Abstract
In pithed rats, 5-HT mediates tachycardia both directly (by 5-HT2 receptors) and indirectly (by a tyramine-like effect). The receptor mediating tachycardia directly has been classified as an ‘atypical' 5-HT2 receptor since it was ‘weakly' blocked by ketanserin. Moreover, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), a 5-HT2 agonist, failed to mimic 5-HT-induced tachycardia. Since 5-HT2 receptors consist of 5-HT2A, 5-HT2B and 5-HT2C subtypes, this study investigated if these subtypes mediate the above response.
In pithed rats, intraperitoneally (i.p.) pre-treated with reserpine (5 mg kg−1), intravenous (i.v.) administration of 5-HT, 5-methoxytryptamine (5-MeO-T), 1-(3-chlorophenyl) piperazine (mCPP) and 5-carboxamidotryptamine (5-CT) (10, 30, 100 and 300 μg kg−1 each), produced dose-dependent tachycardic responses. Interestingly, DOI (10 – 1000 μg kg−1, i.v.) induced only slight, dose-unrelated, tachycardic responses, whilst the 5-HT2C agonist, Ro 60-0175 (10 – 1000 μg kg−1, i.v.), produced a slight tachycardia only at 300 and 1000 μg kg−1. In contrast, sumatriptan and 1-(m-trifluoromethylphenyl)- piperazine (TFMPP) were inactive. The rank order of potency was: 5-HT⩾5-MeO-T> mCPP⩾5-CT⩾DOI>Ro 60-0175.
The tachycardic responses to 5-HT, which remained unaffected after i.v. saline (0.3 and 1 ml kg−1) or propranolol (3 mg kg−1), were selectively blocked by the 5-HT2A antagonists ketanserin (30 and 100 μg kg−1) or spiperone (10 and 30 μg kg−1) as well as by the non-selective 5-HT2 antagonists, ritanserin (10 and 30 μg kg−1) or mesulergine (100 μg kg−1). Remarkably, these responses were unaffected by the antagonists rauwolscine (5-HT2B), SB204741 (5-HT2B/2C) or Ro 04-6790 (5-ht6) (300 and 1000 μg kg−1 each).
These results suggest that the ‘atypical' 5-HT2 receptors mediating tachycardia in reserpinized pithed rats are pharmacologically similar to the 5-HT2A receptor subtype.
Keywords: Ketanserin, 5-HT, 5-HT2A receptors, Ro 04-6790, Ro 60-0175, SB204741, spiperone, cardiac serotonin receptors
Introduction
Serotonin (5-hydroxytryptamine; 5-HT) elicits complex changes in the cardiovascular system of anaesthetized animals comprising bradycardia or tachycardia, hypotension or hypertension and vasodilatation or vasoconstriction (for review see Martin, 1994; Saxena & Villalón, 1990; 1991). In most species, bradycardia induced by 5-HT is mediated by 5-HT3 receptors, via the von Bezold Jarish reflex. In contrast, 5-HT-induced tachycardia is notoriously species-dependent and is mediated, directly or indirectly, either by 5-HT2 (dog), 5-HT3 (rabbit, dog), 5-HT4 (pig, human) and 5-HT7 (cat) receptors or by tyramine-like (guinea-pig) or unidentified mechanisms (Saxena, 1986; Saxena & Villalón, 1990; 1991; Villalón et al., 1997).
The tachycardia induced by 5-HT in the pithed rat has been reported to involve two main mechanisms: (i) a direct stimulation of 5-HT2 receptors at low doses (up to 100 μg kg−1, intravenously, (i.v.)) and (ii) an indirect sympathomimetic (tyramine-like) action at high doses, which can be reduced by desipramine (Göthert et al., 1986), propranolol (Docherty, 1988) or reserpine (Dabiré et al., 1992). The first mechanism was proposed on the basis that the tachycardia to 5-HT is antagonized by non-selective 5-HT2 receptor antagonists, including cyproheptadine (Saxena, 1986; Saxena & Villalón, 1990; 1991), mesulergine (Krstic & Katusic, 1994), LY53857 (Dabiré et al., 1992) or methiothepin (Dabiré et al., 1992; Krstic & Katusic, 1994), but is resistant to blockade by drugs affecting 5-HT1A (spiroxatrine; Dabiré et al., 1992), 5-HT1A/1B (pindolol; Dabiré et al., 1992), 5-HT3 (MDL72222; Dabiré et al., 1992; Krstic & Katusic, 1994), 5-HT4 (metoclopramide; Krstic & Katusic, 1994) and α-adrenoceptors (idazoxan; Dabiré et al., 1992). In addition, the tachycardic response to 5-HT is mimicked by the agonists 5-MeO-T (Göthert et al., 1986), α-methyl-5-HT and mCPP (Chaouché-teyara et al., 1993), but is not mimicked by the agonists 8-OH-DPAT (5-HT1A), RU-24969 (5-HT1A/1B), ipsapirone (5-HT1A), DOI (5-HT2) or 5-CT (5-HT1/7) (Dabiré et al., 1992; Docherty, 1988; Saxena & Lawang, 1985). However, the 5-HT2 receptors mediating this response have been considered ‘atypical' on the basis of: (i) the low antagonist potency of ketanserin, which did not match with its high affinity at 5-HT2 binding sites (see Table 1); and (ii) the weak tachycardic activity of the classical 5-HT2 receptor agonist, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI; see Table 1) (Dabiré et al., 1989; Chaouché-teyara et al., 1993).
Table 1.
