Abstract
Release of acetylcholine from parasympathetic nerves is inhibited by neuronal M2 muscarinic receptors. The effects of streptozotocin-induced diabetes on prejunctional M2 and postjunctional M3 muscarinic receptor function in rat trachea and ileum were investigated in vitro.
Neuronal M2 muscarinic receptor function was tested by measuring the ability of an agonist, pilocarpine, to inhibit and an antagonist, methoctramine, to potentiate electrical field stimulation (EFS)-induced contraction of trachea and ileum. Concentration-response curves to pilocarpine and methoctramine were shifted to the left in both to a greater degree in diabetics than controls.
In trachea, post-junctional M3 muscarinic receptor function was increased since maximum contractile responses to the muscarinic agonists acetylcholine and carbachol were greater in diabetics than controls. This increase offset the increased function of the inhibitory neuronal M2 muscarinic receptors since EFS-induced, frequency-dependent contraction was equal in control and diabetic rats.
In contrast, post-junctional M3 muscarinic receptor function was unchanged by diabetes since concentration-response curves to acetylcholine and carbachol were not different between groups. Thus, EFS-induced contractions of the ileum were decreased in diabetics versus controls.
In conclusion, inhibitory M2 muscarinic receptors on parasympathetic nerves in the trachea and ileum are hyperfunctional in diabetic rats. The function of post-junctional M3 muscarinic receptors in the trachea, but not the ileum, is also increased in diabetes.
The dysfunction of inhibitory, neuronal M2 muscarinic receptors in the airways may protect against hyperreactivity and in the ileum may contribute to gastrointestinal dysmotility associated with diabetes.
Keywords: Asthma, diabetes, dysmotility, gastroparesis, intestine, parasympathetic nerves
Introduction
A low incidence of asthma among patients with diabetes mellitus has been reported in several epidemiological studies (Abrahamson, 1941; Helander, 1958; Szczeklik et al., 1980; EURODIAB substudy 2 study group, 2000). The mechanisms of the negative association between these two diseases are unclear but a role for neuronal M2 muscarinic receptors has been hypothesized (Belmonte et al., 1997). Inhibitory M2 muscarinic receptors are present on parasympathetic nerves in the lungs and decrease the release of acetylcholine from parasympathetic nerve endings (Fryer & Maclagan, 1984). These receptors limit vagally mediated bronchoconstriction.
The importance of these inhibitory neuronal M2 muscarinic receptors in the airways can be demonstrated using agonists, such as pilocarpine, and antagonists, such as gallamine and methoctramine. Stimulating M2 muscarinic receptors with pilocarpine inhibits acetylcholine release from parasympathetic nerves and decreases bronchoconstriction in response to vagal nerve stimulation by more than 80% (Fryer & Maclagan, 1984; Minette & Barnes, 1988; D'agostino et al., 1990). Blocking M2 muscarinic receptors with gallamine or methoctramine enhances acetylcholine release and increases bronchoconstriction in response to vagal nerve stimulation 5 – 10 fold (Fryer & Maclagan, 1984; Minette & Barnes, 1988; Kilbinger et al., 1991; Patel et al., 1995). The neuronal M2 muscarinic receptors are hyperfunctional in the airways of diabetic rats in vivo, which decreases bronchoconstriction in response to electrical stimulation of vagus nerves (Belmonte et al., 1997).
Inhibitory M2 muscarinic receptors are also present on parasympathetic nerves of the gastrointestinal tract (Damann et al., 1989). Increases in M2 muscarinic receptor function may contribute to decreased parasympathetic nerve function in the gastrointestinal tract of diabetics (Dooley et al., 1988). Decreased enteric nerve function occurs in 30 – 40% of diabetic patients (Horowitz et al., 1996) and contributes to gastrointestinal motility dysfunction. Gastrointestinal dysmotility occurs frequently in patients with diabetes (Rundles, 1945; Keshavarzian & Iber, 1986; Parkman & Schwartz, 1987) and ranges from mild, subclinical disease to severe gastrointestinal disturbances. It has long been considered that the gastrointestinal dysmotility that occurs in diabetic patients is a consequence of diabetic neuropathy (Rundles, 1945). However, it remains to be elucidated whether this is due to nerve damage, or simply to changes in nerve function. For example, Yoshida et al. (1988) were unable to detect morphological abnormalities of the neurons of the myenteric plexus of the gastrointestinal tract of diabetic patients, with or without dysmotility.
The aim of this study was to determine whether the function of neuronal M2 muscarinic receptors in the gastrointestinal tract, as well as the airways, are changed by diabetes.
Methods
Animals
Male, Sprague-Dawley, pathogen-free rats (300 – 350 g; supplied by Hilltop Animal farms, Scottsdale, PA, U.S.A.) were used. All rats were handled in accordance with standards established by the U.S.A. Animal Welfare Acts set forth in the National Institutes of Health guidelines and the Policy and Procedures manual published by Johns Hopkins University School of Public Health Animal Care and Use Committee.
