Abstract
The aim of this study was to establish an experimental model of the escape phenomenon, in which plasma cholesterol, initially reduced by a 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase inhibitor such as pravastatin, increases again on long-term administration. We also evaluated the efficacy of YM-53601 ((E)-2-[2-fluoro-2- (quinuclidin-3-ylidene) ethoxy]-9H-carbazole monohydrochloride), a squalene synthase inhibitor, in this model.
Pravastatin inhibited cholesterol biosynthesis in hamster primary hepatocytes (IC50, 14 nM). After pre-treatment with pravastatin, in contrast, almost no effect on cholesterol biosynthesis was seen.
In hamsters fed a high fat diet, 3 mg kg−1 pravastatin for 9 days decreased plasma non-HDL cholesterol (total cholesterol – high density lipoprotein cholesterol) (P<0.01), but this effect was lost between 17 and 27 days of treatment, accompanied by an increase in HMG-CoA reductase activity. No such increase in plasma non-HDL cholesterol was seen with YM-53601 at 30 mg kg−1 after 9 (P<0.001), 17 (P<0.01) or 27 (P<0.001) days of treatment. Replacement of pravastatin with YM-53601 caused a decrease in plasma non-HDL cholesterol by 53% (P<0.001) and in HMG-CoA reductase activity.
This animal model thus satisfactorily replicates the escape phenomenon observed in humans and may therefore be useful in evaluation of lipid-lowering agents, specifically comparison of HMG-CoA reductase inhibitors. Further, YM-53601 may be useful in the treatment of hypercholesterolemia without induction of the escape phenomenon.
Keywords: Escape phenomenon, YM-53601, pravastatin, squalene synthase, HMG-CoA reductase, cholesterol
Introduction
Long-term administration of inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase (EC 1.1.1.34), the rate limiting enzyme in cholesterol biosynthesis, is reported to be associated with the escape phenomenon, in which plasma cholesterol levels are initially reduced but return to pre-treatment levels (Naruse et al., 1994; Pazzucconi et al., 1995). As a result, this effect may be due to the induction of HMG-CoA reductase expression by a feedback system, wherein the inhibition of the enzyme is countered by homeostatic maintenance of the amount of the cholesterol pool in the liver (Stone et al., 1989). From this result, it has been speculated that the efficacy of the compound might be attenuated or lost, and researchers have sought a compound that has both strong efficacy and high tolerance. Although changes in enzyme expression have been examined in animals, the escape phenomenon has only been observed clinically and no animal model is available (Fujioka & Tsujita, 1997; Fujioka et al., 1995). Among rodents, only guinea-pigs show a hypocholesterolemic effect of statins on plasma cholesterol (Conde et al., 1996).
The mechanism by which HMG-CoA reductase inhibitors induce the escape phenomenon is said to involve their inhibition of not only cholesterol but also mevalonate derivatives such as farnesol. Mevalonate derivatives are known to regulate the protein levels of cholesterol biosynthesis enzymes through translation after transcription (Goldstein & Brown, 1990). On this basis, research has focused on new target molecules that do not inhibit the biosynthesis of these derivatives. An example is squalene synthase, which works downstream in the cholesterol biosynthesis pathway.
As no suitable animal model has been available, however, it has not been possible to compare squalene synthase inhibitors with HMG-CoA reductase inhibitors directly. We therefore developed a model using hamsters fed a high-fat diet in which plasma cholesterol increases after reduction by pravastatin, one of the HMG-CoA reductase inhibitors. Using this model, we also compared the effect of YM-53601 ((E)-2-[2-fluoro-2-(quinuclidin-3-ylidene) ethoxy]-9H-carbazole monohydrochloride), a novel squalene synthase inhibitor that shows a strong hypercholesterolemic effect in hamsters (Ugawa et al., 2000), with pravastatin. To our knowledge, the present report is the first to describe an animal model of the escape phenomenon and to compare the effects of inhibitors of HMG-CoA reductase and squalene synthase.
Methods
Materials
YM-53601 and NB-598 (Horie et al., 1990), a squalene epoxidase inhibitor, were synthesized at the Chemistry Laboratories, Yamanouchi Pharmaceutical Co., Ltd. (Tokyo, Japan). The HMG-CoA reductase inhibitor pravastatin was purchased from Sankyo Co., Ltd. (Tokyo, Japan). Farnesyl pyrophosphate (FPP), [3H]-FPP (15 Ci mmol−1) and [14C]-acetate (55 mCi mmol−1) were obtained from American Radiolabelled Chemicals Inc (MO, U.S.A.).