Receptor binding affinity (pKi) of the drugs used in the present study at 5-HT2A, 5-HT2B, 5-HT2C, 5-ht5, 5-ht6 and 5-HT7 receptors
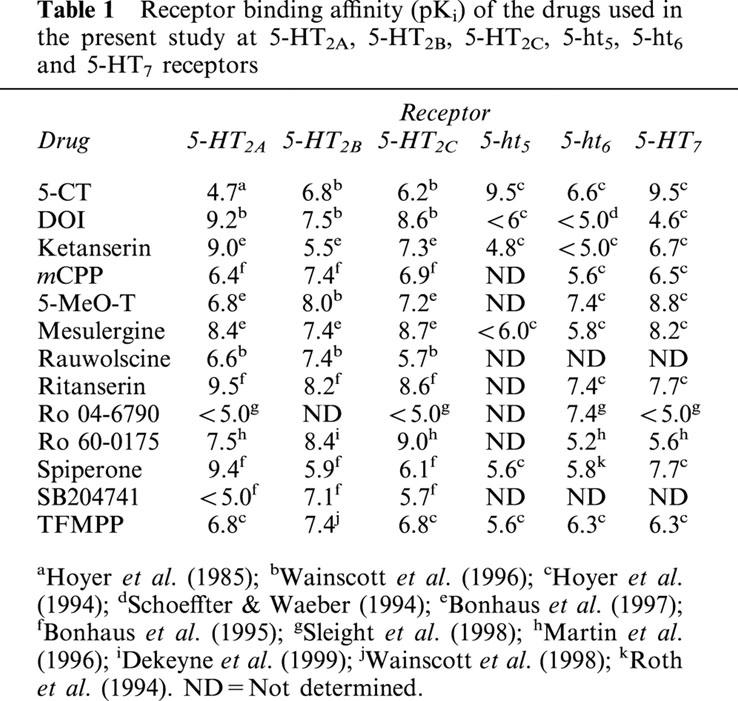
One of the main reasons for the above ‘discrepancy' in the low blocking potency of ketanserin may be that the indirect tyramine-like-component of 5-HT-induced tachycardia in rats could have overshadowed the blockade produced by ketanserin. Hence, it is reasonable to propose that the only way to unequivocally analyse the pharmacological properties of the 5-HT2 receptors involved would be, in principle, the use of catecholamine-depleted rats. Moreover, it is nowadays clear that the 5-HT2 receptor is, in fact, a heterogeneous family consisting of 5-HT2A, 5-HT2B and 5-HT2C receptor subtypes (Hoyer et al., 1994; Martin, 1994). In the light of these findings, the present study set out to further characterize the pharmacological profile of the receptors involved in the tachycardia induced by 5-HT in pithed rats, with particular emphasis on verifying the possible involvement of 5-HT2A, 5-HT2B and/or 5-HT2C receptor subtypes. For this purpose, we made use of rats systematically pre-treated with reserpine to exclude 5-HT-induced indirect mechanisms mediated via the release of catecholamines.
Methods
Animals
Male Wistar normotensive rats (250 – 300 g) were used at 12 – 14 weeks of age. The animals were maintained at a 12 h light – dark cycle, with light beginning at 0700 h. The rats were kept in a special room at constant temperature (22±2°C) and humidity (50%), with food and water freely available in their home cages.
General methods
Experiments were carried out in a total of 85 rats which were divided into two groups (n=74 and 11, respectively).
The first group (n=74) was systematically pre-treated with reserpine (5 mg kg−1) intraperitoneally (i.p.) 19 – 24 h before the experiments, in order to eliminate the tyramine-like action exerted by 5-HT. The second group (n=11) was pre-treated with equivalent volumes of the corresponding vehicle (4% acetic acid, i.p.) following the same schedule (sham group). After anaesthesia with ether and cannulation of the trachea, all rats were pithed by inserting a stainless steel rod through the orbit and foramen magnum into the vertebral foramen (Shipley & Tilden, 1947). The animals were artificially ventilated with room air using an Ideal Palmer pump at 56 strokes min−1 and a stroke volume of 20 ml kg−1, as previously established by Kleinman & Radford (1964). After bilateral vagotomy, catheters were placed in the left and right femoral veins, for the infusion of agonists and for the administration of antagonists respectively, and the left carotid artery, connected to a Statham pressure transducer (P23 ID), for the recording of arterial blood pressure. Heart rate was measured with a tachograph (7P4F, Grass Instrument Co., Quincy, MA, U.S.A.) triggered from the blood pressure signal. Both blood pressure and heart rate were recorded simultaneously by a model 7D Grass polygraph (Grass Instrument Co., Quincy, MA, U.S.A.). Prior to the administration of the agonists, the animals received a single dose of atropine (1.5 mg kg−1, i.p.) as previously reported (Göthert et al., 1986), in order to avoid tracheal secretions.
Experimental protocol
After a stable haemodynamic condition had been observed for at least 30 min, baseline values of mean blood pressure and heart rate were determined. Then, the first group (n=74) was subdivided into five subgroups.
The first subgroup (n=47) received i.v. bolus injections of 5-HT (10, 30, 100 and 300 μg kg−1, given sequentially). At this point, this subgroup was subdivided into nine sets which were treated, using an i.v. cumulative dose schedule, with either: (i) physiological saline (0.3 and 1.0 ml kg−1, n=6); (ii) ritanserin (10 and 30 μg kg−1, n=4); (iii) ketanserin (30 and 100 μg kg−1, n=6); (iv) spiperone (10 and 30 μg kg−1, n=4); (v) rauwolscine (300 and 1000 μg kg−1, n=5); (vi) SB204741 (300 and 1000 μg kg−1, n=5); (vii) Ro 04-6790 (300 and 1000 μg kg−1, n=7); (viii) mesulergine (100 μg kg−1, n=6); or (ix) propranolol (n=4). Subsequently, the responses to the above doses of 5-HT were re-analysed 10 min after each dose of the aforementioned compounds. At the end of these experiments, the rats received i.v. bolus injections of noradrenaline (0.1, 0.18, 0.3, 0.56 and 1.0 μg kg−1, given sequentially) in order to verify the specificity of blockade (if any) by the above compounds.
The second subgroup (n=11) also received i.v. bolus injections of 5-HT (10, 30, 100 and 300 μg kg−1, given sequentially). Then, this subgroup was subdivided into two sets which received sequential i.v. bolus injections of, respectively: (i) DOI (10, 30, 100, 300 and 1000 μg kg−1, n=5); and (ii) Ro 60-0175 (10, 30, 100, 300 and 1000 μg kg−1, n=6). Subsequently, the effects produced by the above doses of 5-HT were re-analysed 10 – 15 min after the last dose of DOI (cumulative dose: 1440 μg kg−1, n=5) or Ro 60-0175 (cumulative dose: 1440 μg kg−1, n=6). At the end of these experiments, the rats received i.v. bolus injections of noradrenaline as previously described for the first subgroup.
The third subgroup (n=5) received consecutive i.v. bolus injections of 5-CT, 5-MeO-T, TFMPP and sumatriptan (10, 30, 100 and 300 μg kg−1 each, given sequentially). The order of administration of these agonists was randomized.
The fourth subgroup (n=6) received sequential i.v. bolus injections of mCPP (10, 30, 100 and 300 μg kg−1).
The fifth subgroup (n=5) received sequential i.v. bolus injections of tyramine (10, 18, 31, 56 and 100 μg kg−1).
Finally, the second group (sham; n=11), which was pretreated with the vehicle of reserpine (4% acetic acid) received sequential i.v. bolus injections of 5-HT (10, 30, 100 and 300 μg kg−1; n=6) or tyramine (10, 18, 31, 56 and 100 μg kg−1; n=5).
The dose-intervals between the different doses of agonists ranged between 1 and 15 min (see Results section), as in each case we waited until the heart rate had returned to baseline values. For physiological saline or the antagonists, a period of 10 min was allowed to elapse before the dose-response curves for the agonists were elicited again. The dosing with 5-HT and the rest of agonists was sequential, whilst that for physiological saline or the antagonists was cumulative. The Ethical Committee of the CINVESTAV-IPN dealing with the use of animals in scientific experiments approved the protocols of the present investigation.