Induction of diabetes mellitus
Diabetes mellitus was induced by injection of 65 mg kg−1 streptozotocin (STZ) into the tail vein of rats lightly anaesthetized with sodium pentobarbitone (30 mg kg−1). Control rats were injected with the same volume (1 ml kg−1) of 0.1 M sodium citrate buffer (pH 4.5). The concentration of glucose in whole blood was measured in every animal immediately before each experiment by a standard glucometer (Accu-Chek Instant™, Roche, Indianapolis, IN, U.S.A.).
Measurement of muscarinic receptor function in isolated trachea and ileum
Seven days after streptozotocin treatment, rats were killed with an overdose of sodium pentobarbitone (60 mg i.p.). The trachea and ileum were removed from each animal and placed in Krebs – Henseleit solution of the following composition (mM): NaCl 117.5, KCl 5.6, MgSO4 1.18, CaCl2.2H20 2.5, NaH2PO4 1.47, NaHCO3 25.0 and dextrose 5.54 containing propranolol (10−6 M) to block the effects of sympathetic nerve stimulation.
The tracheas were cut transversely into four segments consisting of 3 – 5 cartilaginous rings and each segment was cut longitudinally through the cartilage ring to create a strip. The ileum was rinsed with Krebs – Henseleit solution to remove ileal contents and the most distal 10 cm was discarded. The remaining ileum was cut into 1 – 2 cm segments. Tracheal strips and ileal segments were mounted longitudinally between two zig-zag platinum electrodes in a 5 ml water jacketed organ bath that contained Krebs – Henseleit solution, bubbled with 5% CO2 and 95% O2 and kept at 37°C. The strips were placed under 1.0 g isometric tension and allowed to equilibrate.
During a 1 h equilibration period, each tissue was washed every 15 min with Krebs – Henseleit solution. After equilibration, a cumulative concentration-response curve to acetylcholine (10−8 – 10−2 M) or a frequency-response curve to electrical field stimulation (EFS, 1 – 35 Hz, 100 V, 0.2 ms pulse duration for 15 s on trachea and 5 s on ileum) was constructed on each preparation. The preparations were then washed every 15 min for 1 h with Krebs – Henseleit solution to remove acetylcholine from the bath and to allow tissues to re-establish baseline tension. Stable baseline responses to electrical field stimulation (15 Hz for trachea and 5 or 15 Hz for ileum) were then obtained. M2 receptor function was tested by measuring the ability of an agonist, pilocarpine (10−11 – 10−3 M), to inhibit and an antagonist, methoctramine (10−9 – 10−4 M), to potentiate EFS-induced contraction. Pilocarpine and methoctramine were added cumulatively to the bath every 5 min. The pilocarpine concentration-response curve on the trachea was carried out in the presence of pirenzepine (10−7 M) to prevent M1 receptor stimulation by high concentrations of pilocarpine.
Muscarinic receptor antagonists block both pre- and post-junctional M2 and M3 muscarinic receptors. Whilst the antagonist methoctramine has a high degree of selectivity for M2 receptors versus M3 receptors, it can block M3 receptors (Giraldo et al., 1988). In rat trachea, methoctramine has been shown to potentiate EFS-induced contraction at concentrations from 10−9 – 10−6 M, due to blockade of neuronal M2 muscarinic receptors (Aas & Maclagan, 1990). Concurrent with this reduction in EFS-induced contraction, methoctramine reduces muscarinic agonist-induced contraction due to blockade of post-junctional M3 muscarinic receptors (Aas & Maclagan, 1990). Thus, in this study, the effect of methoctramine on post-junctional M3 muscarinic receptors on smooth muscle was determined by testing the ability of methoctramine to inhibit carbachol-induced contraction. The tissues were pre-contracted with the carbachol (10−4 M) and then methoctramine (10−9 – 10−4 M) was added cumulatively to the bath at 5 min intervals.
M3 muscarinic receptor function was tested by measuring the ability of carbachol to contract trachea and ileum. Carbachol (10−8 – 10−2 M) was added cumulatively to the bath.
At the end of each experiment, the muscarinic antagonist atropine (10−4 M) was added to the organ baths. Atropine blocked both carbachol- and EFS-induced contractions, confirming that these contractions were mediated via muscarinic receptors.
Expression and statistical analysis of data
All data are expressed as means±s.e.m. The n values equal the numbers of animals that contributed to the mean. The weights of ileal tissues from rats made diabetic with streptozotocin (52.1±1.75 mg, n=45) were significantly greater than those taken from control rats (44.7±1.21 mg, n=50; P<0.001, Student's t-test). This diabetes-induced increase in ileal weight has been shown by other authors to be due to an increase in both mucosal and smooth muscle mass (Nowak et al., 1990b). Since smooth muscle mass is increased in streptozotocin-treated rats the contractile responses to EFS, carbachol and acetylcholine are expressed as the increase in g tension above baseline per mg tissue. Although tracheal tissues from streptozotocin-treated rats (13.9±0.76 mg, n=48) were not significantly different from control rats (13.5±0.60 mg, n=53; P>0.05, Student's t-test) the data is also expressed as the increase in g tension above baseline per mg tissue.
The concentrations of acetylcholine and carbachol that induced 50% maximum contractile response (EC50) were interpolated from semilogarithimic plots of individual concentration-response curves. EC50 values were first converted to negative logarithmic form then the values from individual experiments were used to calculate means and standard error of means (s.e.m.).