Preparation of hepatocytes
Hepatocyte preparations were produced from male Syrian golden hamsters weighing approximately 80 g. Cells were prepared as described previously (Seglen, 1976) and plated out as a suspension in foetal bovine serum (FBS)-containing Dulbecco's modified eagle medium (DMEM) at a density of 4×105 cells ml−1 in 12-well plates. The cells were allowed to form a mono-layer (2 h) and treated in various ways according to the experimental protocol (see below).
Measurement of cholesterol biosynthesis
After isolation, the cells were plated in 10% FBS-containing DMEM for 24 h and then washed. The medium was replaced with DMEM containing 5% human lipoprotein-deficient serum (LPDS) in the presence or absence of pravastatin (1 – 100 nM final concentration) added as a solution in dimethylsulphoxide (DMSO). After 18 h, pravastatin was added to the medium of cells without pretreatment and [14C]-acetate (1 μCi, 55 mCi mmol−1) was added to the medium of both. The cells were then incubated for 6 h. After removal of the medium, the cells were lysed by adding 15% KOH and the lysate was saponified in 15% KOH in ethanol at 75°C for 1 h. Samples were extracted with petroleum ether under alkaline conditions and the amount of [14C]-cholesterol was measured by scintillation counting following separation by thin layer chromatography in petroleum ether : diethylether : acetate (80 : 20 : 1). The amount of protein was measured by the method of Lowry et al. (1951).
Evaluation of hepatic disorder in hamsters
Male Syrian golden hamsters purchased from Hamri (Ibaraki, Japan) were 13 weeks old and weighed approximately 150 g at the start of the study. A standard low cholesterol diet (CE-2 from CLEA Japan Inc., Tokyo, Japan) and water were provided ad libitum. They were given a 3-, 10- or 30-mg oral dose of pravastatin per kg of body weight once daily for 11 days. Pravastatin was suspended in 0.5% methylcellulose. The no-treatment control group was given an equal volume of the 0.5% methylcellulose vehicle. Blood specimens were obtained 2 h after the last dose from animals which had been fasted 18 h. Plasma samples were analysed for aspartate aminotransferase (AST) and alanine aminotransferase (ALT) using a Hitachi 7250 Automatic analyser (Tokyo, Japan).
Evaluation of hypocholesterolemic effect
Male Syrian golden hamsters weighing approximately 140 g were fed a diet containing 0.5% cholesterol and 10% coconut oil. Hamsters were allotted to one of four groups depending on plasma non-HDL cholesterol level as measured before feeding of the high-fat diet. In the first experiment, pravastatin or YM-53601 at doses of 3 or 30 mg kg−1, respectively, was given once a day for 27 days. Both drugs were suspended in 0.5% methylcellulose. The no-treatment control group was given an equal volume of the 0.5% methylcellulose vehicle. Blood specimens were obtained from the femoral vein using a glass capillary at 2 h after the last compound dose from animals which had been fasted 18 h. Plasma non-HDL cholesterol levels were measured at days 0, 9, 17 and 27 for all animals. Plasma non-HDL cholesterol levels were calculated from total cholesterol and HDL cholesterol as measured enzymatically by the Cholesterol-C Test Wako and HDL Cholesterol Test Wako (Osaka, Japan), respectively.
In the second experiment, pravastatin at a dose of 3 mg kg−1 was given once daily for 17 days, followed by YM-53601 at 30 mg kg−1 body weight orally once daily for a further 10 days. The no-treatment control group was given an equal volume of the 0.5% methylcellulose vehicle. Plasma non-HDL cholesterol levels were measured as above at days 0, 9, 17 and 27.