Drugs
Apart from the anaesthetic (diethyl ether), the drugs used in the present study (obtained from the sources indicated) were: 5-hydroxytryptamine creatinine sulphate, gallamine triethiodide, atropine sulphate, reserpine and noradrenaline bitartrate (Sigma Chemical Co., St. Louis, MO, U.S.A.); 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), 5-methoxytryptamine hydrochloride, 1-(3-chlorophenyl)-piperazine hydrochloride (mCPP), rauwolscine hydrochloride, ritanserin, spiperone hydrochloride and N-(3-trifluoromethylphenyl)piperazine hydrochloride (TFMPP) (Research Biochemicals Int., Natick, MA, U.S.A.); 5-carboxamidotryptamine maleate and sumatriptan succinate (gift: Glaxo Wellcome Research, Stenevage, U.K.); ketanserin (gift: Janssen Pharmaceutica Beerse, Belgium); N-(1-methyl-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea (SB204741; gift: SmithKline Beechman Pharmaceuticals, Harlow, Essex, U.K.); mesulergine hydrochloride (gift: Novartis, Basel, Switzerland); 4-amino-N-(2,6-bis-methylamino-pyridin-4-yl)-benzene sulfonamide (Ro 04-6790) and (S)-2-(chloro-5-fluoro-indol-1-yl)-1-methylethylamine (Ro 60-0175) (gift: Hoffmann-La Roche Ltd., Basel, Switzerland). All compounds were dissolved in distilled water. When needed, 2% (w v−1) ascorbic acid (ritanserin and ketanserin) or 4% (v v−1) acetic acid (reserpine) was added; these vehicles had no effect on mean blood pressure or heart rate. The doses mentioned in the text refer to the free base of substances except in the case of atropine, where it refers to the salt.
Data presentation and statistical analysis
All data in the text and figures are presented as mean±s.e.mean. The peak changes in heart rate produced by i.v. administration of the agonists were determined. The difference between the changes in heart rate within one subgroup of animals was evaluated with Student – Newman – Keuls' test, once an analysis of variance (randomized block design) had revealed that the samples represented different populations (Steel & Torrie, 1980). Moreover, the tachycardic responses to noradrenaline in the different subgroups were compared to those obtained in the saline (control) group by using Student's unpaired t-test. The latter procedure was also used to compare: (i) the tachycardic responses elicited by tyramine or 5-HT in the fifth subgroup and the second (sham) group; and (ii) the effects of saline to those produced by the antagonists on baseline heart rate and mean blood pressure. Statistical significance was accepted at P<0.05 (2-tailed).
Results
Systemic haemodynamic variables
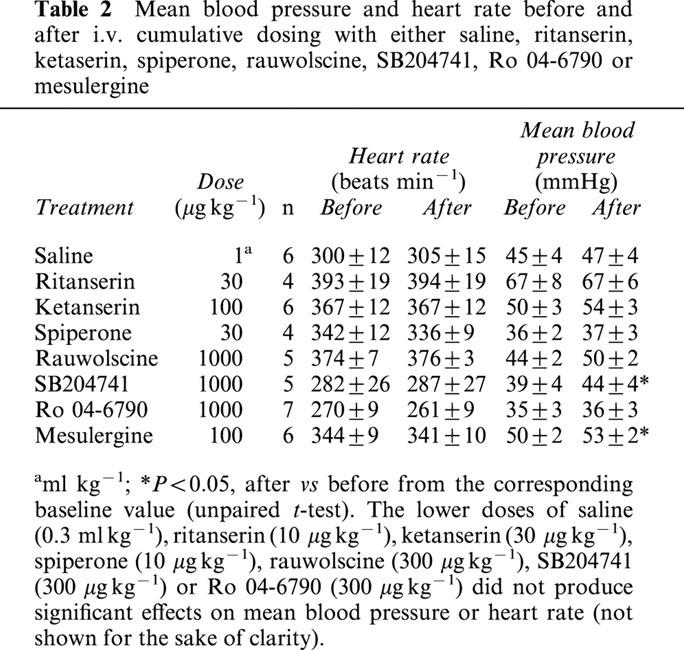
The baseline values of mean blood pressure and heart rate in the 74 pithed rats pretreated with reserpine were, respectively, 54±1 mmHg and 331±6 beats min−1. In animals without reserpine (n=11) the baseline values of the aforementioned variables were, respectively, 60±2 mmHg and 282±11 beats min−1. Although there were statistically significant differences in baseline blood pressure and heart rate between both groups, we do not have a biological explanation for these findings. These haemodynamic variables remained essentially unchanged after two i.v. bolus injections of physiological saline (see Table 2), indicating that no time-dependent changes occurred during the experimental period (210 min) in the animal model used here. Table 2 also shows that the above variables were not significantly changed (P>0.05) after administration of ritanserin, ketanserin, spiperone, rauwolscine or Ro 04-6790; only the highest dose of SB204741 or mesulergine produced a slight (although not biologically relevant), but significant, increase in mean blood pressure.
Table 2.
Mean blood pressure and heart rate before and after i.v. cumulative dosing with either saline, ritanserin, ketaserin, spiperone, rauwolscine, SB204741, Ro 04-6790 or mesulergine
Effects of 5-HT or tyramine on heart rate and blood pressure of vehicle- or reserpine-pretreated rats
The pretreatment schedule with reserpine (5 mg kg−1, i.p., 19 – 24 h before the experiments) used in the present study was validated by investigating the effects of the classical indirect sympathomimetic agent, tyramine (10, 18, 31, 56 and 100 μg kg−1) on mean blood pressure and heart rate in vehicle (4% acetic acid; sham group) and reserpine-pretreated (fifth subgroup) rats. As shown in Figure 1a, reserpine practically abolished the vasopressor and tachycardic responses induced by tyramine when compared with the group pretreated with vehicle (unpaired t-test).
Figure 1.
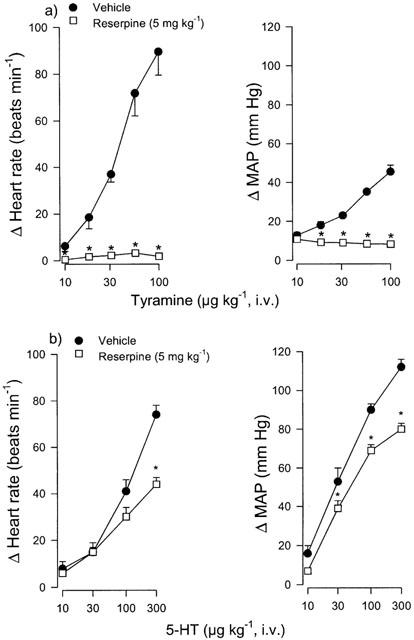
Effect of i.p. administration of the vehicle (acetic acid) or reserpine (5 mg kg−1; 19 – 24 h) on the increases in heart rate and mean arterial blood pressure (MAP) induced by i.v. bolus injections of: (a) Tyramine (n=5; both groups) or (b) 5-HT (n=6; both groups) in pithed rats. *P<0.05 vs vehicle. Each point represents the mean±s.e.mean.
