Abstract
The mechanisms underlying the vasodilator response to urocortin are incompletely understood. The present study was designed to examine the role of endothelial nitric oxide and Ba2+-sensitive K+ channels in the endothelium-dependent component of urocortin-induced relaxation in the rat left anterior descending coronary artery.
Urocortin induced both endothelium-dependent and -independent relaxation with respective pD2 of 8.64±0.03 and 7.90±0.10. Removal of endothelium reduced the relaxing potency of urocortin. In rings pretreated with 10−4 M NG-nitro-L-arginine methyl ester, 10−5 M methylene blue or 10−5 M ODQ, the urocortin-induced relaxation was similar to that observed in endothelium-denuded rings. L-Arginine (5×10−4 M) antagonized the effect of NG-nitro-L-arginine methyl ester.
The relaxant response to urocortin was reduced in endothelium-intact rings preconstricted by 3.5×10−2 M K+ and abolished when extracellular K+ was raised to 5×10−2 M. Pretreatment with 10−4 M BaCl2 significantly inhibited urocortin-induced relaxation. Combined treatment with 10−4 M BaCl2 plus 10−4 M NG-nitro-L-arginine methyl ester did not cause further inhibition. In urocortin (10−8 M)-relaxed rings, BaCl2 induced concentration-dependent reversal in vessel tone. Tertiapin-Q (10−6 M) also attenuated urocortin-induced relaxation. In contrast, BaCl2 did not alter urocortin-induced relaxation in endothelium-denuded rings.
In endothelium-denuded rings, hydroxylamine- and nitroprusside-induced relaxation was inhibited by 10−4 M BaCl2, but not by 10−6 M tertiapin-Q.
The endothelium of the coronary artery was moderately stained with the antiserum against urocortin.
Taken together, the present results indicate that the urocortin-induced endothelium-dependent relaxation of rat coronary arteries is likely attributable to endothelial nitric oxide and subsequent activation of Ba2+- or tertiapin-Q-sensitive K+ channels. The urocortin-induced endothelium-dependent relaxation appears to be mediated by cyclic GMP-dependent mechanisms.
Keywords: Urocortin, K+ channels, barium, nitric oxide, endothelium, coronary artery, rat
Introduction
Corticotropin-releasing factor (CRF), a 41-amino acid peptide produced mainly in the hypothalamus, is believed to be the principal neuromediator of the hypothalamic-pituitary-adrenal stress axis in mammals (Vale et al., 1981). Several structurally related peptides of the CRF family include fish neuropeptide urotensin (Pearson et al., 1980), sauvagine (Erspamer et al., 1980), and urocortin (Vaughan et al., 1995). The action of CRF-related peptides are mediated through binding to two G protein-coupled receptors derived from two distinct genes (Potter et al., 1994; Lovenberg et al., 1995b). There exists at least three alternatively spliced forms of Type-2 CRF receptor, CRF-R2α, CRF-R2β and CRF-R2γ (Lovenberg et al., 1995b; Kishimoto et al., 1995).
Although these receptors are widely distributed throughout the mammalian brain, CRF-R2 is the only type of CRF receptor detected in the heart (Kishimoto et al., 1995; Lovenberg et al., 1995a; Perrin et al., 1995). Two subtypes of CRF-R2 are expressed in cardiac tissues, CRF-R2α in human (Chen et al., 1993) and CRF-R2β in rats (Stenzel et al., 1995). Urocortin II selectively binds to type 2 CRF receptors (Reyes et al., 2001). Urocortin II shares a significant sequence homology to CRF and the coincident expression of CRF-R2 receptors with urocortin ligands in the heart suggests that urocortin may play a physiological role in the cardiac activity. Urocortin or CRF exerted positive ionotropic effect, elevated cardiac output and caused coronary vasodilatation in sheep and rats (Parkes et al., 1997; Terui et al., 2001). This effect may be associated with increased production of cardiac cyclic AMP level resulting from stimulation of adenylyl cyclase (Heldwein et al., 1996). The cardiac effect can be achieved by urocortin within a narrow nanomolar concentration range. Urocortin mRNA was expressed by both cultured neonatal rat cardiomyocytes and the adult rat heart; urocortin transcripts were increased after simulated hypoxia/ischemia (Brar et al., 1999). Urocortin exerted cardioprotective effect against cell death following ischemic and reperfusion injury via a MAPK-dependent signaling pathway (Brar et al., 2000). Besides, urocortin stimulated secretion of atrial natriuretic peptide and brain natriuretic peptide from neonatal rat cardiomyocytes (Ikeda et al., 1998). Both urocortin mRNA and CRF-R2β mRNA are expressed in the cultured rat cardiac myocytes. Urocortin mRNA expression was upregulated in left ventricular hypertrophy (Nishikimi et al., 2000). Urocortin immunoreactivity was found to be more intense in cardiomyocytes from human failing hearts than from normal hearts (Nishikimi et al., 2000). These new findings indicate that urocortin may play a role in the pathophysiology of cardiac hypertrophy or heart failure.
Urocortin and CRF-related peptides are extremely potent vasodilators. Systemic administration of CRF-related peptides lowered blood pressure in human (Kübler et al., 1994) and in experimental animals (Lenz et al., 1985; Lei et al., 1993; Richter & Mulvany, 1995; Vaughan et al., 1995). In vitro studies show that these peptides produced potent vasorelaxation in human foetal vessels (Clifton et al., 1994; Leitch et al., 1998) and in rat cerebral artery (Schilling et al., 1998).
Urocortin and CRF-related peptides were found to decrease coronary perfusion pressure in isolated rat heart via coronary vasodilatation (Terui et al., 2001). The coronary vasodilating and the positive ionotropic effects of urocortin may underpin its potential cardiac protective action. However, the exact mechanisms underlying the urocortin-induced coronary vasodilatation remain unclear. The present study aimed at investigating the role of endothelium and K+ channels in the coronary vasorelaxant response to urocortin by measuring isometric tone of ring segments of the isolated rat left anterior descending coronary artery on small vessel myograph.
