Abstract
The molecular nature and functions of the I2 subtype of imidazoline binding sites are unknown but evidence suggests an association with monoamine oxidase (MAO). Rats can distinguish the selective imidazoline I2-site ligand 2-BFI from vehicle in drug discrimination, indicating functional consequences of occupation of these sites. We have used drug discrimination to investigate the nature of the discriminable stimulus, especially in relation to MAO inhibition.
Following training to distinguish 2-BFI 7 mg kg−1 i.p. from saline vehicle in two-lever operant-chambers, male Hooded Lister rats underwent sessions where test substances were given instead and the proportion of lever presses on the 2-BFI-associated lever (substitution) recorded.
2-BFI; its cogeners BU216, BU224, BU226 and LSL60101; the reversible MAO-A inhibitors moclobemide and RO41-1049; the β-carbolines harmane, norharmane and harmaline which also reversibly inhibit MAO-A, and the anti-addictive substance ibogaine exhibited potent, dose-dependent substitution for 2-BFI.
Agmatine, and LSL60125 substituted at one dose only. The reversible MAO-B inhibitors lazabemide and RO16-1649; the σ2-site ligand SKF10,047 and the I2A-site ligand, amiloride, failed to substitute. The irreversible inhibitor of MAO, deprenyl, substituted for 2-BFI while clorgyline did not.
These results suggest imidazoline I2 site ligands produce a common discriminable stimulus that appears associated with reversible inhibition of MAO-A rather than MAO-B, possibly through increases in extracellular concentration of one or more monoamines. Ibogaine exhibits a commonality in its subjective effects with those of I2-site ligands.
Keywords: 2-BFI, drug discrimination, β-carbolines, MAO inhibitors, agmatine, ibogaine, imidazoline I2-site, rat, in vivo
Introduction
Imidazoline binding sites (I-sites) constitute a unique component of the binding properties of many imidazolines, guanidiniums and structurally related derivatives and have been separated into at least three entities (I1, I2, and I3 sites; Eglen et al., 1998). Autoradiographical studies have indicated imidazoline I2 sites exhibit a discrete regional distribution in rat brain (Lione et al., 1998; Macinnes & Handley, 2001). The functional significance of I2-sites is unknown, since neither their molecular structure(s) nor their second-messenger systems have been elucidated. Recently, ligands have been developed with high selectivity for these sites, notably 2-BFI and its quinoline and isoquinoline analogues BU216, BU224 and BU226 (Nutt et al., 1995; Lione et al., 1998). In vivo studies have indicated these and other I2-site ligands can increase food intake (Jackson et al., 1991; Menargues et al., 1995; Polidori et al., 2000), decrease immobility in the forced-swim test (Nutt et al., 1995) and potentiate morphine analgesia (Kolesnikov et al., 1996; Li et al., 1999; Sánchez-Blázquez et al., 2000) but the agonist/antagonist nature of these properties is yet to be resolved.
The naturally occurring β-carbolines harmane, norharmane and harmaline (Rommelspacher et al., 1991) potently displace [3H]-2-BFI binding to I2-sites in rat brain, whilst having only a weak affinity for I1-sites and α2-adrenoceptors (Hudson et al., 1999b). Drug-discrimination studies (Helsley et al., 1998b) indicate harmane and harmaline substitute for ibogaine, an alkaloid derived from Tabernanthe iboga (Evans-Schultes & Hoffman, 1980) that has attracted recent interest as an anti-addictive agent (Szumlinski et al., 2001). However, in vitro, ibogaine has low affinity for [3H]-harmaline binding sites (Nelson et al., 1979). There is extensive evidence that both imidazolines and beta carbolines reversibly inhibit MAO (Buckholtz & Boggan, 1977; Nelson et al., 1979; Carpéné et al., 1995; Lalies et al., 1999) and an imidazoline I2 binding site exists on MAO at a location distinct from the catalytic site (Alemany et al., 1995; Raddatz et al., 1997; Remaury et al., 2000). However, there is no correlation between affinity for I2-sites and inhibition of MAO (Ozaita et al., 1997; Lalies et al., 1999) and MAO-inhibitors themselves do not necessarily bind to I2-sites (Olmos et al., 1993; Alemany et al., 1995).
Rats can be trained to discriminate 2-BFI from vehicle, as shown by choice of the drug-appropriate lever in a two-lever operant chamber (Jordan et al., 1996). Both pargyline, and moclobemide substituted for 2-BFI, suggesting this model may detect a site of action related to MAO. Drug discrimination allows ligand actions to be analysed at the level of the whole organism with a high neurobiological and pharmacological specificity; its sensitivity to activation of molecular substrates enabling the unravelling of molecular mechanisms of drug action (Colpaert, 1999). We have therefore examined the potency of a range of synthetic I2-site ligands, the β-carbolines harmane, norharmane and harmaline, MAO-inhibitors, ibogaine and the putative endogenous imidazoline-site ligand agmatine to substitute for 2-BFI in a rat 2-lever drug-discrimination paradigm.
Methods
Animals
Six groups of eight pair-housed Hooded Lister rats (Charles River, U.K.), starting weight 100 g, were housed at an ambient temperature of 21°C, humidity 45%, on a 12 h light/dark cycle (lights on at 0800 h) with free access to food and water. All work was performed in conformity with the Animals (Scientific Procedures) Act, 1986.
Experimental procedure
Animals were transferred to an adjacent room for testing daily between 1000 and 1400 h (weekdays only). Eight two-lever operant chambers (Campden Instruments, U.K.) were controlled by ‘Operant Program for the Neurosciences' (Emmett-Oglesby et al., 1982) software. A liquid dipper presented a reward of sweetened condensed milk (0.1 ml; diluted one part milk to two parts water; Nestle, U.K.). Food deprivation was not required as non-deprived rats show a high level of lever pressing for a condensed milk reward (Jordan et al., 1996).