Preparation of microsomes from hamster livers
Microsomes were prepared from the livers of hamsters fed a diet containing 0.5% cholesterol and 10% coconut oil and given pravastatin or YM-53601 at a dose of 3 or 30 mg kg−1, respectively, for 9, 17 and 27 days, or given pravastatin for the first 17 days and YM-53601 for the following 10 days. Livers were also collected from control groups given an equal volume of the 0.5% methylcellulose solution for 0, 9, 17 and 27 days. All livers were prepared as previously described (Burton et al., 1995). The tissues were homogenized in 0.1 M potassium phosphate buffer (pH 7.4) containing (mM): MgCl2 5, nicotinamide 30, using a glass homogenizer. Homogenates were centrifuged at 500×g for 5 min at 4°C and the resulting supernatants further centrifuged at 8000×g for 15 min at 4°C. Microsomes were then isolated from this second supernatant by ultra-centrifugation at 100,000×g for 60 min at 4°C. The microsome precipitates were washed four times and suspended in the above mixed buffer (approximately 25 mg ml−1), and stored in aliquots at −80°C for up to 2 months. Protein was assayed by the method of Lowry et al. (1951).
Measurement of HMG-CoA reductase activity
HMG-CoA reductase activity was measured by a modification of the method of Burton et al. (1995). After pre-incubation of microsomes (200 μg protein) with 10 units inorganic pyrophosphatase from E. coli, the reaction was started by adding 0.1 M potassium phosphate buffer (pH 7.4) containing (mM): EDTA 10, DTT 10, NADPH 5, 144 MBq mmol−1 [14C]-HMG-CoA and HMG-CoA 0.091. The reaction was carried out at 37°C for 60 min and then terminated by the addition of 2N HCl. Synthesized [14C]-mevalonolactone was measured by scintillation counting following separation by thin layer chromatography in benzene : acetone (1 : 1).
Measurement of squalene synthase activity
Squalene synthase activity in microsomes was assayed using the technique of Amin et al. (1992) with modifications. Briefly, the assay was performed in 50 mM HEPES buffer (pH 7.5) containing (mM): NaF 11, MgCl2 5.5, DTT 3, NADPH 1, FPP 5 μM, [3H]-FPP (0.017 μM, 15 Ci mmol−1), 10 μM NB-598 and 1 mM sodium pyrophosphate decahydrate. After pre-incubation of these components at 30°C for 5 min, the reaction was started by the addition of microsomes (10 μg protein), continued at 30°C for 20 min and then terminated by the addition of 100 μl of a 1 : 1 solution of 40% KOH : ethanol. Synthesized [3H]-squalene was extracted in petroleum ether and counted in Aquasol-2 (Packard, Netherlands) using a Beckman liquid scintillation counter.
Statistical analysis
Results are presented as mean±s.e.mean. For study of the effects of drugs on plasma non-HDL cholesterol, AST, ALT and on HMG-CoA reductase and squalene synthase activities, drug-treatment values were compared with those of the vehicle-treatment control using Dunnett's multiple comparison tests. IC50 values were calculated using linear regression analysis by the probit method in a RS/1 computer program (Domain Solutions Corp., U.S.A.). Differences between treatments in cholesterol biosynthesis were estimated by parallel line assay using the Statistical Analysis System (SAS).
Ethical considerations
All experiments were performed in accordance with the regulations of the Animal Ethical Committee of Yamanouchi Pharmaceutical Co., Ltd.
Results
Pravastatin showed little inhibitory effect on cholesterol biosynthesis in vitro with pre-incubation
Figure 1 shows that pravastatin inhibited cholesterol biosynthesis in a dose-dependent manner in hamster primary hepatocytes after 6 h of treatment. The IC50 value for pravastatin was 14 nM. After 18 h pre-treatment with pravastatin, in contrast, no effect on cholesterol biosynthesis was seen at 30 nM. At a pravastatin concentration of 100 nM, the inhibitory effect without 18 h pre-treatment was 89%, but only 17% on pre-treatment for 18 h.
Figure 1.
