Abstract
Previous studies investigating the role of metabotropic glutamate (mGlu) receptors in nociceptive processing have been hampered by the lack of systemically active, selective, ligands. This study investigates the possible analgesic and/or anti-hyperalgesic properties of the most potent compound to date that has systemic agonist activity at group II mGlu receptors, LY379268.
In testing the drug in rats as an analgesic to acute noxious stimuli, LY379268 (in doses up to 3 mg kg−1 i.p.) did not affect withdrawal latencies to either mechanical or thermal stimulation.
However, when a 3 mg kg−1 dose was given prior to an intraplantar injection of carrageenan, the inflammatory hyperalgesia that developed was significantly delayed compared to saline pre-treated controls, without affecting the inflammation of the paw. A similar dose of the mGlu-inactive enantiomer, LY379267, was not anti-hyperalgesic.
In a model of mouse tail withdrawal to warm water, LY379268 (12 mg kg−1 i.p.), given before a subcutaneous tail injection of capsaicin, reduced the subsequent neurogenic hyperalgesia.
Rota-rod testing showed that the drug did not produce a motor impairment in rats at antihyperalgesic doses.
The results indicate that systemic activation of this group of mGlu receptors reduces both inflammatory and neurogenic thermal hyperalgesia.
Keywords: LY379268, group II metabotropic glutamate receptors, inflammatory hyperalgesia, neurogenic hyperalgesia, nociception
Introduction
To date, eight G-protein coupled metabotropic glutamate (mGlu) receptors have been cloned and characterized into three groups (I – III) on the basis of their sequence homology and their biochemical and pharmacological properties. Group II mGlu receptors consist of mGlu2 and mGlu3 that act via negative coupling to adenylate cyclase to inhibit cyclic AMP formation (for reviews see Pin & Duvoisin, 1995; Conn & Pin, 1997; Schoepp et al., 1999).
Both mGlu2 and mGlu3 receptors have been localized in the spinal cord, but mGlu3 receptors have been shown to be more prevalent, particularly in the superficial layers of the dorsal horn (Ohishi et al., 1993a, 1993b; Petralia et al., 1996; Boxall et al., 1998; Yung, 1998; Jia et al., 1999). These group II receptors are distributed widely over pre-synaptic terminals (Petralia et al., 1996; Testa et al., 1998), unlike group I receptors which are clustered at release sites (Luján et al., 1997). Their pre-synaptic position at glutamatergic synapses suggests an autoreceptive role, potentially inhibiting synaptic transmission, as has been shown in isolated spinal cord by Jane et al. (1996) and in locus coeruleus neurones by Dube & Marshall (1997). In addition, group II mGlu receptors have been shown to negatively modulate glutamate release in striatal neurones (Battaglia et al., 1997). Activation of these receptors is thought to suppress transmitter release by inhibiting voltage-sensitive Ca2+ channels (Chavis et al., 1995). This evidence implies that group II mGlu receptors may reduce hyperexcitable states including those underlying hyperalgesia and allodynia. mGlu3 receptors are also found on glial cells (Ohishi et al., 1993b; Tanabe et al., 1993; Petralia et al., 1996).
Intrathecal administration of (1S,3S)-1-aminocyclopentane-1,3-dicarboxylic acid ((1S,3S)-ACPD), a predominantly group II mGlu receptor agonist, provided initial evidence that mGlu receptors are involved in nociceptive processing. (1S,3S)-ACPD potentiated the behavioural response to formalin (Fisher & Coderre, 1996), co-administration of trans-ACPD (in effect 1S,3R-ACPD) with AMPA elicited mechanical hyperalgesia (Meller et al., 1993) and iontophoretic administration of an mGlu receptor antagonist reduced hyperalgesic responses (Neugebauer et al., 1994). In contrast, mGlu receptor agonists inhibited dorsal horn neurones following carrageenan induced peripheral inflammation (Stanfa & Dickenson, 1998). However, (1S,3S)-ACPD has mixed pharmacology, for instance showing affinity for group I mGlu (Thomsen et al., 1994) and mGlu4 (Kozikowski et al., 1998) receptors (for review see Schoepp et al., 1999). Therefore, the availability of a systemically active, selective, agonist at group II receptors would provide an opportunity to test the effect of activating these receptors, and its behavioural specificity, on nociceptive processing in awake animals.
One highly potent and selective agonist of group II mGlu receptors, LY379268, ( – )-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylic acid, is also bioavailable (Bond et al., 1997; Monn et al., 1997; 1999; Schoepp et al., 1997). In characterization studies of LY379268 (Monn et al., 1999), its affinity for group II receptors was shown by displacement of specific binding of the mGlu2/3 antagonist, [3H]-LY341495, with Ki values of 14.1±1.4 nM (mGlu2) and 5.8±0.64 nM (mGlu3). There was no agonist or antagonist activity of the compound at recombinant cells expressing the human mGlu 1a, 4a, 5a or 7a receptors. However, agonist activity was seen at higher concentrations on mGlu6 and mGlu8 receptors. There was no effect of LY379268 on radioligand binding to the glutamate recognition site of ionotropic receptors for NMDA, AMPA or kainate (Monn et al., 1999). Recently LY379268, and the structurally related (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740), have been shown to be neuroprotective in in vitro and in vivo models (Bond et al., 1998; 2000; Kingston et al., 1999). LY354740 has been shown to prevent release of glutamate in the striatum in freely moving rats (Battaglia et al., 1997) and to attenuate morphine withdrawal-associated activation of locus coeruleus neurones, thought to be caused by an increased release of glutamate (Vandergriff & Rasmussen, 1999).
