Abstract
Transcription factors such as NF-κB provide powerful targets for drugs to use in the treatment of cancer. In this report parthenolide (PT), a sesquiterpene lactone of herbal remedies such as feverfew (Tanacetum parthenium) with NF-κB inhibitory activity, markedly increased the degree of human leukaemia HL-60 cell differentiation when simultaneously combined with 5 nM 1α,25-dihydroxyvitamin D3 (1,25-(OH)2D3). PT by itself did not induce HL-60 cell differentiation.
Cytofluorometric analysis indicated that PT stimulated 1,25-(OH)2D3-induced differentiation of HL-60 cells predominantly into monocytes.
Pretreatment of HL-60 cells with PT before the 1,25-(OH)2D3 addition also potentiated the 1,25-(OH)2D3-induced HL-60 cell differentiation in both a dose- and a time-dependent manner, in which the enhanced levels of cell differentiation closely correlated with the inhibitory levels of NF-κB binding activity by PT.
In contrast, santonin, a sesquiterpene lactone without an inhibitory activity of NF-κB binding to the κB sites, did not enhance the 1,25-(OH)2D3-induced HL-60 cell differentiation.
In transfection experiments, PT enhanced 1,25-(OH)2D3-induced VDRE-dependent promoter activity. Furthermore, PT restored 1,25-(OH)2D3-induced VDRE-dependent promoter activity inhibited by TNF-α, an activator of NF-κB signalling pathway.
These results indicate that PT strongly potentiates the 1,25-(OH)2D3-induced HL-60 cell differentiation into monocytes via the inhibition of NF-κB activity and provide evidence that inhibition of NF-κB activation can be a pre-requisite to the efficient entry of promyelocytic leukaemia cells into a differentiation pathway.
Keywords: Differentiation; nuclear factor-κB; parthenolide; sesquiterpene lactone; 1α,25-dihydroxyvitamin D3
Introduction
Most cancer cells exhibit a defect in their capacity to mature into non-replicating adult cells, thereby existing in a highly proliferating state, which results in outgrowing their normal cellular counterparts. The induction of terminal differentiation represents an alternative approach to the treatment of cancer by conventional anti-neoplastic agents since cells exposed to chemical or biological inducers of differentiation do not undergo the cytodestruction produced by cytotoxic agents. Instead they acquire the phenotypic characteristics of endo-stage adult cell forms with no replicative capacity and ultimately undergo programmed cell death. Leukaemia cells can be induced to undergo terminal differentiation by a variety of chemical and biological agents, indicating that the malignant state is not an irreversible process. Certain cancers may eventually be treated with agents that induce terminal differentiation, presumably with less morbidity than that produced by cytodestructive agents (Beere & Hickman, 1993).
Vitamin D3 has been shown to be one of the most potent initiators of the differentiation of HL-60 cells as well as other haematopoietic cell lines. Several studies provide evidence that activation of protein kinase C (PKC) has been shown to be necessary for differentiation of HL-60 cells, especially along the monocytic pathway. Continuous treatment of HL-60 cells with 1α,25-dihdroxyvitamin D3 (1,25-(OH)2D3) increased PKC levels and cell differentiation (Pan et al., 1997), which were significantly inhibited by PKC inhibitors (Martell et al., 1987) or a PKC anti-sense construct (Gamard et al., 1994). Other studies have demonstrated that interference with the activation of NF-κB appears to be a common feature for agents that enhance the differentiation of HL-60 cells produced by 1,25-(OH)2D3 (Sokoloski et al., 1997; 1998).
Clinical trials of 1,25-(OH)2D3 in leukaemia patients, however, have been ineffective, presumably due to hypercalcaemia that limited the therapeutic potential of this agent as a differentiating agent. Since the formation of reactive oxygen species (ROS) appears to be a common denominator in the signals that induce the activation of the transcription factor NF-κB by various stimuli, the potential of several anti-oxidants including curcumin has been evaluated to enhance the differentiation of HL-60 cells when used in combination with 1,25-(OH)2D3. The use of low levels of 1,25-(OH)2D3 in combination with dietary nutrients might well result in a clinical therapy through the induction of terminal differentiation with tolerable side effects.
Tanacetum parthenium (L.), known as ‘feverfew', has been widely used in a traditional indigenous medical practice for migraine, arthritis, and fever (Knight, 1995). Tanacetum parthenium extracts inhibit the generation of oxyradicals (Jodynis-Liebert et al., 2000), prostaglandins (Pugh & Sambo, 1988) and proinflammatory cytokines from activated macrophages and leukocytes (Hwang et al., 1996; Brown et al., 1997). The active compounds from these extracts (Bork et al., 1997) and extracts of other Mexican – Indian medicinal plants used as anti-inflammatory agents (Lyss et al., 1997) appear to be sesquiterpene lactones in the plants. Parthenolide (PT), a predominant sesquiterpene lactone in feverfew, was demonstrated to have significant anti-nociceptive action and anti-inflammatory effect in vitro (Jain & Kulkarni, 1999). PT exerts an anti-inflammatory activity by inhibiting the expression of inducible cyclo-oxygenase, proinflammatory cytokines (Hwang et al., 1996) and inducible nitric oxide synthase (Fukuda et al., 2000). PT also has an anti-ulcerogenic activity in vivo although further work is required to establish its profit/risk ratio, suggesting that this compound may act as an important dietary phytochemical with a potential therapeutic benefit (Tournier et al., 1999). Previously anti-inflammatory agents such as indomethacin were demonstrated to increase the sensitivity of human leukaemia HL-60 cells to physiological differentiating agents (Bunce et al., 1996; Sokoloski & Sartorelli, 1998).
In this report, we investigated the effect of PT on cellular differentiation in the human promyelocytic leukaemia HL-60 cell culture system. We also investigated the effect of PT on the 1,25-(OH)2D3-induced HL-60 cell differentiation. 1,25-(OH)2D3 was chosen for this study because it has been widely used endogenous stimulators of differentiation in HL-60 cells. In addition, analogues of 1,25-(OH)2D3 are used clinically for the treatment of psoriasis (van de kerkhof, 1998).
Methods
Preparation of differentiation inducers
A stock solution of 1 mM 1,25-(OH)2D3 was dissolved in absolute ethanol (Hayman Limited, U.K.) and stored at −80°C. PT was dissolved in dimethylsulphoxide to make a stock solution of 20 mM. The solutions were diluted at least 2000-fold in the growth medium such that the final concentration of ethanol had no effect on the expression of the differentiation. All manipulations were performed in subdued light.
Determination of cell viability and proliferation
Cell viability and proliferation were determined by the trypan blue exclusion assay and the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium (MTT) assay, as previously described (Coligan et al., 1995). In brief, after each treatment, 10 μl of MTT (5 mg ml−1) was added to each well in 96-well plates. After incubation for 4 h at 37°C, the crystals of viable cells were dissolved with 100 μl of 0.04 N HCl in isopropranol. The absorbance of each well was then read at 540 nm using a kinetic microplate reader.
Morphologic studies
Single-cell suspensions were prepared and 2×105 cells were loaded into a cytofunnel and spun at 27 g in a cytospin centrifuge. The slides were fixed with methanol and dried. The slides were stained with Giemsa staining solution for 20 min and rinsed in deionized water, air dried, and observed under a microscope with a camera. The stained cells were assessed for size, regularity of the cell margin, and morphological characteristics of the nuclei.
Immunofluorescent staining and cytofluorometric measurements
Quantitative immunofluorescence measurements were performed in an Epic V flow cytofluorograph (Coulter Electronics, Hialeah, FL, U.S.A.) equipped with a multi-parameter data acquisition and display system (MDADS), as previously described (Kim et al., 1995). Briefly, single-cell suspensions were collected from the various cultures and washed twice with ice-cold phosphate buffered saline (PBS, pH 7.4). Afterwards, the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-human CD14 or anti-human CD11b MAbs (Becton Dickinson, San Jose, CA, U.S.A.) at 4°C for 1 h. After incubation, the cells were washed with PBS and were fixed in PBS containing 1% paraformaldehyde, and cytofluorometric analysis was performed. Background staining was determined by staining the cells with FITC-conjugated isotype control MAbs. One-parameter fluorescence histograms were generated by analysing at least 1×104 cells.