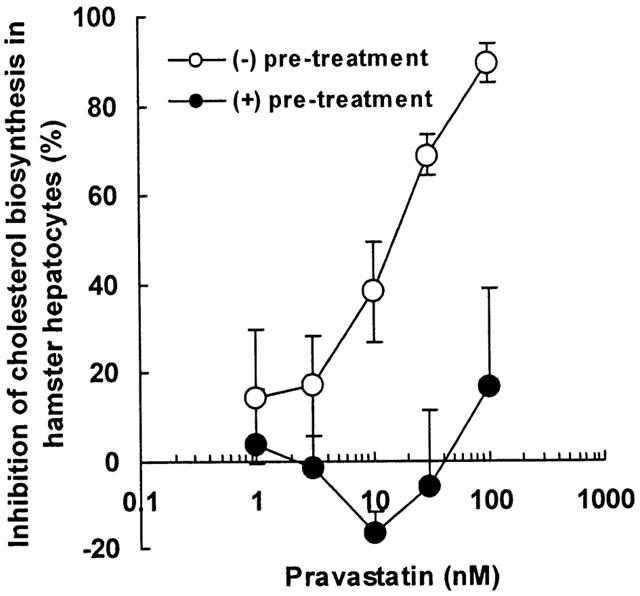
Effect of pravastatin on cholesterol biosynthesis in hamster primary hepatocytes. Hepatocytes were incubated for 18 h in the presence (+) or absence (−) of pravastatin pre-treatment and then labelled with [14C]-acetate (1 μCi) for 6 h with pravastatin at the indicated concentrations. See Methods section for details. Data are presented as the mean of assays performed in triplicate±s.e.mean of per cent inhibition versus the DMSO-treated cells. The IC50 value of (−) pre-treatment was 14 nM by RS/1 computer program. The difference between these treatments was statistically significant by parallel line assay in the Statistical Analysis System (P<0.01).
Pravastatin increases plasma AST and ALT in hamsters
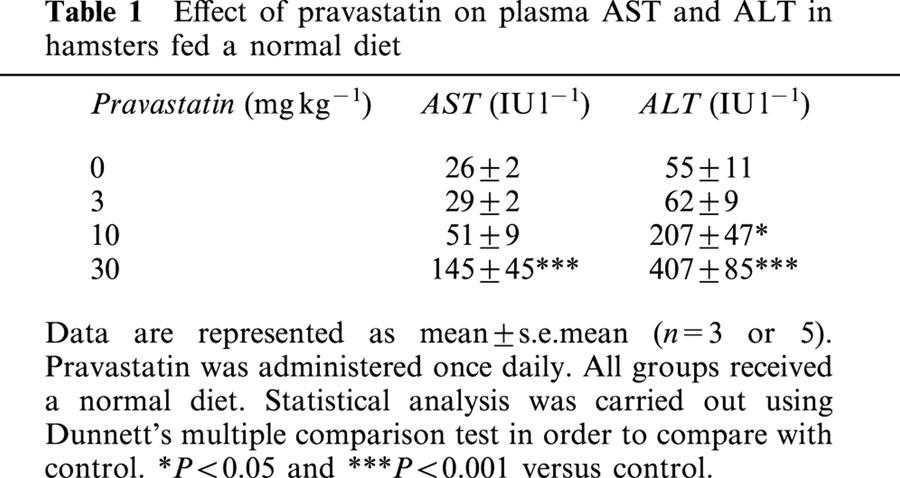
In order to find the highest dosage of pravastatin that could be used without causing hepatic disorder, the effect of pravastatin on plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels was confirmed in hamsters fed a normal diet and orally administered the compound. As shown in Table 1, no difference in plasma AST levels was seen between the control group and animals given pravastatin at 3 mg kg−1 for 11 days. In contrast, animals given pravastatin at 10 and 30 mg kg−1 showed marked and significant increases in plasma AST levels of two and six times the control group values, respectively. With regard to ALT, plasma levels were maintained at the same level as the control group on oral administration at 3 mg kg−1 for 11 days, but, similar to the results with AST, were significantly elevated in hamsters given 10 and 30 mg kg−1 for 11 days, by four and seven times control group values, respectively.
Table 1. Effect of pravastatin on plasma AST and ALT in hamsters fed a normal diet.
Pravastatin initially reduces plasma cholesterol but it increases again on long-term administration, accompanied by induction of HMG-CoA reductase activity
Figure 2A shows the effects of pravastatin and YM-53601 on plasma non-HDL cholesterol levels in hamsters fed a high cholesterol diet. In control hamsters, (open circle), plasma non-HDL cholesterol increased in a time-dependent manner. Pravastatin at 3 mg kg−1 significantly decreased plasma non-HDL cholesterol compared with control by 25% at day 9. Levels increased to those of the control at day 17, and finally tended to be greater than the control at day 27, although the change was not significant. In contrast, non-HDL cholesterol levels with YM-53601 30 mg kg−1 were significantly lower than those of the control throughout administration.
Figure 2.