Methods
Artery preparation and mounting
The experimental protocols were approved by the Animal Research Ethics Committee of the Chinese University of Hong Kong. Male Sprague-Dawley rats (∼300 – 330 g) were killed by cervical dislocation. The heart was rapidly removed and placed in a dissecting plate containing ice-cold Krebs solution. The left anterior descending arteries were carefully dissected out. After the surrounding tissues were cleaned off, each artery was cut into 2 – 3 cylindrical segments ∼1.5 mm in length.
Ring segments were mounted in a Multi Myograph System (Danish Myo Technology A/S, Denmark) and changes in vessel tension were recorded. Briefly, two tungsten wires (each of 40 μm diameter) were passed through the segment's lumen and each wire fixed to jaws of myograph. The chamber bath was filled with 5 ml Krebs solution. The Krebs solution contained (mM): NaCl 119, KCl 4.7, CaCl2 2.5, MgCl2 1, NaHCO3 25, KH2PO4 1.2 and D-glucose 11. Each ring was bathed in Krebs solution that was continually aerated with a gas mixture of 95% O2 plus 5% CO2. The Krebs solution in the chamber was maintained at 37°C using a built-in heat-exchange device. The pH values were between 7.3 and 7.5. The segment was stretched between the wires under a previously determined optimal tension of 0.5 mN. Two ring-segments were prepared from each rat. The rings were allowed to stabilize at a baseline tone for 90 min before the start of the experiments. Each experiment was carried out on rings prepared from different rats. In total, 84 rats were used in this study.
Experimental design
After equilibration in Krebs solution for 30 min, each vessel was initially contracted by 3×10−8 M U46619 to test its contractile capacity followed by addition of 3×10−6 M acetylcholine to test integrity of the endothelium. The ring was then rinsed three times with an interval of 20 min and baseline tone was readjusted when necessary.
In the first set of experiments, 3×10−8 M U46619 was used to generate sustained vessel tone; the relaxant responses to cumulative concentration of urocortin were subsequently studied. To determine the role of the endothelium, the responses to urocortin were examined in endothelium-denuded rings. The urocortin-induced relaxation was also examined in endothelium-intact rings pretreated with 10−4 M L-NAME, 10−5 M methylene blue or 10−5 M ODQ (30-min incubation time) to assess the involvement of nitric oxide or cyclic GMP-dependent pathway. To examine whether the substrate of nitric oxide synthase, L-arginine, would counteract with the effect of L-NAME, some endothelium-intact rings were incubated with 5×10−4 M L-arginine for 10 min before the addition of 10−4 M L-NAME.
In the second group of experiments, the endothelium-intact rings were incubated for 30 min with K+ channel blockers (10−3 M TEA+, 10−4 M BaCl2, or 10−7 M CTX plus 10−7 M apamin) before addition of U46619; urocortin was then cumulatively applied. The relaxant response to urocortin was also tested in rings constricted by high K+ (3.5×10−2 and 5×10−2 M) as compared with the rings constricted by U46619. To study the role of prostacyclin, rings were exposed to 10−5 M indomethacin for 30 min prior to addition of U46619.
In order to localize the site of action for BaCl2, BaCl2 was examined on the relaxation induced by hydroxylamine or nitroprusside in the endothelium-denuded rings. The rings were pretreated for 30 min with various inhibitors (BaCl2, tertiapin-Q, L-NAME, ODQ, methylene blue) before addition of U46619. Once a steady tone was established, the nitric oxide donor was applied cumulatively.
Since pretreatment with inhibitors of nitric oxide-cyclic GMP pathway or of K+ channels, could increase the U46619-induced tone, the concentration of U46619 was lowered between 2×10−8 and 3×10−8 M in order to produce a similar amplitude of vessel tension in different rings.
In some rings, the endothelium was mechanically removed by rubbing the luminal surface of the ring several times with small stainless steel wire (40 μm in diameter). The functional denuation of the endothelium was confirmed if the relaxant response to 3×10−6 M acetylcholine was lacking in the beginning of each experiment. In experiments using high K+-containing solution, an equimolar concentration of K+ replaced Na+ in order to retain a constant ionic strength.
Immunohistochemistry
The anaesthetized male Sprague-Dawley rats of approximately 300 g in weight were perfused with freshly prepared 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) via the left ventricle. The left anterior descending coronary arteries were dissected from the hearts with the coronary arteries en bloc were further fixed overnight in the same fixative and embedded in paraffin. Paraffin sections of cross-sections of coronary arteries were cut. Hydrated sections were treated with 0.3 – 0.5% H2O2 in absolute methanol for 30 min, followed by washing in PBS and incubated in a blocking solution containing 0.1% bovine serum albumin (BSA) and 0.3 – 0.5% Tween-20 in PBS for 15 min. Sections were then incubated with a rabbit antiserum against synthetic rat urocortin (Y360 or Y361, Peptide Institute, Inc., Osaka, Japan), diluted 1 : 200 in the blocking solution, overnight at room temperature in a moist chamber. Control sections were incubated with the blocking solution without primary antiserum. After washing in PBS, sections were incubated with a biotin-labelled goat anti-rabbit IgG (Jackson ImmunResearch Laboratories, Inc., West Grove, PA, U.S.A.) for 1 h at room temperature, followed by 1 h with avidin-biotin-peroxidase complex. The immunoreactive sites were then visualized by a glucose oxidase-diaminobenzidine (DAB)-nickel method (Chan & Choi, 1995) and appeared as dark blue or black in colour.
Chemicals
Phenylephrine hydrochloride, acetylcholine chloride, urocortin (human), indomethacin, NG-nitro-L-arginine methyl ester (L-NAME), L-arginine, charybdotoxin (CTX), apamin, 9,11-dideoxy-11α,9α-epoxy-methanoprostaglandin F2α (U46619), methylene blue, barium chloride, tetraethylammonium ions, hydroxylamine, sodium nitroprusside (Sigma, St. Louis, MO, U.S.A.), 1H-[1,2,4]oxadiazolo[4,2-α]quinoxalin-1-one (ODQ), tertiapin-Q (Tocris, U.K.). Antiserum against rat urocortin was purchased from Peptide Institute, Inc. (Osaka, Japan). Indomethacin U46619 and ODQ were dissolved in dimethyl sulphoxide. 0.2% DMSO did not affect U46619-induced tension. Stock solution of urocortin was prepared in 0.1 N HCl and desired dilution was made daily before experiments. Other chemicals were dissolved in distilled water.