NBT reduction assay
HL-60 cell differentiation was assessed by the NBT reduction assay, as previously described (Collins et al., 1979). The assay is based on the ability of phagocytes to produce superoxide upon stimulation with tissue plasminogen activator (TPA). For this assay, 2×105 cells were harvested by centrifugation and incubated with an equal volume of 0.2% NBT dissolved in PBS containing 1 ng ml−1 of freshly diluted TPA at 37°C for 30 min in the dark. Cytospin slides were prepared and examined for blue – black nitroblue diformazan deposits, an indicative of a TPA-stimulated respiratory burst. At least 200 cells were assessed for each experiment.
Western blot analysis
Cell extracts were separated on SDS-polyacrylamide gel in a Mini-Protein II gel apparatus (Bio-Rad, Richmond, CA, U.S.A.) and the proteins, subsequently, were transferred from the SDS gel onto a nitrocellulose membrane (Millipore, Bedford, MA, U.S.A.) using a semi-dry electroblotting apparatus, as previously described (Coligan et al., 1995). The blots were probed with rabbit anti-IκBα and IκBβ MAbs (Santa Cruz Inc., Santa Cruz, CA, U.S.A.), washed, and exposed to horseradish peroxidase-conjugated anti-rabbit IgG antibody. The blots were then visualized with enhanced chemiluminescence according to the instructions of the manufacturer (Amersham, Arlington Heights, IL, U.S.A.).
Transient transfection
For transfection, HeLa cells or COS-7 cells were grown in 24-well plates with medium supplemented with 10% FBS for 24 h and transfected with indicated plasmids in the presence of Superfectam according to the manufacturer's protocol (Qiagen, Germany). After 16 h, the cells were treated with PT and/or TNF-α for 8 h, and washed and refed with DMEM containing 10% FBS. The cells were harvested 24 h later and luciferase activity was assayed, as previously described (Na et al., 1999), and the results were normalized to the LacZ expression.
Electrophoretic mobility shift assay (EMSA)
The nuclear extracts were prepared from the cells, as previously described (Chung et al., 2000). An oligonucleotide containing an NF-κB-binding site within the Igκ-chain (5′-CCG GTT AAC AGA GGG GGC TTT CCG AG-3′) was used as a probe. Labelled oligonucleotides (10,000 c.p.m.) were incubated for 30 min at room temperature, along with 10 μg of nuclear extracts, in 20 μl of binding buffer ((mM): Tris.HCl 10, pH 7.6, KCl 500, EDTA 10, 50% glycerol, 100 ng of poly (dI-dC), and dithiothreitol 1). The reaction mixture was analysed by electrophoresis on a 4% polyacrylamide gel in 0.5×Tris-borate buffer. Specific binding was confirmed by competition experiments with a 50-fold excess of unlabelled, identical oligonucleotides or cyclic AMP response element-containing oligonucleotides.
Statistical analysis
Student's t-test and one-way analysis of variance (ANOVA) were used to determine the statistical significance of differences between values for various experimental and control groups. P-values <0.05 were considered significant.
Materials
HL-60 cells, HeLa cells and COS-7 cells were obtained from the American Type Culture Collection (ATCC, Rockville, MD, U.S.A.) and maintained in RPMI-1640 medium supplemented with 10% foetal bovine serum (Gibco BRL, Grand Island, NY, U.S.A.). Parthenolide, 1,25-(OH)2D3, Giemsa staining solution, ethanol, methanol-free paraformaldehyde and all other reagents were purchased from the Sigma Chemical Co. (St. Louis, MO, U.S.A.). Chelerythrine (CE), 1-(5-isoquinolinesulphonyl)-2-methylpiperazine dihydrochloride (H-7) and 2-(2′-amino-3′-methoxyphenyl)-oxanaphthalen-4-one (PD-098059) were purchased from the Tocris Cookson Ltd (U.K.).
Results
Treatment with PT enhanced 1,25-(OH)2D3-induced differentiation of HL-60 cells
To determine the effect of PT on 1,25-(OH)2D3-induced cell differentiation, the HL-60 cells were simultaneously treated with 5 nM of 1,25-(OH)2D3 in combination with various concentrations of PT, and the numbers of differentiated cells, as measured by NBT positivity, were determined. As controls, the cells were treated with either 1,25-(OH)2D3 or PT alone. As shown in Figure 1, the addition of PT to cultures exposed to a suboptimal concentration of 1,25-(OH)2D3 (5 nM), which by itself caused a relatively low level of differentiation (less than 27%), resulted in a marked increase in the degree of cell differentiation. PT strongly enhanced 1,25-(OH)2D3-induced HL-60 cell differentiation in both dose- and time-dependent manners. The effects were maximal at 10 μM of PT, with greater than 95% of the treated cells attaining a differentiated state. PT by itself did not induce significant cell differentiation, with less than 5% of the cells attaining a differentiated phenotype. Cells' viability in all cultures remained constant throughout the incubation period in the presence of PT concentrations used in the experiment, as demonstrated by the trypan blue exclusion assay and the MTT assay (data not shown).
Figure 1.
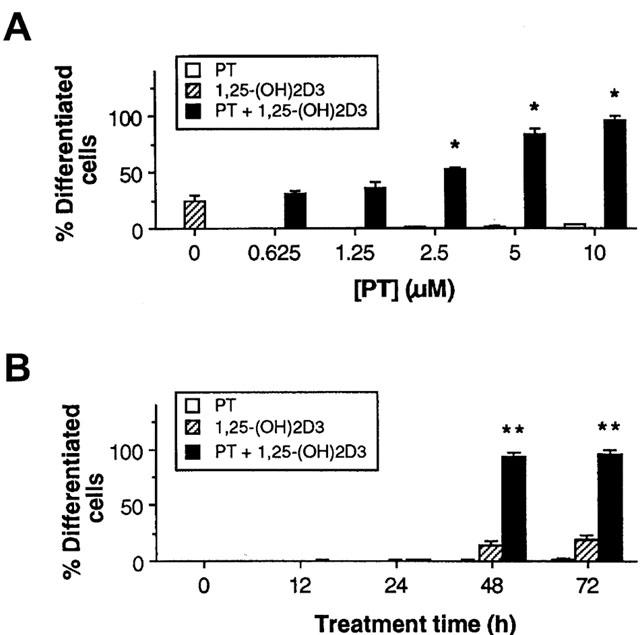
Effect of parthenolide (PT) on the induction of HL-60 cell differentiation produced by 1,25-(OH)2D3. HL-60 leukemia cells were simultaneously treated with 5 nM 1,25-(OH)2D3 alone or in combination with various concentrations of PT for 72 h (A), or with 10 μM PT for various periods (B). Then, the cellular differentiation was assessed by the NBT reduction assay. The results are represented as the mean±s.e.mean (n=3). *P<0.01, relative to a group treated with 1,25-(OH)2D3 alone. **P<0.0001, relative to any other groups.
To further determine the cell differentiation enhanced by PT, the morphologic phenotypes and the expression of cell surface antigens on HL-60 cells were analysed. As shown in Figure 2, Giemsa-stained undifferentiated control HL-60 cells (Figure 2A) were predominantly promyelocytes with round and regular cell margins, and large nuclei, suggesting that the cells were highly active in DNA synthesis and were rapidly proliferating. PT- or 1,25-(OH)2D3-treated cells (Figure 2B,C) exhibited relatively small changes in cell morphology such as irregular cell margins (especially for 1,25-(OH)2D3-treated cells). Combined treatment of HL-60 cells with 1,25-(OH)2D3 plus PT (Figure 2D) resulted in significantly decreased cell size, denser chromatin and an increased cytoplasm to nuclear ratio, which suggested less DNA synthesis. Some cells showed a horseshoe-shaped nucleus, which is a sign of cell differentiation into a monocytic lineage.