This study was performed to test whether LY379268 is antinociceptive or antihyperalgesic in awake animals. Some of these data have been presented in abstract form (Sharpe et al., 1998; 1999).
Methods
Animals
Reflex tests of nociception were performed on 78 male Sprague-Dawley rats (250 – 330 g) and 47 male C57B46 mice (25 – 30 g). The animals were maintained in a controlled environment with food and water ad libitum. All experiments were carried out in accordance with the U.K. Animal (Scientific Procedures) Act, 1986. Rats were acclimatized to handling and the test environment for 3 – 4 days prior to experimentation. All experiments were performed during the light phase of a 12 : 12 h light : dark cycle.
Induction of thermal and neurogenic inflammation
For experiments involving hind paw inflammation, carrageenan (2 mg in 0.2 ml distilled H2O) was injected into the plantar surface of one hind paw of rats under transient halothane anaesthesia. Paw volumes were measured (Plethysmometer, Ugo Basile) before the carrageenan injection and immediately after each test. At the completion of inflammation experiments, animals were killed humanely by an overdose of pentobarbitone (300 mg kg−1 i.p.). In a separate set of experiments, a subcutaneous injection of capsaicin (5 μl; 0.25%) was made into the terminal 3 cm of the tail of mice so as to produce a transient thermal hyperalgesia to warm water.
Nociceptive threshold and motor coordination tests
Thermal paw withdrawal latency
Rats were placed in 10.5×10.5×22 cm Perspex containers positioned on a non-heat retaining glass surface and were allowed to habituate for a period of at least 15 min. Paw withdrawal latencies (PWL) were measured to a movable radiant heat source positioned under the plantar surface of the hind paw. An automatic timer recorded PWL to the nearest 0.1 s, with the maximum cut-off time for stimulation set to 20.5 s. For each time point, PWL was measured three times at 30 s intervals (alternately for each paw) and the mean values calculated. PWL to thermal stimulation was used both in naïve animals and in those that had received an intraplantar injection of carrageenan; thermal rather than mechanical testing was selected for animals with an inflamed paw because the thermal tests do not involve handling a potentially painful limb.
Mechanical paw withdrawal threshold
Rats were tested for hind paw withdrawal threshold (PWT) to mechanical pressure using a Randall-Selitto device (Analgesy-Meter, Ugo Basile). Rats were held manually and one paw was placed in the Randall-Selitto device. A progressive increase in mechanical pressure (at 15 g s−1) was applied alternately to each hind paw, at 30 s intervals, until it evoked a withdrawal or escape response, such as vocalisation or struggling, with a cut-off of 250 g. The mean of 3 PWT readings for each paw was measured consecutively.
Tail thermal withdrawal latency
Mice were used to assess tail withdrawal latency (TWL) by dipping the final 5 cm of tail in either warm or hot water (46.5 – 47.5°C or 49.5 – 50.5°C). The average was calculated for 3 TWLs at 15 s intervals taken to a cut-off time of 20 s.
Motor co-ordination
A rotating rod (Ugo Basile) of 7 cm diameter was used at a constant velocity of 4 r.p.m. Rats used in these experiments had all been trained previously on the apparatus; and on the day of testing all remained on the rotating rod for a ‘baseline' of 5 min. Post-drug testing was taken as the mean duration spent on the rod in three consecutive attempts (without interval), with a cut-off time of 3 min. Other assessments of motor activity and co-ordination were subjective only.
Drugs
LY379268 and its mGlu receptor-inactive enantiomer, LY379267 (Eli Lilly & Co.), were dissolved in equimolar NaOH and adjusted to a pH of 7 – 9 with equimolar HCl. Limits on drug availability precluded more extensive testing of dose-dependence, and caused us to use mouse rather than rat models for some tests. Carrageenan (Sigma) was dissolved in distilled H2O (10 mg ml−1) the day prior to an experiment. Capsaicin (Sigma) was dissolved in 100% ethanol (500 mg ml−1) and made into a stock solution (0.1 mg ml−1) with Tween80 and saline (50 : 50) by slow heating.
Experimental design
The experimenter was ‘blind' to the treatment under test. In all tests, ‘control' readings were taken immediately before i.p. injection of either vehicle (saline) or test agent (LY379268 or LY379267) in a volume of 2 ml kg−1, and before the induction of hyperalgesia.
Protocol 1
The analgesic effects of LY379268 or LY379267 were tested on responses to thermal and mechanical stimuli (PWL and PWT) in rats without hind paw inflammation.
Protocol 2
The analgesic effects of LY379268 were tested in mice on TWL to hot water at 50°C. To match protocol 6 below, tests were repeated four times at 3 min intervals 1 h after drug administration.
Protocol 3
The ability of LY379268 to reverse inflammatory hyperalgesia was tested by i.p. injection 3 h after carrageenan injection. Thermal PWL was tested hourly for a further 3 h.
Protocol 4
The ability of LY379268 to prevent the induction of inflammatory hyperalgesia was assessed by administering drug concurrently with carrageenan, after having made baseline readings of PWL. Measurements of thermal PWL were made hourly for the following 4 h.
Protocol 5
As Protocol 4 but drug was administered 1 h before carrageenan was injected into one hind paw. PWLs were then tested hourly for a further 4 h.
Protocol 6
LY379268 or vehicle was given 1 h prior to an injection of capsaicin into the tail of mice. The degree of neurogenic thermal hyperalgesia was tested by TWL to warm water at 47°C. This lower water temperature than for Protocol 2 gave baseline latencies close to cut off so as to maximize the ability to detect hyperalgesia. TWL tests were repeated before and 3, 6 and 9 min after capsaicin.