In addition, the reserpine-pretreatment significantly blocked the vasopressor responses to 30, 100 and 300 μg kg−1 of 5-HT (Figure 1b), whilst the tachycardic responses to 5-HT were only significantly blocked at the highest dose of 5-HT (300 μg kg−1) when compared with the group pretreated with vehicle.
Effect of propranolol on the tachycardic and vasopressor responses induced by 5-HT in rats pretreated with reserpine
As expected in reserpine-pretreated rats, the tachycardic responses to 5-HT (9±2, 16±2, 33±6 and 42±5 beats min−1; n=4) were not significantly modified after 3 mg kg−1 propranolol (6±1, 16±2, 30±3 and 40±3 beats min−1; n=4) at the doses of 10, 30, 100 and 300 μg kg−1 of 5-HT. A similar lack of effect of propranolol was observed on 5-HT-induced vasopressor responses (not shown).
Initial effects of 5-HT and some agonists on heart rate and blood pressure
Figure 2 shows that i.v. administration of 5-HT, 5-MeO-T, mCPP and 5-CT (10 – 300 μg kg−1 each) produced, in decreasing order of potency, dose-dependent increases in heart rate. Interestingly, DOI (10 – 1000 μg kg−1) produced only slight, though not dose-dependent, increases in heart rate (significant only at the doses of 30, 100 and 300 μg kg−1), whilst the 5-HT2C receptor agonist, Ro 60-0175 (10 – 1000 μg kg−1; see Table 1), produced a slight, but significant, tachycardia only at 300 and 1000 μg kg−1.
Figure 2.
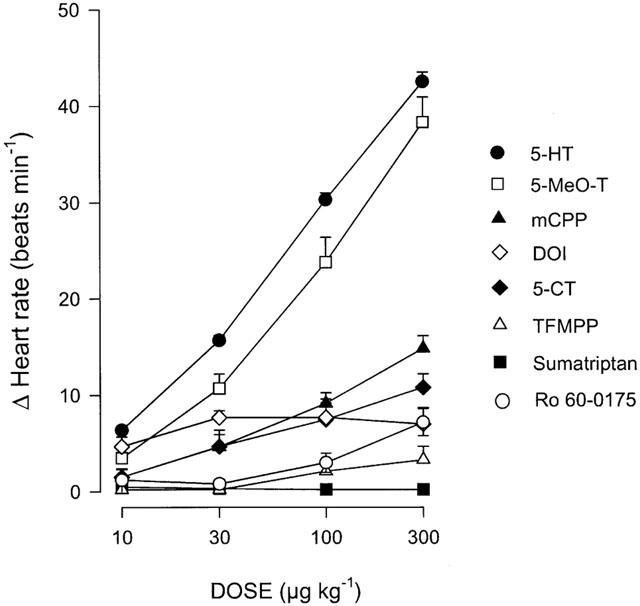
Effect of sequential i.v. bolus injections of 5-HT (n=58), 5-MeO-T (n=5), mCPP (n=6), DOI (n=5), 5-CT (n=5), TFMPP (n=5), sumatriptan (n=5) or Ro 60-0175 (n=6) on heart rate in reserpinized (5 mg kg−1), atropine-pretreated (1.5 mg kg−1) pithed rats. Each point represents the mean±s.e.mean.
In contrast, sumatriptan and 1-(m-trifluoromethylphenyl)-piperazine (TFMPP) were virtually inactive at the doses used. The apparent rank order of agonist potency to produce tachycardia was: 5-HT⩾5-MeO-T> mCPP⩾5-CT⩾DOI>Ro 60-0175.
At the doses used, the duration of the tachycardic responses to DOI (6±1, 9±2, 9±1, 11±2 and 16±1 min; n=5) was longer than that of 5-CT (2±1, 4±1, 8±1 and 8±1 min; n=5), 5-HT (2±1, 4±1, 5±1 and 7±1 min; n=52), 5-MeO-T (2±1, 3±1, 4±1 and 5±1 min; n=5), mCPP (1±1, 2±1, 3±1 and 4±1 min; n=6) or Ro 60-0175 (1±1, 1±1, 4±1, 7±1 and 10±1 min, n=6).
The above tachycardic responses were accompanied by dose-dependent increases in blood pressure. These vasopressor responses induced by the above agonists were: 5-HT (15±1, 44±2, 73±2 and 85±2 mmHg; n=52); 5-MeO-T (7±1, 24±5, 61±6 and 73±7 mmHg; n=50); mCPP (13±2, 18±3, 37±5 and 43±5 mmHg; n=6); 5-CT (4±1, 4±1, 7±2 and 14±3 mmHg, n=5) and Ro 60-0175 (4±1, 5±1, 10±1, 19±2 and 34±6 mmHg, n=6). It is noteworthy that, unlike their capability to produce tachycardia, the same doses of DOI and TFMPP produced significant increases in mean blood pressure: DOI (23±3, 32±4, 29±5, 25±2 and 25±4 mmHg; n=5); TFMPP (5±1, 7±2, 12±3 and 14±4 mmHg; n=5). In contrast, sumatriptan failed to significantly increase this variable (4±1, 5±1, 4±1 and 5±1 mmHg; n=5). The onset of the tachycardic and vasopressor responses induced by the above agonists was immediate.
Potential antagonist properties of DOI and Ro 60-0175
In view that DOI and the selective 5-HT2C receptor agonist, Ro 60-0175 (see Table 1), apparently behaved as weak agonists, we decided to investigate these compounds as potential antagonists of the tachycardic and vasopressor responses to 5-HT. Thus, in control animals where 5-HT had been administered before and after the last set of injections of DOI (10, 30, 100, 300 and 1000 μg kg−1), the tachycardic responses induced by 5-HT (10, 30, 100 and 300 μg kg−1) were significantly antagonized (*P<0.05) after DOI (total dose: 1440 μg kg−1) (7±1, 16±3, 34±2 and 42±4 beats min−1 before and 3±1*, 4±1*, 12±3* and 20±4* beats min−1 after DOI (n=5), respectively). Similarly, the corresponding vasopressor responses to 5-HT were significantly antagonized after DOI (20±5, 56±7, 80±8 and 93±2 mmHg before and 14±2*, 25±3*, 51±6* and 64±6* mmHg after DOI (n=5), respectively). In contrast, after Ro 60-0175 (10, 30, 100, 300 and 1000 μg kg−1; total dose: 1440 μg kg−1), the tachycardic and vasopressor responses to 5-HT remained unaffected (data not shown).