The effects of pilocarpine and methoctramine on EFS-induced contraction are expressed as the ratio of contraction in the presence of drug to the contraction in the absence of drug. Differences in the effects of pilocarpine and methoctramine between control and streptozotocin-treated rats were compared using ANOVA for repeated measures. P<0.05 was considered statistically significant.
Drugs
Acetylcholine, atropine, methoctramine, pilocarpine, pirenzepine, propranolol, sodium pentobarbitone and streptozotocin, all purchased from Sigma (St Louis, MO, U.S.A.). Carbachol was purchased from Calbiochem (San Diego, CA, U.S.A.). All drugs were dissolved in 0.9% sodium chloride solution except streptozotocin, which was dissolved in 0.1 M sodium citrate buffer, pH 4.5.
Results
Effects of diabetes on blood glucose and body weight
Rats treated with streptozotocin had significantly greater blood glucose levels than controls (482±8 mg dl−1, n=13; 173±12 mg dl−1, n=14), indicating that they were diabetic. Diabetic rats also weighed significantly less than controls (312±12 g, n=13; 392±15 g, n=14).
Neuronal M2 muscarinic receptor function
Pilocarpine inhibited EFS-induced contractions in a concentration-related manner in both trachea and ileum (Figures 1 and 2), demonstrating functional M2 muscarinic receptors. In diabetic rats, concentration-response curves to pilocarpine were shifted to left in both trachea and ileum (Figures 1 and 2), demonstrating an increase in M2 muscarinic receptor function.
Figure 1.

Pilocarpine inhibits contraction of airway smooth muscle in vitro in response to electrical field stimulation (EFS, 15 Hz, 100 V, 0.2 ms pulse duration for 15 s at 1 min intervals) of isolated trachea. The pilocarpine concentration response curve was shifted to the left in diabetic rats, indicating that pilocarpine causes greater inhibition of EFS-induced contraction of tissues from diabetic rats than controls, although this did not reach statistical significance (P=0.3161). Data are expressed as the ratio of contraction after pilocarpine divided by the contraction before pilocarpine. Each point represents the mean and vertical bars show standard error of mean (n=8 – 9). The representative traces are from a control rat and show contractions to EFS before and after the addition of pilocarpine to the organ bath (at dots). At concentrations above 10−5 M, pilocarpine caused an increase in basal smooth muscle tone.
Figure 2.

Pilocarpine inhibits the contraction of gastrointestinal smooth muscle in vitro in response to electrical field stimulation (EFS, 100 V, 0.2 ms pulse duration for 5 s at 30 s intervals) of isolated ileum. The frequencies of stimulation were 5 and 15 Hz. The pilocarpine concentration response curves were shifted significantly to the left in diabetic rats, indicating that pilocarpine causes significantly greater inhibition of EFS-induced contraction of tissues from diabetic rats than controls. Data are expressed as the ratio of contraction after pilocarpine divided by the contraction before pilocarpine. Each point represents the mean and vertical bars show standard error of mean (n=6).
The ability of the neuronal M2 muscarinic receptors to respond to endogenous acetylcholine was measured using the selective antagonist methoctramine. In tracheas from control rats methoctramine inhibited nerve-induced (open circles, Figure 3) and carbachol-induced (open triangles, Figure 3) contractions equally. In contrast, in tracheas from diabetic rats methoctramine inhibited nerve-induced contractions (closed circles, Figure 3) significantly less well than the carbachol-induced (closed triangles, Figure 3) contractions. Methoctramine (10−9 – 10−6 M) potentiated nerve-induced contractions of the tracheas only from diabetic rats.
Figure 3.
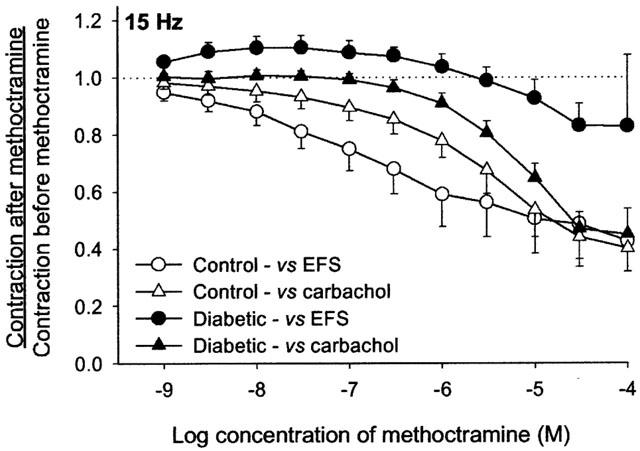
Effects of methoctramine on carbachol- and electrical field stimulation (EFS)-induced contraction of airway smooth muscle in isolated trachea. In control rats, methoctramine inhibited contractions to both EFS and carbachol. In diabetic rats, methoctramine (10−9 – 10−5 M) facilitated EFS-induced contraction but inhibited carbachol-induced contraction. At concentrations of 10−5 M and above, methoctramine inhibited both carbachol- and EFS-induced contraction. Stimulation parameters were the same as described in Figure 1. Data are expressed as the ratio of contraction after methoctramine divided by the contraction before methoctramine. Each point represents the mean and vertical bars show standard error of mean (n=8 – 9).
