoxin at a concentration of 1 or 3 μM (inhibition of 88±12% and 101±1%, n=4 and 6 respectively), or by atropine at a concentration of 1 or 10 μM (inhibition of 84±7% and 89±6%, n=5 and 6 respectively), indicating that they were neuronal in nature, and they involved (at least in part) cholinergic neurotransmission (data not shown).
Application of 5-HT produced a significant, concentration-dependent potentiation of the contractile response to electrical stimulation, with no significant effect on basal tone (Figures 5 and 6). The EC50 for 5-HT was 8.2±0.1 with a Hill slope of 1.3±0.8 (both n=49). There was no evidence of desensitization to increasing concentrations of 5-HT during the cumulative concentration-effect curve, although a small inhibitory effect of 5-HT was evident at higher concentrations (above 10−7 M), in some tissues. A range of 5-HT receptor agonists was also shown to cause potentiation of the contractile response. The order of agonist potency was α-Me-5-HT (5-HT2 receptor agonist)>5-HT (non-selective agonist)>2-Me-5-HT (5-HT3 receptor agonist)⩾5-MeOT (5-HT4 receptor agonist)>cisapride (5-HT4 receptor agonist), which is consistent with a receptor of the 5-HT2 family (Table 1). Application of a range of 5-HT receptor antagonists caused significant, rightward shifts of concentration-response curves to 5-HT, and the profile generated corresponds to the receptors involved being of the 5-HT2B receptor class (Table 2). In particular, application of a highly potent and selective 5-HT2B receptor antagonist, RS-127445 (Bonhaus et al., 1999), caused a rightward displacement of the concentration-effect curve to 5-HT, with no significant alteration of the maximum response to 5-HT (Figure 7). Schild analysis generated a plot with slope not significantly different from unity (0.9±0.2), yielding a pKB of 9.4±0.4 (Figure 8).
Figure 5.
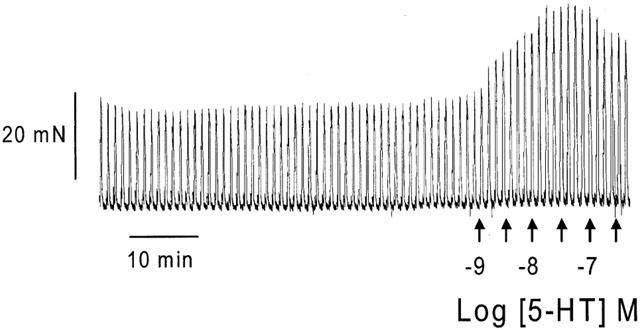
Typical contractile responses to 5-HT in electrically-stimulated human colon longitudinal smooth muscle. Figure shows the transient contractile response to EFS, and the potent, concentration-dependent potentiation of this neurally-mediated response by increasing concentrations of 5-HT (10−9 to 10−6.5 M in half log increments). At concentrations in excess of 10−7 M, 5-HT induces mild inhibition of electrically-stimulated contractions.
Figure 6.
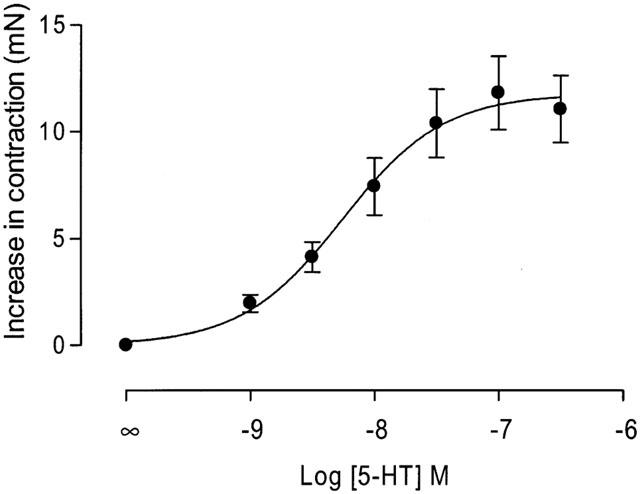
Effect of 5-HT on electrical field stimulation (EFS)-induced contractions of human colon longitudinal smooth muscle. Figure shows mean concentration-effect curve to 5-HT, with data expressed as increase in contractility (mN) over basal EFS-induced contractions. Data are given as mean±s.e.mean for n=49 donors, and have been fitted to the Hill equation according to a three parameter curve fit.
Table 1.