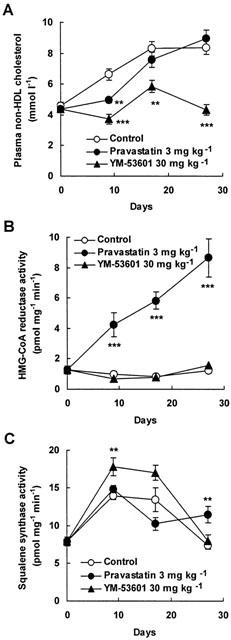
Effect of YM-53601 and pravastatin on plasma concentrations of non-HDL cholesterol and on activity of HMG-CoA reductase and squalene synthase in hamsters fed a high-fat diet. Effects of pravastatin and YM-53601 on plasma non-HDL cholesterol (A) and activities of HMG-CoA reductase (B) and squalene synthase (C) were evaluated. Values are expressed as mean±s.e.mean (n=5 – 7). See Methods section for details. Statistical analysis was carried out using Dunnett's multiple comparison test versus the control group on each indicated day. *P<0.05, **P<0.01, ***P<0.001 versus respective controls.
Next, to understand why pravastatin's effect on plasma non-HDL cholesterol levels changed during administration, we measured HMG-CoA reductase activity of microsomes prepared from livers of hamsters administered pravastatin or YM-53601 at 3 or 30 mg kg−1, respectively, for 0, 9, 17 or 27 days. In control hamsters, activity remained unchanged. In contrast, pravastatin increased activity in a time-dependent manner. YM-53601 did not affect activity (Figure 2B).
Similarly, we measured squalene synthase activity of the above microsomes. As shown in Figure 2C, unlike the case with HMG-CoA reductase activity, no increase by pravastatin was seen in squalene synthase activity up to day 17. At day 27, however, pravastatin increased activity significantly. In contrast, YM-53601 induced squalene synthase activity at day 9, but no effect was seen at days 17 and 27.
Figure 3A shows that pravastatin at a dose of 3 mg kg−1 significantly decreased plasma non-HDL cholesterol levels by 18% of the control value on 9 days' oral administration. At day 17, levels returned to those of the control group. Following pravastatin treatment for 17 days, YM-53601 was substituted at a dose of 30 mg kg−1. At day 27 (i.e. day 10 of YM-53601), plasma non-HDL cholesterol decreased by 53% compared with control. The decrease in the plasma non-HDL cholesterol level with YM-53601 was greater than that with pravastatin.
Figure 3.
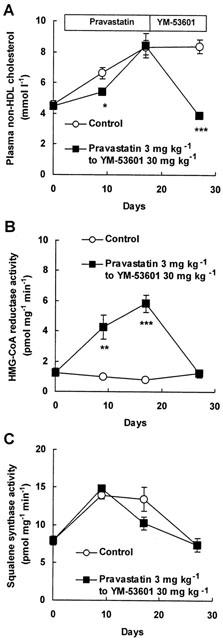
Decrease in plasma non-HDL cholesterol and activities of HMG-CoA reductase and squalene synthase in hamsters pre-treated with pravastatin. Effects of pravastatin and YM-53601 on plasma non-HDL cholesterol (A), activities of HMG-CoA reductase (B) and squalene synthase (C) in hamsters fed a high-fat diet were evaluated. The values are expressed as mean±s.e.mean (n=7). See Methods section for details. Statistical analysis was carried out using Dunnett's multiple comparison test versus control group in each indicated day. *P<0.05, **P<0.01, ***P<0.001 versus each control.
HMG-CoA reductase activity in microsomes from livers of animals treated with pravastatin at 3 mg kg−1 increased in a time-dependent manner until day 17. In contrast, HMG-CoA reductase activity in control microsomes showed similar levels during the experiment. After replacement with YM-53601 at 30 mg kg−1, HMG-CoA reductase activity returned to the same level as in the control group (Figure 3B).
Squalene synthase activity in microsomes prepared from livers from animals treated with pravastatin show no significant changes compared with control group values. At 10 days after replacement with YM-53601, control and YM-53601 group activity was the same (Figure 3C).