Data analysis
Several rings prepared from the same artery were studied in parallel, and a concentration-response curve was established in each ring. The relaxant effect of urocortin was quantified as the percentage reduction of the initial tone in each ring preconstricted by U46619. Concentration-relaxation curves were constructed based on the responses to cumulative concentrations of urocortin and analysed by non-linear curve fitting using Graphpad software (Version 3.0). The negative logarithm of the relaxant concentration that caused 50% of the maximum relaxation (pD2) and the maximum relaxation (Emax) were calculated. For statistical analysis, Student's t-test or one-way analysis of variance followed by Newman-Keuls test was used when more than two treatments were compared. Statistically significance was accepted when P<0.05. The results are mean±s.e.mean and n represents a number of rings prepared from different rats.
Results
The relaxant effect of urocortin
Traces in Figure 1a show that cumulative application of urocortin induced concentration-dependent relaxation in endothelium-intact rings preconstricted with U46619. Urocortin produced significantly less relaxation in the endothelium-denuded rings without an effect on the maximum relaxation (Figure 1b). Figure 1c shows the concentration-response curves for urocortin-induced relaxant response in endothelium-intact and denuded rings with pD2 8.64±0.03 (n=15) and 7.90±0.10 (n=8), respectively (P<0.05, Figure 6). In the endothelium-intact rings preconstricted with 3.5×10−2 M K+, urocortin at the same concentration range induced only partial relaxation with 31.9±5.5% maximal relaxation (n=4) and urocortin failed to relax 5×10−2 M K+-preconstricted rings (n=4, Figure 1d).
Figure 1.

Representative records of the relaxant responses to urocortin in isolated rat coronary artery rings with endothelium (a) and without endothelium (b). Calibration bars apply to both traces. (c) Concentration-response curves for urocortin-induced relaxation in endothelium-intact and -denuded rings preconstricted with U46619. (d) Concentration-response curves for urocortin in endothelium-intact rings preconstricted by 35 mM K+ or 50 mM K+. Results are mean±s.e.mean of n experiments. *Significantly different from control; P<0.05.
Figure 6.
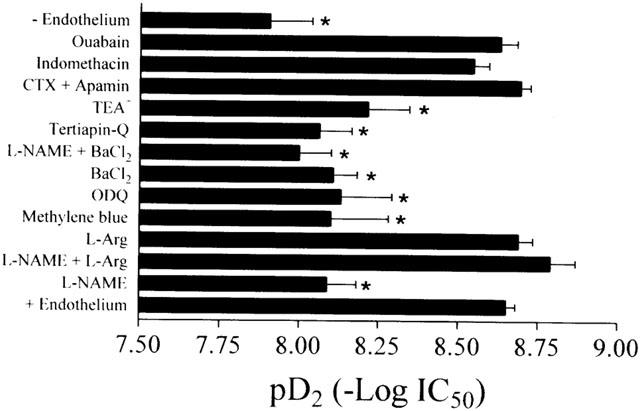
pD2 values were calculated as the negative logarithm of the urocortin concentration inducing 50% maximum relaxation after various pharmacological treatments in isolated rat coronary artery rings. Results are mean±s.e.mean of n experiments. *Indicates a significant difference from the control (with endothelium).
Nitric oxide inhibitors
In the endothelium-intact rings pretreated with 10−4 M L-NAME, urocortin-induced relaxant response was significantly attenuated (n=7, Figure 2a). The pD2 value was 8.09±0.09 (n=7), close to that obtained in endothelium-denuded rings (Figure 6). Pretreatment with nitric oxide precursor, L-arginine (5×10−4 M) did not influence urocortin-induced relaxation (n=4, Figures 2b and 6), but it prevented the inhibitory effect of 10−4 M L-NAME on urocortin-induced relaxation in endothelium-intact rings (n=5, Figure 2b). Methylene blue at 10−5 M or ODQ at 10−5 M inhibited urocortin-induced relaxation with similar potency (n=5, Figures 2c and 6).
Figure 2.

(a) Concentration-relaxation curves for urocortin in control and in the presence of 10−4 M L-NAME. (b) Concentration-relaxation curves for urocortin in control and in the presence of 5×10−4 M L-arginine, 10−4 M L-NAME, or 5×10−4 M L-arginine plus 10−4 M L-NAME. (c) Concentration-relaxation curves for urocortin in control and in the presence of 10−5 M methylene blue or 10−5 M ODQ. All experiments were conducted in endothelium-intact rings. Each ring was exposed to L-NAME, methylene blue or ODQ for 30 min prior to application of U46619. Results are mean±s.e.mean of n experiments. *Significantly different from control; P<0.05.
K+ channel inhibitors
Figure 3a shows that in endothelium-intact rings preconstricted by U46619, urocortin-induced concentration-dependent relaxation was significantly attenuated by pretreatment with 10−4 M BaCl2 or 10−6 M tertiapin-Q (pD2: 8.11±0.07, n=6 in BaCl2 and 8.06±0.10, n=6 in tertiapin-Q, P<0.05 compared with control, Figure 3b). Furthermore, co-treatment with 10−4 M BaCl2 plus 10−4 M L-NAME did not produce additive inhibition of urocortin-induced relaxation (pD2: 8.00±0.10, n=6, Figure 3a) as compared with BaCl2 or L-NAME alone (P>0.05, Figure 6). Besides, following the relaxant response to urocortin (2×10−8 M), BaCl2 (10−6 – 10−4 M) concentration-dependently reversed the urocortin-induced relaxation in endothelium-intact rings (n=5, Figure 3c). In the endothelium-denuded rings, pretreatment with 10−4 M BaCl2 had no effect on urocortin-induced relaxation (pD2: 7.79±0.24, n=6, P>0.05 compared with control, Figures 3d and 6). BaCl2 at 10−4 M did not affect baseline tone in both endothelium-intact and -denuded rings (data not shown).