Effect of physiological saline or some 5-HT receptor antagonists on the increases in heart rate induced by 5-HT
The effects of physiological saline, ritanserin, ketanserin or spiperone on the tachycardic responses induced by 5-HT are depicted in Figure 3. No evidence of tachyphylaxis was observed as the responses to this tryptamine, at the doses and time intervals used here, were reproducible and remained essentially unchanged in control animals receiving two subsequent doses of physiological saline (0.3 and 1 ml kg−1, i.v.) (Figure 3a). Interestingly, low doses of the antagonists with high affinity for 5-HT2A receptors, ritanserin (10 and 30 μg kg−1), ketanserin (30 and 100 μg kg−1) and spiperone (10 and 30 μg kg−1) (see Table 1), produced a dose-dependent blockade of the tachycardic responses to 5-HT (Figure 3b, c and d, respectively). In contrast, Figure 4 shows that the responses to 5-HT remained unaltered after rauwolscine, the 5-HT2B receptor antagonist, SB204741, or the selective 5-ht6 receptor antagonist, Ro 04-6790 (300 and 1000 μg kg−1 each) (Figure 4a, b and c, respectively), but were significantly blocked by mesulergine (100 μg kg−1, see Figure 4d), a 5-HT2A/2B/2C and 5-HT7 receptor ligand (see Table 1).
Figure 3.
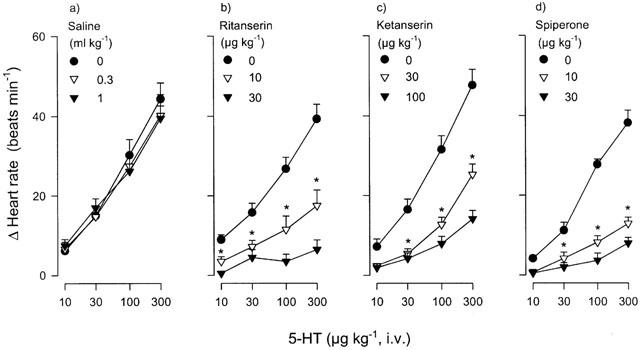
Effect of i.v. cumulative administration of: (a) saline (n=6), (b) ritanserin (n=4), (c) ketanserin (n=6) or (d) spiperone (n=4) on the increases in heart rate induced by i.v. bolus injections of 5-HT in reserpinized (5 mg kg−1), atropine-pretreated (1.5 mg kg−1) pithed rats. Each point represents the mean±s.e.mean. *P<0.05 vs control. All the other points after the starred (*) graph are also significantly different from the control response.
Figure 4.
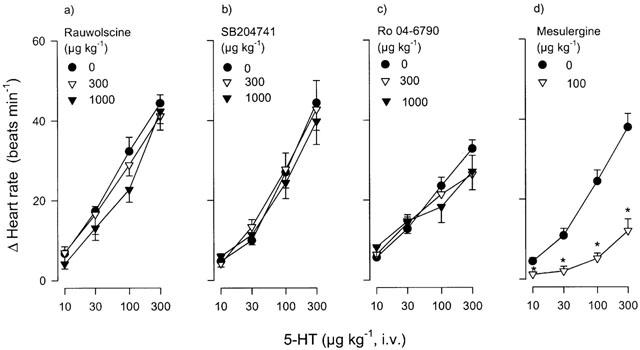
Effect of i.v. cumulative administration of: (a) rauwolscine (n=5), (b) SB204741 (n=5), (c) Ro 04-6790 (n=7) or (d) mesulergine (n=6) on the increases in heart rate induced by i.v. bolus injections of 5-HT in reserpinized (5 mg kg−1), atropine-pretreated (1.5 mg kg−1) pithed rats. *P<0.05 vs control. Each point represents the mean±s.e.mean.
It should be emphasized that ketanserin blocked, with a similar potency, the vasopressor responses to 5-HT (a 5-HT2A receptors-mediated response). Indeed, the vasopressor responses to 5-HT (10, 30, 100 and 300 μg kg−1) before ketanserin (10±2, 34±5, 70±5 and 82±5 mmHg) were significantly blocked after 10 μg kg−1 (6±1, 8±1*, 20±3* and 34±4* mmHg) or 30 μg kg−1 (6±1, 7±1*, 12±2* and 19±3* mmHg) of ketanserin, (*P<0.05 vs the first dose-response curve to 5-HT).
Effect of physiological saline or 5-HT receptor ligands on the increases in heart rate induced by noradrenaline
It must be emphasised that the blockade produced by the above compounds (i.e. DOI, ritanserin, ketanserin, spiperone and mesulergine) on the tachycardic responses to 5-HT was selective, as the tachycardic responses elicited by noradrenaline in saline-treated animals did not significantly differ (P>0.05) from those elicited in the animals that had previously received the corresponding agonist (DOI or Ro 60-0175) or antagonist (ritanserin, ketanserin, spiperone, rauwolscine, SB204741, mesulergine or Ro 04-6790) (see Figure 5).
Figure 5.
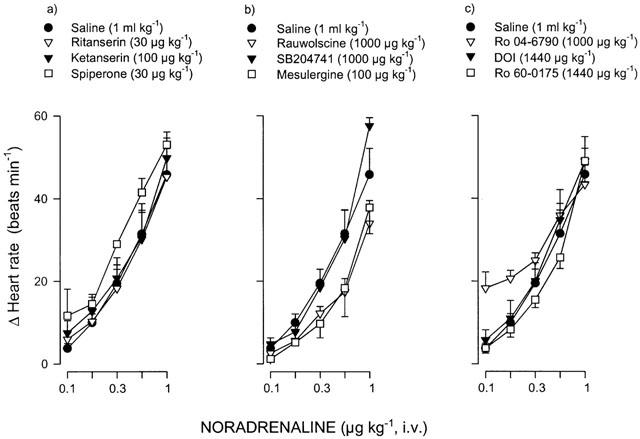
Effects of i.v. saline (1 ml kg−1; n=6) or a number of drugs acting on 5-HT receptors on the increases in heart rate induced by i.v. bolus injections noradrenaline in reserpinized (5 mg kg−1), atropine-pretreated (1.5 mg kg−1) pithed rats. The i.v. cumulative doses of the various drugs used were: (a) ritanserin (30 μg kg−1; n=4), ketanserin (100 μg kg−1; n=6) or spiperone (30 μg kg−1; n=4); (b) rauwolscine (1000 μg kg−1; n=5), SB204741 (1000 μg kg−1; n=5) or mesulergine (100 μg kg−1; n=6); and (c) Ro 04-6790 (1000 μg kg−1; n=6), DOI (1440 μg kg−1; n=5) or Ro 60-0175 (1440 μg kg−1; n=7). Each point represents the mean±s.e.mean. P>0.05 vs saline (with each drug).