In the experiment in Figure 2, plasma HDL cholesterol was significantly increased by pravastatin on day 9 only by 23% compared to control animals. In the experiment in Figure 3, pravastatin tended to increase plasma HDL cholesterol on day 9 only by 11%, although the change was not significant. Except for day 9, pravastatin did not raise this. On the other hand, YM-53601 showed no increase in plasma HDL cholesterol in either experiment (data not shown).
Discussion
This study set out to establish an experimental model of the escape phenomenon and to use this model to evaluate the effect of YM-53601, a squalene synthase inhibitor, in comparison with that of pravastatin, a widely used HMG-CoA reductase inhibitor.
The hamster was selected because plasma lipid composition in this species is similar to that of humans (Spady & Dietschy, 1985). Pravastatin, a HMG-CoA reductase inhibitor (statin) in wide clinical use for the treatment of hypercholesterolemia, was used as reference compound because this drug produces the escape phenomenon in humans, wherein total plasma cholesterol levels may increase after first showing a significant decrease (Naruse et al., 1994).
Plasma non-HDL cholesterol levels in individual hamsters fell during pravastatin treatment but HMG-CoA reductase was up-regulated approximately 7 fold compared to the control group. Continued administration of pravastatin lead to enhanced enzyme activities and subsequently to an increase in plasma non-HDL cholesterol to the same levels of the control group. No re-reduction in plasma cholesterol level compared with control was seen on longer administration; on the contrary, HMG-CoA reductase activity was further enhanced by pravastatin treatment. This animal model thus satisfactorily replicates the escape phenomenon observed in humans. Use of a high-fat diet may result in decreased cholesterol biosynthesis activity through down regulation in response to adequate or excessive diet-derived cholesterol in the liver. This may explain why pravastatin induces a decrease in plasma non-HDL cholesterol. All rodents apart from guinea-pigs normally have high cholesterol biosynthesis activity (Spady & Dietschy, 1983). This activity is easily induced, and would seem to prevent the observation of a cholesterol-lowering effect. The absence of this effect in guinea-pigs may therefore justify their use in the present model.
Pravastatin attenuated inhibition of cholesterol biosynthesis in hamster primary hepatocytes by pre-treatment (IC50 values >100 nM or 14 nM, with or without pre-treatment, respectively). Further, pravastatin is reported to induce HMG-CoA reductase (Fujioka & Tsujita, 1997; Fujioka et al., 1995). The present result may therefore be derived from enzyme induction by the positive feedback system itself. In other words, long-treatment with pravastatin in vitro showed a similar effect to that in vivo and in previous reports. Moreover, the effect of HMG-CoA reductase inhibitors on enzyme expression or activity is also seen in mononuclear leukocytes of normal subjects (Stone et al., 1989). As enzyme induction is known to lead to the escape phenomenon, drugs have been sought that do not produce induction and the escape phenomenon.
Unlike HMG-CoA reductase, squalene synthase acts downstream in cholesterol biosynthesis. YM-53601 belongs to a novel class of lipid-lowering agents that inhibit squalene synthase activity in cholesterol biosynthesis in animal models (Ugawa et al., 2000). In pre-clinical studies in hamsters, guinea-pigs and rhesus monkeys, YM-53601 reduced plasma concentrations of non-HDL cholesterol equally or to a greater degree than pravastatin. YM-53601 thus appears to be a potentially useful agent for the treatment of hypercholesterolemia.
In the present animal model of the escape phenomena, YM-53601 showed a consistent plasma cholesterol-lowering effect during treatment. YM-53601 weakly up-regulated squalene synthase activity and did not affect HMG-CoA reductase. A possible explanation is that, given the level at which squalene synthase inhibitors act in the biosynthesis pathway, the cholesterol inhibition system is less prone to positive regulatory feedback mechanisms as compared with statins. Squalene synthase inhibitors may consequently provide greater lipid lowering efficacy than statins. Accumulation of farnesyl pyrophosphate as a result of squalene synthase inhibition is known to down regulate HMG-CoA reductase mRNA expression (Correll et al., 1994; Meigs et al., 1996). However, a previous report indicates that other squalene synthase inhibitors have a strong effect on HMG-CoA reductase up-regulation (Amin et al., 1997). One possible explanation why YM-53601 did not induce HMG-CoA reductase activity in comparison with other squalene synthase inhibitors might be the difference in their action on squalene synthase. Squalene synthase catalyzes the head to head condensation of two molecules of FPP to form squalene. In the first reaction, two molecules of FPP react to form the stable cyclopropylcarbinyl diphosphate intermediate, presqualene pyrophosphate, with concomitant release of a proton and a molecule of inorganic pyrophosphate. In the second half of the reaction, presqualene pyrophosphate undergoes heterolysis, isomerization, and reduction with NADPH to form squalene (Poulter, 1990; Poulter & Rilling, 1981). A variety of structurally distinct, reversible, competitive, first half-reaction squalene synthase inhibitors such as P-3622, CP-210172, CP-295697 and CP-294838 reduce cholesterogenesis by up to 90% with no appreciable increase in HMG-CoA reductase activity. In contrast, zaragozic acid A, an irreversible squalene synthase inhibitor, exhibits a high degree of HMG-CoA reductase modulation, producing increases in activity at concentrations that produce cholesterolgenesis inhibition (Petras et al., 1999). YM-53601 may belong to the former group showing reversible, competitive, first half-reaction squalene synthase inhibition. These enzyme regulation aspects should be investigated in more detail.