Drug discrimination training
Following preliminary training to ensure rats consistently pressed either lever without bias to obtain one reward for every 10 lever presses (FR10), rats were admitted to daily 15 min training sessions with either 2-BFI 7 mg kg−1 or saline vehicle administered i.p. 20 min before each session. Discrimination training occurred in a repeated sequence, SDDSSDSSDD (S=saline day, D=drug day; weekdays only) and rats were rewarded on the FR10 schedule for pressing the ‘correct' lever for that training day, i.e., either drug or saline. For 50% of rats, the left lever was set to deliver reward (i.e. ‘correct') if the rat had received 2-BFI and the right lever if saline had been administered, with levers reversed for the remaining animals (Sanger, 1989).
Substitution testing
Criteria for entry into test sessions were 10 consecutive training sessions where: (i) seven of the first 10 lever presses of the session were on the correct lever; and (ii) ⩾90% of all responses during the session were on the correct lever. Test days were added into the training cycle: STDTSDTSTD (T=test day). Test sessions ended after 10 responses on one lever or 30 min whichever was sooner; no reward was administered; data from any rat failing to complete 10 lever presses in 30 min was discarded. The number of responses to the 2-BFI associated lever was expressed as per cent total responses. The group mean of these percentages represented the ability of a drug to substitute for 2-BFI. The number of responses (lever-presses) per minute (r.p.m.) was recorded. Drugs were administered i.p. 20 min before testing except for clorgyline which was assessed 1 and 2 h after its administration. Different doses, including vehicle control, were distributed across sessions and rats in pseudorandom order. Drug treatments were distributed between groups as follows: 2-BFI (as a test drug), groups 1 and 2; BU224, BU216, BU226, LSL60101, LSL60125 and agmatine, group 2; amiloride, group 3; moclobemide and lazabemide, group 4; SKF10,047, group 5; harmane, norharmane, harmaline, ibogaine RO41-1049 and RO16-6491, group 6. Deprenyl was administered as the last test dose given to group 2, while clorgyline was the final compound given to group 4.
Statistical analysis
Repeated measures analysis of variance with Dunnet's post-hoc test (GraphPAD Prism version 3) was used after confirming there were no deviations from gaussian distribution. ED50 values with 95% confidence intervals were determined by linear regression.
Materials
2-BFI (2-(-2-benzofuranyl)-2-imidazoline), Pierre Fabre, France; BU224 (2-[4,5-dihydroimidaz-2-yl]-quinoline hydrochloride), BU216 (3-[4,5-dihydroimidaz-2-yl]-quinoline hydrochloride), BU226 (2-[4,5-dihydroimidaz-2-yl]-isoquinoline hydrochloride), Alan Hudson, Bristol University, U.K.; LSL60101 (2-[2-benzofuranyl]-2-imidazole hydrochloride) and LSL60125 (2-[6-methoxybenzofuran-2-yl]imidazole hydrochloride), J. Garcia Sevilla and R. Obach, LASA Laboratories, Spain; moclobemide (P-chloro-N-(2-morpholinoethyl) benzamide), lazabemide (N-(2-aminoethyl)-5-chloro-2-pyridinecarboxamide hydrochloride), Hoffman La Roche, Switzerland; SKF10,047 (2′hydroxy-5,9-dimethyl-2-allyl-6,7-benzomorphan), SmithKline Beecham, U.K. The gift of the compounds above are gratefully acknowledged. Agmatine, ([4-aminobutyl]guanidine sulphate), amiloride (3,5-diamino-N-(aminoiminomethyl)-6-chloropyrazine-carboxamide hydrochloride), Research Biochemicals International (U.K.); clorgyline (N-methyl-N-propargyl-3-(2,4-dichlorophenoxy)-propylamine hydrochloride), deprenyl (R(−)-N-α-Dimethyl-N-2-propynyl-benzene ethanamine hydrochloride), harmane (1-methyl-9H-pyrido(3,4-b) indole hydrochloride), norharmane (9H-pyrido(3,4-b)indole hydrochloride), harmaline (1-methyl-7-methoxy-3,4-dihydro-beta-carboline), ibogaine hydrochloride, RO41-1049 (N-(2-aminoethyl)-5-(3-fluorophenyl)-4-thiazolecarboxamide hydrochloride), RO16-6491 (N-(2-aminoethyl)-4-chlorobenzamide hydrochloride), Sigma (U.K.).
All drugs were dissolved in 0.9% physiological saline, except for RO41-1049, RO16-6491, moclobemide, SKF10,047, harmane, norharmane, harmaline and ibogaine, which were made up in deionized water, and administered i.p. in a dose volume of 1 ml kg−1.
Results
2-BFI
Over all the groups, rats reached criterion to discriminate 2-BFI 7.0 mg kg−1 from saline vehicle in an average of 57±9 training sessions. None failed to reach criterion. Preliminary experiments with a separate group during which training doses of 2-BFI were progressively increased from 3.5 to 5.0 mg kg−1 indicated that this group of eight rats failed to reach criterion in 213 sessions (data not shown). When administered as a test compound, however, lower doses of 2-BFI (1.6 – 4.8 mg kg−1) dose-dependently substituted for the training dose of 7.0 mg kg−1, while, as expected, the training dose itself produced 100% substitution (Figure 1). The ED50 was found to be 2.5 (1.1 – 5.5) mg kg−1. All rats completed the sessions and there were no significant effects on rate of responding (data not shown).