In the ileum, methoctramine inhibited nerve-induced contractions significantly less well than the carbachol-induced contractions in both control and diabetic rats (Figure 4). The effects of methoctramine on carbachol-induced contractions were not different between the control and diabetic rats. However, inhibition of nerve induced contractions in tracheas from diabetic rats was significantly less than in tracheas from controls (Figure 4).
Figure 4.

Effects of methoctramine on contractions of ileum elicited by carbachol or electrical field stimulation (EFS). The frequencies of stimulation were 5 and 15 Hz at the same parameters as described in Figure 2. Methoctramine inhibited carbachol-induced contraction in control and diabetic rats. Methoctramine also inhibited EFS-induced contraction in control and diabetic rats but this was significantly less than the inhibition of carbachol. The methoctramine concentration response curves against EFS were shifted significantly further to the right in diabetic rats, indicating that methoctramine causes significantly greater facilitation of EFS-induced contraction of tissues from diabetic rats than controls. Data are expressed as the ratio of contraction after methoctramine divided by the contraction before methoctramine. Each point represents the mean and vertical bars show standard error of mean (n=6).
Effect of electrical field stimulation (EFS)
EFS of isolated trachea caused frequency-dependent contraction of airway smooth muscle, which was not different between control and diabetic rats (Figure 5). In contrast, EFS-induced contraction of the ileum from diabetic rats was markedly less than controls, although this did not reach statistical significance (P=0.175; Figure 6).
Figure 5.

Electrical field stimulation (EFS) of isolated trachea causes frequency-dependent contraction of airway smooth muscle in vitro. EFS-induced contraction of tissues from diabetic rats was not different from controls. Stimulation parameters were the same as described in Figure 1. Data are expressed as the increase in g tension developed above baseline per mg tissue. Each point represents the mean and vertical bars show standard error of the means (n=8 – 9).
Figure 6.

Electrical field stimulation (EFS) of isolated ileum caused a frequency-dependent contraction of gastrointestinal smooth muscle in vitro. EFS-induced contractions of tissues from diabetic rats were less than control rats, although this trend did not reach statistical significance (P=0.175). Stimulation parameters were the same as described in Figure 2. Data are expressed as the increase in g tension developed above baseline per mg tissue. Each point represents the mean and vertical bars show standard error of the means (n=6).
Post-junctional M3 receptor function
Acetylcholine and carbachol induced concentration-dependent contractions of trachea and ileum (Figures 7 and 8). In the trachea, the EC50 s for acetylcholine and carbachol were not significantly different while maximum responses to both agonists were significantly greater in diabetic rats than controls (Table 1). In the ileum, neither the EC50 s nor maximum responses to acetylcholine or carbachol were significantly different between control and streptozotocin-treated rats (Table 1).
Figure 7.

Acetylcholine and carbachol induce concentration-dependent contraction of airway smooth muscle in vitro. The maximum responses to acetylcholine and carbachol of tissues from diabetic rats were significantly greater than those from control rats. Data are expressed as the increase in g tension developed above baseline per mg tissue. Each point represents the mean and vertical bars show standard error of mean (n=8 – 9).
Figure 8.

Acetylcholine and carbachol induce concentration-dependent contraction of the ileum in vitro. The contractile responses to acetylcholine and carbachol of tissues from diabetic rats were not significantly different from controls. Data are expressed as the increase in g tension developed above baseline per mg tissue. Each point represents the mean and vertical bars show standard error of mean (n=5 – 6).
Table 1.
Maximum responses and −log EC50 values for acetylcholine and carbachol in trachea and ileum from control and diabetic rats

Discussion
In the present study, rats were made diabetic for seven days by a single injection of streptozotocin, a toxin that destroys the β cells of the pancreas (Arison et al., 1967). Diabetes was confirmed in each rat by significant elevation of glucose in whole blood. The seven day duration of diabetes was selected because changes in neuronal M2 muscarinic receptor function occur in the airways within this time period (Belmonte et al., 1997, 1998) and there is also gastrointestinal dysmotility (Chang et al., 1997). Longer periods of diabetes were not investigated because autonomic neuropathy, which can lead to impaired vagal neurotransmission, begins to appear in streptozotocin-treated animals 4 – 6 weeks after treatment (Schmidt et al., 1981, 1983; Yagahashi & Sima, 1986). Furthermore, within a short duration of diabetes, blood pH, blood gas tension, oxygen saturation, plasma electrolyte concentrations and haematocrit values are still within normal range (Vianna & Garcia-Leme, 1995) and weight loss is minimal.
The presence of functional M2 muscarinic receptors on the parasympathetic nerves in the trachea was demonstrated by pilocarpine-induced inhibition of EFS-induced contraction (Figure 1). In control rats, this effect was seen at 10−6 – 10−3 M pilocarpine while an inhibition was seen with as little as 10−9 M pilocarpine in diabetic rats. This suggests that neuronal M2 muscarinic receptors in the trachea are hyperfunctional in diabetic rats. However it should be noted that at concentrations above 10−5 M, pilocarpine increased basal smooth muscle tone due to activation of post-junctional M3 muscarinic receptors (Figure 1 trace). This effect of pilocarpine on the smooth muscle may have dampened contractions to EFS, independent of stimulation of neuronal M2 muscarinic receptors, and account for the marked drop in the pilocarpine concentration-response curves at concentrations above 10−5 M.