Figure 2.
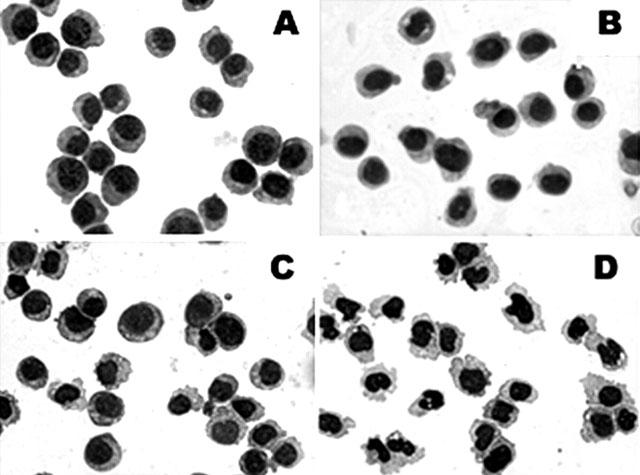
Morphologic analysis of HL-60 cells treated with PT alone or in combination with 1,25-(OH)2D3. HL-60 cells were treated for 72 h with vehicle control (A), 10 μM PT (B), 5 nM 1,25-(OH)2D3 (C), or 10 μM PT plus 5 nM 1,25-(OH)2D3 (D). Cytospin slides were made from HL-60 cells (5×105 cells ml−1) and were stained with Giemsa stain.
Cytofluorometric analysis was also performed to determine the expression of specific surface antigens on HL-60 cells. CD11b (Mac-1) is a cell surface marker for differentiation into either monocytes or granulocytes (Kansas et al., 1990). As shown in Figure 3, PT strongly increased the number of CD11b-positive cells when combined with 5 nM 1,25-(OH)2D3, confirming that PT potentiated 1,25-(OH)2D3-induced HL-60 cell differentiation.
Figure 3.
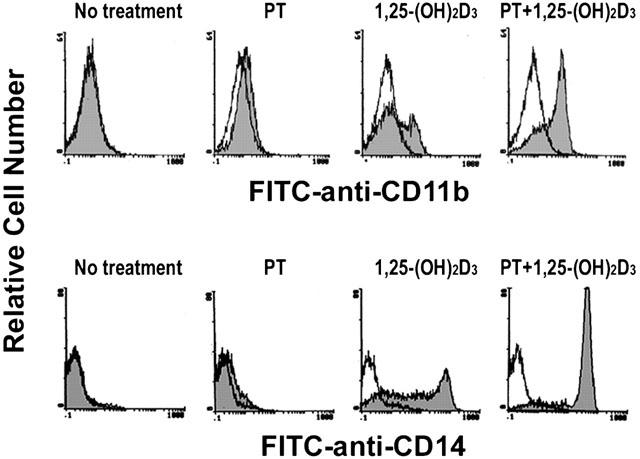
Effect of PT on 1,25-(OH)2D3-induced HL-60 cell differentiation as examined with cell surface marker expression. HL-60 cells were treated for 72 h with medium alone, 10 μM PT, 5 nM 1,25-(OH)2D3, or 10 μM PT in combination with 5 nM 1,25-(OH)2D3. The cells were assessed by cytofluorometric analysis using FITC-conjugated anti-CD11b MAb or anti-CD14 MAb (shaded area), or control MAbs (unshaded area). Data are representative of three independent experiments.
PT enhanced 1,25-(OH)2D3-induced differentiation of HL-60 cells predominantly into monocytes
To determine the differentiation pathway taken by HL-60 cells after treatment with PT and 1,25-(OH)2D3, HL-60 cells were first treated with PT alone or in combination with 1,25-(OH)2D3, and cytofluorometric analysis using mAb for the monocytic surface antigen CD14 was performed. CD14 is expressed exclusively when the cells are differentiated into monocytes (Wright et al., 1990). As shown in Figure 3, HL-60 cells treated with PT in combination with 1,25-(OH)2D3 reacted very strongly with the anti-CD14 MAb. The cells treated with 1,25-(OH)2D3 alone also reacted with the anti-CD14 MAb, but to a lesser extent than those treated with PT in combination with 1,25-(OH)2D3. In contrast, HL-60 cells treated with PT alone did little staining with the anti-CD14 MAb. These results indicate that PT stimulated 1,25-(OH)2D3-induced differentiation of HL-60 cells predominantly into monocytes.
Both PKC and ERK might not be involved in the enhanced HL-60 cell differentiation by PT
Previous studies provide evidence that activation of PKC is necessary for the HL-60 cell differentiation induced by some differentiation-inducing compounds (Martell et al., 1987; Gamard et al., 1994; Pan et al., 1997). To determine any relationship between the effect of PT on cellular differentiation and PKC activation, HL-60 cells were treated with specific PKC inhibitors, chelerythrine or H-7, in the presence of PT alone or in combination with 1,25-(OH)2D3. Thereafter, the degree of cellular differentiation was assessed by the NBT reduction assay. As shown in Figure 4, both PKC inhibitors completely inhibited 1,25-(OH)2D3-induced HL-60 cell differentiation. However, the PKC inhibitors did not inhibit the enhanced cell differentiation by PT. Approximately 75 – 80% of the HL-60 cells treated with PT plus 1,25-(OH)2D3 were still differentiated into monocytes in the presence of PKC inhibitors (at 8 μM for chelerythrine; at 30 μM for H-7), suggesting that PKC may not be involved in the HL-60 cell differentiation enhanced by PT. The PKC inhibitors were not toxic to the HL-60 cells at concentrations used in the experiments, as demonstrated by the trypan blue exclusion assay (data not shown).
Figure 4.
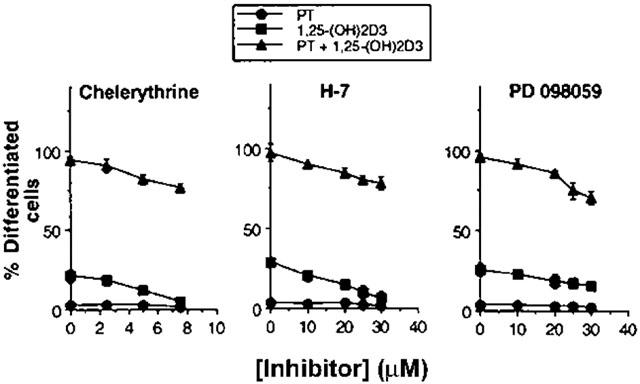
Effect of PKC and ERK inhibitors on HL-60 cell differentiation induced with PT alone or in combination with 1,25-(OH)2D3. HL-60 cells were treated for 40 min with varying concentrations of PKC inhibitors (chelerythrine, H-7) or an ERK inhibitor (PD-098059), followed by incubation with 10 μM, 5 nM 1,25-(OH)2D3, or 10 μM PT in combination with 5 nM PT 1,25-(OH)2D3 for 72 h. The cellular differentiation was assessed by the NBT reduction assay. The results are presented as percentages of the differentiated cells with the mean±s.e.mean (n=3).
ERK/MAP kinase is a downstream element in the PKC signalling pathway of HL-60 cells (Marcinkowska et al., 1997). To determine the involvement of ERK/MAP kinase in 1,25-(OH)2D3-induced cell differentiation enhanced by PT, HL-60 cells were treated with 2-(2′-amino-3′-methoxyphenyl)-oxanaphthalen-4-one (PD-098059), a specific inhibitor of MAP kinase kinase 1 (MEK1), in the presence of PT alone or in combination with 1,25-(OH)2D3. The synthetic compound PD-098059 inhibits the ERK/MAP kinase pathway by preventing the activation of MEK1 by c-Raf (Alessi et al., 1995). As shown in Figure 4, PD-098059 decreased the cell differentiation induced with 1,25-(OH)2D3 alone or 1,25-(OH)2D3 plus PT. However, approximately 70% of the HL-60 cells treated with PT plus 1,25-(OH)2D3 were still differentiated in the presence of PD-098059 (30 μM), indicating that the ERK/MAPK may not also be involved in the HL-60 cell differentiation enhanced by PT. These results suggest that both PKC and ERK may not be involved in 1,25-(OH)2D3-induced HL-60 cell differentiation enhanced by PT.