Figure 1.
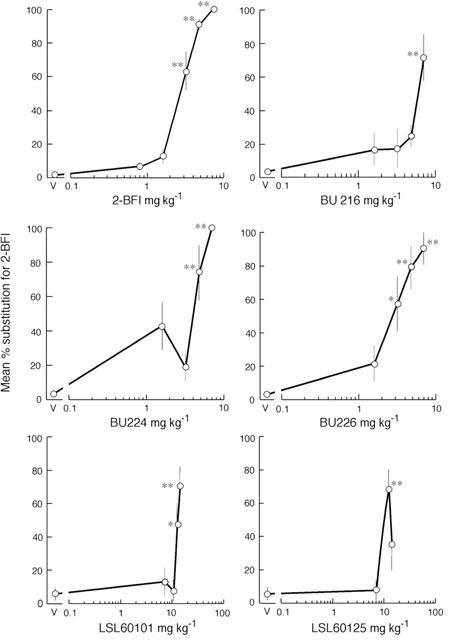
Substitution of I2-site ligands for 2-BFI in drug-discrimination in the rat. The ligands were administered i.p., 20 min before testing in two-lever operant chambers, to a group of eight rats previously trained to discriminate 2-BFI (7 mg kg −1) from vehicle. The dose in mg kg−1 is given on the abscissa. For each rat, the number of lever presses performed on the 2-BFI-appropriate lever was calculated as a percentage of total lever presses and the results are expressed as mean±s.e.mean of these percentages. The asterisks, *P<0.05 and **P<0.01, indicate a statistically significant difference from saline vehicle (V) by Dunnett's test after significant one-way ANOVA.
Synthetic I2 ligands
As shown in Figure 1, BU216, BU224, BU226 (1.6 – 7.0 mg kg−1) and LSL60101 (7.0 – 14.0 mg kg−1) dose-dependently substituted for 2-BFI with similar potency (ED50: 4.4 (0.6 – 8.2); 3.1 (0.8 – 5.4); 3.2 (0.6 – 6.9) and 11.6 (6.9 – 16.2) mg kg−1 respectively). LSL60125 (7.0 – 14.0 mg kg−1) produced peak substitution at 12.25 mg kg−1 while 14.0 mg kg−1 did not significantly substitute. Amiloride (Figure 2) failed to significantly substitute at the only dose tested (7.0 mg kg−1). All rats completed the sessions and there were no significant effects on rate of responding (data not shown).
Figure 2.
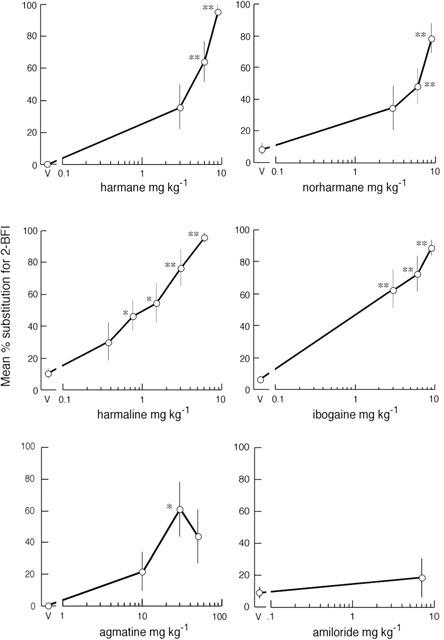
Substitution of β-carbolines, ibogaine agmatine and amiloride for 2-BFI in drug-discrimination in the rat. The ligands were administered i.p., 20 min before testing in two-lever operant chambers, to groups of eight rats previously trained to discriminate 2-BFI (7 mg kg−1) from vehicle. The dose in mg kg−1 is given on the abscissa. For each rat, the number of lever presses performed on the 2-BFI-appropriate lever was calculated as a percentage of total lever presses and the results are expressed as mean±s.e.mean of these percentages. The asterisks, *P<0.05 and **P<0.01, indicate a statistically significant difference from saline vehicle (V) by Dunnett's test after significant one-way ANOVA.
β-Carbolines
Harmane, norharmane (3.0 – 9.0 mg kg−1) and harmaline (0.3 – 6.0 mg kg−1) dose-dependently substituted for 2-BFI (ED50: 4.9 (0.3 – 9.4); 6.0 (1.1 – 10.9); 1.2 (0.3 – 6.0) mg kg−1 respectively) (Figure 2). All rats completed the sessions apart from one in the harmane 9.0 mg kg−1 group. The rate of responding was unchanged except for the group receiving harmane 9.0 mg kg−1 (r.p.m.: 2.3±0.6 (s.e.mean) compared with saline 5.3±0.9; P<0.05).
Agmatine and ibogaine
Agmatine (10.0 – 50.0 mg kg−1) exhibited maximum substitution at 30 mg kg−1, other doses failed to substitute significantly (Figure 2). All rats completed and there were no significant changes in rate of responding (data not shown). Ibogaine (3.0 – 9.0 mg kg−1) significantly and dose-dependently substituted for 2-BFI with an ED50 of 4.7 (1.8 – 12.2) mg kg−1 (Figure 2). SKF10,046 (0.3 – 3.0 mg kg−1) failed to substitute significantly (Figure 4).
Figure 4.