The diabetes-induced increase in neuronal M2 muscarinic receptor function suggested by the pilocarpine results was confirmed with data obtained with the M2 muscarinic receptor antagonist methoctramine. Methoctramine potentiated the response to EFS in diabetic rats while significantly inhibiting this response (due to effects on smooth muscle M3 muscarinic receptors) in control rats.
The increase in neuronal M2 muscarinic receptor function in the trachea of diabetic rats was accompanied by an increase in the function of post-junctional M3 muscarinic receptors. Thus the magnitudes of carbachol- and acetylcholine-induced contractions were significantly greater in trachea from diabetic rats than controls. Diabetes-induced increased M3 muscarinic receptor function is not seen in the whole lung (Belmonte et al., 1997) and may indicate that regulation of M3 muscarinic receptor function differs between the upper and lower airways. In the whole lung, bronchoconstriction reflects contraction of both the upper and lower airways. Hence an increase in M3 receptor function in the trachea may not be observed in vivo if M3 muscarinic receptor function in the lower airways is unchanged.
EFS-induced contraction was equal in control and diabetic rats despite greater M2 muscarinic receptor function in diabetic rats. This occurred because the decreased release of acetylcholine from parasympathetic nerves of diabetic rats was offset by increased smooth muscle contraction in response to stimulation of post-junctional M3 muscarinic receptors. The increased responsiveness of M3 muscarinic receptors occurred without an increase in agonist potency (−log EC50), suggesting that the change occurred beyond the receptor-agonist interaction, perhaps as the result of an alteration in M3 muscarinic receptor coupling to second messenger systems or in the contractile apparatus.
The increased sensitivity of M3 muscarinic receptors on smooth muscle may be an adaptive change in response to upregulation of the inhibitory M2 muscarinic receptors. Increased M2 muscarinic receptor function would result in decreased release of neurotransmitter, which might be equivalent to denervation. Cholinergic denervation has been shown in animal studies to increase M3 muscarinic receptor sensitivity in a variety of peripheral tissues including the urethra (Ekstrom & Malmberg, 1984), vas deferens (Hata et al., 1980) and urinary bladder (Mattiasson et al., 1984). Thus, M3 muscarinic receptor supersensitivity in diabetes, following increased M2 muscarinic receptor function, could occur by mechanisms similar to those that occur following cholinergic denervation.
Diabetes-induced increases in the function of both M2 muscarinic receptors in the airways in vivo (Belmonte et al., 1997, 1998) and M3 muscarinic receptors in the trachea in vitro (Mongold et al., 1988; Cros et al., 1992; Ozansoy et al., 1993) have been previously reported but the mechanisms underlying these changes remain to be elucidated. Normal M2 muscarinic receptor function can be restored by treatment with insulin (Belmonte et al., 1997) without normalization of blood glucose levels, suggesting that the increases in muscarinic receptor function in diabetes are due to insulin.
In the ileum, neuronal M2 muscarinic receptor function was investigated at two frequencies of nerve stimulation, 5 and 15 Hz. At 15 Hz, the frequency used to examine neuronal M2 muscarinic receptor function in the trachea, ileal contraction was maximal. This indicates that acetylcholine release from ileal parasympathetic nerves is maximal at 15 Hz. It was considered possible that in order to observe pilocarpine-induced inhibition or methoctramine-induced potentiation of acetylcholine release it would be necessary to examine them against a frequency that induced less acetylcholine release and, as a result, a smaller contractile response. The frequency selected to induce a submaximal response was 5 Hz. However it was found that the overall effects of pilocarpine and methoctramine were the same at both frequencies of stimulation.
Pilocarpine caused concentration-dependent inhibition of EFS-induced contraction, indicating that the neuronal M2 muscarinic receptors in the ileum are functional. This was confirmed with methoctramine. Methoctramine inhibited EFS-induced contraction significantly less than it inhibited carbachol-induced contraction. This showed that methoctramine potentiated acetylcholine release from the parasympathetic nerves of the ileum in addition to inhibiting post-junctional M3 muscarinic receptors. In diabetic rats, pilocarpine-induced inhibition of EFS-induced contraction was significantly greater than that seen in control rats, demonstrating that the M2 muscarinic receptors are hyperfunctional. This was confirmed with methoctramine data, which showed that methoctramine-induced potentiation of EFS-induced contraction was significantly greater in diabetic rats than controls.