NF-κB is active in HL-60 leukaemia cells, which is inhibited by PT
PT was shown to inhibit activation of NF-κB by preventing the degradation of IκBα and IκBβ in Jurkat T cells stimulated with TNF-α, PMA, or H2O2 (Hehner et al., 1998; 1999). To ascertain whether the levels of PT employed were capable of causing the inhibition of NF-κB in unstimulated HL-60 leukaemia cells, we analysed nuclear extracts of HL-60 cells for NF-κB activity by an EMSA, using a probe specific for the κB DNA-binding motif of NF-κB. As shown in Figure 5, HL-60 cells showed relatively high levels of NF-κB DNA binding activity in an untreated control condition. The binding was specific since it was competed with an unlabelled, identical oligonucleotide, but not with unrelated, non-specific oligonucleotide. A 24-h exposure to PT caused a marked reduction in the NF-κB DNA binding activity both in the presence and absence of 1,25-(OH)2D3, while exposure of the cells to 5 nM 1,25-(OH)2D3 alone for 24 h had no effect on the level of NF-κB complex.
Figure 5.
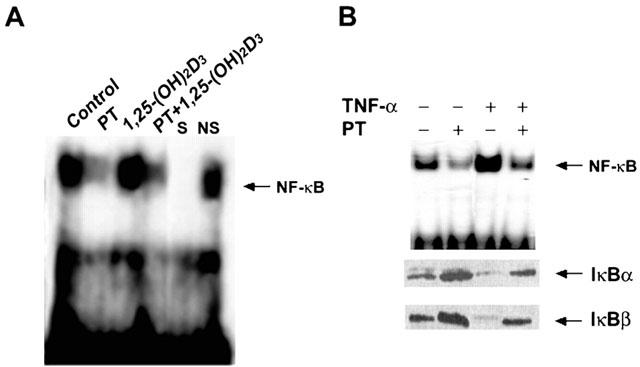
Effect of PT on NF-κB binding to κB sites and degradation of IκB proteins in HL-60 cells. Nuclear extracts from HL-60 cells treated with dimethylsulphoxide as a vehicle (control), 10 μM PT, 5 nM 1,25-(OH)2D3, or 10 μM PT in combination with 1,25-(OH)2D3 for 24 h were analysed by EMSA for NF-κB DNA binding activity using a labelled oligonucleotide containing a consensus I-κB site. S and NS indicate the presence of an unlabelled, identical oligonucleotide and non-specific oligonucleotide, respectively (A). Nuclear extracts from HL-60 cells treated with vehicle or 10 μM PT in the absence or presence of TNF-α (10 ng ml−1) for 24 h were tested by EMSAs for DNA binding of NF-κB and by Western blot experiments for the occurrence of IκB proteins (B). Data are representative of two independent experiments.
Furthermore, to investigate the effect of PT on IκB degradation as an inhibitory mechanism of NF-κB activation, levels of IκBα and IκBβ proteins were determined in HL-60 cells in the absence or presence of PT. NF-κB complexes are generally sequestered in the cytoplasm in an inactive form via interaction with IκB and, after IκB degradation upon addition of NF-κB-inducing stimuli such as lipopolysaccharide or TNF-α, are translocated into the nucleus and induce gene expression (May & Ghosh, 1997). NF-κB inhibitors are known to mainly exert their inhibitory effects by interfering with the induced degradation of IκB-family members (Griscavage et al., 1996) or by scavenging reactive oxygen intermediates (Singh & Aggarwal, 1995). As shown in Figure 5B, preincubation with PT significantly prevented degradation of IκBα and IκBβ in HL-60 cells in the absence or presence of TNF-α as monitored by Western blotting, suggesting that PT inhibits the activation of NF-κB by suppressing the degradation of IκB proteins.
Inhibition of NF-κB activity by PT is crucial for the enhanced induction of HL-60 cell differentiation
To determine when the inhibition of NF-κB binding activity by PT was needed to obtain the optimum enhancement of cell differentiation induced by 1,25-(OH)2D3, HL-60 cells were exposed to 1,25-(OH)2D3 along with PT at various treatment schedules. As shown in Figure 6, pretreatment with PT enhanced 1,25-(OH)2D3-induced cell differentiation in both time-dependent and dose-dependent manners in which the enhanced levels of cell differentiation closely correlated with the inhibitory levels of NF-κB binding activity. Curcumin, a well-known NF-κB inhibitor, also enhanced 1,25-(OH)2D3-induced cell differentiation in a dose-dependent manner when the cells were pretreated (Figure 6C).
Figure 6.
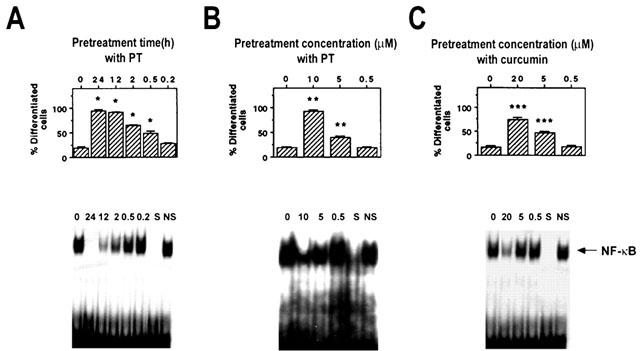
Pretreatment effect of PT and curcumin on 1,25-(OH)2D3-induced HL-60 cell differentiation. HL-60 cells were pretreated with 10 μM PT for various periods (24, 12, 2, 0.5, 0.2 h, respectively) or not pretreated (A), or with PT at various doses (10, 5, 0.5 μM, respectively) for 24 h (B), or with curcumin at various doses (20, 5, 0.5 μM, respectively) for 24 h (C). After washing with DMEM, the cells were treated with 5 nM 1,25-(OH)2D3 for 48 h, and analysed by the NBT reduction assay for cellular differentiation and by the EMSA for NF-κB DNA binding activity. S and NS indicate the presence of an unlabelled, identical oligonucleotide and non-specific oligonucleotide, respectively. Data are representative of two independent experiments. *P<0.001, relative to an untreated group with PT. **P<0.005, relative to an untreated group with PT. ***P<0.005, relative to an untreated group with curcumin.
Importantly, pretreatment of leukaemia cells with PT reduced the dosage and treatment time of 1,25-(OH)2D3, which was required to reach 50% of the treated cells attaining a differentiated state (Figure 7), suggesting that inhibition of NF-κB by PT increases the sensitivity of HL-60 leukaemia cells to a differentiating agent, 1,25-(OH)2D3.
Figure 7.
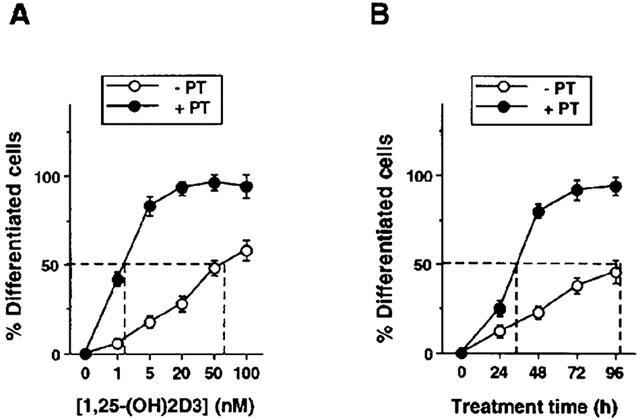
Pretreatment with PT increases the sensitivity of leukaemia cells to 1,25-(OH)2D3. HL-60 cells were pretreated with 10 μM PT for 24 h or not pretreated. After washing with DMEM, the cells were incubated with varying concentrations of 1,25-(OH)2D3 for 48 h (A), or with 5 nM 1,25-(OH)2D3 for 24, 48, 72 and 96 h (B). The cellular differentiation was determined by the NBT reduction assay. The results are represented as the mean±s.e.mean of triplicate determinations. The experiment was repeated twice with similar results.