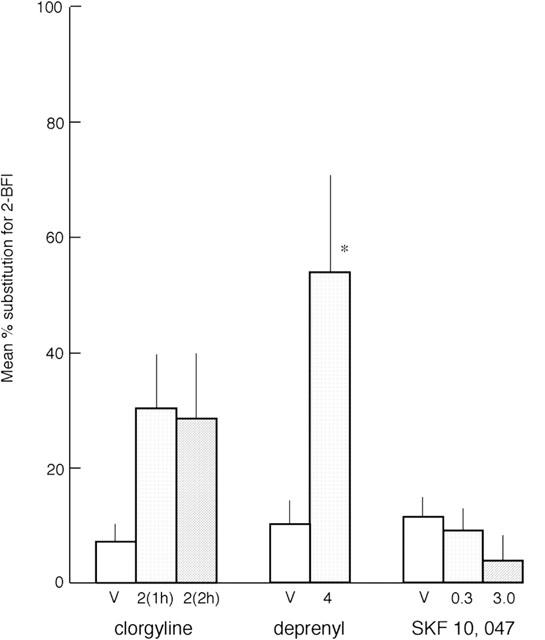
Substitution of the irreversible monoamine oxidase inhibitors clorgyline and deprenyl, and the σ2-site ligand SKF10,047, for 2-BFI in drug-discrimination in the rat. Deprenyl and SKF10,047 were administered i.p., 20 min, and clorgyline was administered i.p. 1 or 2 h, before testing in two-lever operant chambers, to groups of eight rats previously trained to discriminate 2-BFI (7 mg kg−1) from vehicle. The dose in mg kg−1 is given on the abscissa. For each rat, the number of lever presses performed on the 2-BFI-appropriate lever was calculated as a percentage of total lever presses and the results are expressed as mean±s.e.mean of these percentages. The asterisks, *P<0.05 and **P<0.01, indicate a statistically significant difference from saline vehicle (V) by Dunnett's test after significant one-way ANOVA.
MAO inhibitors
The reversible MAO-A inhibitors, RO41-1049 (3.0 – 9.0 mg kg−1) and moclobemide (5.0 – 20.0 mg kg−1) dose-dependently substituted for 2-BFI (Figure 3) with ED50 values of 6.6 (2.0 – 11.2) and 8.0 (3.5 – 18.5) mg kg−1 respectively. The reversible MAO-B inhibitors RO16 6491 (3.0 – 9.0 mg kg−1) and lazabemide (0.75 – 12.0 mg kg−1) were less potent (Figure 3): RO16-6491 failed to substitute significantly while lazabemide exhibited a maximum of 47% substitution at 3.0 mg kg−1, other doses failing to substitute significantly. All rats completed and there were no significant effects on response rates (data not shown).
Figure 3.
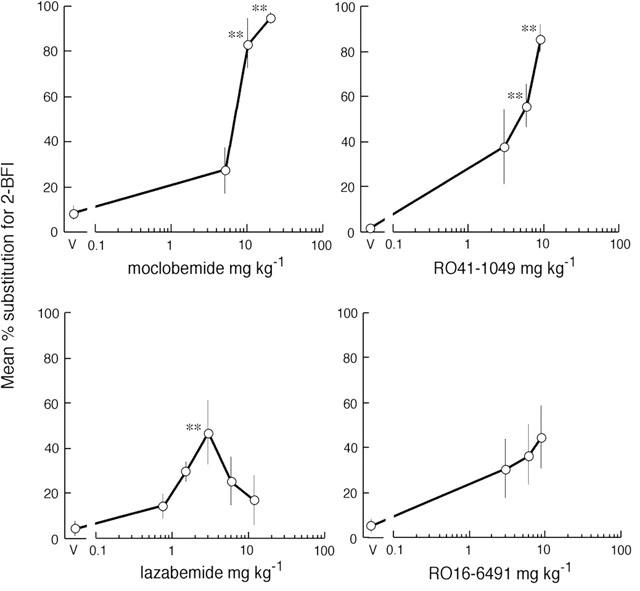
Substitution of reversible inhibitors of monoamine oxidase for 2-BFI in drug-discrimination in the rat. The ligands were administered i.p., 20 min before testing in two-lever operant chambers, to groups of eight rats previously trained to discriminate 2-BFI (7 mg kg−1) from vehicle. The dose in mg kg−1 is given on the abscissa. For each rat, the number of lever presses performed on the 2-BFI-appropriate lever was calculated as a percentage of total lever presses and the results are expressed as mean±s.e.mean of these percentages. The asterisks, *P<0.05 and **P<0.01, indicate a statistically significant difference from saline vehicle (V) by Dunnett's test after significant one-way ANOVA.
Irreversible inhibitors were administered in a single dose as the last injection for each experimental group (Figure 4). The MAO-A inhibitor clorgyline 2 mg kg−1 was administered at either 1 or 2 h before testing and failed to substitute significantly, while the MAO-B inhibitor deprenyl (4 mg kg−1) did substitute significantly but two rats failed to complete the session. Neither agent significantly altered rate of responding (data not shown).
Discussion
2-BFI produced a discriminable stimulus, or cue, as demonstrated by the consistent ability of rats to distinguish 7 mg kg−1 2-BFI from saline. Although rats could not be trained to recognize lower doses of 2-BFI, lower doses did substitute dose-dependently for 2-BFI 7 mg kg−1 when administered as test compounds. I2-site ligands appear to share a common discriminable stimulus, since the other substances tested that have high affinity for I2-sites: BU216, BU224 and BU226 (Lione et al., 1998), harmane, norharmane and harmaline (Hudson et al., 1999b) substituted potently and dose-dependently for 2-BFI. The imidazoles LSL60101 and LSL60125 are less potent in their binding to I2-sites than the above substances (Alemany et al., 1995, 1997) and potential differences in pharmacokinetic properties may explain why the former exhibited a potency approaching that of 2-BFI itself while the latter did not exhibit dose-dependent substitution. Agmatine, which has micromolar affinity for I2-sites and is not I2-selective (Lione et al., 1998), only substituted at the high dose of 30 mg kg−1, while 50 mg kg−1 failed to substitute. Previous work has demonstrated that substances such as clonidine, which bind more potently to I1- than I2-sites do not substitute for 2-BFI (Jordan et al., 1996). None of the agents investigated had any effects on rate of responding or session completion, confirming the absence of adverse effects at the doses used.