Figure 3.
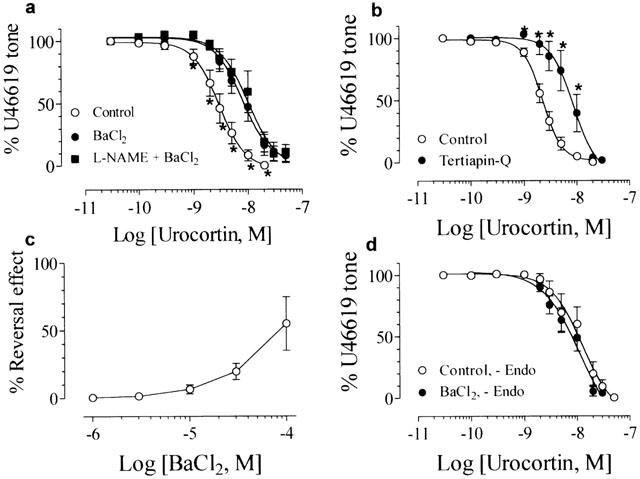
Concentration-relaxation curves for urocortin in control and in the presence of (a) 10−4 M BaCl2 or 10−4 M L-NAME plus 10−4 M BaCl2, or (b) 10−6 M tertiapin-Q. (c) The reversal effect by BaCl2 on 10−8 M urocortin-induced relaxation. All these experiments were conducted in endothelium-intact rings. (d) Lack of effect of 10−4 M BaCl2 on urocortin-induced relaxation in endothelium-denuded rings. Results are mean±s.e.mean of n experiments. *Significantly different from control; P<0.05.
The concentration-dependent response to urocortin was inhibited by 10−3 M TEA+ (pD2: 8.21±0.12, n=6, P<0.05 compared with control, Figures 4a and 6), but was unaffected by a combination of 10−7 M CTX and 10−7 M apamin (pD2: 8.69±0.03, n=6, P>0.05 compared with control, Figures 4b and 6).
Figure 4.
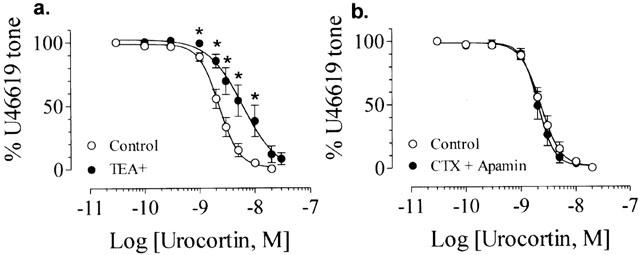
(a) Effect of 10−3 M TEA+ on urocortin-induced relaxation. (b) Lack of effect of 10−7 M CTX plus 10−7 M apamin on urocortin-induced relaxation. All experiments were performed in endothelium-intact rings. Results are mean±s.e.mean of n experiments.
Indomethacin or ouabain
In endothelium-intact rings preconstricted by U46619, neither indomethacin (10−5 M) nor ouabain (10−4 M) affected the relaxation of U46619-contracted endothelium-intact rings induced by urocortin (pD2: 8.55±0.05, n=4 in indomethacin and 8.63±0.05, n=5 in ouabain, P<0.05 compared with control, Figures 5 and 6).
Figure 5.
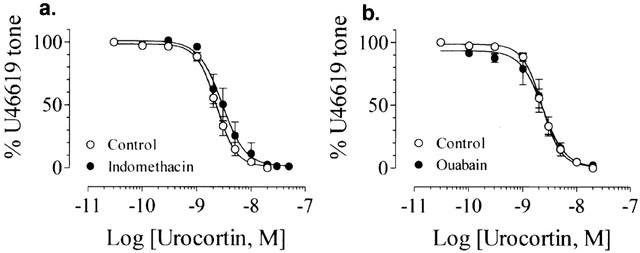
Lack of effect of 10−5 M indomethacin (a) or 10−4 M ouabain (b) on the urocortin-induced relaxation in endothelium-intact rings. Results are mean+s.e.mean of n experiments.
Nitric oxide donors-induced relaxation
In the endothelium-denuded rings preconstricted by 2×10−8 M U46619, hydroxylamine induced concentration-dependent relaxation with pD2 of 6.48±0.06 (n=8, Figure 7c). Trace in Figure 7a shows the control relaxant response to hydroxylamine. Pretreatment with BaCl2 (10−4 M) significantly attenuated the hydroxylamine-induced relaxation without affecting the maximal response (pD2: 6.06±0.07, n=8 in BaCl2, P<0.05 compared with control, Figure 7b,d). But tertiapin-Q (10−6 M) had no effect (pD2: 6.36±0.04, n=8, Figure 7d). Similarly, Ba2+ (10−4 M) but not tertiapin-Q (10−6 M) inhibited nitroprusside-induced relaxation in the endothelium-denuded rings (pD2: 8.03±0.03 in control, 7.49±0.09 in Ba2+, and 7.95±0.06 in tertiapin-Q, n=6 in each case). Methylene blue (10−5 M, n=5) but not L-NAME (10−4 M, n=5) significantly reduced the relaxant response to hydroxylamine (Figure 7c). ODQ at 10−5 M abolished the relaxation induced by hydroxylamine (n=4, Figure 7c) or by nitroprusside (n=6).
Figure 7.
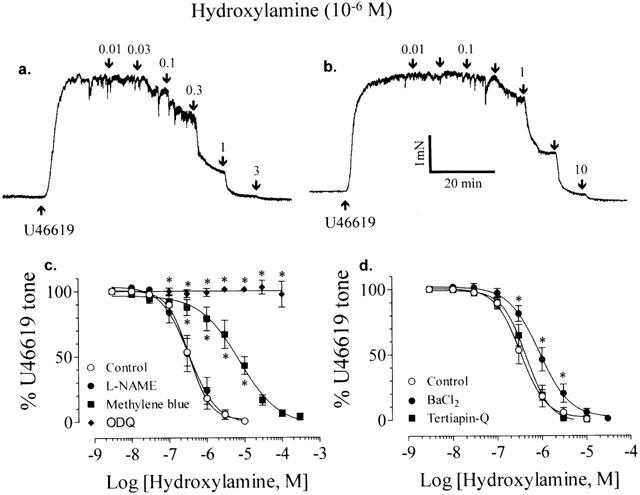
Traces showing the relaxant response of endothelium-denuded rings induced by hydroxylamine in control (a) and in the presence of 10−4 M BaCl2 (b). (c) Effects of methylene blue (10−5 M), L-NAME (10−4 M) and 10−5 M ODQ on hydroxylamine-induced relaxation. (d) The effects of BaCl2 (10−4 M) and tertiapin-Q (10−6 M) on the relaxation induced by hydroxylamine. All experiments were performed in the endothelium-denuded rings. Results are mean±s.e.mean of n experiments. *Significantly different from control; P<0.05.