In conclusion, we have developed an animal model of the escape phenomenon observed in humans using hamsters fed a high-fat diet. In this model, YM-53601 consistently decreased plasma non-HDL cholesterol without inducing HMG-CoA reductase activity. YM-53601 also decreased plasma non-HDL cholesterol in hamsters whose enzyme activities had been induced by pravastatin and returned HMG-CoA reductase activity to the control level. It should be noted that the substition of pravastatin by YM-53601 was effective in decreasing plasma cholesterol and HMG-CoA reductase activity, suggesting that an increase in HMG-CoA reductase activity might counteract the lipid-lowering effect of pravastatin. We therefore suggest that, because it does not induce HMG-CoA reductase activity, YM-53601 may decrease plasma cholesterol levels in both hypercholesterolemic patients who have been affected by the escape phenomenon as well as those who have not.
Acknowledgments
We would like to express our gratitude to Drs Yuichi Iizumi, Osamu Inagaki, Koyo Matsuda and Shin Naganuma for helpful contributions. We would also like to thank Mr Tsukasa Ishihara for synthesizing YM-53601, and Dr Guy Harris for his assistance in the preparation of this manuscript.
Glossary
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- DTT
dithiothreitol
- EDTA
ethylenediaminetetraacetic acid
- FPP
farnesyl pyrophosphate
- HDL
high density lipoprotein
- HMG-CoA
3-hydroxy-3-methylglutaryl coenzyme A
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
References
- AMIN D., CORNELL S.A., GUSTAFSON S.K., NEEDLE S.J., ULLRICH J.W., BILDER G.E., PERRONE M.H. Bisphosphonates used for the treatment of bone disorders inhibit squalene synthase and cholesterol biosynthesis. J. Lipid Res. 1992;33:1657–1663. [PubMed] [Google Scholar]
- AMIN D., RUTLEDGE R.Z., NEEDLE S.N., GALCZENSKI H.F., NEUENSCHWANDER K., SCOTESE A.C., MAGUIRE M.P., BUSH R.C., HELE D.J., BILDER G.E., PERRONE M.H. RPR 107393, a potent squalene synthase inhibitor and orally effective cholesterol-lowering agent: comparison with inhibitors of HMG-CoA reductase. J. Pharmacol. Exp. Ther. 1997;281:746–752. [PubMed] [Google Scholar]
- BURTON P.M., SWINNEY D.C., HELLER R, DUNLAP B., CHIOU M., MALONZO E., HALLER J., WALKER K.A.M., SALARI A., MURAKAMI S., MENDIZABAL G., TOKES L. Azalanstat (RS-21607), a lanosterol 14-demethylase inhibitor with cholesterol-lowering activity. Biochem. Pharmacol. 1995;50:529–544. doi: 10.1016/0006-2952(95)00152-p. [DOI] [PubMed] [Google Scholar]
- CONDE K., VERGARA-JIMENEZ M., KRAUSE B.R., NEWTON R.S., FERNANDEZ M.L. Hypocholesterolemic actions of atorvastatin are associated with alterations on hepatic cholesterol metabolism and lipoprotein composition in the guinea-pig. J. Lipid Res. 1996;37:2372–2382. [PubMed] [Google Scholar]
- CORRELL C.C., NG L., EDWARDS P.A. Identification of farnesol as the non-sterol derivative of mevalonic acid required for the accelerated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 1994;269:17390–17393. [PubMed] [Google Scholar]
- FUJIOKA T., NARA F., TSUJITA Y., FUKUSHIGE J., FUKAMI M., KURODA M. The mechanism of lack of hypocholesterolemic effects of pravastatin sodium, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, in rats. Biochim. Biophys. Acta. 1995;1254:7–12. doi: 10.1016/0005-2760(94)00154-q. [DOI] [PubMed] [Google Scholar]