The agonist or antagonist nature of substances that bind to I2-sites is not known. BU224 and idazoxan have been suggested as antagonists, since they did not potentiate morphine in the mouse tail-flick assay but were able to prevent potentiation caused by other I2 site ligands (Sánchez-Blázquez et al., 2000). In the present study, BU224 resembled the other I2-site ligands tested, in that it substituted potently and dose-dependently for 2-BFI. Idazoxan also substitutes potently for 2-BFI (Jordan et al., 1996). Thus, it appears that, in this drug-discrimination model, either the I2-site ligands tested are all full agonists, or they are all antagonists acting on a system that is tonically active under these experimental conditions. The former may be more likely, since agmatine and β-carbolines are endogenous substances (Rommelspacher et al., 1991) and thus likely to be agonists. The difference between models suggests that there may be more than one site, location or mechanism of I2-site ligand action.
I2-sites may be heterogeneous. Species differences in the affinity of amiloride have led to classification into I2A and I2B subtypes (Diamant et al., 1992), with the rat predominantly exhibiting I2B (low amiloride affinity) type binding (see Alemany et al., 1997). This may account for the failure of amiloride to substitute for 2-BFI in the present study. A further distinction has been made based on the resolution of the binding of many imidazoline I2 site ligands into two distinct components of high- and low-affinity respectively, although whether this is due to interconvertible affinity states or the existence of different molecular species is controversial. The variability of drug potencies for the low-affinity component (Wikberg et al., 1992; Lione et al., 1998), together with the relatively narrow range of ED50 values reported here, preclude attribution of the 2-BFI cue to specific actions at either the putative high- or low-affinity component of imidazoline I2-site binding.
The ability of ibogaine to substitute potently and dose-dependently for 2-BFI may be significant for developing further understanding of its anti-addictive properties (Szumlinski et al., 2001), since it indicates a commonality in the subjective effects of ibogaine and I2-site ligands. This is particularly interesting since BU224 and norharmane resemble ibogaine in reducing morphine withdrawal signs in rodents (Cappendijk et al., 1994; Hudson et al., 1999a). Ibogaine appears to have negligible affinity for imidazoline I2-sites (Dr Alan Hudson, personal communication) and evidence suggests it does not inhibit MAO (Nelson et al., 1979). It has low affinity for harmaline, serotonin (5-HT2 and 5-HT1A), dopamine (D2), nicotinic acetylcholine, μ-opioid and benzodiazepine receptors, but nanomolar affinity for σ2-sites (Nelson et al., 1979; Bowen et al., 1995; Mah et al., 1998; Glennon et al., 2000) and ibogaine-trained rats recognize the σ2 ligand SKF10,047 in drug-discrimination (Helsley et al., 1998a). However, SKF10,047 failed to substitute for 2-BFI in the present experiments over a similar dose-range, suggesting this property does not form part of the 2-BFI cue.
What could be the nature of the discriminable stimulus generated by 2-BFI? Ability to substitute for 2-BFI appears associated with reversible inhibition of MAO-A rather than MAO-B. The substances tested that reversibly inhibit MAO-A substituted potently for 2-BFI. In the case of 2-BFI analogues and β-carbolines, this effect is consistent with their affinity for I2-sites; however moclobemide and RO41-1049 have very low affinity for I2-sites (Olmos et al., 1993; Alemany et al., 1995). In contrast, selective reversible inhibitors of MAO-B exhibited little or no substitution: RO16-6491 failed to substitute and lazabemide substituted significantly at one dose only, indicating that inhibition of MAO-B does not generate a discriminable stimulus resembling that of 2-BFI. Recent knockout studies have shown that the high-affinity component of idazoxan binding to I2-sites is associated exclusively with MAO-B, while the low affinity component is associated exclusively with MAO-A (Remaury et al., 2000). Although these authors suggest it may prove appropriate to define I2-sites by the high affinity component of idazoxan binding, the apparent association observed here, between substitution for 2-BFI and reversible inhibition of MAO-A, raises the possibility of functional activity of the low-affinity component of imidazoline I2-site binding.
The pattern of substitution for 2-BFI suggests the possibility that increases in extracellular concentrations of one or more of the monoamines may be important in generating its discriminable stimulus. A number of I2-site ligands have been found to increase extracellular monoamines during acute microdialysis studies, including 2-BFI and BU224 (Lalies & Nutt, 1993; Hudson et al., 1999a), harmane (Adell et al., 1996; Baum et al., 1996) and norharmane (Baum et al., 1995). Microdialysis studies indicate ibogaine increases extracellular serotonin to a much greater extent than can be accounted for by its weak affinity for the serotonin transporter (Wei et al., 1998). The 2-BFI cue has been tentatively linked to increases in synaptic noradrenaline (Jordan et al., 1996). Deprenyl (4 mg kg−1), although a selective irreversible MAO-B inhibitor, causes an immediate increase in extracellular noradrenaline by an as yet unknown mechanism (Lalies et al., 2000) and did substitute for 2-BFI, whereas the selective irreversible MAO-A inhibitor clorgyline at 2 mg kg−1, produced only a slight increase in noradrenaline after 2 h (Lalies et al., 2000) and failed to substitute for 2-BFI at either 1 or 2 h after its administration. Re-testing of clorgyline at shorter time-intervals would be worthwhile since clorgyline potently displaces [3H]-2-BFI from high-affinity I2-sites while showing only weak effects at low-affinity I2-sites defined by [3H]-2-BFI (Lione et al., 1998) and this could be taken as further evidence for the importance of low-affinity I2-site binding to the 2-BFI cue. Further studies are in progress to determine whether agonists at monoamine receptors substitute for 2-BFI.