Immunochemistry
The endothelium of the coronary artery moderately reacted with the antiserum against urocortin (Figure 8b,c). The smooth muscle cell layer was not stained. No positive staining was seen when the primary antiserum was replaced by the blocking solution (Figure 8c).
Figure 8.

(a – c). Immunohistochemistry of urocortin in rat left anterior descending coronary artery. (a) Control section for figures (b) and (c). There is absence of staining over the endothelial layer of the coronary artery (×100). (b) The endothelium of the coronary artery is positively stained (×100). (c) A higher magnification of (b). However, no reaction is seen in the smooth muscle cell layer (×200). The calibration bar indicates 100 μm in (a) and (b) and 50 μm in (c).
Discussion
This study characterizes some mechanisms by which urocortin acts as a potent dilator of the rat left anterior descending coronary arteries. The major and new findings of this study include: (1) urocortin induces both endothelium-dependent and -independent coronary relaxation and endothelial nitric oxide is the primary mediator of the endothelium-dependent response; (2) urocortin-induced endothelium-dependent relaxation is likely mediated via a cyclic GMP-related mechanism; and (3) urocortin-induced endothelium-dependent relaxation is inhibited by BaCl2 and tertiapin-Q at concentrations used to block inwardly rectifier K+ (KIR) channels in the vascular cells.
Role of the endothelium
A recent study has shown that CRF and urocortin decreased coronary perfusion pressure (CPP) at a constant flow rate in the isolated rat heart, indicating that both peptides possess a coronary vasodilator effect (Terui et al., 2001). Our results demonstrate a very potent vasorelaxant effect of urocortin with an IC50 of 2.24 nM in the isolated rat coronary arteries with intact endothelium.
A significant decrease in the potency of urocortin is observed in the endothelium-denuded rings (IC50 of 12.5 nM) even though the maximum relaxation to urocortin remains unchanged. However, it was previously shown that a small vasodilator effect of urocortin (4% reduction of CPP) started to occur only at 86 nM and a moderate decrease in CPP (27%) was obtained with urocortin at 206 nM (Terui et al., 2001). This value (206 nM) is approximately 16 fold the IC50 value calculated for urocortin-induced coronary relaxation in the isolated endothelium-denuded rings. It is likely that other factors may affect the coronary response to urocortin in the intact heart. For example, urocortin was also found to exert positive ionotropic action that may complicate its vasodilator effect. It is not known whether regional differences in the response to urocortin exist in coronary circulation, which could partly explain the discrepancy in the relaxing potency of urocortin between the isolated rings and the whole vasculature. However, the endothelium was found to play little role in urocortin-induced relaxation in the isolated rat mesenteric artery (Lei et al., 1993) and rat basilar artery (Schilling et al., 1998). In contrast, CRF-induced relaxation was markedly blunted by endothelium removal in rat uterine artery (Jain et al., 1999) and rat aorta (Jain et al., 1997). These data indicate that the endothelium may have differential role in different vessels that are chosen for testing the vascular effect of urocortin.
Inhibitors of nitric oxide-dependent vasodilation
Removal of the endothelium attenuates the relaxant effect of urocortin, indicating that the endothelial-derived factors are involved. It is well known that various physiological stimuli including endogenous neuropeptides which trigger Ca2+ influx in the endothelial cells, regulate the contractility of vascular smooth muscle by liberating a number of vasoactive agents, both relaxing and constricting from the endothelium. The balance between the two opposing forces determines the net effect of these factors on vasomotor activity. Nitric oxide, prostacyclin and hyperpolarizing factor are the three major types of vasodilators produced in the endothelium, which act via a variety of cellular mechanisms. Pretreatment with L-NAME, an inhibitor of nitric oxide synthase, or with methylene blue and ODQ, inhibitors of guanylyl cyclase, inhibited the urocortin-induced relaxation to the same degree as observed in the endothelium-denuded rings. In contrast, indomethacin, an inhibitor of prostacyclin formation, had not effect. These results point to a primary role of nitric oxide/cyclic GMP-dependent pathway but not the relaxing prostanoids in the endothelium-dependent relaxant response to urocortin. Similarly, NG-nitro-L-arginine, another inhibitor of nitric oxide synthase, reduced the vasodilator effect of CRF in the isolated rat heart (Grunt et al., 1993) and in human foetal-placental vessels (Clifton et al., 1995).
Our results, however, do not support a recent report that in the isolated rat heart L-NAME did not affect urocortin-induced decrease in CPP (Terui et al., 2001). This difference may be partly due to the different concentrations of urocortin used in the two studies. Our study shows that urocortin caused endothelial nitric oxide-mediated relaxation at concentrations between 1 and 30 nM. Urocortin at 30 nM or higher induces only the endothelium-independent response. On the other hand, in the study using the isolated heart, the concentrations used to dilate the intact coronary vasculature were between 86 and 660 nM. If all vessels in the heart respond to urocortin in a similar manner as that in the isolated left anterior descending coronary artery, it is reasonable to suggest that the endothelial nitric oxide-dependent component of the relaxation may have been masked by higher concentrations of urocortin.