PT activates VDRE-dependent reporter activity
To evaluate whether PT activates VDRE-dependent reporter activity, HeLa cells were transfected with a VDRE reporter vector and a CMV-driven expression vector encoding VDR in the absence or presence of 1,25-(OH)2D3 and PT. As shown in Figure 8, the cells treated with 1,25-(OH)2D3 had comparable 6.3-fold increase in VDRE-promoter activity. PT enhanced 1,25-(OH)2D3-induced VDRE-promoter activity in a dose-dependent manner. PT enhanced 1,25-(OH)2D3-induced VDRE-promoter activity in a dose-dependent manner. PT also increased 1,25-(OH)2D3-induced VDRE-promoter activity in COS-7 cells (data not shown).
Figure 8.
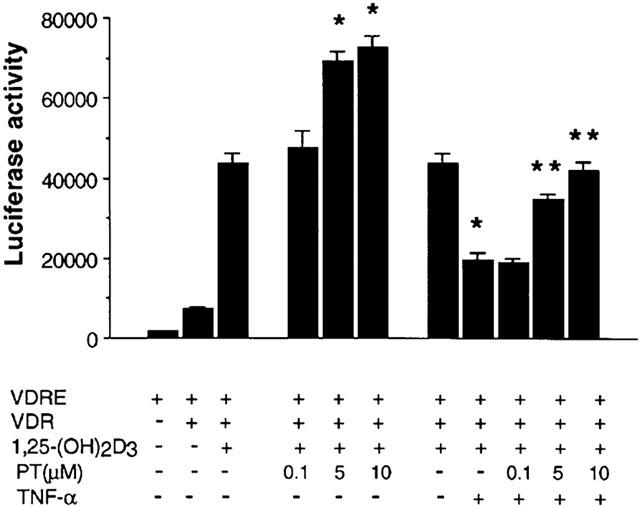
Effect of PT on VDRE reporter activity in HeLa cells. HeLa cells (3×104 cells well−1 in 24 well plate) were transiently transfected with 200 ng VDRE promoter/luciferase report construct and 100 ng VDR expression construct or empty vector as a control. The transfected cells were treated with 10 μM PT and/or 10 ng ml−1 TNF-α and, 7 h later, treated with 5 nM 1,25-(OH)2D3 for 24 h. Normalized luciferase expressions from triplicate samples are presented relative to the LacZ expressions. The results are represented as the mean±s.e.mean of triplicate determinations. The experiment was repeated four times with similar results. *P<0.001, relative to a group of VDRE(+), VDR(+) and 1,25-(OH)2D3. **P<0.001, relative to a group of VDRE(+), VDR(+), 1,25-(OH)2D3 and TNF-α(+).
TNF-α is known to suppress VDRE-promoter activity through NF-κB activation (Farmer et al., 2000). To investigate whether PT restored TNF-α-mediated inhibition of the VDRE-promoter activity, HeLa cells were transfected with plasmids of VDRE and VDR, and treated with 1,25-(OH)2D3 and TNF-α in the absence or presence of PT. As expected, TNF-α significantly inhibited 1,25-(OH)2D3-stimulated increase in VDRE-promoter activity by 55%. Importantly treatment with PT restored the inhibitory activity of TNF-α on VDRE-promoter activity in a dose-dependent manner, suggesting that PT may activate VDRE-promoter activity via inhibition of NF-κB activation.
Santonin, a sesquiterpene lactone without an inhibitory activity of NF-κB DNA binding, does not enhance 1,25-(OH)2D3-induced HL-60 cell differentiation
To further demonstrate the inhibitory role of NF-κB DNA binding activity by PT for enhancing 1,25-(OH)2D3-induced cell differentiation, HL-60 cells were treated with 1,25-(OH)2D3 along with santonin, a sesquiterpene lactone compound without an inhibitory activity of NF-κB DNA binding, or along with PT as a positive control, and the degree of cell differentiation and the NF-κB DNA binding activity were determined. As shown in Figure 9, santonin did not enhance 1,25-(OH)2D3-induced cell differentiation even at 100 μM, while PT strongly enhanced the cell differentiation even at 5 and 10 μM. As expected, santonin did not inhibit the NF-κB binding activity while PT significantly inhibited the activity in a dose-dependent manner. Therefore, these results indicate that the enhanced effects on 1,25-(OH)2D3-induced cell differentiation by PT resulted from the inhibition of NF-κB DNA binding activity by PT.
Figure 9.
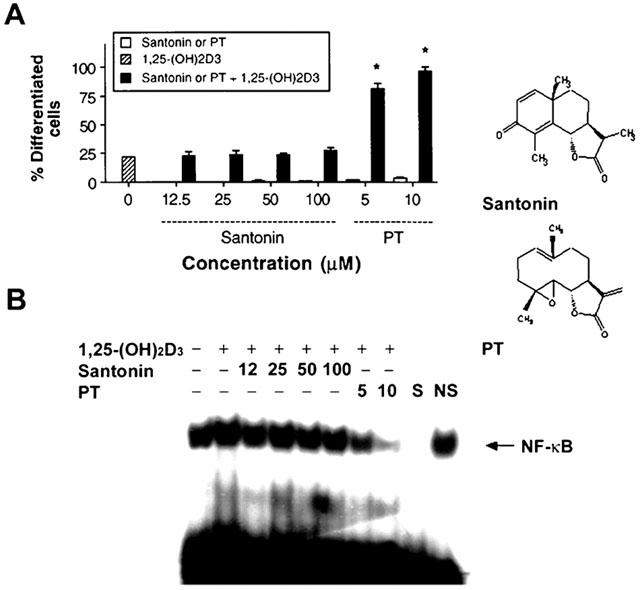
Effect of santonin, a sesquiterpene lactone without inhibitory activity of NF-κB DNA binding, on 1,25-(OH)2D3-induced HL-60 cell differentiation. The cells were treated with 5 nM 1,25-(OH)2D3 alone or in combination with either santonin or PT for 72 h. As controls, the cells were treated with either santonin or PT alone. The percentage of differentiated cells from each treatment was determined by the NBT reduction assay (A), and the NF-κB DNA binding activity was analysed by the EMSA (B). S and NS indicate the presence of an unlabelled, identical oligonucleotide and non-specific oligonucleotide, respectively. *P<0.001, relative to any other groups. Data are representative of two independent experiments.
Discussion
In this study, we have demonstrated that PT, an active sesquiterpene lactone of herbal remedies such as feverfew (Tanacetum parthenium), potentiated 1,25-(OH)2D3-induced differentiation of HL-60 promyelocytic leukaemia cells into a monocytic lineage although this agent alone did not induce the cell differentiation. The enhanced degree of cell differentiation closely correlated with the inhibitory levels of NF-κB binding activity by PT. Furthermore, untreated HL-60 cells showed significantly high levels of NF-κB binding activity and pretreatment of these cells with PT before the 1,25-(OH)2D3 addition also showed enhancing effects on HL-60 cell differentiation in both a time- and a dose-dependent manner. In addition, pretreatment with PT decreased the concentration and treatment time of 1,25-(OH)2D3 which induced 50% of the treated cells with a differentiation phenotype, suggesting that the down-regulation of NF-κB in tumour cells with constitutively active NF-κB is crucial for sensitizing the cells to a differentiation-inducing agent, 1,25-(OH)2D3 (Figures 6 and 7). Previous studies have reported some chemicals that were capable of enhancing the differentiation of HL-60 cells produced by low levels of 1,25-(OH)2D3. These include butyrate (Yoshida et al., 1992), capsaicin (Kang et al., 2001a), silibin (Kang et al., 2001b) and nonsteroidal anti-inflammatory agents such as acetylsalicyclic acid (Sokoloski & Sartorelli, 1998).