The present work indicates that drug-discrimination is a valuable tool with which to investigate the in vivo pharmacological properties of imidazoline I2-site ligands. It supports molecular studies that indicate a link between I2-sites and MAO, highlighting for the first time the potential importance of MAO-A. The ability of ibogaine to substitute for 2-BFI indicates the possibility of commonalities in their in vivo actions that may lead to greater understanding of the anti-addictive properties of ibogaine.
Acknowledgments
We would like to thank the BBSRC who supported this work.
Abbreviations
- 2-BFI
2-(-2-benzofuranyl)-2-imidazoline
- BU216
3-[4,5-dihydroimidaz-2-yl]-quinoline hydrochloride
- BU224
2-[4,5-dihydroimidaz-2-yl]-quinoline hydrochloride
- BU226
2-[4,5-dihydroimidaz-2-yl]-isoquinoline hydrochloride
- LSL60101
2-[2-benzofuranyl]-2-imidazole hydrochloride
- LSL60125
2-[6-methoxybenzofuran-2-yl] imidazole hydrochloride
- MAO
monoamine oxidase
- RO16-1649
N-(2-aminoethyl)-4-chlorobenzamide hydrochloride
- RO41-1049
N-(2-aminoethyl)-5-(3-fluorophenyl)-4-thiazolecarboxamide hydrochloride
- SKF10,047
2′hydroxy-5,9-dimethyl-2-allyl-6,7-benzomorphan
References
- ADELL A., BIGGS T.A., MYERS R.D. Action of harman (1-methyl-beta-carboline on the brain: body temperature and in vivo efflux of 5-HT from the hippocampus of the rat. Neuropharmacology. 1996;35:1107–1101. doi: 10.1016/s0028-3908(96)00043-3. [DOI] [PubMed] [Google Scholar]
- ALEMANY R., OLMOS G., GARCIA-SEVILLA J.A. The effects of phenelzine and other monoamine oxidase inhibitor antidepressants on brain and liver I2 imidazoline-preferring receptors. Br. J. Pharmacol. 1995;114:837–845. doi: 10.1111/j.1476-5381.1995.tb13280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEMANY R., OLMOS G., GARCIA-SEVILLA J.A. Labelling of I2B-imidazoline receptors by [3H]2-(2-benzofuranyl)-2-imidazoline (2-BFI) in rat brain and liver: characterisation, regulation and relation to monoamine oxidase enzymes. Naunyn Schmiedebergs Arch. Pharmacol. 1997;356:39–47. doi: 10.1007/pl00005026. [DOI] [PubMed] [Google Scholar]
- BAUM S.S., HILL R., ROMMELSPACHER H. Norharman-induced changes of extracellular concentrations of dopamine in the nucleus accumbens of rats. Life Sci. 1995;56:1715–1720. doi: 10.1016/0024-3205(95)98578-4. [DOI] [PubMed] [Google Scholar]
- BAUM S.S., HILL R., ROMMELSPACHER H. Harman-induced changes of extracellular concentrations of neuroransmitters in the nucleus accumbens of rats. Eur. J. Pharmacol. 1996;314:75–82. doi: 10.1016/s0014-2999(96)00543-2. [DOI] [PubMed] [Google Scholar]
- BOWEN W.D., VILNER B.J., WILLIAMS W., BERTHA C.M., KUEHNE M.E., JACOBSON A.E. Ibogaine and its congeners are sigma2 receptor-selective ligands with moderate affinity. Eur. J. Pharmacol. 1995;279:R1–R3. doi: 10.1016/0014-2999(95)00247-i. [DOI] [PubMed] [Google Scholar]
- BUCKHOLTZ N.S., BOGGAN W.O. Monoamine oxidase inhibition in brain and liver produced by β-carbolines: structure-activity relationships and substrate specificity. Biochem. Pharmacol. 1977;26:1991–1996. doi: 10.1016/0006-2952(77)90007-7. [DOI] [PubMed] [Google Scholar]
- CAPPENDIJK S.L., FEKKES D., DZOLJIC M.R. The inhibitory effect of norharman on morphine withdrawal syndrome in rats: comparison with ibogaine. Behav. Brain. Res. 1994;65:117–119. doi: 10.1016/0166-4328(94)90080-9. [DOI] [PubMed] [Google Scholar]
- CARPENE C., COLLON P., REMAURY A., CORDI A., HUDSON A., NUTT D., LAFONTAN M. Inhibition of amine oxidase activity by derivatives that recognize imidazoline I2 sites. J. Pharmacol. Exp. Ther. 1995;272:681–688. [PubMed] [Google Scholar]
- COLPAERT F.C. Drug discrimination in neurobiology. Pharmacol. Biochem. Behav. 1999;64:337–345. doi: 10.1016/s0091-3057(99)00047-7. [DOI] [PubMed] [Google Scholar]
- DIAMANT S., ELDAR-GEVA T., ATLAS D. Imidazoline binding sites in human placenta: Evidence for the heterogeneity and a search for physiological function. Br. J. Pharmacol. 1992;106:101–108. doi: 10.1111/j.1476-5381.1992.tb14300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EGLEN R.M., HUDSON A.L., KENDALL D.A., NUTT D.J., MORGAN N.G., WILSON V.G., DILLON M.P. ‘Seeing through a glass darkly': casting a light on imidazoline ‘I' sites. Trends Pharmacol. Sci. 1998;19:381–390. doi: 10.1016/s0165-6147(98)01244-9. [DOI] [PubMed] [Google Scholar]