Potencies of some 5-HT receptor agonists in human colon longitudinal muscle
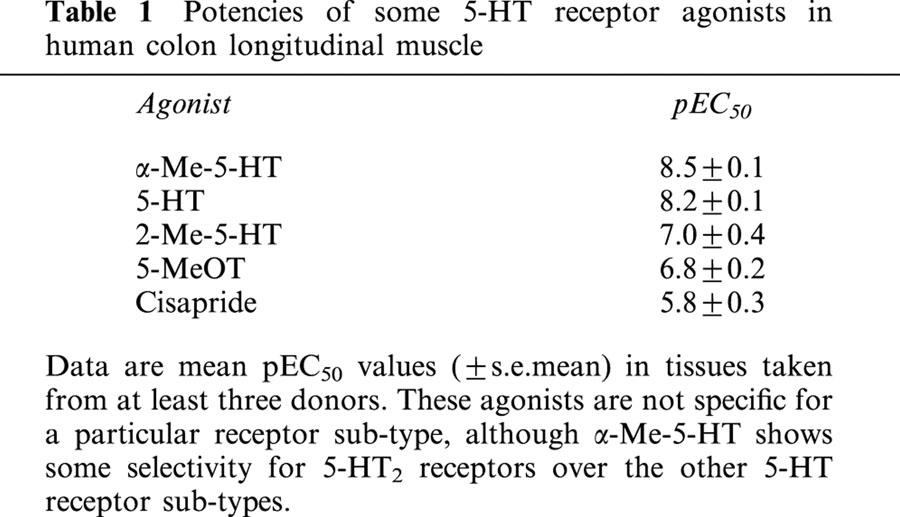
Table 2.
Affinities of some 5-HT receptor antagonists against 5-HT-induced responses in human colon longitudinal muscle
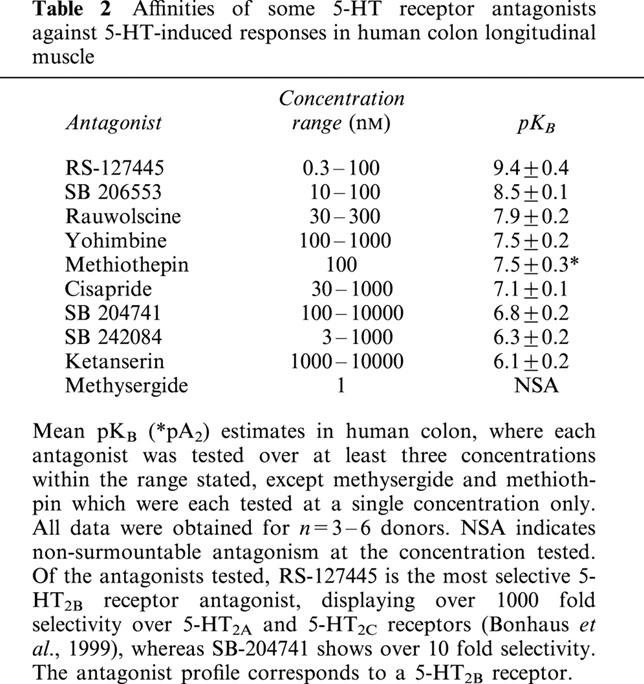
Figure 7.
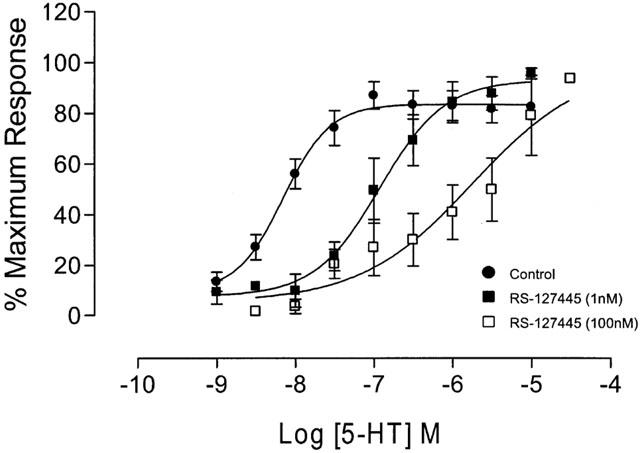
Antagonist effect of RS-127445 on the concentration-effect curves to 5-HT in human colon longitudinal smooth muscle. Figure shows mean concentration-effect curves to 5-HT in the absence and presence of RS-127445. Additional concentration-effect curves to 5-HT, in the presence of different concentrations of RS-127445 (see Figure 8), have been omitted for clarity. Data are expressed as percentage of the maximum response to 5-HT in the absence of antagonist, and are given as mean±s.e.mean for n>4 donors.
Figure 8.