The mechanism by which PT potentiates 1,25-(OH)2D3-induced HL-60 cell differentiation is not clear. Previous studies have shown that 1,25-(OH)2D3 may mediate biological responses including cell differentiation as a consequence of a genomic pathway signalling through the vitamin D receptor to modulate gene transcription (Haussler et al., 1998) and of a non-genomic pathway through a putative cell membrane receptor to generate rapid effects (Norman et al., 1997), including the opening of voltage-gated calcium and chloride channels (Zanello & Norman, 1997), and activation of PKC and MAPK (Pan et al., 1997; Song et al., 1998). In our study, both PKC inhibitors and ERK inhibitor completely inhibited the 1,25-(OH)2D3-induced HL-60 cell differentiation. In contrast, the inhibitors did not decrease the enhanced cell differentiation by PT in the presence of low levels of 1,25-(OH)2D3. Approximately 70 – 80% of the cells treated with PT plus 1,25-(OH)2D3 were still differentiated into monocytes in the presence of the inhibitors, suggesting that both PKC and ERK may not be involved in the HL-60 cell differentiation enhanced by PT.
PT may enhance the sensitivity of HL-60 leukaemia cells to a differentiating agent 1,25-(OH)2D3 by activating a genomic pathway of 1,25-(OH)2D3-induced differentiation via inhibition of NF-κB activity. Several lines of evidence support this point. First, the NF-κB EMSA indicated that HL-60 leukaemia cells showed relatively high levels of NF-κB activity that was not affected by 1,25-(OH)2D3 treatment (Figure 5A). PT, a sesquiterpene lactone with an inhibitory activity of NF-κB, enhanced 1,25-(OH)2D3-induced cell differentiation while santonin, a sesquiterpene lactone without an inhibitory activity of NF-κB, did not (Figure 9). PT significantly inhibited the DNA binding activity of NF-κB by suppressing degradation of IκB proteins, IκBα and IκBβ, in HL-60 cells (Figure 5B). Second, the pretreatment experiments showed that the enhanced levels of cellular differentiation by PT closely correlated with the inhibitory levels of NF-κB binding activity (Figure 6). Furthermore, PT enhanced 1,25-(OH)2D3-induced VDRE-promoter activity in a dose-dependent manner. PT restored the inhibitory action of TNF-α on 1,25-(OH)2D3-induced VDRE-promoter activity (Figure 8). TNF-α is known to suppress VDRE-promoter activity through NF-κB activation (Farmer et al., 2000; Nanes et al., 1994). Therefore, the genomic pathway of cell differentiation may still be under a suppressed state in HL-60 leukaemia cells with the active NF-κB when treated with low levels of 1,25-(OH)2D3. Treatment with PT possibly relieved the suppressed genomic pathway of cellular differentiation via inhibition of the NF-κB activity, leading to the marked increase of 1,25-(OH)2D3-induced HL-60 cell differentiation. The high levels of NF-κB activity in HL-60 leukaemia cells may be involved in the inhibition of a genomic pathway of cellular differentiation when treated with low levels of 1,25-(OH)2D3. Others have reported that some anti-oxidants including curcumin were found to be capable of enhancing the differentiation of HL-60 cells produced by vitamin D3 (Sokoloski et al., 1997). Since these anti-oxidants are known to inhibit the activation of NF-κB (Surh et al., 2000), the current findings provide support for the possible involvement of NF-κB in regulating growth and differentiation.
The mechanism of how inhibition of NF-κB activity enhances HL-60 cell differentiation induced by low levels of 1,25-(OH)2D3 is not fully understood. The transcription factor NF-κB might inhibit a VDR-mediated genomic pathway of cell differentiation induced by 1,25-(OH)2D3. Previous reports showed that NF-κB activated by TNF-α inhibited the transcriptional potency of the VDR at its cognate cis regulatory sites (Farmer et al., 2000). Osteoblastic ROS17/2.8 cells transfected with a NF-κB expression plasmid decreased 1,25-(OH)2D3-stimulated transcription. The over-expression of p65, not p50, completely abrogated enhanced VDRE-mediated transcriptional activity in response to 1,25-(OH)2D3, indicating that NF-κB has inhibitory effects on 1,25-(OH)2D3 receptor function.
The quenching of hormone-stimulated transcription by p65 is consistent with a mechanism in which p65 competes for and sequesters an essential cofactor or activator required for VDR-mediated transactivation. Support for this hypothesis comes from a steadily increasing body of recent experimental evidence. In several studies, affinity ‘pull-down' experiments were employed to show that transcription factors such as SREBP-1α, Sp1, VP16, and the p65 NF-κB subunit interact with one or more proteins within a large activator-recruited cofactor (ARC) complex consisting of multiple proteins. Similarly, a truncated peptide containing the ligand-binding domain and α-helical region corresponding to the activation function-2 of VDR has been shown to account for ligand-dependent association of VDR with proteins within a large VDR-interacting protein (DRIP) complex (Rachez et al., 1999). Some DRIPs have been found to be identical to the independently cloned steroid/thyroid receptor coactivators p100 and p205. In addition, equivalent electrophoretic mobility assays for the majority of ARC and DRIP proteins suggest that these two complexes are essentially the same. This large complex may bridge DNA-bound transcription factors to the RNA polymerase II preinitiation complex. VDR and p65 both form associations with proteins within these complexes and may compete for common factors that confer optimum transcriptional potency. This model of competitive sequestration of cofactors is consistent with other reports showing p65 squenches glucocorticoid-dependent transcription by competing for limited amounts of the coactivators (CBP/p300 and SRC-1 (Sheppard et al., 1998).
1,25-(OH)2D3 and some of its analogues are used for the treatment of psoriasis (Kragballe, 1992). It is possible that many dietary chemicals such as curcuminoids, tocopherols, carotenoids, and other edible plants can prevent human cancer in part by synergizing with endogenously produced stimulators of differentiation such as retinoic acids and 1,25-(OH)2D3. Epidemiological studies suggest that people who eat large amounts of fruit and some vegetables have a lower risk of many kinds of cancer (Negri et al., 1991). The results presented here suggest that treatment of patients with combinations of PT and 1,25-(OH)2D3 may provide a greater therapeutic response than 1,25-(OH)2D3 alone. In addition, these results suggest that the targeting of the NF-κB transcription factor could be important in the treatment of cancer with constitutively active NF-κB, including some breast cancer (Patel et al., 2000).
Acknowledgments
We thank Drs J.W. Lee, J.H. Cheong, B.M. Spiegelman and S.Y. Hwang for providing valuable reagents and helpful discussion. This work was supported by the research grant from the Ministry of Health and Welfare in Korea (01-PJ10-PG6-01GN16-0005).