Data analysis
Data are shown as means±s.e.m. Two-way analysis of variance (ANOVA) was followed by Mann-Whitney U-statistic or Wilcoxon signed rank tests. The Friedman test was used on data from contralateral non-inflamed paws. All analyses were made using Prism 2.01 (GraphPad Software).
Results
Effects of the group II mGlu receptor agonist LY379268 on acute algesic responses
In rats without peripheral inflammation (Protocol 1), neither LY379268 nor its (S)-enantiomer, LY379267, had any effect on the latency of paw withdrawal from either thermal or mechanical stimuli. Figure 1A shows that there was no effect of vehicle (n=7) or 3 mg kg−1 of either LY379268 (n=12) or LY379267 (n=6) on PWL to radiant heat tested over the 2 h after drug injection. The same dose of LY379268 had no effect on PWT to mechanical stimulation (Figure 1B) or on rota-rod ability (not illustrated). LY379268 at 6 mg kg−1 (n=9) caused an exaggerated startle response in rats that precluded further testing. In mice (Protocol 2), LY379268 did not detectably alter resting behaviour at higher doses (24 mg kg−1; n=7), and showed no analgesic effect on TWL from water (50±0.5°C) (control TWL 8.3±1.0 s; TWL 1 h after 12 mg kg−1 LY379268 10.6±1.9 s; P=0.72; 2-way ANOVA; data not shown).
Figure 1.
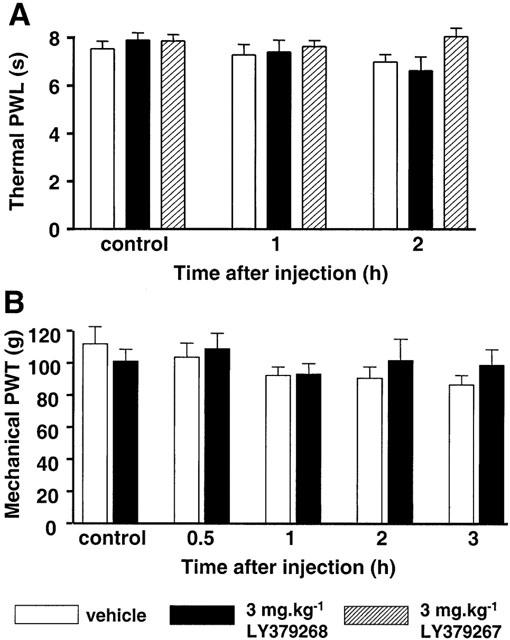
Lack of effect of a systemically-administered mGlu group II agonist, LY379268, on either hind paw withdrawal latency (PWL) to thermal (A), or paw withdrawal threshold (PWT) to mechanical (B), noxious stimuli in naïve rats. All values are given as mean±s.e.m. Data are shown for baseline (pre-drug) and at different times after i.p. injection of either vehicle (saline; open bars; n=7), 3 mg kg−1 LY379268 (solid bars; n=12) or 3 mg kg−1 of the mGlu group II receptor inactive enantiomer LY379267 (hatched bars; n=6).
Time-dependent effects of LY379268 on inflammatory hyperalgesia in rats
Intraplantar injection of the pro-inflammatory agent, carrageenan, into one hind paw of rats caused thermal hyperalgesia that developed over 3 h (Figure 2A,B), shown as a PWL reduction in vehicle-treated animals. When LY379268 was given 3 h after carrageenan (Protocol 3, Figure 2A), at a time at which inflammation was well developed, there was no effect on the established thermal hyperalgesia (P=0.43; 2-way ANOVA; n=7). The same was the case when LY379268 was given at the same time as the carrageenan injection (Protocol 4, P=0.22; 2-way ANOVA; n=8), although the onset of hyperalgesia appeared somewhat slowed (Figure 2B). In both protocols, the slight increase in contralateral PWL observed in vehicle-treated rats was not significant (Protocol 3: P=0.19, n=4 – 5; Protocol 4: P=0.49, n=6; Friedman test). In contrast, pre-dosing of rats with LY379268 (3 mg kg−1, n=9), 1 h prior to the injection of carrageenan, delayed the development of hyperalgesia, compared with vehicle-treated rats (Protocol 5, Figure 2C, n=8). Two hours after carrageenan there was no reduction at all of PWL in drug-treated rats, compared to a 47% reduction of PWL in vehicle pre-dosed rats; this prevention of hyperalgesia was significant (P=0.001, 2-way ANOVA; P=0.015, Mann-Whitney test at 2 h; n=9). At a lower dose of LY379268 (1 mg kg−1) there was no significant reduction of thermal hyperalgesia (P=0.30, 2-way ANOVA; n=6; Figure 4A).
Figure 2.
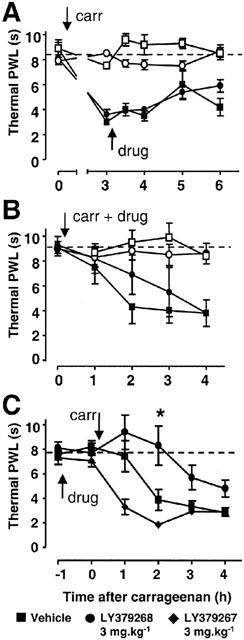
Effects of LY379268, administered at different time points, on inflammatory hyperalgesia. Thermal paw withdrawal latency (PWL) was tested in rats in which LY379268 (3 mg kg−1; circles; n=7 – 9) or vehicle (saline; squares; n=5 – 8) was injected i.p. either 3 h after (A), concurrently with (B), or 1 h before (C), intraplantar carrageenan injection (carr). All values are given as mean±s.e.m. PWL of the paw injected with carrageenan is shown by closed symbols, and the contralateral PWL is shown in A and B by open symbols. Baseline PWL is indicated by a dotted line. In (C) LY379268 is contrasted with its enantiomer LY379267 (3 mg kg−1; diamonds; n=8). *=P<0.05, vehicle vs LY379268, Mann – Whitney test.