Inhibitors of K+ channels
The other novel finding of the present investigation is that activation of the K+ channels is likely involved in the urocortin-induced endothelium-dependent coronary vasorelaxation. Firstly, urocortin-induced relaxation was markedly reduced and almost abolished in rings preconstricted with 35 and 50 mM K+, respectively. One major consequence of raising extracellular K+ concentration is to narrow the electrochemical driving force for K+ efflux, so that the effect of K+ channel activation is minimized. Secondly, the urocortin-mediated relaxant effect was significantly inhibited by BaCl2 or tertiapin-Q, at concentrations used to selectively block the activity of KIR channels in coronary arterial smooth muscle cells (Robertson et al., 1996; Xu et al., 1999) or in cardiac myocyte (Jin et al., 1999; Kitamura et al., 2000). In the urocortin-relaxed rings, BaCl2 induces concentration-dependent (1 – 100 μM) contraction. In contrast, BaCl2 did not affect urocortin-induced relaxation in endothelium-denuded rings, indicating a BaCl2-sensitive element in urocortin-induced relaxation in endothelium-intact vessels but not in endothelium-denuded vessels. This contention is supported by our observation that combined treatment with L-NAME and BaCl2 did not produce additive effect. It appears that urocortin stimulates release of endothelial nitric oxide which then acts on the plasma membrane Ba2+-sensitive K+ channels of the underlying arterial smooth muscle cells. In isolated rabbit middle cerebral arteries, BaCl2 (10 – 300 μM) or inhibition of nitric oxide synthase reduced flow-induced relaxation only in the endothelium-intact preparations; BaCl2 had no effect following endothelium removal or nitric oxide synthase inhibition (Wellman & Bevan, 1995).
The present study indicates that BaCl2 probably acts on arterial smooth muscle cells instead of the endothelium. This is suggested by the observation that BaCl2 at the same concentration also inhibits the relaxant response of endothelium-denuded rings to hydroxylamine or nitroprusside, the nitric oxide donors (Huang, 1998). A similar observation was also made in endothelium-denuded canine cerebral arteries in which BaCl2 (300 μM) significantly reduced relaxation induced by the exogenous nitric oxide donors, 3-morpholinosydnonimine and nitroprusside (Onoue & Katusic, 1997).
It is interesting to note that BaCl2 but not tertiapin-Q inhibited hydroxylamine- or nitroprusside-induced relaxation, while both blocking agents reduced the urocortin-mediated relaxation to the same extent. It is at present unknown what had caused this discrepancy. It can not be ruled out that the mode of action of these two blockers against the nitric oxide-mediated response may be different between naturally occurring nitric oxide from the endothelium and nitric oxide released from its exogenous donors.
Urocortin-induced relaxation was unaffected by glibenclamide at a concentration that selectively inhibited arterial KATP channels (Standen et al., 1989), indicating that KATP channels are not involved. Similarly, glibenclamide did not affect the relaxation induced by CRF-related peptides in rat uterine artery (Jain et al., 1999) or cerebral artery (Schilling et al., 1998).
Direct activation of KCa channels by nitric oxide was reported in vascular smooth muscle cells (Mistry & Garland, 1998). In non-vascular smooth muscle cells, urocortin produces hyperpolarization via stimulation of KCa currents (Petkova-Kirova et al., 2000). Although urocortin-induced relaxation is partially inhibited by tetraethylammonium ions, a non-selective blocker of K+ channels, our data do not indicate that urocortin could activate KCa channels since urocortin-induced relaxation was unaffected by inhibition of both large- and small-conductance KCa channels. Besides, BaCl2 has little effect on arterial KCa channels (Nelson & Quayle, 1995).
In resistance-sized rat vessels, acetylcholine-induced opening of endothelial KCa channels allows efflux of K+ ions that diffuse between endothelium and vascular smooth muscle layers. Local K+ buildup (acting as an endothelium-derived hyperpolarizing factor) then activates two ion transporting proteins, KIR channel and Na+-K+-ATPase in vascular smooth muscle (Edwards et al., 1998). Increased extracellular [K+] is also found to activate KIR currents in coronary myocytes and this effect is sensitive to inhibition by BaCl2 (Xu et al., 1999). However, it is unlikely that urocortin activates endothelial KCa channels since its effect is not influenced by a combined treatment with charybdotoxin plus apamin, a recipe used to block K+ efflux through endothelial KCa channels (Edwards et al., 1998). Lack of effect of ouabain suggests that Na+-K+-ATPase is not the possible target of action for urocortin.
Summary
The present study is the first attempt to examine the mechanisms by which urocortin relaxes rat coronary arteries. Taken together, the results of this investigation suggest that both endothelial nitric oxide and subsequent activation of BaCl2-sensitive K+ channel in arterial smooth muscle cells mediates the endothelium-dependent component of urocortin-induced coronary relaxation. Cyclic GMP-dependent mechanism seems to be involved. We also detected immunoreactivity of urocortin in the endothelial layer of the rat coronary arteries. This suggests that urocortin may be locally produced in blood vessels. If this occurs in the endothelial cells, urocortin may function as a new member of endothelium-derived vasoactive agents.
Acknowledgments
The study was supported by CUHK Direct Grant and CUHK Mainline Research Grant. Authors thank Professor P.Y.D. Wong for critically reading the manuscript and Ms H.L. Choi for her excellent technical assistance in immunochemistry.