Abbreviations
- DRIP
VDR-interacting protein
- EMSA
electrophoretic mobility shift assay
- ERK
extracellular signal-regulated kinase
- FITC
fluorescein isothiocyanate
- MAb
monoclonal antibody
- MAPK
mitogen-activated protein kinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium
- NBT
nitroblue tetrazolium
- NF-κB
nuclear factor-kappaB
- 1,25-(OH)2D3
1α,25-dihydroxyvitamin D3
- PKC
protein kinase C
- PT
parthenolide
- TNF
tumour necrosis factor
- VDRE
vitamin D receptor response element
References
- ALESSI D.R., CUENDA A., COHEN P., DUDLEY D.T., SALTIEL A.R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- BEERE H.M., HICKMAN J.A. Differentiation: a suitable strategy for cancer chemotherapy. Anti-Cancer Drug Design. 1993;8:299–322. [PubMed] [Google Scholar]
- BORK P.M., SCHMITZ M.L., KUHNT M., ESCHER C., HEINRICH M. Sesquiterpene lactone containing Mexican Indian medicinal plants and pure sesquiterpene lactones as potent inhibitors of transcription factor NF-κB. FEBS Lett. 1997;402:85–90. doi: 10.1016/s0014-5793(96)01502-5. [DOI] [PubMed] [Google Scholar]
- BROWN A.M., EDWARDS C.M., DAVEY M.R., POWER J.B., LOWE K.C. Pharmacological activity of feverfew (Tanacetum pathenium (L.) Schultz-Bip.): assessment by inhibition of human polymorphonuclear leukocyte chemiluminesence in vitro. J. Pharm. Pharmacol. 1997;49:558–561. doi: 10.1111/j.2042-7158.1997.tb06841.x. [DOI] [PubMed] [Google Scholar]
- BUNCE C.M., MOUNTFORD J.C., FRENCH P.J., MOLE D.J., DURHAM J., MICHELL R.H., BROWN G. Potentiation of myeloid differentiation by anti-inflammatory agents, by steroids and by retinoic acid involves a single intracellular target, probably an enzyme of the aldoketoreductase family. Biochim. Biophys. Acta. 1996;1311:189–198. doi: 10.1016/0167-4889(96)00005-5. [DOI] [PubMed] [Google Scholar]
- CHUNG S.W., KANG B.Y., KIM S.H., PAK Y.K., CHO D., TRINCHIERI G., KIM T.S. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-γ and nuclear factor-κB. J. Biol. Chem. 2000;275:32681–32687. doi: 10.1074/jbc.M002577200. [DOI] [PubMed] [Google Scholar]
- COLIGAN J.E., KRUISBEEK A.M., MARGULIES D.H., SHEVACH E.M., STROBER W. Current Protocols in Immunology. New York: Wiley; 1995. Isolation and analysis of proteins. [Google Scholar]
- COLLINS S.J., RUSCETTI F.W., GALLAGHER R.E., GALLO R.C. Normal functional characteristics of cultured human promyelocytic leukemia cells (HL-60) after induction of differentiation by dimethylsulfoxide. J. Exp. Med. 1979;149:969–974. doi: 10.1084/jem.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARMER P.K., HE X., SCHMITZ M.L., RUBIN J., NANES M.S. Inhibitory effect of NF-κB on 1,25,dihydroxyvitamin D3 and retinoid X receptor function. Am. J. Physiol. Endocrinol. Metab. 2000;279:213–220. doi: 10.1152/ajpendo.2000.279.1.E213. [DOI] [PubMed] [Google Scholar]
- FUKUDA K., HIBIYA Y., MUTOH M., OHNO Y., YAMASHITA K., AKAO S., FUJIWARA H. Inhibition by parthenolide of phorbol ester-induced transcriptional activation of inducible nitric oxide synthase gene in a human monocytic cell line THP-1. Biochem. Pharmacol. 2000;60:595–600. doi: 10.1016/s0006-2952(00)00340-3. [DOI] [PubMed] [Google Scholar]
- GAMARD C.J., BLOBE G.C., HANNUN Y.A., OBEID L.M. Specific role for protein kinase C beta in cell differentiation. Cell Growth Differ. 1994;5:405–409. [PubMed] [Google Scholar]
- GRISCAVAGE J.M., WILK S., IGNARRO L.J. Inhibitors of the proteosome pathway interfere with induction of nitric oxide synthesis in macrophages by blocking activation of transcription factor NF-κB. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3308–3312. doi: 10.1073/pnas.93.8.3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUSSLER M.R., WHITFIELD G.K., HAUSSLER C.A., HSIEH J.C., THOMPSON P.D., SELZNICK S.H., DOMINGUEZ C.E., JURUTKA P.W. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J. Bone Miner. Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- HEHNER S.P., HEINRICH M., BORK P.M., BOGT M., RATTER F., LEHMANN V., SCHULZE-OSTHOFF K., DROGE W., SCHMITZ M.L. Sesquiterpene lactones specifically inhibit activation of NF-κB by preventing the degradation of IκB-α and IκBβ. J. Biol. Chem. 1998;273:1288–1297. doi: 10.1074/jbc.273.3.1288. [DOI] [PubMed] [Google Scholar]
- HEHNER S.P., HOFMANN T.G., DROGE W., SCHMITZ M.L. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-κB by targeting the IκB kinase complex. J. Immunol. 1999;163:5671–5623. [PubMed] [Google Scholar]
- HWANG D., FISCHER N.H., JANG B.C., TAK H., KIM J.K., LEE W. Inhibition of the expression of inducible cyclooxygenase and proinflammatory cytokines by sesquiterpene lactones in macrophages correlates with the inhibition of MAP kinases. Biochem. Biophys. Res. Commun. 1996;226:810–818. doi: 10.1006/bbrc.1996.1433. [DOI] [PubMed] [Google Scholar]
- JAIN N.K., KULKARNI S.K. Antinociceptive and anti-inflammatory effects of Tanacetum parthenium L. extract in mice and rats. J. Ethnopharmacol. 1999;68:251–259. doi: 10.1016/s0378-8741(99)00115-4. [DOI] [PubMed] [Google Scholar]
- JODYNIS-LIEBERT J., MURIAS M., BLOSZYK E. Effect of sesquiterpene lactones on antioxidant enzymes and some drug-metabolizing enzymes in rat liver and kidney. Planta Med. 2000;66:199–205. doi: 10.1055/s-2000-8566. [DOI] [PubMed] [Google Scholar]
- KANG S.N., CHUNG S.W., KIM T.S. Capsaicin potentiates 1,25-dihydroxyvitamin D3- and all-trans retinoic acid-induced differentiation of human promyelocytic leukemia HL-60 cells. Eur. J. Pharmacol. 2001a;420:83–90. doi: 10.1016/s0014-2999(01)00994-3. [DOI] [PubMed] [Google Scholar]
- KANG S.N., LEE M.H., KIM K.-M., CHO D., KIM T.S. Induction of human promyelocytic leukemia HL-60 cell differentiation into monocytes by silibinin: involvement of protein kinase C. Biochem. Pharmacol. 2001b;61:1487–1495. doi: 10.1016/s0006-2952(01)00626-8. [DOI] [PubMed] [Google Scholar]
- KANSAS G.S., MUIRHEAD M.J., DAILEY M.O. Expression of the CD11/CD18, leukocyte adhesion molecule 1, and CD44 adhesion molecules during normal myeloid and erythroid differentiation in humans. Blood. 1990;76:2483–2492. [PubMed] [Google Scholar]
- KIM T.S., XU W.S., SUN T., COHEN E.P. Immunization with interleukin-2/interferon-γ double cytokine-secreting allogeneic fibroblasts prolongs the survival of mice with melanoma. Melanoma Res. 1995;5:217–227. doi: 10.1097/00008390-199508000-00003. [DOI] [PubMed] [Google Scholar]
- KNIGHT D.W. Feverfew: chemistry and biological activity. Nat. Prod. Rep. 1995;12:271–276. doi: 10.1039/np9951200271. [DOI] [PubMed] [Google Scholar]
- KRAGBALLE K. Vitamin D3 and skin diseases. Arch. Dermatol. Res. 1992;284:30–36. doi: 10.1007/BF00638238. [DOI] [PubMed] [Google Scholar]