Figure 4.
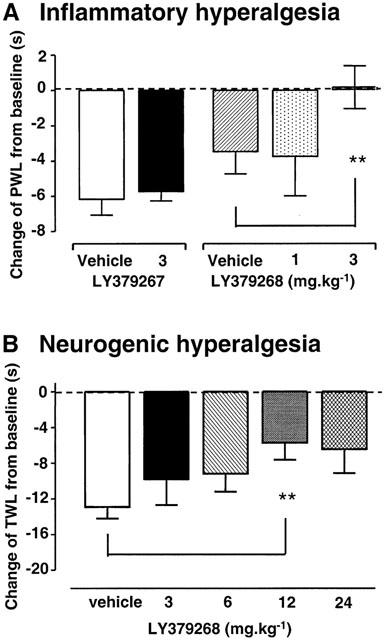
Effects of LY379268, or its inactive enantiomer LY379267, on the development of thermal hyperalgesia induced 1 h after drug. (A): Thermal paw withdrawal latency (PWL) measured in rats 2 h after intraplantar carrageenan injection, shown as a change from pre-carrageenan baseline value; n=6 – 9 per group. **=P<0.01, vehicle vs LY379268 3 mg kg−1 i.p. (B): Tail withdrawal latencies (TWL) in mice 3 min after capsaicin injection into the tail, also expressed as a change from baseline; n=7 – 10 per group. **=P<0.01, vehicle vs LY379268 12 mg kg−1 i.p. All values are mean±s.e.m.
The effects of the mGlu-inactive enantiomer of LY379268, LY379267, were also assessed using Protocol 5, with separate vehicle (saline) controls. Pre-treatment with LY379267 1 h prior to the induction of inflammation had an unexpected pro-algesic effect (P<0.001; 2-way ANOVA, vehicle (not shown) vs LY379267; n=8; Figure 2C). This effect was most significant 2 h after carrageenan injection when the reduction in threshold was by 77% compared to 68% in vehicle-treated rats (P=0.002, Mann-Whitney).
Effects of LY379268 on paw inflammation in rats
One hour after carrageenan injection there was significant inflammation, with paw volumes, measured by a plethysmometer, greater than 150% of the pre-carrageenan baseline volume. Paw volumes remained above 150% control for the whole experimental period, in all treatment groups. Pre-treatment with vehicle or LY379268 did not significantly affect the degree of paw inflammation (P=0.8; 2-way ANOVA vehicle vs 3 mg kg−1 LY379268). Two hours after carrageenan injection, when significant differences in thermal hyperalgesia were observed, paw volumes for the vehicle group and LY379268 (3 mg kg−1) treated group were not significantly different (inflamed paw: vehicle 182±4%, LY379268 167±9% control; contralateral paw: vehicle 98±3%, LY379268 102±3% control; P=0.2; Mann-Whitney; vehicle vs 3 mg kg−1 LY379268 inflamed paws).
Effects of LY379268 on neurogenic hyperalgesia in mice
LY379268 or vehicle was injected i.p. 1 h before a subcutaneous injection of saline or capsaicin into the tail (Protocol 6). Tail injection of saline did not elicit hyperalgesia. Tail injection of capsaicin caused significant thermal hyperalgesia after 3 min (P=0.002; Wilcoxon; vehicle-treated baseline vs 3 min post-capsaicin; n=9; Figure 3) which lasted for up to 9 min (P=0.03). LY379268 dose dependently reduced the hyperalgesia (P<0.01; Mann – Whitney; vehicle vs 12 mg kg−1 LY379268; n=7). LY379268 did not result in complete anti-hyperalgesia in this model; the maximum reduction was by 50% at 12 mg kg−1 (Figure 4B), with no further reduction after 24 mg kg−1. Subjective observation did not reveal any obvious impairment of motor function at these doses.
Figure 3.
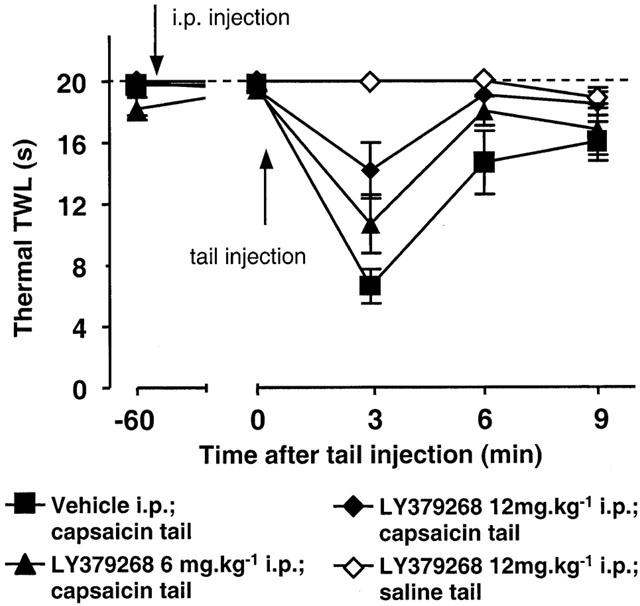
Effects of LY379268 on neurogenic hyperalgesia assessed as mouse tail withdrawal latencies (TWLs) from water at 47°C. LY379268 (6 mg kg−1, closed triangles, n=8; or 12 mg kg−1, closed diamonds, n=7) or vehicle (closed squares, n=10) was injected i.p. 1 h before subcutaneous injection of capsaicin into the tail. The open diamonds show data from mice dosed with LY379268 (at 12 mg kg−1, n=4) followed by a saline injection in the tail. All values are mean±s.e.m.