Methoctramine was remarkably potent at inhibiting carbachol-induced contraction of the ileum, with concentrations of 10−9 M methoctramine inducing up to 30% inhibition of the carbachol contraction. This suggests that post-junctional M2 muscarinic receptors play a role in mediating carbachol-induced contraction of ileal smooth muscle. Receptor-binding studies have shown that the majority of muscarinic receptors on ileal smooth muscle are M2, with a smaller proportion of M3 receptors (Brunner, 1989; Lazareno & Roberts, 1989; Kurjak et al., 1999). Despite the preponderance of M2 receptors in the ileum, the contractile response to muscarinic agonists is largely due to M3 receptor activation. In the guinea-pig ileum, the exact role of post-junctional M2 receptors is still a matter of debate. Activation of M2 receptors in the ileum has no direct contractile response (Eglen & Harris, 1993), although there may be an indirect effect on contraction via inhibition of β-adrenoceptor-mediated relaxation (Reddy et al., 1995). In contrast, in rat ileum, muscarinic agonist-induced contraction is mediated by a heterogeneous population of receptors including both M2 and M3 (Elorriaga et al., 1996; Kurjak et al., 1999).
EFS-induced contraction of the ileum was frequency-dependent and maximal at 15 Hz. In contrast to the trachea, the magnitude of EFS-induced contraction of ileum from diabetic rats was less than that of controls (Figure 6). This reflects the decreased release of acetylcholine from parasympathetic nerves of the ileum, due to increased neuronal M2 muscarinic receptor function. There was no change in the function of the post-junctional M3 muscarinic receptor to compensate for the decreased acetylcholine release (Figure 8).
The increase in neuronal M2 muscarinic receptor function reported in our study may explain diabetes-induced decreases in cholinergic neurotransmission observed in other studies on the ileum (Nowak et al., 1986). Reduced cholinergic neurotransmission in the ileum of diabetic rats is probably not be due to neuropathy since histochemical studies of the ileum cannot demonstrate cholinergic nerve degeneration (Lincoln et al., 1984). Thus, decreased neurotransmission might be due to increased neuronal M2 muscarinic receptor function.
Increased M3 muscarinic receptor function has been reported in the ileum of rats three months after streptozotocin treatment (Carrier & Aronstam, 1990). It is possible that over time the ileum adapts to the reduced release of acetylcholine from parasympathetic nerves by increasing the responsiveness of the smooth muscle to acetylcholine. This would account for the restoration of cholinergic neurotransmission that has been reported to occur in the ileum three months after streptozotocin treatment (Nowak et al., 1990a).
In conclusion, the function of inhibitory neuronal M2 muscarinic receptors is increased in the airways and gastrointestinal tract of diabetic rats in vitro. The increase in M2 muscarinic receptor activity decreases acetylcholine release from parasympathetic nerves. In the trachea, smooth muscle contraction in response to nerve stimulation was maintained, despite decreased acetylcholine release, because M3 muscarinic receptor function was also increased. In the ileum, decreased acetylcholine release in diabetic rats was reflected in decreased smooth muscle contraction to nerve stimulation. In contrast to the trachea, there was no change in M3 muscarinic receptor function in the ileum. Increases in M2 muscarinic receptor function may contribute to the decreased autonomic function that occurs in the gastrointestinal system of diabetic patients and contributes to gastrointestinal dysmotility.
Acknowledgments
This work was funded by the National Institutes of Health grants HL-55543 (A.D. Fryer), HL-54659 (J.B. Jacoby), HL-61013 (D.B. Jacoby), HL-10342 (A.D. Fryer) and by a grant from the American Heart Association (A.D. Fryer).
Abbreviations
- EFS
electrical field stimulation
References
- AAS P., MACLAGAN J. Evidence for prejunctional M2 muscarinic receptors in pulmonary cholinergic nerves in the rat. Br. J. Pharmacol. 1990;101:73–76. doi: 10.1111/j.1476-5381.1990.tb12091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABRAHAMSON E.M. Asthma, diabetes mellitus and hyperinsulinism. J. Clin. Endocrinol. 1941;1:402–406. [Google Scholar]
- ARISON R.N., CIACCIO E.I., GLITZNER M.S., CASSARO J.A., PRUSS M.P. Light and electron microscopy of lesions in rats rendered diabetic with streptozotocin. Diabetes. 1967;16:51–56. doi: 10.2337/diab.16.1.51. [DOI] [PubMed] [Google Scholar]