- EMMETT-OGLESBY M.W., SPENCER D.G., ARNOULT D.E. A TRS-80 based system for the control of behavioural experiments. Pharmacol. Biochem. Behav. 1982;17:584–587. doi: 10.1016/0091-3057(82)90322-7. [DOI] [PubMed] [Google Scholar]
- EVANS-SCHULTES R., HOFFMAN A.Tabernanthe iboga The Botany and Chemistry of Hallucinogens 1980Springfield, IL: Charles C. Thomas Publisher; 235–239.ed. Evans Schultes R, Hofmann A. pp [Google Scholar]
- GLENNON R.A., DUKAT M., GRELLA B., HONG S.O., COSTANTINO L., TEITLER M., SMITH C., EGAN C., DAVIS K., MATTSON M.V. Binding of β-carbolines and related agents at serotonin (5-HT2 and 5-HT1A), dopamine (D2) and benzodiazepine receptors. Drug Alcohol Depand. 2000;60:121–132. doi: 10.1016/s0376-8716(99)00148-9. [DOI] [PubMed] [Google Scholar]
- HELSLEY S., FILIPINK R.A., BOWEN W.D., RABIN R.A., WINTER J.C. The effects of sigma, PCP, and opiate receptor ligands in rats trained with ibogaine as a discriminative stimulus. Pharmacol. Biochem. Behav. 1998a;59:495–503. doi: 10.1016/s0091-3057(97)00454-1. [DOI] [PubMed] [Google Scholar]
- HELSLEY S., FIORELLA D., RABIN R.A., WINTER J.C. Behavioural and biochemical evidence for a non-essential 5-HT2A component of the ibogaine-induced discriminative stimulus. Pharmacol. Biochem. Behav. 1998b;59:419–425. doi: 10.1016/s0091-3057(97)00451-6. [DOI] [PubMed] [Google Scholar]
- HUDSON A.L., GOUGH R.E., TYACKE R.J., LIONE L., LALIES M., LEWIS J., HUSBANDS S., KNIGHT P., MURRAY F., HUTSON P., NUTT D.J. Novel selective compounds for the investigation of imidazoline receptors. Annals N.Y. Acad. Sci. 1999a;881:81–91. doi: 10.1111/j.1749-6632.1999.tb09344.x. [DOI] [PubMed] [Google Scholar]
- HUDSON A.L., PRICE R.E., TYACKE R.J., LALIES M.D., PARKER C.A., NUTT D.J. Harmane, norharmane and tetrahydro β-carboline have high affinity for rat imidazoline binding sites. Br. J. Pharmacol. 1999b;126:P2. [Google Scholar]
- JACKSON H.C., GRIFFIN I.J., NUTT D.J. The effects of idazoxan and other α2-adrenoceptor antagonists on food and water intake in the rat. Br. J. Pharmacol. 1991;104:1258–1262. doi: 10.1111/j.1476-5381.1991.tb12416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN S., JACKSON H.C., NUTT D.J., HANDLEY S.L. Discriminative stimulus produced by the imidazoline I2 site ligand, 2-BFI. J. Psychopharmacol. 1996;10:273–278. doi: 10.1177/026988119601000403. [DOI] [PubMed] [Google Scholar]
- KOLESNIKOV Y., JAIN S., PASTERNAK G.W. Modulation of opioid analgesia by agmatine. Eur. J. Pharmacol. 1996;296:17–22. doi: 10.1016/0014-2999(95)00669-9. [DOI] [PubMed] [Google Scholar]
- LALIES M.D., HIBELL A., HUDSON A.L., NUTT D.J. Inhibition of central monoamine oxidase by imidazoline2 site selective ligands. Annals N.Y. Acad. Sci. 1999;881:114–117. doi: 10.1111/j.1749-6632.1999.tb09350.x. [DOI] [PubMed] [Google Scholar]
- LALIES M., HUDSON A.L., NUTT D.J. Acute peripheral administration of (−) deprenyl elevates extracellular noradrenaline in rat frontal cortex. J. Psychopharmacol. 2000;14:A35. [Google Scholar]
- LALIES M.D., NUTT D.J. A microdialysis study of the neurochemical effects in rat striatum of RX 821039, a selective ligand for non-adrenoceptor idazoxan binding sites. Br. J. Pharmacol. 1993;109:85P. [Google Scholar]
- LI J., LI X., PEI G., QIN B.Y. An analgesic effect of agmatine and its enhancement on morphine analgesia in mice and rats. Acta Pharmacologica Sinica. 1999;20:81–85. [PubMed] [Google Scholar]
- LIONE L.A., NUTT D.J., HUDSON A.L. Characterisation and localisation of [3H]2-(2-benzofuranyl)-2-imidazoline binding in the rat brain: a selective ligand for imidazoline I2 receptors. Eur. J. Pharmacol. 1998;353:123–135. doi: 10.1016/s0014-2999(98)00389-6. [DOI] [PubMed] [Google Scholar]
- MACINNES N., HANDLEY S.L. Region-dependent effects of acute and chronic tranylcypromine in vivo on [3H]2-BFI binding to brain imidazoline I2 sites. Eur. J. Pharmacol. 2001;428:221–225. doi: 10.1016/s0014-2999(01)01259-6. [DOI] [PubMed] [Google Scholar]