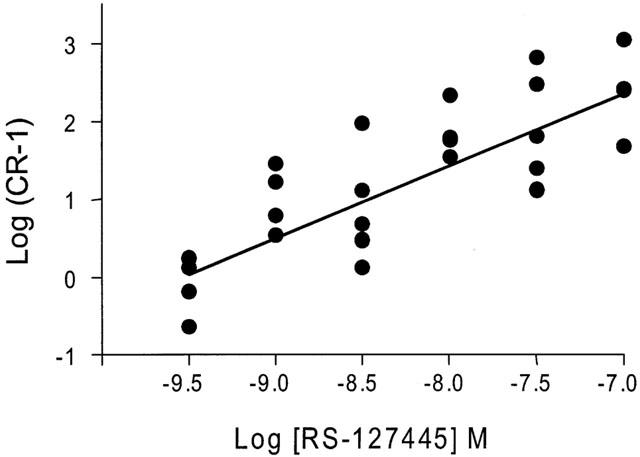
Schild plot of the antagonist effect of RS-127445 on responses to 5-HT in human colon longitudinal smooth muscle. The antagonism generated a pKB of 9.5±0.4 and slope of 0.9±0.2. Data are for n>4 donors.
Discussion
In the present study, we have investigated the role of 5-HT2B receptors in controlling human colonic motility. The mRNA for this receptor is highly expressed throughout the human gastrointestinal tract, including colon. The protein for the receptor is localized within the smooth muscle of human colon, and in particular within the longitudinal smooth muscle and on the myenteric plexus, in accordance with a role in controlling colonic motility. In human colon longitudinal smooth muscle, we demonstrated that electrical stimulation of smooth muscle strips evoked a transient contractile response that could be inhibited by either atropine or tetrodotoxin, implicating the involvement of cholinergic nerves in the response. Exogenously applied 5-HT caused an increase in the magnitude of these neuronally-mediated contractions, probably by either increasing the release of excitatory neurotransmitters such as acetylcholine, or by effects on the smooth muscle itself. The agonist profile of the response, and in particular the high potency of α-Me-5-HT, suggested that the response was mediated by a receptor of the 5-HT2 family. Using a range of selective antagonists, the receptors were characterized as being of the 5-HT2B sub-type. In particular, the highly potent and selective 5-HT2B receptor antagonist, RS-127445, antagonized the response to 5-HT, with a pKB in accordance with its reported affinity at the human 5-HT2B receptor (Bonhaus et al., 1999). Schild analysis yielded a plot with unit slope, indicating that the antagonism was competitive in nature, and that the response is likely to be mediated by a homogenous receptor population.
There are considerable differences in the nature of the receptor populations mediating the excitatory effects of 5-HT in human and animal colon. In the present study, we have demonstrated that in human colon the excitatory effects of 5-HT are mediated by 5-HT2B receptors. In studies in laboratory animals, however, it has been shown that these receptors do not play a significant role in the excitatory effects of 5-HT. In contrast, 5-HT3 and/or 5-HT4 receptors are responsible for 5-HT-induced excitatory effects in guinea-pig colon (Jin et al., 1999), and 5-HT2A and/or 5-HT4 receptors have been proposed to be involved in canine colon (Prins et al., 1997; 2000). In rat colon, contractions to 5-HT have been reported that are mediated either by 5-HT1-like and putative 5-HT3 receptors (Gelal & Guven, 1998), or by 5-HT1 and 5-HT4 receptor sub-types (Grider et al., 1996). Although no selective antagonists of 5-HT3 or 5-HT4 receptors were tested in the present study, the fact that all the 5-HT2B receptor antagonists tested inhibited 5-HT-induced effects, with potencies consistent with 5-HT2B receptor antagonism, supports the involvement of a 5-HT2B receptor. Of these antagonists, RS-127445 is known to be highly selective for 5-HT2B receptors with at least 1000 fold selectivity over other 5-HT receptors (Bonhaus et al., 1999). Furthermore, the unit slope of the Schild plot for the antagonism of 5-HT by RS-127445 makes it extremely unlikely that more than one receptor sub-type is responsible for the effects of 5-HT. It is therefore clear that caution must be exercised when extrapolating from data obtained in animal studies, to predict properties of human tissues.
The precise mechanism by which 5-HT can potentiate neuronal responses in human colonic smooth muscle has yet to be determined. In animals, application of exogenous 5-HT has been shown to induce the release of acetylcholine from myenteric nerves (Yau et al., 1990). Although our data are consistent with such a mechanism, there is as yet no direct evidence that increased neurotransmitter release is responsible for the effects of 5-HT in human colon. We have shown in the present study that 5-HT2B receptors are localized both on colon smooth muscle, and on the nerves of the myenteric plexus. It is therefore possible that 5-HT may be exerting effects on the smooth muscle directly, instead of (or in addition to) increasing the release of excitatory neurotransmitters.