Discussion
Agonists at group II mGlu receptors can reduce spinal excitatory neurotransmission (Jane et al., 1996) and are therefore potentially anti-nociceptive. During periods of hyperexcitability and sustained nociception, group II mGlu receptor agonists can indeed have anti-nociceptive effects by local administration (Young et al., 1994; 1995; Neugebauer et al., 2000). However, investigation of the potential of such compounds as analgesics has been hampered by the lack of selective agonists that cross the blood-brain barrier and that can therefore be given systemically. Consistent with previous studies on neuroprotection (Bond et al., 1999; Cai et al., 1999; Kingston et al., 1999), in the present study LY379268, a group II mGlu agonist, was active by systemic administration in vivo. Pharmacokinetic studies in gerbils have demonstrated that the compound shows a peak brain concentration within 30 min post intraperitoneal administration, and that a dose of 10 mg kg−1 maintains receptor active concentrations over 24 h (Bond et al., 2000). This profile is representative of the pharmacokinetics of the compound in rats (unpublished data). In mice, i.p. doses of 10 – 100 mg kg−1 maintain anticonvulsive activity over at least 4 h, without evidence of sedation or ataxia (Monn et al., 1999).
Effect of LY379268 on acute nociceptive responses
The lack of LY379268 activity on nociceptive withdrawal reflexes in the non-inflammatory state suggests that the group II mGlu agonist was not an analgesic; nor did it have any effects on motor co-ordination (see also Simmons et al., 1998). Other studies have also shown a lack of effect of less selective group II mGlu agonists, applied locally, on basal levels of nociceptive and non-nociceptive spinal processing (Stanfa & Dickenson, 1998; Neugebauer et al., 2000). These findings may relate to the observation that these receptors are not concentrated at transmitter release sites (Luján et al., 1997) suggesting that under (patho)physiological conditions they are activated preferentially by intense presynaptic activity that causes transmitter overspill from release sites. The addition of exogenous agonist at doses not causing overt behavioural effects might not attain adequate concentrations alone to have this effect, but might be additive with endogenously released transmitter; this would result in preferential effectiveness under conditions of intense presynaptic activity, such as during enhanced pain states.
Effect of LY379268 on pathophysiological hyperalgesia
LY379268 delayed the development of thermal hyperalgesia when given before the onset of peripheral inflammation or capsaicin-induced sensitisation (see also Simmons et al., 1998). It did not, however, attenuate the final degree of hyperalgesia. This suggests a role for mGlu2 and/or mGlu3 receptors in the development rather than the maintenance of inflammatory hyperalgesia, although established neurogenic neuronal sensitisation can be reversed by localized administration of LY379268 (Neugebauer et al., 2000). It is notable that mGlu3 mRNA expression is increased in the dorsal spinal cord during the development phase, but not during the later stages, of peripheral inflammation (Boxall et al., 1998). Systemic LY379268 did not affect the degree of inflammation of the paw induced by carrageenan. This suggests that the cascade of events that lead to inflammation at the site of injury is not being modulated, and that the drug target is on neuronal processing.
Whether the lack of efficacy when given 3 h after carrageenan injection would also be seen following a longer term inflammatory hyperalgesia remains to be tested. The lack of significant anti-hyperalgesia with administration at the same time as carrageenan dosing implies that the mGlu receptor mediated effect is on the earliest stages of the sensitization process. Pre-emptive agonist activity at these receptors would presumably reduce the escalation of excitatory transmission induced by peripheral inflammation (Sluka & Westlund, 1992) or neurogenic sensitisation (Dougherty & Willis, 1992). Pre-treatment with another mGlu II agonist, (2R,4R)-APDC, has also been shown to reduce (RS)-DHPG (group I mGlu agonist)-induced spontaneous nociceptive behaviours, suggested as being due to inhibition of glutamate release from capsaicin-sensitive primary afferent terminals (Fisher et al., 1998). Although reversal of hyperalgesia was observed following topical administration of (1S,3S)-ACPD to the spinal cord 3 h after inflammation (Stanfa & Dickenson, 1998), this may be due to the high concentration administered topically (5.8 mM) and the consequent possibility of lack of selectivity at the target receptors.
In this study it is not possible to define where in the nociceptive pathway the drug was acting. mGlu agonists, by activating presynaptic autoreceptors of glutamate, would be expected to reduce glutamate release, and thereby affect the generation of both spinal and supraspinal plastic changes that are thought to be largely mediated by NMDA receptor activation (Headley & Grillner, 1990). Presynaptic mGluR can also activate heteroreceptors to reduce GABA release (Ohishi et al., 1994; Stefani et al., 1994). Indeed, group II agonists are known also to modulate sensory responses of thalamic and periaqueductal grey neurones, presumably by such mechanisms (Salt & Turner, 1998; Maione et al., 2000). It is also possible that there is a peripheral neuronal site of action (see Bhave et al., 2001).
In addition, mGlu3 receptors are located on glial cells (Ohishi et al., 1993b; Tanabe et al., 1993; Petralia et al., 1996). It has been demonstrated that there is a glial role in hyperalgesia (Meller et al., 1994; Sweitzer et al., 1999). Activation of mGlu3 receptors expressed on glia that envelop glutamatergic synapses may aid the termination of neurotransmission by promoting glutamate removal from the synaptic area (Ohishi et al., 1994).