Discussion
General
As previously considered, the tachycardia induced by 5-HT in rats has been reported to involve ‘atypical' 5-HT2 receptors and an indirect mechanism via the release of catecholamines from sympathetic neurons (see Introduction section). The objective of the present study was 2 fold: (i) to analyse whether the apparently lower potency of ketanserin to block the tachycardic responses to 5-HT was due to an interference from the tyramine-like action; and (ii) to investigate in detail the involvement of the 5-HT2 receptor subtypes mediating 5-HT-induced tachycardia. With these objectives in mind, the rats of the present study were systematically pretreated with reserpine.
The main findings of this study were that 5-HT produced tachycardic responses in pithed rats pretreated with reserpine and atropine, which were: (i) unaffected by saline (0.3 and 1 ml kg−1), rauwolscine (300 and 1000 μg kg−1), SB204741 (300 and 1000 μg kg−1) or Ro 04-6790 (300 and 1000 μg kg−1); and (ii) significantly and dose-dependently antagonized by ritanserin (10 and 30 μg kg−1), ketanserin (30 and 100 μg kg−1), spiperone or mesulergine (100 μg kg−1 each) (see Figures 2 and 3). The above blockade was selective since the tachycardic responses to noradrenaline were not blocked by the above antagonists (Figure 5). Apart from the implications discussed below, these results suggest that the 5-HT receptor mediating the tachycardic responses to 5-HT closely resembles the 5-HT2A receptor subtype.
Blockade of the tyramine-like action of the tachycardic responses to 5-HT in pithed rats: an experimental condition required to pharmacologically identify the 5-HT receptors involved
Since 5-HT can produce increases in heart rate by a tyramine-like action in pithed rats (Dabiré et al., 1992; Göthert et al., 1986), we decided to exclude this component by systematically pre-treating the rats with reserpine (5 mg kg−1 i.p., 19 – 24 h before the experiments). Indeed, this indirect mechanism of 5-HT was blocked by reserpine (Figure 1b), as previously shown by Dabiré et al. (1992). Furthermore, the vasopressor effects of 5-HT were significantly blocked, possibly because of the blockade of an indirect mechanism activated by 5-HT as well. The dose of reserpine was high enough to abolish the tyramine-induced increases in heart rate in pithed rats (Figure 1a) but not those induced by noradrenaline (see above). Under these experimental conditions, no time-dependent changes were observed since 5-HT produced reproducible tachycardic responses in pithed rats after saline (Figure 3a). These responses were not blocked by propranolol (see Results section) at a dose (3 mg kg−1) that abolished the noradrenaline-induced tachycardic effects (unpublished results); this finding suggests that the above dose-schedule of reserpine, indeed, completely eliminated the indirect (tyramine-like) component of the responses to 5-HT.
Systemic haemodynamic changes
The fact that administration of two subsequent i.v. bolus injections of physiological saline produced no significant changes in baseline mean blood pressure or heart rate (Table 2) suggests that no time-dependent changes occurred in our experiments. Furthermore, our findings showing that the above variables remained unchanged after ritanserin, ketanserin, spiperone, rauwolscine or Ro 04-6790 (Table 2) are particularly relevant. Thus, any effect of a given antagonist on the tachycardic responses to 5-HT ought to be explained in terms of a direct interaction of the antagonist with its respective receptor and not in terms of a change in baseline systemic haemodynamic values. In this respect neither did SB204741 or mesulergine significantly modify baseline heart rate; however, it is admittedly difficult to explain the slight, though significant, increase in mean blood pressure produced by these two drugs (see Table 2). In any case, this effect seems to be of little biological relevance within the context of the present study.
Rank order of agonist potency of the 5-HT receptor agonists
The rank order of agonist potency, which was 5-HT⩾5-MeO-T>mCPP⩾5-CT⩾DOI, may suggest in its own right, but does not categorically prove, that 5-HT2A receptors could be involved since: (i) 5-MeO-T, a 5-HT2 receptor agonist, mimicked the tachycardic effect of 5-HT; (ii) 5-CT and mCPP, which have low affinity for 5-HT2A receptors (Table 1), weakly mimicked the positive chronotropic effect of 5-HT (see Figure 2); and (iii) TFMPP, which has low affinity for 5-HT2A receptors, did not produce changes in heart rate. Taken together, the above rank order of agonist potency, with the exception of DOI, correlates in principle with the binding affinity for the 5-HT2A receptor. This notion, indeed, was reinforced with the results obtained using more selective antagonists (see below). Although DOI, a 5-HT2 receptor agonist, only produced slight increases in heart rate at high doses (see Figure 2), it should be pointed out that this drug significantly blocked the tachycardic responses to 5-HT (see Results section); this may suggest that DOI behaved as a partial agonist. In agreement with this line of reasoning, the vasopressor responses induced by 5-HT, a response typically mediated by 5-HT2A receptors, were also significantly blocked by DOI. Therefore, the lack of correlation between the high affinity of DOI for 5-HT2A receptors (see Table 1) and its weak ability to stimulate tachycardic ‘5-HT2A receptors' in the rat (Figure 2) could most likely be explained in terms of DOI acting as a partial agonist. On the other hand, the weak tachycardic activity elicited by very high doses of Ro 60-0175 (see Figure 2), a selective 5-HT2C receptor agonist (see Table 1), does not support the possible involvement of 5-HT2C receptors. Most likely, this weak tachycardic activity induced by Ro 60-0175 may be explained by its moderate affinity (pKi: 7.5) at the 5-HT2A receptor subtype (see Table 1).
Lack of resemblance of the tachycardic 5-HT receptors with 5-HT1, 5-HT3, 5-HT4, 5-ht5, 5-ht6 and 5-HT7 receptors
The involvement of 5-HT1, 5-HT3, 5-HT4, 5-ht5, 5-ht6 and 5-HT7 receptors seems unlikely since: (i) sumatriptan, a 5-HT1B/1D/1F receptor agonist, failed to increase heart rate; (ii) 5-MeO-T, which does not interact with 5-HT3 receptors, increased the heart rate in a dose-dependent manner; (iii) ketanserin and spiperone, two antagonists with low affinity for 5-HT4, 5-ht5 and 5-ht6 receptors (Hoyer et al., 1994), potently blocked the 5-HT-induced increases in heart rate; (iv) methiothepin, which does not block 5-HT4 tachycardic receptors in the pig (Saxena & Villalón, 1990), abolished the tachycardic responses to 5-HT in the rat (Krstic & Katusic, 1994); (v) 5-CT, a potent agonist at tachycardic 5-HT7 receptors in the cat (Villalón et al., 1997), was a very weak agonist in the present study; (vi) DOI, which has very low affinity for 5-HT7 receptors (see Table 1), blocked the tachycardic effect of 5-HT. Although, admittedly, the rank order of agonist potency of 5-HT⩾5-MeO-T>mCPP⩾5-CT⩾DOI may suggest the involvement of 5-ht6 receptors (see Hoyer et al., 1994), this seems unlikely since spiperone, ketanserin and mesulergine, which have very low affinity for 5-ht6 receptors (see Table 1), potently blocked the responses to 5-HT. This suggestion is strengthened when considering that the selective 5-ht6 receptor antagonist, Ro 04-6790 (see Table 1), failed to antagonize the tachycardic responses to 5-HT (Figure 4c).