Abbreviations
- CPP
coronary perfusion pressure
- CRF
corticotropin-releasing factor
- CTX
charybdotoxin
- L-NAME
NG-nitro-L-arginine methyl ester
- ODQ
1H-[1,2,4]oxadiazolo[4,2-α]quinoxalin-1-one
- TEA+
tetraethylammonium
- U46619
9,11-dideoxy-11α,9α-epoxy-methanoprostaglandin F2α
References
- BRAR B.K., JONASSEN A.K., STEPHANOU A., SANTILLI G., RAILSON J., KNIGHT R.A., YELLON D.M., LATCHMAN D.S. Urocortin protects against ischemic and reperfusion injury via a MAPK-dependent pathway. J. Biol. Chem. 2000;275:8508–8514. doi: 10.1074/jbc.275.12.8508. [DOI] [PubMed] [Google Scholar]
- BRAR B.K., STEPHANOU A., OKOSI A., LAWRENCE K.M., KNIGHT R.A., MARBER M.S., LATCHMAN D.S. CRH-like peptides protect cardiac myocytes from lethal ischaemic injury. Mol. Cell. Endocrinol. 1999;158:55–63. doi: 10.1016/s0303-7207(99)00183-5. [DOI] [PubMed] [Google Scholar]
- CHAN F.L., CHOI H.L. Proteoglycans associated with the ciliary zonule of the rat eye: a histochemical and immunocytochemical study. Histochem. Cell Biol. 1995;104:369–381. doi: 10.1007/BF01458131. [DOI] [PubMed] [Google Scholar]
- CHEN R, LEWIS K.A., PERRIN M.H., VALE W.W. Expression cloning of a human corticotropin-releasing-factor receptor. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8967–8971. doi: 10.1073/pnas.90.19.8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLIFTON V.L., READ M.A., LEITCH I.M., BOURA A.L., ROBINSON P.J., SMITH R. Corticotropin-releasing hormone-induced vasodilatation in the human fetal placental circulation. J. Clin. Endocrinol. Metab. 1994;79:666–669. doi: 10.1210/jcem.79.2.8045990. [DOI] [PubMed] [Google Scholar]
- CLIFTON V.L., READ M.A., LEITCH I.M., GILES W.B., BOURA A.L., ROBINSON P.J., SMITH R. Corticotropin-releasing hormone-induced vasodilatation in the human fetal-placental circulation: involvement of the nitric oxide-cyclic guanosine 3′,5′-monophosphate-mediated pathway. J. Clin. Endocrinol. Metab. 1995;80:2888–2893. doi: 10.1210/jcem.80.10.7559870. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- ERSPAMER V., ERSPAMER G.F., IMPROTA G., NEGRI L D.E., CASTIGLIONE R. Sauvagine, a new polypeptide from Phyllomedusa sauvagei skin. Occurrence in various Phyllomedusa species and pharmacological actions on rat blood pressure and diuresis. Naunyn Schmiedebergs Arch. Pharmacol. 1980;312:265–270. doi: 10.1007/BF00499156. [DOI] [PubMed] [Google Scholar]
- GRUNT M., GLASER J., SCHMIDHUBER H., PAUSCHINGER P., BORN J. Effects of corticotropin-releasing factor on isolated rat heart activity. Am. J. Physiol. 1993;264:H1124–H1129. doi: 10.1152/ajpheart.1993.264.4.H1124. [DOI] [PubMed] [Google Scholar]
- HELDWEIN K.A., REDICK D.L., RITTENBERG M.B., CLAYCOMB W.C., STENZEL-POORE M.P. Corticotropin-releasing hormone receptor expression and functional coupling in neonatal cardiac myocytes and AT-1 cells. Endocrinology. 1996;137:3631–3639. doi: 10.1210/endo.137.9.8756527. [DOI] [PubMed] [Google Scholar]
- HUANG Y. Hydroxylamine-induced relaxation inhibited by K+ channel blockers in rat aortic rings. Eur. J. Pharmacol. 1998;349:53–60. doi: 10.1016/s0014-2999(98)00178-2. [DOI] [PubMed] [Google Scholar]
- IKEDA K., TOJO K., SATO S., EBISAWA T., TOKUDOME G., HOSOYA T., HARADA M., NAKAGAWA O., NAKAO K. Urocortin, a newly identified corticotropin-releasing factor-related mammalian peptide, stimulates atrial natriuretic peptide and brain natriuretic peptide secretions from neonatal rat cardiomyocytes. Biochem. Biophys. Res. Commun. 1998;250:298–304. doi: 10.1006/bbrc.1998.9297. [DOI] [PubMed] [Google Scholar]
- JAIN V., VEDERNIKOV Y.P., SAADE G.R., CHWALISZ K., GARFIELD R.E. The relaxation responses to corticotropin-releasing factor in rat aorta are endothelium dependent and gestationally regulated. Am. J. Obstet. Gynecol. 1997;176:234–240. doi: 10.1016/s0002-9378(97)80042-7. [DOI] [PubMed] [Google Scholar]
- JAIN V., VEDERNIKOV Y.P., SAADE G.R., CHWALISZ K., GARFIELD R.E. Endothelium-dependent and -independent mechanisms of vasorelaxation by corticotropin-releasing factor in pregnant rat uterine artery. J. Pharmacol. Exp. Ther. 1999;288:407–413. [PubMed] [Google Scholar]
- JIN W., KLEM A.M., LEWIS J.H., LU Z. Mechanisms of inward-rectifier K+ channel inhibition by tertiapin-Q. Biochemistry. 1999;38:14294–14301. doi: 10.1021/bi991206j. [DOI] [PubMed] [Google Scholar]
- KISHIMOTO T., PEARSE R.V., II, LIN C.R., ROSENFELD M.G.A. Sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc. Natl. Acad. Sci. U.S.A. 1995;92:1108–1112. doi: 10.1073/pnas.92.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KITAMURA H., YOKOYAMA M., AKITA H., MATSUSHITA K., KURACHI Y., YAMADA M. Tertiapin potently and selectively blocks muscarinic K+ channels in rabbit cardiac myocytes. J. Pharmacol. Exp. Ther. 2000;293:196–205. [PubMed] [Google Scholar]
- KUBLER A., ROTHACHER G., KNAPPERTZ V.A., KRAMER G., NINK M., BEYER J., LEHNERT H. Intra- and extracerebral blood flow changes and flushing after intravenous injection of human corticotropin-releasing hormone. Clin. Invest. 1994;72:331–336. doi: 10.1007/BF00252822. [DOI] [PubMed] [Google Scholar]
- LEI S., RICHTER R, BIENERT M., MULVANY M.J. Relaxing actions of corticotropin-releasing factor on rat resistance arteries. Br. J. Pharmacol. 1993;108:941–947. doi: 10.1111/j.1476-5381.1993.tb13490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEITCH I.M., BOURA A.L., BOTTI C., READ M.A., WALTERS W.A., SMITH R. Vasodilator actions of urocortin and related peptides in the human perfused placenta in vitro. J. Clin. Endocrinol. Metab. 1998;83:4510–4513. doi: 10.1210/jcem.83.12.5356. [DOI] [PubMed] [Google Scholar]