The present study investigated the effects of 5-HT on the longitudinal muscle layer that runs between the taenia coli. Previous studies have investigated 5-HT-induced inhibitory responses in the circular muscle of human colon, and excitatory effects of 5-HT on the taenia coli itself. Whilst the precise roles of these different muscle layers in controlling human colonic motility has thus far not been elucidated, it is believed that colonic motility is a result of the co-ordinated contraction of both circular and longitudinal muscle layers. The present study has used inter-taenial longitudinal smooth muscle strips as a model for human colonic motility, although it is appreciated that contraction of this smooth muscle layer is not the only factor that contributes to colonic motility.
In summary, the results of the present study indicate that in human colon, the excitatory effects of 5-HT are mediated by 5-HT2B receptors. This contrasts with findings from animal studies, where there is substantial evidence for a role for 5-HT3 and/or 5-HT4 receptors. Indeed, based on such data from animal studies, ligands acting at 5-HT3 and/or 5-HT4 receptors have been proposed (and developed) for the treatment of IBS, but their therapeutic efficacy remains to be fully established. In the light of the present data, it would therefore seem that a 5-HT2B receptor antagonist approach may be beneficial in the treatment of IBS.
Acknowledgments
The authors would like to thank Mozam Ali for technical assistance in carrying out the tissue bath experiments.
Abbreviations
- 2-Me-5-HT
2-methyl-5-hydroxytryptamine
- 5-MeOT
5-methoxytryptamine
- 5-HT
5-hydroxytryptamine
- 5-HT2B R-ir
5-HT2B receptor-like immunoreactivity
- α-Me-5-HT
alpha-methyl-5-hydroxytryptamine
- BSA
Bovine serum albumin
- CR
Concentration-ratio
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- IBS
Irritable bowel syndrome
- PBS
Phosphate-buffered saline
- RS-127445
2-amino-4-(4-fluoronaphth-1-yl)-6-isopropylpyrimidine
- RT – PCR
Reverse-transcriptase polymerase chain reaction
- SB-204741
N-(1-methyl-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea
- SB-206553
5-methyl-1-(3-pyridylcarbamoyl)-1,2,3,5-tetrahydropyrrolo[2,3-f]indole
- SB-242084
6-chloro-5-methyl-1-[6-(2-methylpyridin-3-yloxy)pyridin-3-yl-carbamoyl]indoline
References
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative use of drug antagonists. Br. J. Pharmacol. Chemother. 1959;257:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEARCROFT C.P., PERRETT D., FARTHING M.J. 5-hydroxytryptamine release into human jejunum by cholera toxin. Gut. 1996;39:528–531. doi: 10.1136/gut.39.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEARCROFT C.P., PERRETT D., FARTHING M.J. Postprandial plasma 5-hydroxytryptamine in diarrhoea predominant irritable bowel syndrome: a pilot study. Gut. 1998;42:42–46. doi: 10.1136/gut.42.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONHAUS D.W., FLIPPIN L.A., GREENHOUSE R.J., JAIME S., ROCHA C., DAWSON M., VAN NATTA K., CHANG L.K., PULIDO-RIOS T., WEBBER A., LEUNG E., EGLEN R.M., MARTIN G.R. RS-127445: a selective, high affinity, orally bioavailable 5-HT2B receptor antagonist. Br. J. Pharmacol. 1999;127:1075–1082. doi: 10.1038/sj.bjp.0702632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORMAN R.A., BURLEIGH D.E. Evidence for the involvement of a 5-HT receptor in the secretory response of human small intestine to 5-HT. Br. J. Pharmacol. 1993;110:927–928. doi: 10.1111/j.1476-5381.1993.tb13901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORMAN R.A., BURLEIGH D.E. Heterogeneity of 5-HT receptors in human sigmoid colon. Br. J. Pharmacol. 1994;112:558P. [Google Scholar]