Resemblance of the receptors mediating tachycardia in pithed rats with the 5-HT2A subtype
Since the involvement of the 5-HT1 and 5-HT3 – 5-HT7 receptors seems improbable, the possibility has finally to be discussed that the 5-HT receptors mediating tachycardia in pithed reserpinized rats resemble the 5-HT2A receptor subtype. In principle, the fact that the 5-HT2 receptor agonists, 5-MeO-T (this study) and α-methyl-5-HT (Chaouché-teyara et al., 1993) produced dose-dependent increases in heart rate confirm the involvement of 5-HT2 receptors. This view is reinforced by the partial agonist properties displayed by the 5-HT2 receptor agonist, DOI (see above). Moreover, it had previously been shown that only high doses of ketanserin were capable of blocking the tachycardic responses to 5-HT in rats (Docherty, 1988; Saxena, 1986); however, these rats had not been depleted of catecholamines, nor had they been pithed. Subsequently, when using pithed rats (without reserpine-pretreatment), it was reported that ketanserin (at doses of 100 and 300 μg kg−1) did not antagonize the tachycardic responses to 5-HT (Chaouché-teyara et al., 1993; Dabiré et al., 1992; Göthert et al., 1986). This discrepancy may be explained on the basis that the blockade of the tachycardic responses to 5-HT could have been masked by a tyramine-like action. In keeping with this view, our results using pithed rats systematically pretreated with reserpine (5 mg kg−1) show that low doses of ketanserin (30 and 100 μg kg−1) specifically antagonized the tachycardic responses to 5-HT (Figure 3c). Considering the binding profile of ketanserin (see Table 1), it is tempting to suggest the involvement of 5-HT2A receptors. This suggestion is reinforced when considering that spiperone and ritanserin, which display very high affinity for 5-HT2A receptors (see Table 1), blocked the responses to 5-HT. Interestingly, the doses of the aforementioned compounds antagonized the increases in blood pressure induced by 5-HT (see results section), a response typically mediated by 5-HT2A receptors (Hoyer et al., 1994). The involvement of 5-HT2B and 5-HT2C receptors seems unlikely since: (i) rauwolscine, a 5-HT2B receptor antagonist (Wainscott et al., 1998) or SB204741, a 5-HT2B/2C receptor antagonist (Bonhaus et al., 1997), did not block the 5-HT-induced tachycardic responses; (ii) the 5-HT2C receptor agonist, Ro 60-0175 (Dekeyne et al., 1999), failed to produce increases in heart rate (Figure 2) or to block the tachycardic responses to 5-HT (see Results); (iii) ketanserin, which displays low affinity at the 5-HT2B receptor (see Table 1), antagonized the responses to 5-HT (Figure 3c); and (iv) spiperone, which has low affinity at 5-HT2B/2C receptors and very high affinity at 5-HT2A receptors (Bonhaus et al., 1995), potently antagonized the responses to 5-HT (Figure 3d).
In conclusion, the above findings show that the 5-HT receptors mediating the increases in heart rate induced by 5-HT in pithed rats pre-treated with reserpine closely resemble the 5-HT2A receptor subtype. Therefore, the present experimental model, which is not complicated by the presence of other 5-HT receptors, can be utilized to characterize and develop new drugs with potential agonist or antagonist properties at functional 5-HT2A receptors. The weak antagonism of ketanserin observed in previous studies without reserpine pretreatment (e.g. Docherty, 1988) could be explained in terms that 5-HT activates a concomitant tyramine-like action (to release noradrenaline from sympathetic neurons with stimulation of cardiac β adrenoceptors), which overshadows the potent blockade of 5-HT2A receptors.
Acknowledgments
The skilful technical assistance of Mr Arturo Contreras Bustos is acknowledged. The authors also thank CONACyT (México) and the pharmaceutical companies (see Drugs section) for their support.
Abbreviations
- 5-CT
5-carboxamidotryptamine
- DOI
1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane
- 5-HT
5-hydroxytryptamine
- mCPP
1-(m-chlorophenyl)piperazine
- 5-MeO-T
5-methoxytryptamine
- 8-OH-DPAT
8-hydroxy-2-(di-N-propylamino)tetralin
- Ro 04-6790
4-amino-N-(2,6-bis-methylamino-pyridin-4-yl)-benzene sulfonamide
- Ro-60-0175
(S)-2-(chloro-5-fluoro-indol-1-yl)-1-methylethylamine
- RU24969
5-methoxy-3(1,2,3,6-tetrahydro-4-pyridinyl)-1H-indole
- SB204741
N-(1-methyl-5-indolyl)-N′-(3-methyl-5-isothiazolyl)urea
- TFMPP
1-(m-trifluoromethylphenyl)piperazine
References
- BONHAUS D.W., BACH C., DESOUZA A., SALAZAR F.H., MATSUOKA B.D., ZUPPAN P., CHAN H.W., EGLEN R.M. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br. J. Pharmacol. 1995;115:622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONHAUS D.W., WEINHARDT K.K., TAYLOR M., DESOUZA A., MCNEELEY P.M., SZCZEPANSKI K., FONTANA D.J., TRINH J., ROCHA C.L., DAWSON M.W., FLIPPIN L.A., EGLEN R.M. RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:621–629. doi: 10.1016/s0028-3908(97)00049-x. [DOI] [PubMed] [Google Scholar]
- CHAOUCHÉ-TEYARA K., FOURNIER B., SAFAR M., DABIRÉ H. Vascular and cardiac effects of alpha-methyl-5-HT and DOI are mediated by different 5-HT receptors in the pithed rat. Eur. J. Pharmacol. 1993;250:67–75. doi: 10.1016/0014-2999(93)90622-o. [DOI] [PubMed] [Google Scholar]