In rats, the dose of LY379268 that was anti-hyperalgesic had no effect on rota-rod performance (see also Simmons et al., 1998). However, examination of LY379268 at higher doses was limited due to the induction of exaggerated startle responses. Given that other compounds in this structural series do not show the same effects (unpublished observations), this effect may reflect activity either at other glutamate receptors (perhaps mGlu8, at which LY379268 has activity in vitro; Monn et al., 1999) or at non-glutamate receptors. LY379267, the enantiomer of LY379268, has no agonist or antagonist activity at any mGlu receptor subtype (unpublished data) and in the present study had no anti-hyperalgesic effects, although it did cause an unexplained increase in carrageenan hyperalgesia without affecting baseline values; this is presumably due to an unidentified non-mGlu receptor interaction.
In conclusion, this study shows that the systemic activation of group II mGlu receptors can reduce the induction phase of hyperalgesia at doses that do not have motor or other obvious side effects. This lends impetus to the further dissection of the relative roles of mGlu2 and mGlu3 receptor subtypes in the central sensitization process associated with persistent pain.
Acknowledgments
We would like to thank Simon Lishman for valued technical assistance. E.F. Sharpe was supported by the M.R.C.
Abbreviations
- ANOVA
analysis of variance
- ACPD
1-aminocyclopentane-1,3-dicarboxylic acid
- LY354740
(+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid
- LY379268
(−)-2-Oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylic acid
- mGlu
metabotropic glutamate
- PWL
paw withdrawal latency
- PWT
paw withdrawal threshold
- TWL
tail withdrawal latency
References
- BATTAGLIA G., MONN J.A., SCHOEPP D.D. In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II metabotropic glutamate receptor agonist LY354740 in rats. Neurosci. Lett. 1997;229:161–164. doi: 10.1016/s0304-3940(97)00442-4. [DOI] [PubMed] [Google Scholar]
- BHAVEG G., KARIMF F., CARLTONS S.M., GEREAUR R.W. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat. Neurosci. 2001;4:417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- BOND A., JONES N.M., HICKS C.A., WHIFFIN G.M., WARD M.A., O'NEILL M.F., KINGSTON A.E., MONN J.A., ORNSTEIN P.L., SCHOEPP D.D., LODGE D., O'NEILL M.J. Neuroprotective effects of LY379268, a selective mGlu2/3 receptor agonist: Investigations into possible mechanism of action in vivo. J. Pharmacol. Exp. Ther. 2000;294:800–809. [PubMed] [Google Scholar]
- BOND A., MONN J.A., LODGE D. A novel orally active group 2 metabotropic glutamate receptor agonist: LY354740. Neuroreport. 1997;8:1463–1466. doi: 10.1097/00001756-199704140-00027. [DOI] [PubMed] [Google Scholar]
- BOND A., O'NEILL M.J., HICKS C.A., MONN J.A., LODGE D. Neuroprotective effects of a systemically active group II metabotropic glutamate receptor agonist LY354740 in a gerbil model of global ischaemia. Neuroreport. 1998;9:1191–1193. doi: 10.1097/00001756-199804200-00042. [DOI] [PubMed] [Google Scholar]
- BOND A., RAGUMOORTHY N., MONN J.A., HICKS C.A., WARD M.A., LODGE D., O'NEILL M.J. LY379268, a potent and selective Group II metabotropic glutamate receptor agonist, is neuroprotective in gerbil global, but not focal, cerebral ischaemia. Neurosci. Letts. 1999;273:191–194. doi: 10.1016/s0304-3940(99)00663-1. [DOI] [PubMed] [Google Scholar]
- BOXALL S.J., BERTHELE A., LAURIE D.J., SOMMER B., ZIEGLGÄNSBERGER W., URBAN L., TÖLLE T.R. Enhanced expression of metabotropic glutamate receptor 3 messenger RNA in the rat spinal cord during ultraviolet irradiation induced peripheral inflammation. Neuroscience. 1998;82:591–602. doi: 10.1016/s0306-4522(97)00246-7. [DOI] [PubMed] [Google Scholar]
- CAI Z., XIAO F., FRATKIN J.D., RHODES P.G. Protection of neonatal rat brain from hypoxic-ischemic injury by LY379268, a Group II metabotropic glutamate receptor agonist. Neuroreport. 1999;10:3927–3931. doi: 10.1097/00001756-199912160-00037. [DOI] [PubMed] [Google Scholar]
- CHAVIS P., FAGNI L., BOCKAERT J., LANSMAN J.B. Mod-ulation of calcium channels by metabotropic glutamate receptors in cerebellar granule cells. Neuropharmacology. 1995;34:929–937. doi: 10.1016/0028-3908(95)00082-h. [DOI] [PubMed] [Google Scholar]
- CONN P.J., PIN J.-P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- DOUGHERTY P.M., WILLIS W.D. Enhanced responses of sponothalamic tract neurons to excitatory amino acids accompany capsaicin-induced sensitization in the monkey. J. Neurosci. 1992;12:883–894. doi: 10.1523/JNEUROSCI.12-03-00883.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBE G.R., MARSHALL K.C. Modulation of excitatory synaptic transmission in locus coeruleus by multiple presynaptic metabotropic glutamate receptors. Neuroscience. 1997;80:511–521. doi: 10.1016/s0306-4522(97)00004-3. [DOI] [PubMed] [Google Scholar]