- BORMAN R.A., BURLEIGH D.E. Functional evidence for a 5-HT2B receptor mediating contraction of longitudinal muscle in human small intestine. Br. J. Pharmacol. 1995;114:1525–1527. doi: 10.1111/j.1476-5381.1995.tb14935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORMAN R.A., BURLEIGH D.E. Human colonic mucosa possesses a mixed population of 5-hydroxytryptamine receptors. Eur. J. Pharmacol. 1996;309:271–274. doi: 10.1016/0014-2999(96)00466-9. [DOI] [PubMed] [Google Scholar]
- BORMAN R.A., BURLEIGH D.E. 5-HT1D and 5-HT2B receptors mediate contraction of smooth muscle in human small intestine. Ann. New York Acad. Sci. 1997a;812:327–328. doi: 10.1111/j.1749-6632.1997.tb48182.x. [DOI] [PubMed] [Google Scholar]
- BORMAN R.A., BURLEIGH D.E. Heterogeneity of 5-HT receptors mediating secretion in the human intestine. Ann. New York Acad. Sci. 1997b;812:329–330. doi: 10.1111/j.1749-6632.1997.tb48183.x. [DOI] [PubMed] [Google Scholar]
- BUDHOO M.R., HARRIS R.P., KELLUM J.M. The role of the 5-HT4 receptor in Cl− secretion in human jejunal mucosa. Eur. J. Pharmacol. 1996;314:109–114. doi: 10.1016/s0014-2999(96)00474-8. [DOI] [PubMed] [Google Scholar]
- GELAL A., GUVEN H. Characterization of 5-HT receptors in rat proximal colon. Gen. Pharmacol. 1998;30:343–346. doi: 10.1016/s0306-3623(97)00096-7. [DOI] [PubMed] [Google Scholar]
- GERSHON M.D. Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel. Aliment. Pharmacol, Ther. 1999;13:15–30. [PubMed] [Google Scholar]
- GRIDER J.R., KUEMMERLE J.F., JIN J.G. 5-HT released by mucosal stimuli initiates peristalsis by activating 5-HT4/5-HT1p receptors on sensory CGRP neurons. Am. J. Physiol. 1996;270:G778–G782. doi: 10.1152/ajpgi.1996.270.5.G778. [DOI] [PubMed] [Google Scholar]
- HILLIER K., TAM F.S.-F., BUNCE K.T., GROSSMAN C. Inhibition of motility induced by the activation of 5-HT1-like and 5-HT4-like receptors in isolated human colon smooth muscle. Br. J. Pharmacol. 1994;112:102P. [Google Scholar]
- JIN J-G., FOXX-ORENSTEIN A.E., GRIDER J.R. Propulsion in guinea pig colon induced by 5-hydroxytrptamine (HT) via 5-HT4 and 5-HT3 receptors. J. Pharmacol. Exp. Ther. 1999;288:93–97. [PubMed] [Google Scholar]
- MACKAY D. How should values of pA2 and affinity constants for pharmacological competitive antagonists be estimated. J. Pharm. Pharmacol. 1978;30:312–313. doi: 10.1111/j.2042-7158.1978.tb13237.x. [DOI] [PubMed] [Google Scholar]
- PRINS N.H., AKKERMANS L.M.A., LEFEBVRE R.A., SCHUURKES J.A.J. 5-HT4 receptors on cholinergic nerves involved in contractility of canine and human large intestine longitudinal muscle. Br. J. Pharmacol. 2000;131:927–932. doi: 10.1038/sj.bjp.0703615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS N.H., BRIEJER M.R., SCHUURKES J.A.J. Characterization of the contraction to 5-HT in the canine colon longitudinal muscle. Br. J. Pharmacol. 1997;120:714–720. doi: 10.1038/sj.bjp.0700954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINS N.H., BRIEJER M.R., VAN BERGEN P.J., AKKERMANS L.M., SCHUURKES J.A. Evidence for 5-HT7 receptors mediating relaxation of human colonic circular smooth muscle. Br. J. Pharmacol. 1999;128:849–852. doi: 10.1038/sj.bjp.0702762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- READ N.W., GWEE K.-A. The importance of 5-hydroxytryptamine receptors in the gut. Pharmacol. Ther. 1994;62:159–173. doi: 10.1016/0163-7258(94)90009-4. [DOI] [PubMed] [Google Scholar]
- SANGER G.J. 5-Hydroxytryptamine and functional bowel disorders. Neurogastroenterol. Motil. 1996;8:319–331. doi: 10.1111/j.1365-2982.1996.tb00270.x. [DOI] [PubMed] [Google Scholar]
- TAM F.S., HILLIER K., BUNCE K.T. Characterization of the 5-hydroxytryptamine receptor type involved in inhibition of spontaneous activity of human isolated colonic circular muscle. Br. J. Pharmacol. 1994;113:143–150. doi: 10.1111/j.1476-5381.1994.tb16186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAU W.M., DORSETT J.A., YOUTHER M.L. Modulation of submucosal cholinergic neurons by 5-hydroxytryptamine and neuropeptides. Am. J. Physiol. 1990;259:G1019–G1024. doi: 10.1152/ajpgi.1990.259.6.G1019. [DOI] [PubMed] [Google Scholar]