- DABIRÉ H., CHAOUCHE-TEYARA K., CHERQUI C., FOURNIER B., LAUBIE M., SCHMITT H. Characterization of DOI, a putative 5-HT2 receptor agonist in the rat. Eur. J. Pharmacol. 1989;168:369–374. doi: 10.1016/0014-2999(89)90799-1. [DOI] [PubMed] [Google Scholar]
- DABIRÉ H., CHAOUCHE-TEYARA K., CHERQUI C., FOURNIER B., SCHMITT H. Pharmacological analysis of the cardiac effects of 5-HT and some 5-HT receptor agonists in the pithed rat. Fundam. Clin. Pharmacol. 1992;6:237–245. doi: 10.1111/j.1472-8206.1992.tb00116.x. [DOI] [PubMed] [Google Scholar]
- DEKEYNE A., GIRARDON S., MILLAN M.J. Discriminative stimulus properties of the novel serotonin (5-HT)2C receptor agonist, RO 60-0175: a pharmacological analysis. Neuropharmacology. 1999;38:415–423. doi: 10.1016/s0028-3908(98)00203-2. [DOI] [PubMed] [Google Scholar]
- DOCHERTY J.R. Investigations of cardiovascular 5-hydroxytryptamine receptor subtypes in the rat. Naunyn Schmiedeberg's Arch. Pharmacol. 1988;337:1–8. doi: 10.1007/BF00169468. [DOI] [PubMed] [Google Scholar]
- GÖTHERT M., SCHLICKER E., KOLLECKER P. Receptor-mediated effects of serotonin and 5-methoxytryptamine on noradrenaline release in the rat vena cava and in the heart of the pithed rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 1986;332:124–130. doi: 10.1007/BF00511401. [DOI] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA P.R., HUMPHREY P.P. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- HOYER D., ENGEL G., KALKMAN H.O. Molecular pharmacology of 5-HT1 and 5-HT2 recognition sites in rat and pig brain membranes: radioligand binding studies with [3H]5-HT, [3H]8- OH-DPAT, (−)[125I]iodocyanopindolol, [3H]mesulergine and [3H]ketanserin. Eur. J. Pharmacol. 1985;118:13–23. doi: 10.1016/0014-2999(85)90658-2. [DOI] [PubMed] [Google Scholar]
- KLEINMAN L.I., RADFORD E.P. Ventilation standards for small mammals. J. Appl. Physiol. 1964;19:360–362. doi: 10.1152/jappl.1964.19.2.360. [DOI] [PubMed] [Google Scholar]
- KRSTIC M.K., KATUSIC Z.S. Further evidence that the tachycardiac response to 5-hydroxytryptamine in rats is not mediated via typical 5-HT2 receptors. Gen. Pharmacol. 1994;25:917–921. doi: 10.1016/0306-3623(94)90096-5. [DOI] [PubMed] [Google Scholar]
- MARTIN G.R. Vascular receptors for 5-hydroxytryptamine: distribution, function and classification. Pharmacol. Ther. 1994;62:283–324. doi: 10.1016/0163-7258(94)90048-5. [DOI] [PubMed] [Google Scholar]
- MARTIN J.R., BÖS M., JENCK F., MOREAU J.-L., MUTEL V., SLEIGHT A.J., WICHMANN J., ANDREWS J.S., BERENDENSEN H.H.G., BROEKKAMP C.L.E., RUIGT G.S.F., KÖLHER C., VAN DELFT A.M.L. 5-HT2C receptor agonists: pharmacological characteristics and therapeutic potential. J. Pharmacol. Exp. Ther. 1996;286:913–924. [PubMed] [Google Scholar]
- ROTH B.L., CRAIGO S.C., CHOUDHARY M.S., ULUER A., MONSMA F.J., JR, SHEN Y., MELTZER H.Y., SIBLEY D.R. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J. Pharmacol. Exp. Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- SAXENA P.R. Nature of the 5-hydroxytryptamine receptors in mammalian heart. Prog. Pharmacol. 1986;6:173–185. [Google Scholar]
- SAXENA P.R., LAWANG A. A comparison of cardiovascular and smooth muscle effects of 5- hydroxytryptamine and 5-carboxamidotryptamine, a selective agonist of 5- HT1 receptors. Arch. Int. Pharmacodyn. Ther. 1985;277:235–252. [PubMed] [Google Scholar]
- SAXENA P.R., VILLALÓN C.M. Cardiovascular effects of serotonin agonists and antagonists. J. Cardiovasc. Pharmacol. 1990;15:S17–S34. [PubMed] [Google Scholar]
- SAXENA P.R., VILLALÓN C.M. 5-Hydroxytryptamine: a chameleon in the heart. Trends Pharmacol. Sci. 1991;12:223–227. doi: 10.1016/0165-6147(91)90556-8. [DOI] [PubMed] [Google Scholar]
- SCHOEFFTER P., WAEBER C. 5-Hydroxytryptamine receptors with a 5-HT6 receptor-like profile stimulating adenylyl cyclase activity in pig caudate membranes. Naunyn-Schmiedeberg's Arch. Pharmacol. 1994;350:356–360. doi: 10.1007/BF00178951. [DOI] [PubMed] [Google Scholar]
- SHIPLEY R.E., TILDEN J.H. A pithed rat preparation suitable for assaying pressor substances. Proc. Soc. Exp. Biol. Med. 1947;64:453–455. doi: 10.3181/00379727-64-15828. [DOI] [PubMed] [Google Scholar]
- SLEIGHT A., BOESS F., BOS M., LEVET-TRAFIT B., RIEMER C., BOURSON A. Characterization of Ro 04-6790 and Ro 63-0563:potent and selective antagonists at human and rat 5-HT6 receptors. Br. J. Pharmacol. 1998;124:556–562. doi: 10.1038/sj.bjp.0701851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEEL R.G.D., TORRIE J.H. Principles and procedures of statistics. A biomedical approach 1980Tokyo: McGraw-Hill Kogakusha Ltd; 2nd edn [Google Scholar]
- VILLALÓN C.M., HEILIGERS J.P.C., CENTURIÓN D., DE VRIES P., SAXENA P.R. Characterization of putative 5-HT7 receptors mediating tachycardia in the cat. Br. J. Pharmacol. 1997;121:1187–1195. doi: 10.1038/sj.bjp.0701260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAINSCOTT D.B., KRUSHINSKI J.H., SCHAUS J.M., KALDOR S.W., DRESSMAN B.A., AUDIA J.E., ADHAM N., NELSON D.L.[3H]LY334370, a selective radioligand for labeling the serotonin1F (5-HT1F) receptor 1996Washington D.C; Poster at the Neuroscience Meeting 1996 [Google Scholar]
- WAINSCOTT D.B., SASSO D.A., KURSAR J.D., BAEZ M., LUCAITES V.L., NELSON D.L. [3H]Rauwolscine: an antagonist radioligand for the cloned human 5-hydroxytryptamine2b (5-HT2B) receptor. Naunyn-Schmiedebergs Arch. Pharmacol. 1998;357:17–24. doi: 10.1007/pl00005133. [DOI] [PubMed] [Google Scholar]