- LYSS G., SCHMIDT T.J., MERFORT I., PAHL H.L. Helenalin, an anti-inflammatory sesquiterpene lactone from Arnica, selectively inhibits transcription factor NF-κB. J. Biol. Chem. 1997;378:951–961. doi: 10.1515/bchm.1997.378.9.951. [DOI] [PubMed] [Google Scholar]
- MARCINKOWSKA E., WIEDLOCHA A., RADZIKOWSKI C. 1,25-Dihydroxyvitamin D3 induced activation and subsequent nuclear translocation of MAPK is upstream regulated by PKC in HL-60 cells. Biochem. Biophys. Res. Commun. 1997;241:419–426. doi: 10.1006/bbrc.1997.7832. [DOI] [PubMed] [Google Scholar]
- MARTELL R.E., SIMPSON R.U., TAYLOR J.M. 1,25-Dihydroxyvitamin D3 regulation of phorbol ester receptors in HL-60 leukemia cells. J. Biol. Chem. 1987;262:5570–5575. [PubMed] [Google Scholar]
- MAY M.J., GHOSH S. Rel/NF-κB and IκB proteins: an overview. Semin. Cancer Biol. 1997;8:63–73. doi: 10.1006/scbi.1997.0057. [DOI] [PubMed] [Google Scholar]
- NA S.-Y., KANG B.Y., CHUNG S.W., HAN S.J., MA X., TRINCHIERI G., IM S.-Y., LEE J.W., KIM T.S. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NF-κB. J. Biol. Chem. 1999;274:7674–7680. doi: 10.1074/jbc.274.12.7674. [DOI] [PubMed] [Google Scholar]
- NANES M.S., KUNO H., DEMAY M.B., KURIAN M., HENDY G.N., DELUCA H.F., TITUS L., RUBIN J. A single up-stream element confers responsiveness to 1,25-dihydroxyvitamin D3 and tumor necrosis factor-α in the rat osteocalcin gene. Endocrinology. 1994;134:1113–1120. doi: 10.1210/endo.134.3.8119149. [DOI] [PubMed] [Google Scholar]
- NEGRI E., LA VECCHIA C., FRANCESCHI S., D'AVANZO B., PARAZZINI F. Vegetable and fruit consumption and cancer risk. Int. J. Cancer. 1991;48:350–354. doi: 10.1002/ijc.2910480307. [DOI] [PubMed] [Google Scholar]
- NORMAN A.W., OKAMURA W.H., HAMMOND M.W., BISHOP J.E., DORMANEN M.C., BOUILLON R., VAN BAELEN H., RIDALL A.L., DAANE E., KHOURY R., FARACH-CARSON M.C. Comparison of 6-s-cis- and 6-s-trans-locked analogs of 1α,25-dihydroxyvitamin D3 indicates that the 6-s-cis conformation is preferred for rapid nongenomic biological responses and that neither 6-s-cis- nor 6-s-trans-locked analogs are preferred for genomic biological responses. Mol. Endocrinol. 1997;11:1518–1531. doi: 10.1210/mend.11.10.9993. [DOI] [PubMed] [Google Scholar]
- PAN Q., GRANGER J., O'CONNELL T.D., SOMERMAN M.J., SIMPSON R.U. Promotion of HL-60 cell differentiation by 1,25-dihydroxyvitamin D3 regulation of protein kinase C levels and activity. Biochem. Pharmacol. 1997;54:909–915. doi: 10.1016/s0006-2952(97)00286-4. [DOI] [PubMed] [Google Scholar]
- PATEL N.M., NOZAKI S., SHORTLE N.H., BHAT-NAKSHATRI P., NEWTON T.R., RICE S., GELFANOV V., BOSWELL S.H., GOULET R.J., SLEDGE G.W., NAKSHATRI H. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-κB is enhanced by IκBα super-repressor and parthenolide. Oncogene. 2000;19:4159–4169. doi: 10.1038/sj.onc.1203768. [DOI] [PubMed] [Google Scholar]
- PUGH W.J., SAMBO K. Prostaglandin synthetase inhibitors in feverfew. J. Pharm. Pharmacol. 1988;40:743–745. doi: 10.1111/j.2042-7158.1988.tb07010.x. [DOI] [PubMed] [Google Scholar]
- RACHEZ C., LEMON B.D., SULDAN Z., BROMLEIGH V., GAMBLE M., NAAR A.M., ERDJUMENT-BROMAGE H., TEMPST P., FREEDMAN L.P. Ligand-dependent transcription activation by nuclear receptors requires the DRIP complex. Nature. 1999;398:824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- SHEPPARD K.A., PHELPS K.M., WILLIAMS A.J., THANOS D., GLASS C.K., ROSENFELD M.G., GERRITSEN M.E., COLLINS T. Nuclear integration of glucocorticoid receptor and nuclear factor-κB signaling by CREB-binding protein and steroid receptor coactivator-1. J. Biol. Chem. 1998;273:29291–29294. doi: 10.1074/jbc.273.45.29291. [DOI] [PubMed] [Google Scholar]
- SINGH S., AGGARWAL B.B. Activation of transcription factor NF-κB is suppressed by curcumin(diferuloylmethane) J. Biol. Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- SOKOLOSKI J.A., HODNICK W.F., MAYNE S.T., CINQUINA C., KIM C.S., SARTORELLI A.C. Induction of the differentiation of HL-60 promyleocytic leukemia cells by vitamin E and other antioxidants in combination with low levels of vitamin D3: possible relationship to NF-κB. Leukemia. 1997;11:1546–1553. doi: 10.1038/sj.leu.2400786. [DOI] [PubMed] [Google Scholar]
- SOKOLOSKI J.A., NARAYANAN R., SARTORELLI A.C. Enhancement by antisense oligonucleotides to NF-κB of the differentiation of HL-60 promyelocytic leukemia cells induced by vitamin D3. Cancer Lett. 1998;125:157–164. doi: 10.1016/s0304-3835(97)00505-3. [DOI] [PubMed] [Google Scholar]
- SOKOLOSKI J.A., SARTORELLI A.C. Induction of the differentiation of HL-60 promyelocytic leukemia cells by nonsteroidal anti-inflammatory agents in combination with low levels of vitamin D3. Leuk. Res. 1998;22:153–161. doi: 10.1016/s0145-2126(97)00156-2. [DOI] [PubMed] [Google Scholar]
- SONG X., BISHOP J.E., OKAMURA W.H., NORMAN A.W. Stimulation of phosphorylation of mitogen-activated protein kinase by 1α,25-dihydroxyvitamin D3 in promyelocytic NB4 leukemia cells: a structure-function study. Endocrinology. 1998;139:457–465. doi: 10.1210/endo.139.2.5747. [DOI] [PubMed] [Google Scholar]
- SURH Y.J., HAN S.S., KEUM Y.S., SEO J.J., LEE S.S. Inhibitory effects of curcumin and capsaicin on phorbol ester-induced activation of eukaryotic transcription factors, NF-κB and AP-1. Biofactors. 2000;12:107–112. doi: 10.1002/biof.5520120117. [DOI] [PubMed] [Google Scholar]
- TOURNIER H., SCHINELLA G., DE BALSA E.M., BUSCHIAZZO H., MANEZ S., MORDUJOVICH DE BUSCHIAZZO P. Effect of the chloroform extract of Tanacetum vulgare and one of its active principles, parthenolide, on experimental gastric ulcer in rats. J. Pharm. Pharmacol. 1999;51:215–219. doi: 10.1211/0022357991772169. [DOI] [PubMed] [Google Scholar]
- VAN DE KERKHOF P.C. An update on vitamin D3 analogues in the treatment of psoriasis. Skin Pharmacol. Appl. Skin Physiol. 1998;11:2–10. doi: 10.1159/000029803. [DOI] [PubMed] [Google Scholar]
- WRIGHT S.D., RAMOS R.A., TOBIAS P.S., ULEVITCH R.J., MATHISON J.C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- YOSHIDA M., TANAKA Y., EGUCHI T., IKEKAWA N., SAIJO N. Effect of hexafluoro-1,25-dihydroxyvitamin D3 and sodium butyrate combination on differentiation and proliferation of HL-60 leukemia cells. Anticancer Res. 1992;12:1947–1952. [PubMed] [Google Scholar]
- ZANELLO L.P., NORMAN A.W. Stimulation by 1α,25(OH)2-vitamin D3 of whole cell chloride currents in osteoblastic ROS 17/2.8 cells. A structure-function study. J. Biol. Chem. 1997;272:22617–22622. doi: 10.1074/jbc.272.36.22617. [DOI] [PubMed] [Google Scholar]