- FUJIOKA T., TSUJITA Y. Effects of single administration of pravastatin sodium on hepatic cholesterol metabolism in rats. Eur. J. Pharmacol. 1997;323:223–228. doi: 10.1016/s0014-2999(97)00033-2. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN J.L., BROWN M.S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- HORIE M., TSUCHIYA Y., HAYASHI M., IIDA Y., IWASAWA Y., NAGATA Y., SAWASAKI Y., FUKUZUMI H., KITANI K., KAMEI T. NB-598, a potent competitive inhibitor of squalene epoxidase. J. Biol. Chem. 1990;265:18075–18078. [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MEIGS T.E., ROSEMAN D.S., SIMONI R.D. Regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase degradation by the nonsterol mevalonate metabolite farnesol in vivo. J. Biol. Chem. 1996;271:7916–7922. doi: 10.1074/jbc.271.14.7916. [DOI] [PubMed] [Google Scholar]
- NARUSE M., YOSHIMOTO T., NARUSE K., TANAKA M., HASE M., DEMURA H. Effects of simvastatin on the escape phenomenon following pravastatin therapy in patients with hypercholesterolemia. Domyaku Koka. 1994;22:301–306. [Google Scholar]
- PAZZUCCONI F., DORIGOTTI F., GIANFRANCESCHI G., CAMPAGNOLI G., SIRTORI M., FRANCESCHINI G., SIRTORI C.R. Therapy with HMG CoA reductase inhibitors: characteristics of the long-term permanence of hypocholesterolemic activity. Atherosclerosis. 1995;117:189–198. doi: 10.1016/0021-9150(95)05571-d. [DOI] [PubMed] [Google Scholar]
- PETRAS S.F., LINDSEY S., HARWOOD H.J., JR HMG-CoA reductase regulation: use of structurally diverse first half-reaction squalene synthetase inhibitors to characterize the site of mevalonate-derived nonsterol regulator production in cultured IM-9 cells. J. Lipid Res. 1999;40:24–38. [PubMed] [Google Scholar]
- POULTER C.D. Biosynthesis of non-head-to-tail terpenes. Formation of 1′-1 and 1′-3 linkages. Acc. Chem. Res. 1990;23:70–77. [Google Scholar]
- POULTER C.D., RILLING H.C.1981Conversion of farnesyl pyrophosphate to squalene Biosynthesis of Isoprenoid Compoundsed. Porter, J.W., Spurgeon, S.L. Vol. IChapter 8, pp. 413–441.New York: J. Wiley and Sons [Google Scholar]
- SEGLEN P.O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- SPADY D.K., DIETSCHY J.M. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J. Lipid Res. 1983;24:303–315. [PubMed] [Google Scholar]
- SPADY D.K., DIETSCHY J.M. Dietary saturated triacylglycerols suppress hepatic low density lipoprotein receptor activity in the hamster. Proc. Natl. Acad. Sci. U.S.A. 1985;82:4526–4530. doi: 10.1073/pnas.82.13.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STONE B.G., EVANS C.D., PRIGGE W.F., DUANE E.C., GEBHARD R.L. Lovastatin treatment inhibits sterol synthesis and induces HMG-CoA reductase activity in mononuclear leukocytes of normal subjects. J. Lipid Res. 1989;30:1943–1952. [PubMed] [Google Scholar]
- UGAWA T., KAKUTA H., MORITANI H., MATSUDA K., ISHIHARA T., YAMAGUCHI M., NAGANUMA S., IIZUMI Y., SHIKAMA H. YM-53601, a novel squalene synthase inhibitor, reduces plasma cholesterol and triglyceride levels in several animal species. Br. J. Pharmacol. 2000;131:63–70. doi: 10.1038/sj.bjp.0703545. [DOI] [PMC free article] [PubMed] [Google Scholar]