- FISHER K., CODERRE T.J. The contribution of metabotropic glutamate receptors (mGluRs) to formalin-induced nociception. Pain. 1996;68:255–263. doi: 10.1016/s0304-3959(96)03212-5. [DOI] [PubMed] [Google Scholar]
- FISHER K., LEFEBVRE C., CAHILL C.M., CODERRE T.J. (RS)-DHPG-induced nociception depends on the release of glutamate from primary-afferent C-fibers. Soc. Neurosci. Abstr. 1998;24:742–747. [Google Scholar]
- HEADLEY P.M., GRILLNER S. Excitatory amino acids and synaptic transmission: the evidence for a physiological function. Trends Pharmacol. Sci. 1990;11:205–211. doi: 10.1016/0165-6147(90)90116-p. [DOI] [PubMed] [Google Scholar]
- JANE D.E., THOMAS N.K., TSE H.-W., WATKINS J.C. Potent antagonists at the L-AP4- and (1S,3S)-ACPD-sensitive presynaptic metabotropic glutamate receptors in the neonatal rat spinal cord. Neuropharmacology. 1996;35:1029–1035. doi: 10.1016/s0028-3908(96)00048-2. [DOI] [PubMed] [Google Scholar]
- JIA H., RUSTIONI A., VALTSCHANOFF J.G. Metabotropic glutamate receptors in superficial laminae of the rat dorsal horn. J. Comp. Neurol. 1999;410:627–642. [PubMed] [Google Scholar]
- KINGSTON A.E., O'NEILL M.J., LAM A., BALES K.R., MONN J.A., SCHOEPP D.D. Neuroprotection by metabotropic glutamate receptor agonists: LY354740, LY379268 and LY389795. Eur. J. Pharmacol. 1999;377:155–165. doi: 10.1016/s0014-2999(99)00397-0. [DOI] [PubMed] [Google Scholar]
- KOZIKOWSKI A.P., STEENSMA D., LUCA-ARALDI G., TÜCKMANTEL W., WANG S., PSHENICHKIN S., SURINA E., WROBLEWSKI J.T. Synthesis and biology of the conformationally restricted ACPD analog, 2-aminobicyclo[2.1.1]hexane-2,5-dicarboxylic acid-I, a potent mGluR agonist. J. Med. Chem. 1998;41:1641–1650. doi: 10.1021/jm970719q. [DOI] [PubMed] [Google Scholar]
- LUJÁN R., ROBERTS D.B., SHIGEMOTO R., OHISHI H., SOMOGYI P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1a, mGluR2 and mGluR5, relative to neurotransmitter release sites. J. Chem. Neuroanatomy. 1997;13:219–241. doi: 10.1016/s0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- MAIONE S., OLIVA P., MARABESE I., PALAZZO E., ROSSI F., BERRINO L., FILIPPELLI A. Periaqueductal gray matter metabotropic glutamate receptors modulate formalin-induced nociception. Pain. 2000;85:183–189. doi: 10.1016/s0304-3959(99)00269-9. [DOI] [PubMed] [Google Scholar]
- MELLER S.T., DYKSTRA C., GEBHART G.F. Acute mechanical hyperalgesia is produced by coactivation of AMPA and metabotropic glutamate receptors. Neuroreport. 1993;4:879–882. doi: 10.1097/00001756-199307000-00010. [DOI] [PubMed] [Google Scholar]
- MELLER S.T., DYKSTRA C., GRZYBYCKI D., MURPHY S., GEBHART G.F. The possible role of glia in nociceptive processing and hyperalgesia in the spinal cord of the rat. Neuropharmacology. 1994;33:1471–1478. doi: 10.1016/0028-3908(94)90051-5. [DOI] [PubMed] [Google Scholar]
- MONN J.A., VALLI M.J., MASSEY S.M., HANSEN M.M., KRESS T.J., WEPSIEC J.P., HARKNESS A.R., GRUTSCH J.L., WRIGHT R.A., JOHNSON B.G., ANDIS S.L., KINGSTON A.E., TOMLINSON R., LEWIS R., GRIFFEY K.R., TIZZANO J.P., SCHOEPP D.D. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid ( LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J. Med. Chem. 1999;42:1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- MONN J.A., VALLI M.J., MASSEY S.M., WRIGHT R.A., SALHOFF C.R., JOHNSON B.G., HOWE T., ALT C.A., RHODES G.A., ROBEY R.L., GRIFFEY K.R., TIZZANO J.P., KALLMAN M.J., HELTON D.R., SCHOEPP D.D. Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid ( LY354740): A potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J. Med. Chem. 1997;40:528–537. doi: 10.1021/jm9606756. [DOI] [PubMed] [Google Scholar]
- NEUGEBAUER V., LUCKE T., SCHAIBLE H.-G. Requirement of metabotropic glutamate receptors for the generation of inflammation-evoked hyperexcitability in rat spinal cord neurons. Eur. J. Neurosci. 1994;6:1179–1186. doi: 10.1111/j.1460-9568.1994.tb00616.x. [DOI] [PubMed] [Google Scholar]
- NEUGEBAUER V., CHEN P.S., WILLIS W.D. Groups II and III metabotropic glutamate receptors differentially modulate brief and prolonged nociception in primate STT cells. J. Neurophysiol. 2000;84:2998–3009. doi: 10.1152/jn.2000.84.6.2998. [DOI] [PubMed] [Google Scholar]
- OHISHI H., SHIGEMOTO R., NAKANISHI S., MIZUNO N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993a;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- OHISHI H., SHIGEMOTO R., NAKANISHI S., MIZUNO N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in rat brain: An in situ hybridization study. J. Comp. Neurol. 1993b;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- OHISHI H., OGAWA-MEGURO R., SHIGEMOTO R., KANEKO T., NAKANISHI S., MIZUNO N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR2 and mGluR3, in rat cerebellar cortex. Neuron. 1994;13:55–66. doi: 10.1016/0896-6273(94)90459-6. [DOI] [PubMed] [Google Scholar]