- MAH S.J., TANG Y., LIAUW P.E., NAGEL J.E., SCHNEIDER A.S. Ibogaine acts at the nicotinic acetylcholine receptor to inhibit catecholamine release. Brain Res. 1998;797:173–180. doi: 10.1016/s0006-8993(98)00207-8. [DOI] [PubMed] [Google Scholar]
- MENARGUES A., CEDO M., ARTIGA O., OBACH R., GARCIA-SEVILLA J.A. Effects of the I2-imidazoline receptor ligand LSL 60101 on various models of anorexia in rats. Annals N.Y. Acad. Sci. 1995;763:494–496. doi: 10.1111/j.1749-6632.1995.tb32439.x. [DOI] [PubMed] [Google Scholar]
- NELSON D.L., HERBET A., PETILLOT Y., PICHANT L., GLOWINSKI J., HAMON M. [3H]harmaline as a specific ligand of MAO-A I. Properties of the active site of MAO A from rat and bovine brain. J. Neurochem. 1979;32:1817–1827. doi: 10.1111/j.1471-4159.1979.tb02296.x. [DOI] [PubMed] [Google Scholar]
- NUTT D.J., FRENCH N., HANDLEY S.L., HUDSON A., HUSBANDS S., JACKSON H., JORDAN S., LALIES M.D., LEWIS J., LIONE L.A., MALLARD N., PRATT J. Functional studies of specific imidazoline-2 receptor ligands. Annals N.Y. Acad. Sci. 1995;763:125–139. doi: 10.1111/j.1749-6632.1995.tb32397.x. [DOI] [PubMed] [Google Scholar]
- OLMOS G., GABILONDO A.M., MIRALLES A., ESCRIBA P.V., GARCIA-SEVILLA J.A. Chronic treatment with the monoamine oxidase inhibitors clorgyline and pargyline down-regulates non-adrenoceptor [3H]-idazoxan binding sites in the rat brain. Br. J. Pharmacol. 1993;108:597–603. doi: 10.1111/j.1476-5381.1993.tb12848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OZAITA A., OLMOS G., BORONAT M.A., LIZCANO J.M., UNZETA M., GARCIA-SEVILLA J.A. Inhibition of monoamine oxidase A and B activities by imidazol(ine)/guanidine drugs, nature of the interaction and distinction from I2-imidazoline receptors in rat liver. Br. J. Pharmacol. 1997;121:901–912. doi: 10.1038/sj.bjp.0701214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POLIDORI C., GENTILI F., PIGINI M., QUAGLIA W., PANOCKA I., MASSI M. Hyperphagic effect of novel compounds with high affinity for imidazoline I2 binding sites. Eur. J. Pharmacol. 2000;392:41–49. doi: 10.1016/s0014-2999(00)00014-5. [DOI] [PubMed] [Google Scholar]
- RADDATZ R., PARINI A., LANIER S.M. Localization of the imidazoline binding domain on monoamine oxidase B. Mol. Pharmacol. 1997;52:549–553. doi: 10.1124/mol.52.4.549. [DOI] [PubMed] [Google Scholar]
- REMAURY A., RADDATZ R., ORDENER C., SAVIC S., SHIH J.C., CHE K., SEIF I., DE MAEYER E., LANIER S.M., PARINI A. Analysis of the pharmacological and molecular heterogeneity of I2-imidazoline-binding proteins using monoamine oxidase-deficient mouse models. Mol. Pharmacol. 2000;58:1085–1090. doi: 10.1124/mol.58.5.1085. [DOI] [PubMed] [Google Scholar]
- ROMMELSPACHER H., MAY T., SUSILO R. Beta-carbolines and tetrahydroisoquinolines: detection and function in mammals. Plant Med. 1991;57:S85–S92. [PubMed] [Google Scholar]
- SÁNCHEZ-BLÁZQUEZ P., ASSUMPICO BORONAT M., OLMOS G., GARCIA-SEVILLA J.A., GARZON J. Activation of I2-imidazoline receptors enhances supraspinal morphine analgesia in mice: a model to detect agonist and antagonist activities at these receptors. Br. J. Pharmacol. 2000;130:146–152. doi: 10.1038/sj.bjp.0703294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER D.J. Discriminative stimulus effects of the α2-adrenoceptor antagonist idazoxan. Psychopharmol. 1989;99:117–121. doi: 10.1007/BF00634464. [DOI] [PubMed] [Google Scholar]
- SZUMLINSKI K.K., MAISONNEUVE I.M., GLICK S.D. Iboga interactions with psychomotor stimulants: panacea in the paradox. Toxicon. 2001;39:75–86. doi: 10.1016/s0041-0101(00)00158-6. [DOI] [PubMed] [Google Scholar]
- WEI D., MAISONNEUVE I.M., KUEHNE M.E., GLICK S.D. Acute iboga alkaloid effects on extracellular serotonin (5-HT) levels in the nucleus accumbens and striatum in rats. Brain Res. 1998;800:260–268. doi: 10.1016/s0006-8993(98)00527-7. [DOI] [PubMed] [Google Scholar]
- WIKBERG J.E.S., UHLEN S., CHHAJLANI V. Evidence that drug binding to non-adrenergic [3H]-idazoxan binding sites (I-receptors) occurs to interacting or interconvertible affinity forms of the receptor. Pharmacol. Toxicol. 1992;70:208–219. doi: 10.1111/j.1600-0773.1992.tb00459.x. [DOI] [PubMed] [Google Scholar]