- LENZ H.J., FISHER L.A., VALE W.W., BROWN M.R. Corticotropin-releasing factor, sauvagine, and urotensin I: effects on blood flow. Am. J. Physiol. 1985;249:R85–R90. doi: 10.1152/ajpregu.1985.249.1.R85. [DOI] [PubMed] [Google Scholar]
- LOVENBERG T.W., CHALMERS D.T., LIU C., DE SOUZA E.B. CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology. 1995a;136:4139–4142. doi: 10.1210/endo.136.9.7544278. [DOI] [PubMed] [Google Scholar]
- LOVENBERG T.W., LIAW C.W., GRIGORIADIS D.E., CLEVENGER W., CHALMERS D.T., DE SOUZA E.B., OLTERSDORF T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. U.S.A. 1995b;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MISTRY D.K., GARLAND C.J. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BKCa) in smooth muscle cells isolated from the rat mesenteric artery. Br. J. Pharmacol. 1998;124:1131–1140. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIKIMI T., MIYATA A., HORIO T., YOSHIHARA F., NAGAYA N., TAKISHITA S., YUTANI C., MATSUO H., MATSUOKA H., KANGAWA K. Urocortin, a member of the corticotropin-releasing factor family, in normal and diseased heart. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H3031–H3039. doi: 10.1152/ajpheart.2000.279.6.H3031. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- ONOUE H., KATUSIC Z.S. Role of potassium channels in relaxations of canine middle cerebral arteries induced by nitric oxide donors. Stroke. 1997;28:1264–1270. doi: 10.1161/01.str.28.6.1264. [DOI] [PubMed] [Google Scholar]
- PARKES D.G., VAUGHAN J., RIVIER J., VALE W., MAY C.N. Cardiac inotropic actions of urocortin in conscious sheep. Am. J. Physiol. 1997;272:H2115–H2122. doi: 10.1152/ajpheart.1997.272.5.H2115. [DOI] [PubMed] [Google Scholar]
- PEARSON D., SHIVELY J.E., CLARK B.R., GESCHWIND II., BARKLEY M., NISHIOKA R.S., BERN H.A. Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc. Natl. Acad. Sci. U.S.A. 1980;77:5021–5024. doi: 10.1073/pnas.77.8.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRIN M., DONALDSON C., CHEN R, BLOUNT A., BERGGREN T., BILEZIKJIAN L., SAWCHENKO P., VALE W. Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc. Natl. Acad. Sci. U.S.A. 1995;92:2969–2973. doi: 10.1073/pnas.92.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETKOVA-KIROVA P.S., GAGOV H.S., DURIDANOVA D.B. Urocortin hyperpolarizes stomach smooth muscle via activation of Ca2+-sensitive K+ currents. J. Muscle Res. Cell Motil. 2000;21:639–645. doi: 10.1023/a:1005653218639. [DOI] [PubMed] [Google Scholar]
- POTTER E., SUTTON S., DONALDSON C., CHEN R, PERRIN M., LEWIS K., SAWCHENKO P.E., VALE W. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc. Natl. Acad. Sci. U.S.A. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYES T.M., LEWIS K., PERRIN M.H., KUNITAKE K.S., VAUGHAN J., ARIAS C.A., HOGENESCH J.B., GULYAS J., RIVIER J., VALE W.W., SAWCHENKO PE. Urocortin II: A member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHTER R.M., MULVANY M.J. Comparison of hCRF and oCRF effects on cardiovascular responses after central, peripheral, and in vitro application. Peptides. 1995;16:843–849. doi: 10.1016/0196-9781(95)00035-i. [DOI] [PubMed] [Google Scholar]
- ROBERTSON B.E., BONEV A.D., NELSON M.T. Inward rectifier K+ currents in smooth muscle cells from rat coronary arteries: block by Mg2+, Ca2+, and Ba2+ Am. J. Physiol. 1996;271:H696–H705. doi: 10.1152/ajpheart.1996.271.2.H696. [DOI] [PubMed] [Google Scholar]
- SCHILLING L., KANZLER C., SCHMIEDEK P., EHRENREICH H. Characterization of the relaxant action of urocortin, a new peptide related to corticotropin-releasing factor in the rat isolated basilar artery. Br. J. Pharmacol. 1998;125:1164–1171. doi: 10.1038/sj.bjp.0702182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANDEN N.B., QUAYLE J.M., DAVIES N.W., BRAYDEN J.E., HUANG Y., NELSON M.T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- STENZEL P., KESTERSON R, YEUNG W., CONE R.D., RITTENBERG M.B., STENZEL-POORE M.P. Identification of a novel murine receptor for corticotropin-releasing hormone expressed in the heart. Mol. Endocrinol. 1995;9:637–645. doi: 10.1210/mend.9.5.7565810. [DOI] [PubMed] [Google Scholar]
- TERUI K., HIGASHIYAMA A., HORIBA N., FURUKAWA K.I., MOTOMURA S., SUDA T. Coronary vasodilation and positive inotropism by urocortin in the isolated rat heart. J. Endocrinol. 2001;169:177–183. doi: 10.1677/joe.0.1690177. [DOI] [PubMed] [Google Scholar]
- VALE W., SPIESS J., RIVIER C., RIVIER J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- VAUGHAN J., DONALDSON C., BITTENCOURT J., PERRIN M.H., LEWIS K., SUTTON S., CHAN R, TURNBULL A.V., LOVEJOY D., RIVIER C., RIVIER I., SAWCHENKO P.E., VALE W. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- WELLMAN G.C., BEVAN J.A. Barium inhibits the endothelium-dependent component of flow but not acetylcholine-induced relaxation in isolated rabbit cerebral arteries. J. Pharmacol. Exp. Ther. 1995;274:47–53. [PubMed] [Google Scholar]
- XU X., RIALS S.J., WU Y., MARINCHAK R.A., KOWEY P.R. The properties of the inward rectifier potassium currents in rabbit coronary arterial smooth muscle cells. Pflugers Arch. 1999;438:187–194. doi: 10.1007/s004240050897. [DOI] [PubMed] [Google Scholar]