- BELMONTE K.E., FRYER A.D., COSTELLO R.W. Role of insulin in antigen-induced airway eosinophilia and neuronal M2 muscarinic receptor dysfunction. J. Appl. Physiol. 1998;85:1708–1718. doi: 10.1152/jappl.1998.85.5.1708. [DOI] [PubMed] [Google Scholar]
- BELMONTE K.E., JACOBY D.B., FRYER A.D. Increased function of inhibitory neuronal M2 muscarinic receptors in diabetic rat lungs. Br. J. Pharmacol. 1997;121:1287–1294. doi: 10.1038/sj.bjp.0701274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUNNER F. Subclassification of atrial and intestinal muscarinic receptors of the rat: direct binding studies with agonists and antagonists. Br. J. Pharmacol. 1989;97:572–578. doi: 10.1111/j.1476-5381.1989.tb11987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARRIER G.O., ARONSTAM R.S. Increased muscarinic responsiveness and decreased muscarinic receptor content in ileal smooth muscle in diabetes. J. Pharmacol. Exp. Ther. 1990;254:445–449. [PubMed] [Google Scholar]
- CHANG F.Y., DOONG M.L., CHEN T.S., LEE S.D., WANG P.S. Altered intestinal transit is independent of gastroparesis in the early diabetic rats. Chin. J. Physiol. 1997;40:31–35. [PubMed] [Google Scholar]
- CROS G., GIES J-P., CAHARD D., COHEN P., FILIPEK B., MONGOLD J-J., SERRANO J-J. Impairment of contractility associated with muscarinic supersensitivity in trachea isolated from diabetic rats: lack of correlation with ultrastructural changes or quinuclidinyl benzylate binding to lung membranes. Mol. Cell. Biochem. 1992;109:181–183. doi: 10.1007/BF00229774. [DOI] [PubMed] [Google Scholar]
- D'AGOSTINO G., CHIARI M.C., GRANA E., SUBISSI A., KILBINGER H. Muscarinic inhibition of acetylcholine release from a novel in vitro preparation of the guinea-pig trachea. Naunyn Schmiedeberg's Arch. Pharmacol. 1990;342:141–145. doi: 10.1007/BF00166956. [DOI] [PubMed] [Google Scholar]
- DAMANN F., FUDER H., GIACHETTI A., GIRALDO E., KILBINGER H., MICHELETTI R. AF-DX 116 differentiates between prejunctional muscarine receptors located on noradrenergic and cholinergic nerves. Naunyn-Schmiedeberg's Arch. Pharmacol. 1989;339:268–271. doi: 10.1007/BF00173576. [DOI] [PubMed] [Google Scholar]
- DOOLEY C.P., EL NEWIHI H.M., ZEIDLER A., VALENZUELA J.E. Abnormalities of the migrating motor complex in diabetics with autonomic neuropathy and diarrhea. Scand. J. Gastroenterol. 1988;23:217–223. doi: 10.3109/00365528809103971. [DOI] [PubMed] [Google Scholar]
- EKSTROM J., MALMBERG L. On a cholinergic motor innervation of the rat urethra. Acta Physiol. Scand. 1984;120:237–242. doi: 10.1111/j.1748-1716.1984.tb00129.x. [DOI] [PubMed] [Google Scholar]
- ELORRIAGA M., ANSELMI E., HERNANDEZ J.M., D'OCON P., IVORRA D. The sources of Ca2+ for muscarinic receptor-induced contraction in the rat ileum. J. Pharm. Pharmacol. 1996;48:817–819. doi: 10.1111/j.2042-7158.1996.tb03980.x. [DOI] [PubMed] [Google Scholar]
- EURODIAB SUBSTUDY 2 STUDY GROUP Decreased prevalence of atopic diseases in children with diabetes. J. Pediatr. 2000;137:470–474. doi: 10.1067/mpd.2000.109109. [DOI] [PubMed] [Google Scholar]
- FRYER A.D., MACLAGAN J. Muscarinic inhibitory receptors in pulmonary parasympathetic nerves in the guinea-pig. Br. J. Pharmacol. 1984;83:973–978. doi: 10.1111/j.1476-5381.1984.tb16539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIRALDO E., MICHELETTI R, MONTAGNA E., GIACHETTI A., VIGANO M.A., LADINSKY H., MELCHIORRE C. Binding and functional characterization of the cardioselective muscarinic antagonist methoctramine. J. Pharmacol. Exp. Ther. 1988;244:1016–1020. [PubMed] [Google Scholar]
- HATA F., TAKEYASU K., MORIKAWA Y., LAI R.T., ISHIDA H., YOSHIDA H. Specific changes in the cholinergic system in guinea-pig vas deferens after denervation. J. Pharmacol. Exp. Ther. 1980;215:716–722. [PubMed] [Google Scholar]
- HELANDER E. Asthma and diabetes. Acta Med. Scand. 1958;162:165–174. doi: 10.1111/j.0954-6820.1958.tb01762.x. [DOI] [PubMed] [Google Scholar]
- HOROWITZ M., WISHART J.M., JONES K.L., HEBBARD G.S. Gastric emptying in diabetes: an overview. Diab. Med. 1996;13:S16–S22. [PubMed] [Google Scholar]
- KESHAVARZIAN A., IBER F.L. Intestinal transit in insulin-requiring diabetics. Am. J. Gastroenterol. 1986;81:257–260. [PubMed] [Google Scholar]
- KILBINGER H., SCHNEIDER R, SIEFKEN H., WOLF D., D'AGOSTINO G. Characterization of prejunctional muscarinic autoreceptors in the guinea-pig trachea. Br. J. Pharmacol. 1991;103:1757–1763. doi: 10.1111/j.1476-5381.1991.tb09859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURJAK M., SATTLER D., SCHUSDIAZZA V., ALLESCHER H.D. Characterization of prejunctional and postjunctional muscarinic receptors of the ascending reflex contraction in rat ileum. J. Pharmacol. Exp. Ther. 1999;290:893–900. [PubMed] [Google Scholar]