- PETRALIA R.S., WANG Y.-X., NIEDZIELSKI A.S., WENTHOLD R.J. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- PIN J.-P., DUVOISIN R. Review: Neurotransmitter receptors I. The metabotropic glutamate receptors: Structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- SALT T.E., TURNER J.P. Modulation of sensory inhibition in the ventrobasal thalamus via activation of group II metabotropic glutamate receptors by 2R,4R-aminopyrrolidine-2,4-dicarboxylate. Exp. Brain Res. 1998;121:181–185. doi: 10.1007/s002210050450. [DOI] [PubMed] [Google Scholar]
- SCHOEPP D.D., JANE D.E., MONN J.A. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- SCHOEPP D.D., JOHNSON B.G., WRIGHT R.A., SALHOFF C.R., MAYNE N.G., WU S., COCKERHAM S.L., BURNETT J.P., BELEGAJE R., BLEAKMAN D., MONN J.A. LY354740 is a potent and highly selective group II metabotropic glutamate receptor agonist in cells expressing human glutamate receptors. Neuropharmacology. 1997;36:1–11. doi: 10.1016/s0028-3908(96)00160-8. [DOI] [PubMed] [Google Scholar]
- SHARPE E.F., KINGSTON A.E., LODGE D., MONN J., HEADLEY P.M. Effects of the selective group II agonist, LY379268, on carrageenan-induced hyperalgesia. Soc. Neurosci. Abst. 1998;24:232.7. [Google Scholar]
- SHARPE E.F., KINGSTON A.E., LODGE D., MONN J., HEADLEY P.M. Group II metabotropic glutamate receptors and behavioural tests of nociception. J. Physiol. 1999;515:94P. [Google Scholar]
- SIMMONS R.M.A., MONN J.A., LI D.L., SCHOEPP D.D., TIZZANO J.P., IYENGAR S. LY379268, a novel mGlu2/3 receptor agonist, mediates nociceptive responses in vivo in rats. Soc. Neurosci. Abst. 1998;24:232.8. [Google Scholar]
- SLUKA K.A., WESTLUND K.N. An experimental arthritis in rat: dorsal horn aspartate and glutamate increases. Neurosci. Lett. 1992;145:141–144. doi: 10.1016/0304-3940(92)90006-s. [DOI] [PubMed] [Google Scholar]
- STANFA L.C., DICKENSON A.H. Inflammation alters the effects of mGlu receptor agonists on spinal nociceptive neurones. Eur. J. Pharmacol. 1998;347:165–172. doi: 10.1016/s0014-2999(98)00098-3. [DOI] [PubMed] [Google Scholar]
- STEFANI A., PISANI A., MERCURI N.B., BERNARDI G., CALABRESI P. Activation of metabotropic glutamate receptors inhibits calcium currents and GABA-mediated synaptic potentials in striatal neurons. J. Neurosci. 1994;14:6734–6743. doi: 10.1523/JNEUROSCI.14-11-06734.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWEITZER S.M., COLBURN R.W., RUTKOWSKI M., DELEO J.A. Acute peripheral inflammation induces moderate glial activation and spinal IL-1β expression that correlates with pain behavior in the rat. Brain Res. 1999;829:209–221. doi: 10.1016/s0006-8993(99)01326-8. [DOI] [PubMed] [Google Scholar]
- TANABE Y., NOMURA A., MASU M., SHIGEMOTO R., MIZUNO N., NAKANISHI S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J. Neurosci. 1993;13:1372–1378. doi: 10.1523/JNEUROSCI.13-04-01372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TESTA C.M., FRIBERG I.K., WEISS S.W., STANDAERT D.G. Immunohistochemical localization of metabotropic glutamate receptors mGluR1a and mGluR2/3 in the rat basal ganglia. J. Comp. Neurol. 1998;390:5–19. [PubMed] [Google Scholar]
- THOMSEN C., HANSEN L., SUZDAK P.D. L-Glutamate up-take inhibitors may stimulate phosphoinositide hydrolysis in baby kidney cells expressing mGluR1a via heteroexchange with L-glutamate without direct activation of mGluR1a. J. Neurochem. 1994;63:2038–2047. doi: 10.1046/j.1471-4159.1994.63062038.x. [DOI] [PubMed] [Google Scholar]
- VANDERGRIFF J., RASMUSSEN M. The selective mGluR2/3 receptor agonist LY354740 attenuates morphine-withdrawal-induced activation of locus coeruleus neurones and behavioral signs of morphine withdrawal. Neuropharmacology. 1999;38:217–222. doi: 10.1016/s0028-3908(98)00196-8. [DOI] [PubMed] [Google Scholar]
- YOUNG M.R., FLEETWOOD-WALKER S.M., MITCHELL R., DICKENSON T. The involvement of metabotropic glutamate receptors and their intracellular signalling pathways in sustained nociceptive transmission in rat dorsal horn neurons. Neuropharmacology. 1995;34:1033–1041. doi: 10.1016/0028-3908(95)00071-d. [DOI] [PubMed] [Google Scholar]
- YOUNG M.R., FLEETWOOD-WALKER S.M., MITCHELL R., MUNRO F.E. Evidence for a role of metabotropic glutamate receptors in sustained nociceptive inputs to rats dorsal horn neurons. Neuropharmacology. 1994;33:141–144. doi: 10.1016/0028-3908(94)90109-0. [DOI] [PubMed] [Google Scholar]
- YUNG K.K.L. Localization of glutamate receptors in dorsal horn of rat spinal cord. Neuroreport. 1998;9:1639–1644. doi: 10.1097/00001756-199805110-00069. [DOI] [PubMed] [Google Scholar]