- LAZARENO S., ROBERTS F.F. Functional and binding studies with muscarinic M2-subtype antagonists. Br. J. Pharmacol. 1989;98:309–317. doi: 10.1111/j.1476-5381.1989.tb16896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINCOLN J., BOKOR J.T., CROWE R, GRIFFITH S.G., HAVEN A.J., BURNSTOCK G. Myenteric plexus in streptozotocin-treated rats. Neurochemical and histochemical evidence for diabetic neuropathy in the gut. Gastroenterol. 1984;86:654–661. [PubMed] [Google Scholar]
- MATTIASSON A., ANDERSSON K.E., SJOGREN C., SUNDIN T., UVELIUS B. Supersensitivity to carbachol in parasympathetically decentralized feline urinary bladder. J. Urol. 1984;131:562–565. doi: 10.1016/s0022-5347(17)50504-2. [DOI] [PubMed] [Google Scholar]
- MINETTE P.A., BARNES P.J. Prejunctional inhibitory muscarinic receptors on cholinergic nerves in human and guinea pig airways. J. Appl. Physiol. 1988;64:2632–2537. doi: 10.1152/jappl.1988.64.6.2532. [DOI] [PubMed] [Google Scholar]
- MONGOLD J.J., CROS G.H., MICHGEL A., MCNEILL J.H., SERRANO J.J. Diabetes-induced rat tracheal segment supersensitvity to carbachol. Can. J. Physiol. Pharmacol. 1988;66:660–662. doi: 10.1139/y88-103. [DOI] [PubMed] [Google Scholar]
- NOWAK T.V., HARRINGTON B., KALBFLEISCH J.H., AMATRUDA J.M. Evidence for abnormal cholinergic neuromuscular transmission in diabetic rat small intestine. Gastroenterol. 1986;91:124–132. doi: 10.1016/0016-5085(86)90448-8. [DOI] [PubMed] [Google Scholar]
- NOWAK T.V., HARRINGTON B., KALBFLEISCH J.H., AMATRUDA J.M. Adaptation of cholinergic enteric neuromuscular transmission in diabetic rat small intestine. Diabetes. 1990a;39:891–897. doi: 10.2337/diab.39.8.891. [DOI] [PubMed] [Google Scholar]
- NOWAK T.V., HARRINGTON B., WEISBRUCH J.P., KALBFLEISCH J.H. Structural and functional characteristics of muscle from diabetic rodent small intestine. Am. J. Physiol. 1990b;258:G690–G698. doi: 10.1152/ajpgi.1990.258.5.G690. [DOI] [PubMed] [Google Scholar]
- OZANSOY G., KARAU C., OZCELIKAY A.T. The effect of oral vandyl treatment on the reactivity of tracheal smooth muscle obtained from insulin-dependent diabetic rats. Gen. Pharmacol. 1993;24:115–119. doi: 10.1016/0306-3623(93)90020-x. [DOI] [PubMed] [Google Scholar]
- PARKMAN H.P., SCHWARTZ S.S. Esophagitis and gastroduodenal disorders associated with diabetic gastroparesis. Arch. Intern. Med. 1987;147:1477–1480. [PubMed] [Google Scholar]
- PATEL H.J., BARNES P.J., TAKAHASHI T., TADJKARIMI S., YACOUB M.H., BELVISI M.G. Evidence for prejunctional muscarinic autoreceptors in human and guinea pig trachea. Am. J. Resp. Crit. Care Med. 1995;152:872–878. doi: 10.1164/ajrccm.152.3.7663798. [DOI] [PubMed] [Google Scholar]
- REDDY H., WATSON N., FORD A.P.D.W., EGLEN R.M. Characterization of the interaction between muscarinic M2 receptors and β-adrenoceptor subtypes in guinea-pig isolated ileum. Br. J. Pharmacol. 1995;114:49–56. doi: 10.1111/j.1476-5381.1995.tb14904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUNDLES R.W. Diabetic neuropathy general review with report of 125 cases. Medicine. 1945;24:111–160. [Google Scholar]
- SCHMIDT R.E., NELSON J.S., JOHNSON E.M. Experimental diabetic autonomic neuropathy. Am. J. Pathol. 1981;103:210–225. [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT R.E., PLURAD S.B., MODERT C.W. Experimental diabetic neuropathy characterization in steptozotocin-diabetic Sprague-Dawley rats. Lab. Invest. 1983;49:538–552. [PubMed] [Google Scholar]
- SZCZEKLIK A., PIETON R., SIERADZKI J. Alteration in both insulin release and its hypoglycemic effects in atopic bronchial asthma. J. Allergy Clin. Immunol. 1980;66:424–427. doi: 10.1016/0091-6749(80)90123-2. [DOI] [PubMed] [Google Scholar]
- VIANNA E.O., GARCIA-LEME J. Allergen-induced airway inflammation in rats: role of insulin. Am. J. Crit. Care Med. 1995;151:809–815. doi: 10.1164/ajrccm/151.3_Pt_1.809. [DOI] [PubMed] [Google Scholar]
- YAGAHASHI S., SIMA A.A.F. Diabetic autonomic neuropathy in BB rat: ultrastructural and morphometric changes in parasympathetic nerves. Diabetes. 1986;35:733–743. doi: 10.2337/diab.35.7.733. [DOI] [PubMed] [Google Scholar]
- YOSHIDA M.M., SCHUFFLER M.D., SUMI S.M. There are no morphologic abnormalities of the gastric wall or abdominal vagues in patients with diabetic gastroparesis. Gastroenterol. 1988;94:907–914. doi: 10.1016/0016-5085(88)90546-x. [DOI] [PubMed] [Google Scholar]


